Abstract
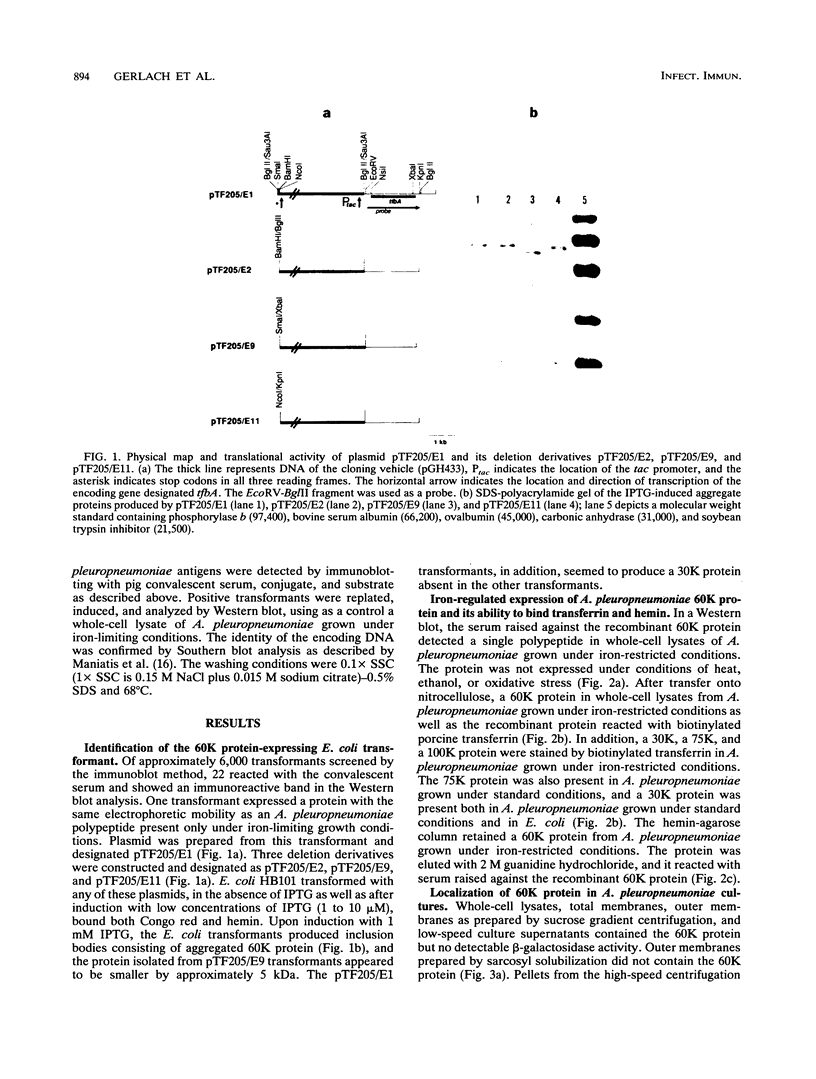
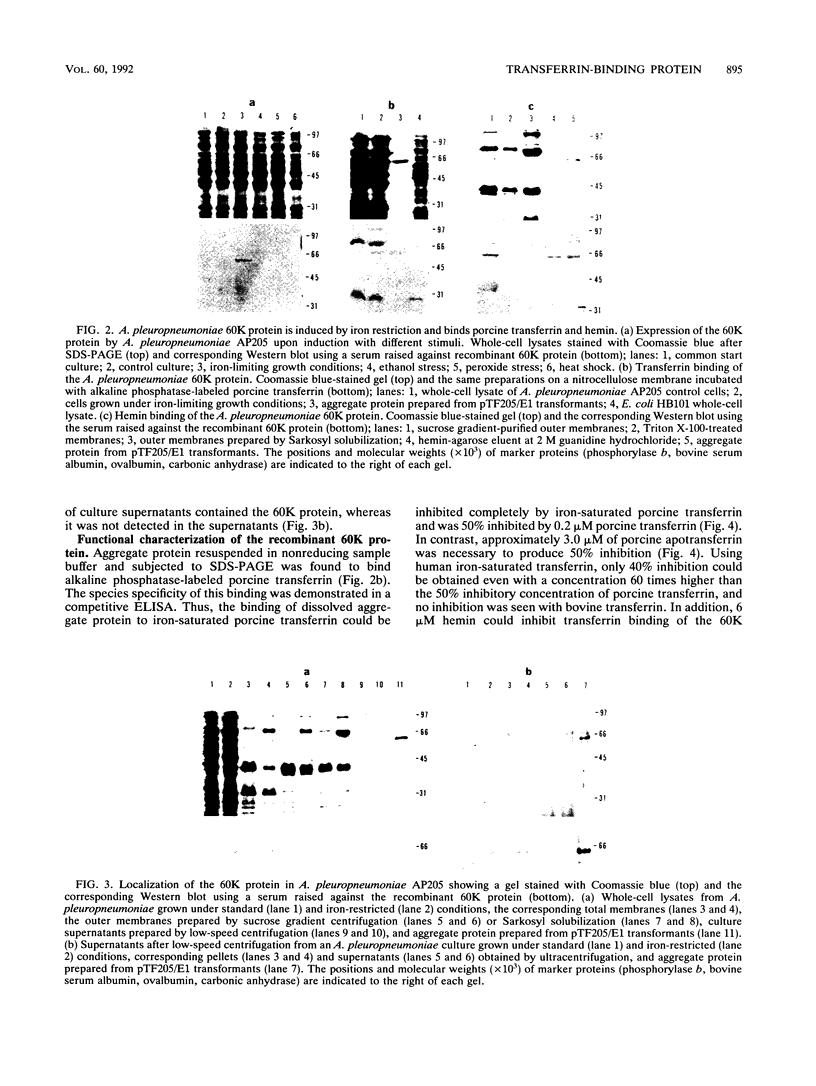
An expression library was constructed from Actinobacillus pleuropneumoniae serotype 7. Escherichia coli transformants expressing recombinant proteins were identified by immunoscreening with porcine convalescent serum. One transformant expressing a 60-kDa protein (60K protein) in aggregated form was identified. Serum raised against the recombinant protein recognized a polypeptide with an indistinguishable electrophoretic mobility in the A. pleuropneumoniae wild type after iron-restricted growth only. The recombinant protein bound transferrin after blotting onto nitrocellulose. Using a competitive enzyme-linked immunosorbent assay (ELISA), the specificity of this binding for the amino-terminal half of iron-saturated porcine transferrin was established. Also, the 60K wild-type protein bound hemin as assessed by hemin-agarose chromatography. Hemin could inhibit transferrin binding of the recombinant protein in the competitive ELISA, whereas hemoglobin and synthetic iron chelators failed to do so. Southern blot analysis of several other A. pleuropneumoniae strains indicated that highly homologous sequence is present in eight of eight isolates of serotype 7 and in some isolates of serotypes 2, 3, and 4.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C., Potter A. A., Gerlach G. F. Isolation and molecular characterization of spontaneously occurring cytolysin-negative mutants of Actinobacillus pleuropneumoniae serotype 7. Infect Immun. 1991 Nov;59(11):4110–4116. doi: 10.1128/iai.59.11.4110-4116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald F. S., DeVoe I. W. Iron acquisition by Neisseria meningitidis in vitro. Infect Immun. 1980 Feb;27(2):322–334. doi: 10.1128/iai.27.2.322-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee-Bhatnagar N., Frasch C. E. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect Immun. 1990 Sep;58(9):2875–2881. doi: 10.1128/iai.58.9.2875-2881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward D. W., Garon C. F., Judd R. C. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989 May;171(5):2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Hansen E. J. Molecular cloning, partial purification, and characterization of a haemin-binding lipoprotein from Haemophilus influenzae type b. Mol Microbiol. 1991 Feb;5(2):267–278. doi: 10.1111/j.1365-2958.1991.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Sparling P. F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981 Oct;34(1):120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lineback-Zins J., Brew K. Preparation and characterization of an NH2-terminal fragment of human serum transferrin containing a single iron-binding site. J Biol Chem. 1980 Jan 25;255(2):708–713. [PubMed] [Google Scholar]
- Mazurier J., Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta. 1980 May 7;629(2):399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Morton D. J., Williams P. Siderophore-independent acquisition of transferrin-bound iron by Haemophilus influenzae type b. J Gen Microbiol. 1990 May;136(5):927–933. doi: 10.1099/00221287-136-5-927. [DOI] [PubMed] [Google Scholar]
- Ogunnariwo J. A., Schryvers A. B. Iron acquisition in Pasteurella haemolytica: expression and identification of a bovine-specific transferrin receptor. Infect Immun. 1990 Jul;58(7):2091–2097. doi: 10.1128/iai.58.7.2091-2097.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A. D., Saunders J. R., Sebunya T. K., Willson P., Green G. H. A simple aerosol chamber for experimental reproduction of respiratory disease in pigs and other species. Can J Comp Med. 1985 Oct;49(4):434–435. [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J., Dyer D. W., Sparling P. F. Loss of transferrin receptor activity in Neisseria meningitidis correlates with inability to use transferrin as an iron source. Infect Immun. 1988 Dec;56(12):3132–3138. doi: 10.1128/iai.56.12.3132-3138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]