Abstract
The inner ear contains the developmentally related cochlea and peripheral vestibular labyrinth. Given the similar physiology between these two organs, hearing loss and vestibular dysfunction may be expected to occur simultaneously in individuals segregating mutations in inner ear genes. Twenty-two different genes have been discovered that when mutated lead to non-syndromic autosomal dominant hearing loss. A review of the literature indicates that families segregating mutations in 13 of these 22 genes have undergone formal clinical vestibular testing. Formal assessment revealed vestibular dysfunction in families with mutations in ten of these 13 genes. Remarkably, only families with mutations in the COCH and MYO7A genes self-report considerable vestibular challenges. Families segregating mutations in the other eight genes do not self-report significant balance problems and appear to compensate well in everyday life for vestibular deficits discovered during formal clinical vestibular assessment. An example of a family (referred to as the HL1 family) with progressive hearing loss and clinically-detected vestibular hypofunction that does not report vestibular symptoms is described in this review. Notably, one member of the HL1 family with clinically-detected vestibular hypofunction reached the summit of Mount Kilimanjaro.
Keywords: Non-sydromic deafness (DFN), calorics, ocular motor, vestibulo-ocular reflex, velocity step test, cervico-ocular reflex, computerized dynamic posturography, vestibular evoked myogenic potential
1. Introduction
Given the common embryonic origins and biology of the auditory and vestibular systems within the inner ear, it might be anticipated that single gene mutations known to cause inherited hearing loss would also lead to vestibular dysfunction. Hearing loss is classified as either syndromic or non-syndromic. When hearing loss is coupled with diagnoses affecting body systems other than the inner ear (e.g. diabetes, retinitis pigmentosa, heart arrhythmias) the hearing impairment is considered syndromic. More commonly, hearing loss is found as a single entity and therefore referred to as non-syndromic.
Genes underlying non-syndromic deafness (DFN) can be inherited in an autosomal dominant (DFNA), autosomal recessive (DFNB), or X-linked (DFN) manner. A number following the DFNA, DFNB, or DFN designation indicates the order in which the genetic locus was discovered (e.g. DFNA1, DFNA2) and each locus refers to a specific chromosomal location. Non-syndromic hearing loss can also be inherited maternally due to mitochondrial mutations. The vast majority of deafness genes have been discovered by collaborating with large hearing impaired human pedigrees where the inheritance pattern of the hearing loss in the family is compared to the segregation pattern of genetic markers in DNA from these same individuals. Twenty-two different genes have been discovered that lead to non-syndromic autosomal dominant hearing loss.
In this reveiw, we describe a human pedigree (the HL1 family) segregating non-syndromic autosomal dominant sensorineural hearing loss. Clinical testing in the HL1 family revealed vestibular hypofunction, however the family appears to have adapted well to this lack of vestibular information from their inner ears. In an effort to interpret the HL1 vestibular findings in the context of other DFNA pedigrees we conducted a review of the DFNA vestibular literature.
2. The HL1 family
2.1 Autosomal dominant inheritance of progressive hearing loss
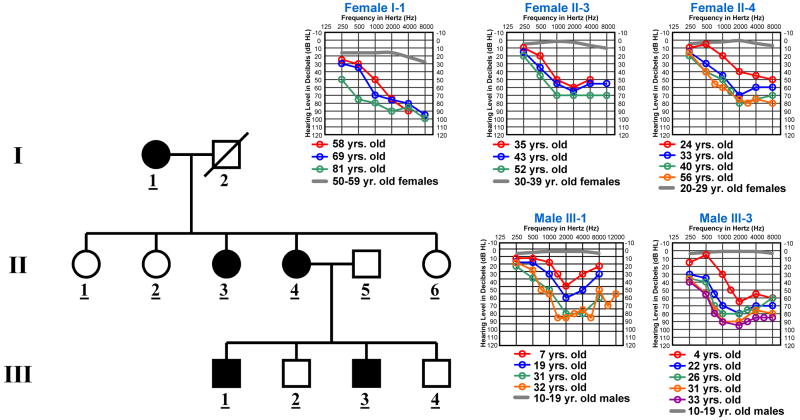
The HL1 family is an American pedigree of Irish decent (Fig. 1). Written informed consent was obtained from the HL1 family members for all study procedures under a protocol approved by the Institutional Review Board of the University of Washington. Linkage to the X-chromosome was excluded by genetic marker analysis as male-to-male transmission was not observed in the pedigree. We also analyzed the segregation pattern of genetic markers tightly flanking the COCH (D14S1021-COCH-D14S54) and MYO7A (D11S1314-MYO7A-D11S937) genes in the HL1 family. These markers did not co-segregate with hearing loss in the HL1 family. Mitochondrial inheritance from the mother can not be ruled out as father-to-child transmission has not been observed in the HL1 pedigree. However, the HL1 family members do not have mutations in the two mitochondrial genes, 12S rRNA (bp 648-1601) and tRNASer (UCN) (bp 7446-7516), known to harbor alterations associated with non-syndromic hearing impairment. In addition, the auditory findings in the HL1 family are not consistent with mitochondrial inheritance which is correlated generally with a variable phenotype due to heteroplasmy. Therefore, the auditory phenotype in the HL1 family most likely segregates as an autosomal dominant trait. The gene mutation in the HL1 family has not yet been discovered. Affected HL1 family members demonstrate progressive hearing loss with a tendency for a notch at 2000 Hz (Fig. 1). All molecular genetic and auditory analyses were conducted as described previously [29].
Fig. 1.
Audiologic and haplotype characterization of the HL1 Pedigree. (A) Each individual in the pedigree is assigned a number by generation. Underlined numbers indicate that auditory evaluations were performed for that person. Affected individuals are denoted by blackened symbols, males are denoted by squares, females are denoted by circles, and deceased persons are indicated by a diagonal line through the symbol. Symmetrical hearing loss was detected in all affected HL1 family members and therefore only right ear threshold values are plotted on the audiogram. Frequency in Hertz (Hz) is plotted on the x-axis and hearing level in decibels (dB HL) on the y-axis. Plotted on each audiogram (gray line) are the average pure-tone air conduction thresholds for a person with normal hearing matched in age to the earliest audiogram collected for the family member.
2.2 Vestibular hypofunction
Based on the hearing and balance questionnaires and discussions with the HL1 family members, complaints of significant vestibular problems were not elicited. To determine if clinical vestibular problems could be detected, testing was conducted with two HL1 family members that both demonstrated hearing loss, female II-4 (at the age of 56 years) and her son, male III-3 (at the age of 32 years). Vestibular test parameters and normal values have been described previously [29]. Oculomotor and caloric test results are not available for female II-4. Oculomotor testing was normal for male III-3.
Computerized dynamic posturography
Sensory organization and motor control testing
Male III-3 generated an overall normal sensory organization test (SOT) composite score of 84. During the test, the COG (center of gravity) alignment indicated that male III-3 put more weight on his left than right foot. This uneven weight distribution may have impacted the motor control test (MCT) results which scored prolonged latencies on the backward translations on the right side. Female II-4 gave an overall abnormal SOT composite score of 65 with her poorest performance under platform condition 6. Her COG alignment favored weight to her left foot, but not to the extent of her son. On the MCT, a prolonged latency was scored on the backward translation on the left side.
Rotational testing
Velocity Step Test
For the velocity step test, the mother and son pair displayed abnormal gain and response time constants under almost all conditions (Table 1).
Table 1.
Velocity Step Test
| Subject | Velocity Step Test
|
|||||||
|---|---|---|---|---|---|---|---|---|
| CW
|
CW stop
|
CCW
|
CCW stop
|
|||||
| Gain | RTC (sec) | Gain | RTC (sec) | Gain | RTC (sec) | Gain | RTC (sec) | |
| II-4 | 0.41 | 6* | 0.35* | 8* | 0.17* | 5* | 0.26* | 3* |
| III-3 | 0.22* | 7* | 0.22* | 3* | 0.26* | 4* | 0.28* | 8* |
indicates abnormal response
Normal gain (>0.40), normal RTC (≥10 sec)
CW, clockwise
CCW, counter clockwise
RTC, response time constant
Sinusoidal oscillation test
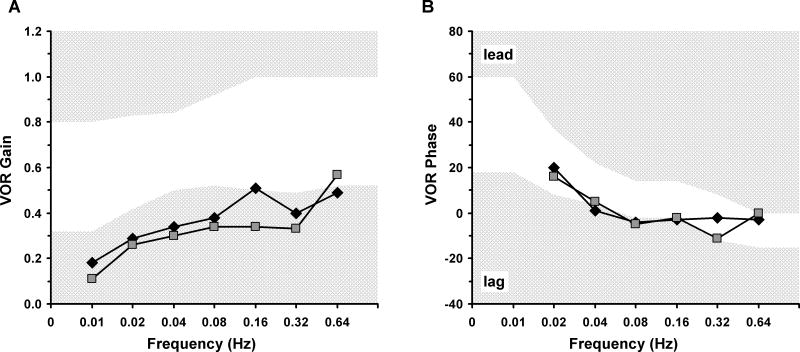
During the oscillation test, male III-3 demonstrated abnormal gains across all frequencies (Fig. 2A) with a phase angle lag at 0.04 and 0.08 Hz (Fig. 2B). Female II-4 displayed abnormal gains across all frequencies except 0.64 Hz (Fig. 2A) and a phase lag at 0.08 Hz (Fig. 2B).
Fig. 2.
Sinusoidal Oscillation Test Results. The mother (II-4) and son (III-3) are plotted with a square and diamond, respectively. For both graphs (A, B) the frequency in Hertz (Hz) is shown on the x-axis. For both graphs the abnormal response range defined by cutting scores (2SD) is denoted by hatched light gray regions. (A) The gain (peak eye velocity/peak head velocity) is plotted on the y-axis. (B) The phase relationship between chair stimulus and eye response is plotted on the y-axis. An abnormal phase angle would be a lag or lead in degrees between eye velocity and chair velocity. VOR = vestibule-ocular reflex.
Caloric testing
Caloric testing in male III-3 detected a 30% unilateral weakness in the right ear indicating that male III-3 is receiving more vestibular information from his left inner ear than his right.
3. Vestibular literature review for known DFNA gene mutations
To appreciate how often vestibular symptoms and/or abnormal findings from formal clinical vestibular assessment are reported for families with mutations in known DFNA genes, we conducted a review of the DFNA literature.
3.1 Compensation for vestibular dysfunction in families with DFNA mutations
Families with alterations in 13 of the 22 DFNA genes had undergone formal clinical vestibular testing (Table 2) consisting of one or more of the following evaluations: calorics, ocular motor, vestibulo-ocular reflex (VOR) in most cases by the rotary chair velocity step test, cervico-ocular reflex (COR), computerized dynamic posturography (CDP), and vestibular evoked myogenic potential (VEMP). Caloric testing was the most common type of vestibular assessment in these families. Formal vestibular testing yielded abnormal results in at least some family members for 10 of these 13 DFNA genes. For two of these 10 genes, COCH and MYO7A, abnormal clinical vestibular tests manifested as vestibular symptoms in at least some family members. This is particularly evident in families with COCH mutations where Meniere-like features are not unusual (Table 2). Therefore, genetic screening of COCH and MYO7A is clearly indicated in families with abnormal clinical vestibular test results. Families segregating mutations in the other eight DFNA genes yield at yeast some abnormal clinical vestibular test results, but these family members either do not complain of vestibular problems or the problems are considered mild and may also be found in a random sample of individuals. In most cases, family members affected by a DFNA mutation seem to compensate for their clinically-detected vestibular loss fairly well in everyday life.
Table 2.
Vestibular Findings in Families with Known DFNA Gene Mutations
| Locus/Gene | Reference | Mutation | Testing Method & # of Individuals Tested | Findings | Author Conclusion/Comment |
|---|---|---|---|---|---|
| CRYM | 1 | p.K314T, p.X315Y | Caloric on 1 affected person with the X315Y mutation. | Normal caloric. | No vestibular involvement for all affected individuals. |
| ESPN | 11 | p.S719R
p.D744N p.delK848 |
Routine vestibular tests. 1–2 individuals tested per mutation. | No vestibular involvement. | No vestibular involvement. |
| DFNA1, DIAPH1 | 20 | Splice donor, gt-to-gg, FS | Caloric, VAT, & oculomotor. 3 affected individuals tested. | All normal, except 1 person with unilateral caloric weakness. | None of the affected members report vestibular disturbances. |
| DFNA2, KCNQ4 | 21 | p.W276S | Caloric, VST, & oculomotor.
37 affected individuals tested. |
3 of 37 showed unilateral caloric weakness.
13 of 37 showed VOR hyperactivity, 4 of these 13 reported motion sickness, 1 of these 13 reported transient vertigo. 1 of the other 37 also reported transient vertigo. Oculomotor normal except for 1 of 37. |
Vestibular system was functional throughout life of these patients. No bilateral caloric weakness. VOR hyperactivity is puzzling, may be a central abnormality. |
| 2 | p.W276S | Caloric.
5 affected individuals tested. |
1 of 5 showed unilateral caloric weakness.
2 of 5 reported vertigo. 1 of 5 reported an epilepsy episode. |
Further discussion needed to reach a conclusion as whether epilepsy is component of KCNQ4 mutation. | |
| 7 | p.W276S | Caloric & VST.
11 affected individuals tested. |
3 of 11 showed VOR hyperactivity, 2 of 3 reported motion sickness. 2 of 11 showed VOR hypoactivity but had normal calorics. | VOR hyperactivity may be explained by release from central inhibition. In mice, KCNQ4 is expressed in vestibular nuclei. | |
| DFNA3, GJB2 | 31 | 5 different mutations | Caloric & VEMP.
1 person w/each mutation tested |
Normal calorics, 3 of 5 with absent VEMP, 1 of these 3 reported unsteadiness. | In most cases, saccular defect is well compensated for by patient. |
| 22 | T55N | Caloric & VEMP.
6 affected individuals tested. |
5 of 6 with abnormal caloric.
4 of 6 with abnormal VEMP. |
Vestibular dysfunction associated with Cx26 mutation for first time. | |
| DFNA4, MYH14 | 23 | p.S120L | Caloric.
5 generation family. |
Normal caloric findings. | Normal caloric findings. |
| 10 | p.G376C, p.R726S, p.L976F | Do not indicate testing method.
Individuals from 3 different families. |
Hearing loss without vestibular involvement. | Hearing loss without vestibular involvement. | |
| DFNA5 | 8 | Exon 8 skip, premat. stop | Caloric & VST.
4 affected individuals tested. |
Response parameter values of 4 patients were normal w/few exceptions. | No vestibular symptoms. |
| 3 | Exon 8 skip, premat. stop | VOR.
6 affected individuals tested. |
Vestibular symptoms not self reported.
1 of 6 with hyperactive VOR. |
Vestibular function was generally normal. | |
| DFNA6/14, WFS1 | 13 | p.L829P | Caloric & oculomotor.
4 generation family. |
Oculomotor & caloric tests did not show any consistent deficits. | No significant abnormalities. |
| 19 | p.T699M | Caloric, VST, & oculomotor.
20 affected family members. |
19 of 20 w/normal oculomotor, 15 of 17 w/normal caloric, 1 of 15 w/VOR hyporeflexia, 6 of 15 showed VOR hyperreflexia. | With few exceptions, vestibular function was intact. | |
| 27 | p.G674V | Caloric, VST, & oculomotor.
2 affected family members. |
Normal oculomotor and vestibular responses. | No substantial vestibular symptoms. | |
| 32 | p.T699M | Caloric & oculomotor.
4 affected individuals tested. |
Normal oculomotor and caloric responses. | Normal vestibular responses. | |
| DFNA8/12, TECTA | 14 | p.R2021H | Caloric.
4 affected individuals tested. |
All affected members had normal vestibular function. | All affected members had normal vestibular function. |
| 28 | p.R1890C, p.T83M | VST & oculomotor.
4 affected individuals tested. |
Intact horizontal VOR, 2 people w/long time constant. Normal oculomotor.
No vestibular symptoms. |
Given TECTA is expressed in otolith membrane, otolith-testing paradigms should be considered. | |
| DFNA9, COCH | 6 | p.P51S | Do not indicate testing method.
6 affected individuals tested. |
Vestibular tests disclosed complete areflexia in 5 individuals & severe hyporeflexia in 1. | Patients experienced instability esp in dark & head movement-dependent oscillopsia. |
| 17 | p.V66G | Caloric, 3 affected individuals. | 2 with absent bithermal caloric responses.
1 with unilaterally absent caloric responses. |
Vestibular complaints. | |
| 33 | p.A119T | Caloric, 1 affected individual. | Bilateral decreased response. | Recurrent dizziness & vertigo. | |
| 24 | p.V104del | Do not indicate testing method.
1 affected individual. |
Total cochleovestibular areflexia in both ears. | Patient reports severe vertigo, nausea, & vomiting. | |
| 15 | p.G88E | Caloric, oculomotor, VST.
10 affected individuals. |
2 showed vestibular areflexia, 1 showed bilateral caloric weakness, 1 showed asymmetric responses to caloric testing. | Instability especially in dark, vertigo, & tendency to fall. | |
| 29 | p.C542F | Caloric, oculomotor, VST, SOT, VEMP, CDP
3 affected individuals tested. |
Central oculomotor dysfunction, progressive vestibular hypofunction, absent VEMP responses. | Vestibular symptoms not noticed until after the 4th decade of life. | |
| 25 | p.G87W | Caloric, oculomotor, VST.
12 affected individuals. |
2 of 10 affected individuals showed vestibular areflexia. | Instability especially in dark, vertigo, & tendency to fall. | |
| 26 | p.I109T | Caloric, oculomotor, VST.
7 affected individuals. |
1 of 7 showed complete vestibular areflexia. | Instability especially in dark, vertigo, & tendency to fall. | |
| DFNA11, MYO7A | 30 | del p.A886, K887, K888 | Caloric & spontaneous nystagmus.
5 affected individuals tested. |
3 of 5 showed caloric hyporeflexia.
3 of 5 showed spontaneous nystagmus. |
No vestibular symptoms. |
| 5 | p.R853C | Do not indicate testing method.
5 affected individuals tested. |
Mild vestibular dysfunction found in 1 of 5 patients. | Mild vestibular dysfunction found in 1 of 5 patients. | |
| 4 | N458I | Caloric & VST.
6 affected individuals tested. |
Vestibular symptoms and dysfunction reported & found in 4 of 6 patients. | Vestibular dysfunction not fully penetrant & likely progressive. | |
| 9 | p.A230V | Do not indicate testing method.
3 affected individuals tested. |
Vestibular bilateral areflexia in all 3 patients. | Minor vestibular problems. | |
| DFNA13, COL11A2 | 18 | p.G323E | Caloric & VST.
17 affected individuals tested. |
Abnormal caloric in 8 of 17, 4 of 17 w/VOR hyperactivity, all w/normal oculomotor. | No substantial vestibular impairment symptoms. |
| DFNA20/26, ACTG1 | 12 | p.T89I | Interview & neurologic exam. | No evidence of vestibular dysfunction was observed during neurologic exam. | Neither proband nor relatives report vestibular problems. |
| 16 | p.T278I | Caloric, VST, oculomotor, COR.
7 affected individuals. |
Normal calorics, 2 people with VOR hyporeflexia, 1 with enhanced COR. | No or very limited vestibular involvement. |
FS, frameshift; VAT, vestibular autorotation; VST, vestibular step test; VOR, vestibulo-ocular reflex; VEMP, vestibular evoked myogenic potential; Cx26; connexin-26; Premat., premature; SOT, sinusoidal oscillation test; CDP, computerized dynamic posturography; COR, cervico-ocular reflex
3.2 Notes regarding Table 2
A few points of clarification regarding Table 2 follow. The article reference indicates where the vestibular data was presented, not necessarily where mutation findings were first documented for the family. The “affected individual” notation in Table 2 refers to the finding of hearing loss, but not necessarily vestibular dysfunction in those individuals. In most reports, family members with normal hearing are not included in the vestibular testing. If an article could not be located describing clinical vestibular testing, the cloned DFNA locus was not listed in Table 2, for example DFNA genes GJB3 (DFNA2), GJB6 (DFNA3), EYA4 (DFNA10), POU4F3 (DFNA15), MYH9 (DFNA17), MYO6 (DFNA22), TFCP2L3 (DFNA28), TMC1 (DFNA36), and MYO1A (DFNA48). The two DFNA5 mutations leading to skipping of exon 8 are different genomic alterations. The three p.W276S KCNQ4 mutations are thought to be from unrelated families as is the case for the two p.T669M WFS1 alterations. The ESPN gene study [11], indicated that routine vestibular tests were conducted including one or more of the following tests: caloric, rotatory, optokinetic, swinging torsion, statokinesimetric, and vestibulo-vegetative. Some of the abnormal vestibular findings may be specific to a particular mutation within the DFNA gene. The DFNA9 family segregating the p.C542F cochlin alteration demonstrated abnormal central oculomotor test results, suggesting the need for a study addressing cochlin expression in the human central nervous system.
4. Summary
As seen in the HL1 family, the DFNA literature review indicates that in most cases vestibular symptoms are not a major complaint of families with mutations in the known DFNA genes even if a vestibular loss is detected in these individuals through formal clinical evaluation. Therefore, the lack of self-reported vestibular symptoms may not accurately convey the amount of vestibular information contributed by the inner ear to families segregating DFNA mutations. The HL1 family members do not self-report vestibular problems perhaps because they have adapted to a lack of vestibular information from their inner ears from an early age. Female II-4 and male III-3 appear to compensate well for their clinically-detected vestibular loss in every-day life. This is evident particularly in male III-3 who demonstrated vestibular hypofunction with caloric and rotary chair testing, but generated normal SOT scores on the balance platform and during the same year as these vestibular evaluations reached the summit of Mount Kilimanjaro.
Acknowledgments
We are grateful to the HL1 family for their cooperation. The HL1 family data presented in this review has not been published previously. This work was funded by NIH grants DC04945 (V.A.S), DC006901 (V.A.S.) and P30 DC04661 (V. M. Bloedel Core).
References
- 1.Abe S, Katagiri T, Saito-Hisaminato A, Usami S, Inoue Y, Tsunoda T, Nakamura Y. Identification of CRYM as a candidate responsible for nonsyndromic deafness, through cDNA microarray analysis of human cochlear and vestibular tissues. Am J Hum Genet. 2003;72:73–82. doi: 10.1086/345398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akita J, Abe S, Shinkawa H, Kimberling WJ, Usami S. Clinical and genetic features of nonsyndromic autosomal dominant sensorineural hearing loss: KCNQ4 is a gene responsible in Japanese. J Hum Genet. 2001;46:355–361. doi: 10.1007/s100380170053. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff AM, Luijendijk MW, Huygen PL, van Duijnhoven G, DeLeenheer EM, Oudesluijs GG, Van Laer L, Cremers FP, Cremers CW, Kremer H. A novel mutation identified in the DFNA5 gene in a Dutch family: a clinical and genetic evaluation. Audiol Neurootol. 2004;9:34–46. doi: 10.1159/000074185. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff AM, Pennings RJ, Huygen PL, Luijendijk MW, Van Wijk E, Cruysberg JR, Kremer H, Cremers CW. Cochleovestibular and ocular features in a Dutch DFNA11 family. Otol Neurotol. 2006;27:323–331. doi: 10.1097/00129492-200604000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bolz H, Bolz SS, Schade G, Kothe C, Mohrmann G, Hess M, Gal A. Impaired calmodulin binding of myosin-7A causes autosomal dominant hearing loss (DFNA11) Hum Mutat. 2004;24:274–275. doi: 10.1002/humu.9272. [DOI] [PubMed] [Google Scholar]
- 6.de Kok YJ, Bom SJ, Brunt TM, Kemperman MH, van Beusekom E, van der Velde-Visser SD, Robertson NG, Morton CC, Huygen PL, Verhagen WI, Brunner HG, Cremers CW, Cremers FP. A Pro51Ser mutation in the COCH gene is associated with late onset autosomal dominant progressive sensorineural hearing loss with vestibular defects. Hum Mol Genet. 1999;8:361–366. doi: 10.1093/hmg/8.2.361. [DOI] [PubMed] [Google Scholar]
- 7.DeLeenheer EM, Huygen PL, Coucke PJ, Admiraal RJ, van Camp G, Cremers CW. Longitudinal and cross-sectional phenotype analysis in a new, large DFNA2/KCNQ4 family. Ann Otol Rhinol Laryngol. 2002;111:267–274. doi: 10.1177/000348940211100312. [DOI] [PubMed] [Google Scholar]
- 8.DeLeenheer EM, van Zuijlen DA, Van Laer L, Van Camp G, Huygen PL, Huizing EH, Cremers CW. Clinical features of DFNA5. Adv Otorhinolaryngol. 2002;61:53–59. doi: 10.1159/000066800. [DOI] [PubMed] [Google Scholar]
- 9.Di Leva F, D’Adamo P, Cubellis MV, D’Eustacchio A, Errichiello M, Saulino C, Auletta G, Giannini P, Donaudy F, Ciccodicola A, Gasparini P, Franze A, Marciano E. Identification of a novel mutation in the myosin VIIA motor domain in a family with autosomal dominant hearing loss (DFNA11) Audiol Neurootol. 2006;11:157–164. doi: 10.1159/000091199. [DOI] [PubMed] [Google Scholar]
- 10.Donaudy F, Snoeckx R, Pfister M, Zenner HP, Blin N, DiStazio M, Ferrara A, Lanzara C, Ficarella R, Declau F, Pusch CM, Nurnberg P, Melchionda S, Zelante L, Ballana E, Estivill X, Van Camp G, Gasparini P, Savoia A. Nonmuscle myosin heavy-chain gene MYH14 is expressed in cochlea and mutated in patients affected by autosomal dominant hearing impairment (DFNA4) Am J Hum Genet. 2004;74:770–776. doi: 10.1086/383285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donaudy F, Zheng L, Ficarella R, Ballana E, Carella M, Melchionda S, Estivill X, Bartles JR, Gasparini P. Espin gene (ESPN) mutations associated with autosomal dominant hearing loss cause defects in microvillar elongation or organisation. J Med Genet. 2006;43:157–161. doi: 10.1136/jmg.2005.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elfenbein JL, Fisher RA, Wei S, Morell RJ, Stewart C, Friedman TB, Friderici K. Audiologic aspects of the search for DFNA20: a gene causing late-onset, progressive, sensorineural hearing loss. Ear Hear. 2001;22:279–288. doi: 10.1097/00003446-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 13.T.V.U.H.D.S. Group. Dominantly inherited low-frequency hearing loss. Arch Otolaryngol. 1968;88:242–250. [PubMed] [Google Scholar]
- 14.Iwasaki S, Harada D, Usami S, Nagura M, Takeshita T, Hoshino T. Association of clinical features with mutation of TECTA in a family with autosomal dominant hearing loss. Arch Otolaryngol Head Neck Surg. 2002;128:913–917. doi: 10.1001/archotol.128.8.913. [DOI] [PubMed] [Google Scholar]
- 15.Kemperman MH, De Leenheer EM, Huygen PL, van Duijnhoven G, Morton CC, Robertson NG, Cremers FP, Kremer H, Cremers CW. Audiometric, vestibular, and genetic aspects of a DFNA9 family with a G88E COCH mutation. Otol Neurotol. 2005;26:926–933. doi: 10.1097/01.mao.0000185062.12458.87. [DOI] [PubMed] [Google Scholar]
- 16.Kemperman MH, DeLeenheer EM, Huygen PL, van Wijk E, van Duijnhoven G, Cremers FP, Kremer H, Cremers CW. A Dutch family with hearing loss linked to the DFNA20/26 locus: longitudinal analysis of hearing impairment. Arch Otolaryngol Head Neck Surg. 2004;130:281–288. doi: 10.1001/archotol.130.3.281. [DOI] [PubMed] [Google Scholar]
- 17.Khetarpal U. DFNA9 is a progressive audiovestibular dysfunction with a microfibrillar deposit in the inner ear. Laryngoscope. 2000;110:1379–1384. doi: 10.1097/00005537-200008000-00030. [DOI] [PubMed] [Google Scholar]
- 18.Kunst H, Huybrechts C, Marres H, Huygen P, Van Camp G, Cremers C. The phenotype of DFNA13/COL11A2: nonsyndromic autosomal dominant mid-frequency and high-frequency sensorineural hearing impairment. Am J Otol. 2000;21:181–187. doi: 10.1016/s0196-0709(00)80006-x. [DOI] [PubMed] [Google Scholar]
- 19.Kunst H, Marres H, Huygen P, Van Camp G, Joosten F, Cremers C. Autosomal dominant non-syndromal low-frequency sensorineural hearing impairment linked to chromosome 4p16 (DFNA14): statistical analysis of hearing threshold in relation to age and evaluation of vestibulo-ocular functions. Audiology. 1999;38:165–173. doi: 10.3109/00206099909073018. [DOI] [PubMed] [Google Scholar]
- 20.Lalwani AK, Jackler RK, Sweetow RW, Lynch ED, Raventos H, Morrow J, King MC, Leon PE. Further characterization of the DFNA1 audiovestibular phenotype. Arch Otolaryngol Head Neck Surg. 1998;124:699–702. doi: 10.1001/archotol.124.6.699. [DOI] [PubMed] [Google Scholar]
- 21.Marres H, van Ewijk M, Huygen P, Kunst H, van Camp G, Coucke P, Willems P, Cremers C. Inherited nonsyndromic hearing loss. An audiovestibular study in a large family with autosomal dominant progressive hearing loss related to DFNA2. Arch Otolaryngol Head Neck Surg. 1997;123:573–577. doi: 10.1001/archotol.1997.01900060015002. [DOI] [PubMed] [Google Scholar]
- 22.Melchionda S, Bicego M, Marciano E, Franze A, Morgutti M, Bortone G, Zelante L, Carella M, D’Andrea P. Functional characterization of a novel Cx26 (T55N) mutation associated to non-syndromic hearing loss. Biochem Biophys Res Commun. 2005;337:799–805. doi: 10.1016/j.bbrc.2005.09.116. [DOI] [PubMed] [Google Scholar]
- 23.Mirghomizadeh F, Bardtke B, Devoto M, Pfister M, Oeken J, Konig E, Vitale E, Riccio A, DeRienzo A, Zenner HP, Blin N. Second family with hearing impairment linked to 19q13 and refined DFNA4 localisation. Eur J Hum Genet. 2002;10:95–99. doi: 10.1038/sj.ejhg.5200769. [DOI] [PubMed] [Google Scholar]
- 24.Nagy I, Horvath M, Trexler M, Repassy G, Patthy L. A novel COCH mutation, V104del, impairs folding of the LCCL domain of cochlin and causes progressive hearing loss. J Med Genet. 2004;41:e9. doi: 10.1136/jmg.2003.012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauw RJ, Collin RW, Huygen PL, Hoefsloot LH, Kremer H, Cremers CW. Clinical characteristics of a Dutch DFNA9 family with a novel COCH mutation, G87W. Audiol Neurootol. 2007;12:77–84. doi: 10.1159/000097794. [DOI] [PubMed] [Google Scholar]
- 26.Pauw RJ, Huygen PL, Collin RW, Cruysberg JR, Hoefsloot LH, Kremer H, Cremers CW. Phenotype description of a novel DFNA9/COCH mutation, I109T. Ann Otol Rhinol Laryngol. 2007;116:349–357. doi: 10.1177/000348940711600506. [DOI] [PubMed] [Google Scholar]
- 27.Pennings RJ, Bom SJ, Cryns K, Flothmann K, Huygen PL, Kremer H, Van Camp G, Cremers CW. Progression of low-frequency sensorineural hearing loss (DFNA6/14-WFS1) Arch Otolaryngol Head Neck Surg. 2003;129:421–426. doi: 10.1001/archotol.129.4.421. [DOI] [PubMed] [Google Scholar]
- 28.Plantinga RF, de Brouwer AP, Huygen PL, Kunst HP, Kremer H, Cremers CW. A novel TECTA mutation in a Dutch DFNA8/12 family confirms genotype-phenotype correlation. J Assoc Res Otolaryngol. 2006;7:173–181. doi: 10.1007/s10162-006-0033-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Street VA, Kallman JC, Robertson NG, Kuo SF, Morton CC, Phillips JO. A novel DFNA9 mutation in the vWFA2 domain of COCH alters a conserved cysteine residue and intrachain disulfide bond formation resulting in progressive hearing loss and site-specific vestibular and central oculomotor dysfunction. Am J Hum Genet A. 2005;139:86–95. doi: 10.1002/ajmg.a.30980. [DOI] [PubMed] [Google Scholar]
- 30.Tamagawa Y, Ishikawa K, Ishikawa K, Ishida T, Kitamura K, Makino S, Tsuru T, Ichimura K. Phenotype of DFNA11: a nonsyndromic hearing loss caused by a myosin VIIA mutation. Laryngoscope. 2002;112:292–297. doi: 10.1097/00005537-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Todt I, Hennies HC, Basta D, Ernst A. Vestibular dysfunction of patients with mutations of Connexin 26. Neuroreport. 2005;16:1179–1181. doi: 10.1097/00001756-200508010-00009. [DOI] [PubMed] [Google Scholar]
- 32.Toth T, Pfister M, Zenner HP, Sziklai I. Phenotypic characterization of a DFNA6 family showing progressive low-frequency sensorineural hearing impairment. Int J Pediatr Otorhinolaryngol. 2006;70:201–206. doi: 10.1016/j.ijporl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Usami S, Takahashi K, Yuge I, Ohtsuka A, Namba A, Abe S, Fransen E, Patthy L, Otting G, Van Camp G. Mutations in the COCH gene are a frequent cause of autosomal dominant progressive cochleo-vestibular dysfunction, but not of Meniere’s disease. Eur J Hum Genet. 2003;11:744–748. doi: 10.1038/sj.ejhg.5201043. [DOI] [PubMed] [Google Scholar]