Summary
Hypertonia, which is characterized by stiff gait, abnormal posture, jerky movements, and tremor, is associated with a number of neurological disorders, including cerebral palsy, dystonia, Parkinson’s disease, stroke, and spinal cord injury. Recently, a spontaneous mutation in the gene encoding trafficking protein, kinesin-binding 1 (Trak1) was identified as the genetic defect for causing hypertonia in mice. The subcellular localization and biological function of Trak1 remain unclear. Here we report that Trak1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs), an essential component of the endosomal sorting and trafficking machinery. Double-label immunofluorescence confocal studies show that the endogenous Trak1 protein partially colocalizes with Hrs on early endosomes. Like Hrs, both overexpression and siRNA-mediated knockdown of Trak1 inhibit degradation of internalized epidermal growth factor receptors through a block in endosome-to-lysosome trafficking. Our findings support a role for Trak1 in the regulation of Hrs-mediated endosomal sorting and have important implications for understanding hypertonia associated with neurological disorders.
Keywords: Endosome, Hrs, Trak1, Trafficking, Hypertonia
Introduction
Endosome-to-lysosome trafficking is a crucial step in the endocytic pathway that not only controls degradation of cell surface receptors, but also regulates intracellular signaling.1; 2 Receptors at the cell surface are endocytosed either constitutively or in response to binding of their ligands and delivered to early endosomes. At the early endosome, receptors are rapidly and specifically sorted between recycling and lysosomal degradation pathways. Lysosome-bound receptors, such as the epidermal growth factor receptor (EGFR) are recruited into invaginations of the early endosome’s limiting membrane that then bud into the endosome lumen. The luminal contents of the endosome are delivered to the lysosome for degradation.1; 3 Recent studies have identified several components of the endosomal sorting machinery, including hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) and the endosomal sorting complexes required for transport (ESCRTs).4; 5; 6 However, the molecular mechanisms that control endosomal sorting and trafficking are not fully understood.
Trafficking protein, kinesin-binding 1 (Trak1), also known as OGT-interacting protein with a molecular mass of 106 kDa (OIP106), is a 939-amino-acid protein initially identified as a binding partner for the enzyme β-O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT). 7; 8 Subsequently, Trak1 has been shown to interact with kinesin heavy chain,9 γ-amino-n-butyric acid A (GABAA) receptor α1 subunit,10 and mitochondrial Rho GTPases (Miro-1 and Miro-2).11 However, the functional roles of these interactions have not yet been examined. Recently, a homozygous frameshift mutation in the mouse Trak1 gene, which produces a protein truncated at amino acid 824, was found to cause a recessively transmitted form of hypertonia, a neurological dysfunction characterized by postural abnormalities, jerky movements, and tremor.10 Hypertonia is observed in a variety of neurological disorders, including cerebral palsy, dystonia, Parkinson’s disease, stroke, and spinal cord injury.10 Despite the genetic evidence indicating the importance of Trak1 in normal physiology, the cellular localization and biological function of Trak1 remain unclear.
In this study, we investigated the subcellular distribution and functional role of Trak1. Our results reveal that the endogenous Trak1 protein partially localizes to early endosomes, interacts with the endosomal sorting machinery component Hrs, and plays an essential role in the regulation of Hrs-mediated endosome-to-lysosome trafficking.
Results
Trak1 is a member of the HAPN family
Mouse Trak1 is a 939-amino-acid protein that contains three putative coiled-coil domains (Fig. 1a).12; 13 The mouse hypertonia-associated Trak1 mutation10 produces a mutant Trak1 protein truncated at residue 824 (Fig. 1a). Sequence analysis indicates that the mouse Trak1 protein shares 92% overall amino acid identity with human Trak1 (Fig. 1b). The main difference between the 953-amino-acid human and 939-amino-acid mouse Trak1 sequences is an insertion of 12 residues (TVTSAIGGLQLN) after residue 896 in the human Trak1 sequence. Aside from this insertion, the human and mouse Trak1 C-termini are virtually identical. As shown in Fig. 1b, Trak1 contains a HAP1 N-terminal homologous domain (HAPN), which encompasses the first two coiled-coil domains. 14 The HAPN domain is also found in two other mammalian proteins, Huntingtin-associated protein-1 (HAP1) and GABAA receptor interacting factor-1 (GRIF1), as well as their Drosophila homologue, Milton (Fig. 1b).
Fig. 1. Characterization of anti-Trak1 antibodies.
(a) Domain structure of full-length mouse Trak1 (top) and the truncated Trak1 produced in the hyrt mutant mice (bottom). The white boxes indicate the location of the predicted coiled-coil regions for mouse Trak1. (b) Domain structure of Trak1 and its homologues. Accession numbers are as follows: Mm Trak1, NP_780323; Hs Trak1, NP_001036111; Hs GRIF1, NP_055864; Hs HAP1, NP_003940; and Dm Milton, NP_723249. The amino acid identity and similarity of each protein relative to the protein sequence of human Trak1 are indicated. Each protein contains three predicted coiled-coil domains shown as white boxes. Mm Trak1: 103–185, 207–356, 489–529; Hs Trak1: 104–186, 207–356, 492–532; Hs GRIF1: 126–170, 198–354, 507–519; Hs HAP1: 212–293, 307–427, 431–460, 593–606; Dm Milton: 133–209, 226–377, 1021–1034. The bracket indicates the location of the HAPN domain. Mm Trak1: 46–353; Hs Trak1: 47–354; Hs GRIF1: 47–354; Hs HAP1: 106–460; Dm Milton: 75–376. Mm, Mus musculus; Hs, Homo sapiens; Dm, Drosophila melanogaster. (c) Specificity of the anti-Trak1 antibody. Western blot analysis of cell lysates from SH-SY5Y, HeLa, and pGFP-Trak1 transfected HeLa cells using anti-Trak1 and anti-EEA1 antibodies. The asterisk indicates a band that appears specific to the anti-Trak1 antibody. (d) HeLa cells were transiently transfected with 100 nM of the indicated siRNA constructs. Whole cell lysates were subjected to SDS-PAGE followed by immunoblotting with anti-Trak1 antibody. Equal loading was confirmed by immunoblotting with anti-DJ1 antibody. The asterisk indicates a band that appears specific to the anti-Trak1 antibody. (e) Upper panel, HeLa cells were transfected with GFP-Trak1 WT (green), and then immunostained using anti-Trak1 antibody (red). Arrowhead indicates an untransfected cell. Lower panel, HeLa cells were transfected with Trak1 siRNA-2 and then immunostained using anti-Trak1 antibody (green). Nuclei were stained using DAPI. Arrow indicates a transfected cell. Bar, 10µm.
In order to characterize the Trak1 protein, we generated a rabbit polyclonal anti-Trak1 antibody against residues 935–953 of human Trak1. To investigate the specificity of our anti-Trak1 antibody, immunoblot analysis was performed using cell lysates prepared from untransfected SH-SY5Y and HeLa cells, as well as transfected HeLa cells expressing GFP-tagged Trak1 (Fig. 1c). The anti-Trak1 antibody specifically recognized recombinant GFP-tagged Trak1 in transfected HeLa cell lysates as well as the 115-kDa endogenous Trak1 protein in untransfected SH-SY5Y and HeLa cell lysates, consistent with a previous report.7 In addition, the anti-Trak1 antibody also recognized a band at ~106 kDa (asterisk, Fig. 1c), which might represent a Trak1 degradation product because its relative intensity as compared with the 115-kDa band varied from preparation to preparation. To further confirm the specificity of our anti-Trak1 antibody, we used two distinct siRNA duplexes, Trak1 siRNA-1 and Trak1 siRNA-2, which specifically target different regions of the Trak1 mRNA, to deplete endogenous Trak1 protein in HeLa cells. Immunoblot analysis revealed that both Trak1-immunoreactive 115-kDa and 106-kDa bands, but not the DJ-1-immunoreactive band, disappeared on treatment of cells with Trak1 siRNA-1 or Trak1 siRNA-2 (Fig. 1d). These results provide additional support for the specificity of our anti-Trak1 antibody and are consistent with the possibility that the 106-kDa band is a degradation product of Trak1.
The localization of Trak1 protein is poorly characterized and remains controversial. Iyer et al. reported Trak1 immunoreactivity was present in the nucleus as well as in the cytoplasm of HeLa cells with a punctate pattern.7 In contrast, Gilbert et al. found that Trak1 immunoreactivity was absent from nuclei and exhibited a diffuse cytosolic staining pattern in mouse brain tissue sections.10 To determine whether our anti-Trak1 antibodies could be used for immunocytochemistry and to clarify the localization of Trak1, we performed immunofluorescence confocal microscopic analysis to determine the intracellular distribution of endogenous Trak1 and GFP-tagged Trak1 WT in HeLa cells (Fig. 1e). We found extensive overlap between the staining pattern detected by the anti-Trak1 antibody and the distribution pattern of GFP-tagged Trak1 WT visualized by the green fluorescence emitted by the GFP tag (Fig. 1e, upper panel), indicating that our anti-Trak1 antibody is able to recognize Trak1 protein by immunostaining. The endogenous Trak1 in untransfected HeLa cells, detected by the anti-Trak1 antibody, displays a punctate staining pattern that is excluded from the nucleus (Fig. 1e, upper panel, arrowhead), suggesting a vesicular localization for Trak1. In contrast, overexpression of Trak1 in transfected cells results in a tubular staining pattern (Fig. 1e, upper panel), consistent with previous reports9; 11. This pattern is different from the punctate staining pattern of endogenous Trak1 (Fig. 1e, upper panel, arrowhead), suggesting that overexpression of Trak1 induces a change in Trak1 intracellular distribution.
To further confirm the specificity of our anti-Trak1 antibody in immunostaining, we transfected HeLa cells with Trak1 siRNA-2 or non-targeting control siRNA and then double-labeled the cells with anti-Trak1 antibody to detect endogenous Trak1 protein and with DAPI to stain the nuclei. We observed the presence of Trak1-immunonegative cells only in Trak1 siRNA-transfected cultures (Fig. 1e, lower panel), but not in control siRNA-transfected cultures or in untransfected HeLa cell cultures (data not shown), suggesting that Trak1 siRNA treatment resulted in the loss of Trak1 immunoreactivity. These data, together with the result of immunostaining in GFP-Trak1-transfected cells (Fig. 1e, upper panel) and the results of our antibody characterization by Western blot analysis (Fig. 1, c and d), provide strong evidence supporting the specificity of our anti-Trak1 antibody.
A population of endogenous Trak1 is localized to early endosomes
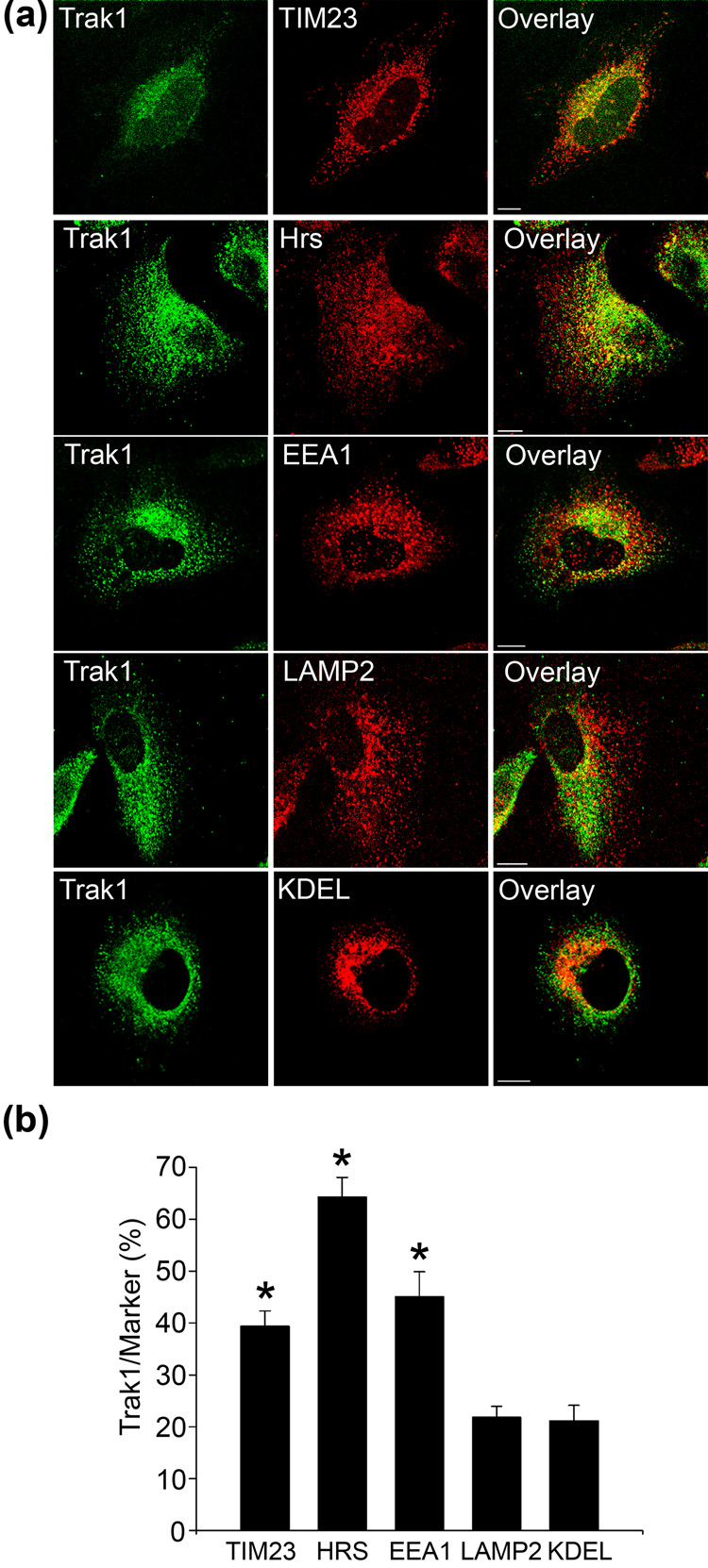
To further characterize the subcellular distribution of Trak1, we compared the distribution of endogenous Trak1 with that of various organelle marker proteins using double-label immunofluorescence confocal microscopy (Fig. 2). Previous studies reported the presence of epitope-tagged Trak1 in mitochondria;9; 11 however it is unclear whether the endogenous Trak1 protein also localizes to mitochondria. To test this possibility, we first compared endogenous Trak1 staining with that of the mitochondrial marker translocase of inner membrane 23 (TIM23). We observed a partial overlap (39.4 ± 3.0%) between Trak1 and TIM23 immunoreactivity (Fig. 2) that indicates a subset of Trak1 localizes to mitochondria. The lack of complete overlap suggested that Trak1 is not restricted to the mitochondrial compartment. Given the reported localization of other HAPN proteins to early endosomes,14; 15 we next compared Trak1 staining with that of the early endosomal markers, Hrs and early endosome antigen 1 (EEA1). Endogenous Trak1 overlaps 64.3 ± 3.7% with Hrs and 45.1 ± 4.8% with EEA1 (Fig. 2), indicating a population of Trak1 is associated with early endosomes. In contrast, we observed 21.8 ± 2.1% overlap between Trak1 and the late endosome/lysosome marker lysosomal-associated membrane protein 2 (LAMP2); and 21.2 ± 3.0% of Trak1 overlapped with the endoplasmic reticulum marker KDEL (Fig. 2). Together these findings indicate that Trak1 is associated with both mitochondria and endosomes; however our data suggests that Trak1 is predominantly associated with early endosomes (Fig. 2b).
Fig. 2. Endogenous localization of Trak1.
(a) HeLa cells were double-immunostained using anti-Trak1 antibody and anti-TIM23, anti-Hrs, anti-EEA1, anti-LAMP2, and anti-KDEL antibodies. Bar,10µm. (b) Quantification of Trak1 localization in HeLa cells. Images were processed and analyzed as described in Methods. The percentage of Trak1 that overlaps with the indicated marker proteins is presented as the mean ± s.e.m. The asterisks indicate a statistically significant difference (p < 0.05) from the KDEL results. Data are the result of 3–5 separate experiments.
Trak1 interacts with Hrs in vivo and in vitro
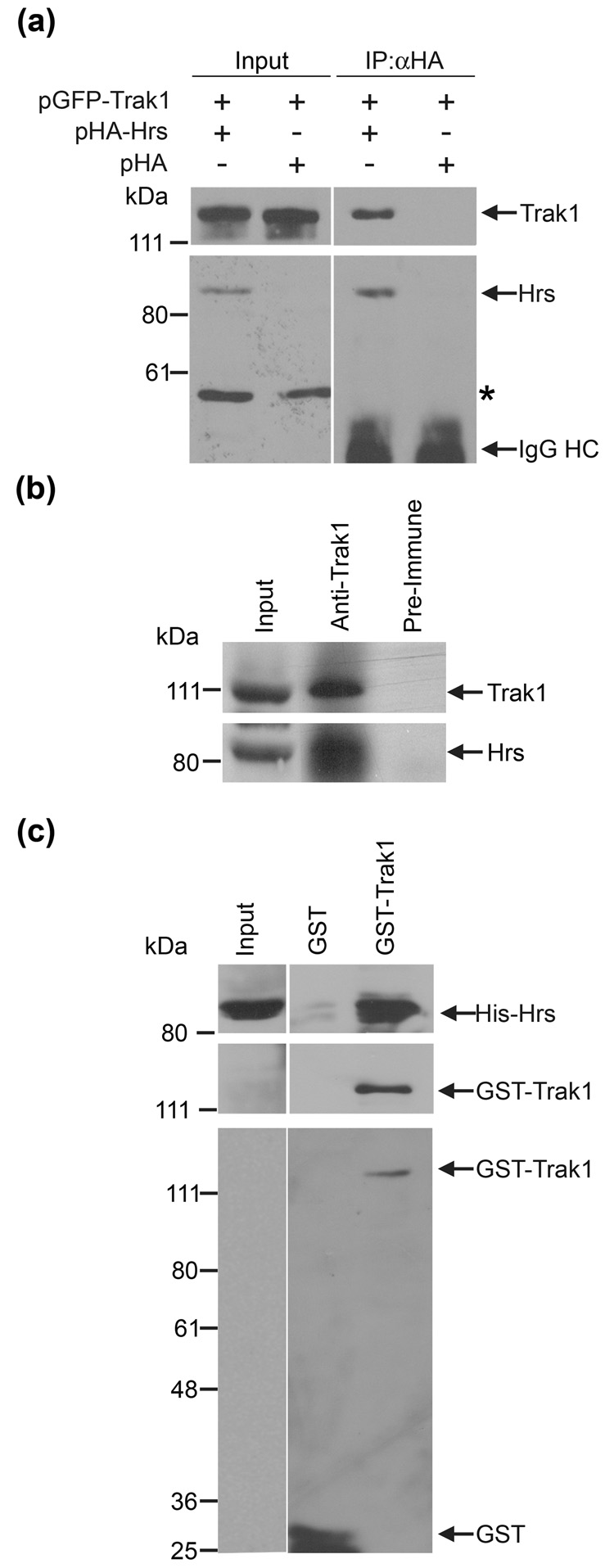
Trak1 is a member of the HAPN family, which also includes the Hrs-binding proteins, HAP1 and GRIF1.14; 15 The Hrs-binding region of rat GRIF1 (residues 359–507)14 shows 50% amino-acid similarity to the corresponding region (residues 359–507) of human Trak1. This observation along with our data showing that Trak1 localizes to early endosomes (Fig. 2) raises the possibility that Trak1 may associate with Hrs. To examine this possibility, we first performed co-immunoprecipitation experiments using lysates from HeLa cells co-transfected with pGFP-Trak1 and pHA-Hrs or pHA vector (Fig. 3a). Immunoprecipitation of the lysates with an anti-HA antibody revealed that GFP-Trak1 was specifically co-immunoprecipitated with HA-Hrs, but not with the HA vector control. We then performed additional co-immunoprecipitation experiments to examine the association of endogenous Trak1 and Hrs in HeLa cells (Fig. 3b). The anti-Trak1 antibody, but not the pre-immune serum, was able to co-immunoprecipitate Trak1 and Hrs from HeLa cell lysates, demonstrating the existence of an endogenous Hrs-Trak1 complex (Fig. 3b). To verify that the interaction between Hrs and Trak1 is direct, we performed in vitro binding assays using recombinant Hrs and Trak1 proteins. GST or GST-Trak1 fusion proteins immobilized on glutathione-agarose beads were incubated with soluble His-tagged Hrs. As shown in Fig. 3c, His-Hrs is specifically bound by GST-Trak1 and not the GST control, indicating a direct and specific interaction between recombinant Hrs and Trak1.
Fig. 3. Trak1 and Hrs associate in vivo and in vitro.
(a) Co-immunoprecipitation of Trak1 with Hrs in transfected HeLa cells. HeLa cells were co-transfected with pGFP-Trak1 in combination with pHA-Hrs or pHA vector. Lysates were immunoprecipitated with anti-HA antibody, followed by immunoblotting with anti-GFP and anti-HA antibodies. The asterisk indicates a non-specific band. (b) Co-immunoprecipitation of endogenous Hrs with Trak1 in HeLa cells. HeLa cell lysates were immunoprecipitated with anti-Trak1 antibody or pre-immune serum, followed by immunoblotting for Hrs and Trak1. (c) In vitro association between Hrs and Trak1. Soluble His-tagged Hrs was incubated with equal amounts of immobilized GST or GST-Trak1 fusion proteins. Bound His-Hrs and immobilized GST-fusion proteins were detected by immunoblotting with anti-Hrs, anti-Trak1 and anti-GST antibodies.
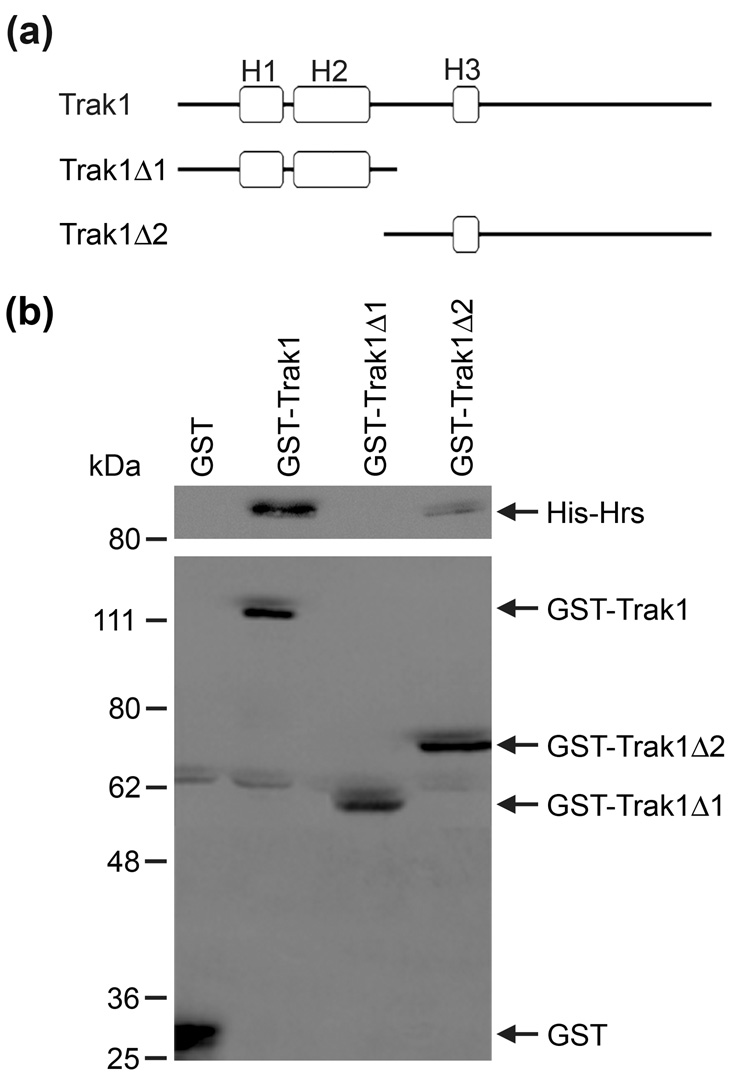
To further define the interaction between Trak1 and Hrs, we generated two GST-tagged Trak1 deletion constructs (Fig. 4a), Trak1Δ1, which encodes the N-terminal region, and Trak1Δ2, which encodes the C-terminal region that includes the predicted Hrs-binding domain (residues 359–507). Only the GST fusion proteins containing the predicted Hrs binding region (Trak1 and Trak1Δ2) were capable of binding Hrs (Fig. 4b). The GST-Trak1Δ1 fusion protein did not interact with Hrs, suggesting that the conserved Hrs-binding region in Trak1, which was first identified in GRIF114, is required for the interaction between Hrs and Trak1.
Fig. 4. Mapping the Hrs binding region of Trak1.
(a) Domain structure of Trak1 and its deletion mutants encoded by GST-tagged cDNA constructs. (b) Soluble His-tagged Hrs was incubated with equal amounts of immobilized GST or GST fusion proteins. Bound His-Hrs and immobilized GST fusion proteins were detected by immunoblotting with anti-Hrs and anti-GST antibodies, respectively.
Localization of Trak1 to early endosomes is dependent on the presence of the Hrs-binding domain
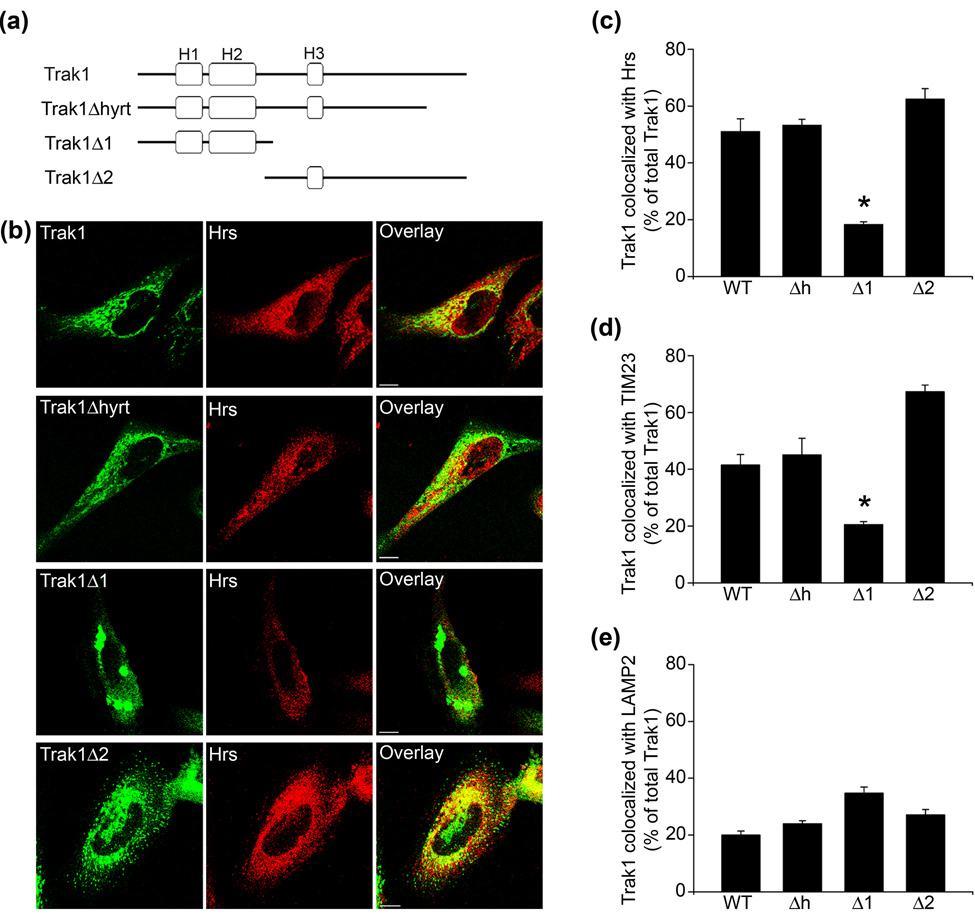
As a first step to investigate the pathogenic effects of hypertonia-associated Trak1 mutation, we generated a truncated Trak1Δhyrt (residues 1–824) construct to assess whether the Trak1 truncation mutation found in the hyrt mice10 would impact the subcellular localization of Trak1 (Fig. 5a). Using double-label immunofluorescence microscopy, we compared the distribution of the Trak1 WT and Trak1Δhyrt mutant with Hrs (Fig. 5b). An overlap of 51.1 ± 4.5% between GFP-tagged Trak1 WT and Hrs staining was observed (Fig. 5b and c). The GFP-tagged Trak1Δhyrt mutant showed 53.3 ± 2.1% overlap with Hrs, which appears to be indistinguishable from that of GFP-Trak1 WT (Fig. 5b and c), suggesting that the hyrt mutation does not affect the localization of Trak1 to Hrs-positive early endosomes.
Fig. 5. The C-terminal region of Trak1 is required for the early endosomal and mitochondrial localization of Trak1.
(a) Domain structure of Trak1 and its deletion mutants encoded by GFP-tagged cDNA constructs. (b) HeLa cells were transiently transfected with pGFP-Trak1, pGFP-Trak1Δhyrt, pGFP-Trak1Δ1, or pGFP-Trak1Δ2. GFP-Trak1 was visualized by the green fluorescence emitted by the GFP tag, and endogenous Hrs was detected using an anti-Hrs antibody (red). Bar, 10 µm. (c–e) HeLa cells were transiently transfected with pGFP-Trak1 (WT), pGFP-Trak1Δhyrt (Δh), pGFP-Trak1Δ1 (Δ1), or pGFP-Trak1Δ2 (Δ2). GFP-Trak1 was visualized by the green fluorescence emitted by the GFP tag, and cells were immunostained using anti-Hrs (c), anti-TIM23 (d), and anti-LAMP2 (e) antibodies. Images were processed and analyzed as described in Methods. The percentage of Trak1 that overlaps with the indicated marker proteins is presented as the mean ± s.e.m. The asterisks indicate a statistically significant difference (p < 0.05) from the WT results. Data are the result of 3–5 separate experiments.
Next, we used the N-terminal region construct (Trak1Δ1) and the C-terminal region construct (Trak1Δ2) to test the effect of the Hrs-binding region on Trak1 localization (Fig. 5a). The C-terminal GFP-Trak1Δ2 mutant did not exhibit the WT staining pattern; however an overlap of 62.5 ± 3.7% between GFP-Trak1Δ2 and Hrs was still observed (Fig. 5b and c). In contrast, GFP-tagged Trak1Δ1 only displayed 18.3 ± 0.9% overlap with Hrs staining (Fig. 5b and c). These findings suggest that the presence of the Hrs-binding domain is necessary to target Trak1 to Hrs-positive early endosomes.
To further characterize the intracellular distribution of the Trak1 deletion mutants, we quantified the overlap between our GFP-tagged Trak1 proteins and TIM23 (Fig. 5d) and LAMP2 (Fig. 5e). Full-length GFP-tagged Trak1, Trak1Δhyrt, and Trak1Δ2 overlap 41.5 ± 3.7%, 45.1 ± 5.8%, and 67.4 ± 2.3% with TIM23, respectively (Fig. 5d). GFP-tagged Trak1Δ1 only showed 20.1 ± 1.0% overlap with TIM23 (Fig. 5d). Endogenous Trak1 labeling was not observed to significantly overlap with LAMP2 (Fig. 2b) and in agreement with this we did not observe any statistically significant changes in the overlap observed between full-length GFP-tagged Trak1, Trak1Δhyrt, Trak1Δ1, or Trak1Δ2 (Fig. 5e). However, we did observe a trend, which failed to reach statistical significance, of colocalization between Trak1Δ1 and LAMP2 suggesting an increased localization to late endosomes/lysosomes. Overexpressed Trak1Δ1 forms inclusions (Fig. 5b) that may be processed through autophagy,16; 17 hence the increased localization of Trak1Δ1 to lysosomes (Fig. 5e). These results suggest that, although the deletion of the N-terminal region in Trak1Δ2 produces a staining pattern that is distinct from that of full-length Trak1 (Fig. 5b), it is similarly distributed to early endosomes and mitochondria (Fig. 5c and d). Furthermore, the ability of Trak1Δ2, but not Trak1Δ1, to localize to early endosomes and mitochondria supports the idea that the C-terminal region of Trak1 contains the conserved Hrs-14 and Miro-18 binding domains.
Overexpression of Trak1 and its mutants inhibit EGF-induced EGFR degradation, but not endocytosis
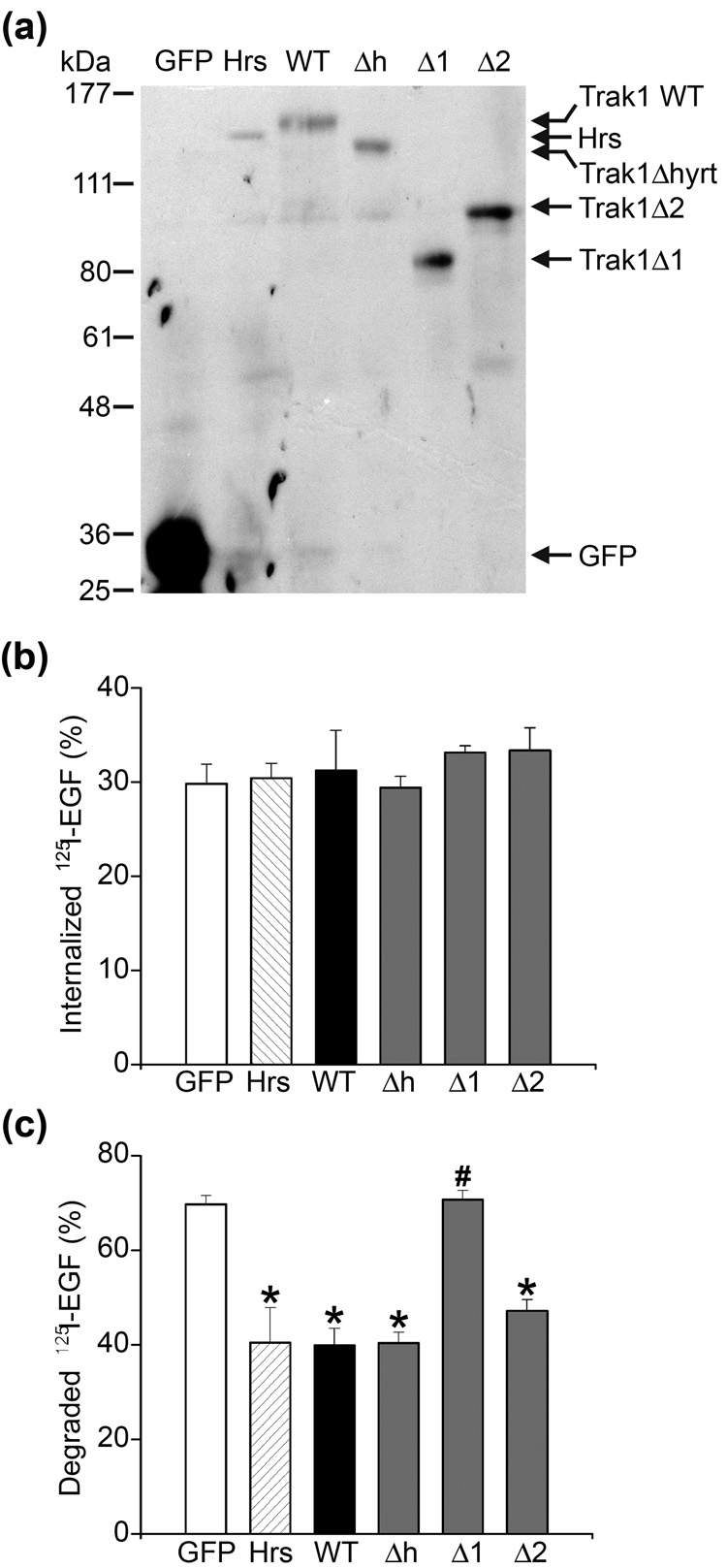
EGF-induced degradation of the EGFR is a widely used model for studying endocytic trafficking. Binding of EGF to the EGFR at the cell surface triggers rapid internalization of the EGF-EGFR complex and subsequent sorting at the early endosome for lysosomal degradation.19; 20; 21 The role of Hrs in regulating endosomal trafficking of EGFR has been well established; both overexpression and knockdown of Hrs inhibit ligand-induced EGFR degradation.22; 23 The observed interaction and colocalization of Trak1 with Hrs thus raise the possibility that Trak1 may participate in regulation of EGF-induced endocytic trafficking of EGFR to the lysosome for degradation. To test this possibility, we assessed the effects of overexpressing wild-type and mutant Trak1 on EGF-induced EGFR internalization and degradation, using the overexpression of Hrs as a positive control. HeLa cells were transfected with the indicated GFP-tagged Trak1 wild-type and deletion constructs, or GFP-Hrs, and the expression of these proteins were confirmed by Western blot analysis (Fig. 6a). Biochemical analysis of endocytosis using a quantitative 125I-EGF uptake assay showed that cells overexpressing wild-type or mutant Trak1 internalized a similar amount of 125I-EGF compared to the GFP-or GFP-Hrs-transfected controls (Fig. 6b). In addition, cell-based assays of endocytosis with Texas Red (TR)-conjugated EGF or TR-transferrin also showed that cells overexpressing wild-type or mutant Trak1 internalized similar amounts of TR-EGF and TR-transferrin compared to the GFP-or GFP-Hrs-transfected controls (data not shown). Together, these results indicate that, like Hrs,22; 23 overexpression of wild-type or mutant Trak1 had no significant effect on constitutive or regulated endocytosis.
Fig. 6. Trak1 inhibits the degradation of EGF-EGFR complexes and this action depends on the interaction with Hrs.
(a) Lysates from HeLa cells transfected with the indicated GFP-tagged expression constructs were subjected to SDS-PAGE followed by immunoblotting with anti-GFP antibody. (b) Trak1 overexpression has no effect on 125I-EGF uptake. HeLa cells were transiently transfected with GFP vector, GFP-tagged Hrs (Hrs) or the following GFP-tagged Trak1 constructs: Trak1 (WT), Trak1Δhyrt (Δh), Trak1Δ1 (Δ1), or Trak1Δ2 (Δ2). Cells were incubated with 125I-EGF for 10 minutes at 37°C. The internalized 125I-EGF is expressed as a percentage of the initially bound 125I-EGF. (c) HeLa cells transfected with the indicated constructs were allowed to internalized 125I-EGF for 10 minutes and then chased for 1 hour at 37°C. The degraded 125I-EGF is expressed as a percentage of the initially internalized 125I-EGF. Data represent mean ± s.e.m. from three independent experiments. The asterisks indicate a statistically significant difference (p < 0.05) from the vector-transfected cells, whereas the pound sign indicates a statistically significant difference (p < 0.05) from the GFP-Trak1 WT transfected cells.
We next examined and compared the effects of overexpressing wild-type or mutant Trak1 and Hrs on EGF-induced EGFR degradation using a quantitative 125I-EGF degradation assay. In this assay, cells were allowed to internalize 125I-EGF for 10 minutes. After washing to remove extracellular and surface-bound 125I-EGF, cells were chased at 37°C for 1, 2, or 3 hours to allow degradation. As shown in Fig. 6c, in the GFP-transfected control cells, 69.7 ± 1.9% (n = 3) of internalized 125I-EGF was degraded after the 1-hour chase. Overexpression of full-length Trak1 significantly decreased the degradation of 125I-EGF to 39.9 ± 3.6% (n = 3; p < 0.001), which is similar to the effect exerted by overexpression of Hrs (40.5 ± 7.4%, n = 3; p < 0.001). A similar extent of reduction in the 125I-EGF degradation was also observed in cells overexpressing Trak1 deletion mutants that contain the Hrs-binding domain, specifically Trak1Δhyrt (40.3 ± 2.2%, n = 3; p < 0.001) and Trak1Δ2 (47.1 ± 2.4%, n = 3; p < 0.001). In contrast, the amount of degraded 125I-EGF in cells overexpressing Trak1Δ1 (70.8 ± 1.9%, n = 3) was not significantly different from that in the GFP-transfected control cells. Similar effects of overexpressing wild-type and mutant Trak1 on 125I-EGF degradation were also seen at the 2-hour and 3-hour chase time points (data not shown). These data, together with the result obtained from analysis of effects of Trak1 deletions on the colocalization with Hrs (Fig. 5), suggest that the association of Trak1 with Hrs-positive early endosomes is required for the inhibitory effect of Trak1 overexpression on EGF-induced EGFR degradation.
Trak1 regulates endosome-to-lysosome trafficking of internalized EGFR
To assess the effect of Trak1 overexpression on endosome-to-lysosome trafficking of EGF-EGFR complexes, we used a "pulse-chase" trafficking assay.14; 15 In this assay, HeLa cells were allowed to internalize TR-EGF for 10 minutes, and the fate of internalized TR-EGF after a 1, 2, or 3-hour chase period was monitored by fluorescence confocal microscopy (Fig. 7a). We found that the TR-EGF fluorescence signal in untransfected cells was dramatically reduced after the 1-hour chase, indicating that most of the internalized TR-EGF had been degraded (Fig. 7a, asterisks). In comparison, cells overexpressing Trak1 retained a significant amount of the internalized TR-EGF after the same 1-hour chase period (Fig. 7a, arrowheads), suggesting the trafficking from the early endosome to the lysosome is impeded by Trak1 overexpression. Quantification analysis (Fig. 7b) revealed that, in untransfected cells, 10.9 ± 1.2% of the internalized TR-EGF remained after the 1-hour chase, which is similar to the amount of remaining TR-EGF (10.5 ± 0.8%) found in GFP-transfected cells. The amount of remaining TR-EGF was significantly increased (p < 0.001) in Trak1-overexpressing cells (27.0 ± 3.3%) as well as in Hrs-overexpressing cells (25.9 ± 5.3%), suggesting that Trak1 overexpression inhibits endosome-to-lysosome trafficking to a similar extent compared to Hrs overexpression. Moreover, we found that the amount of remaining TR-EGF was increased in cells expressing Trak1Δ hyrt (24.8 ± 6.9%) and Trak1Δ2 (30.2 ± 1.5%), but not in cells expressing Trak1Δ1 (10.5 ± 0.5%), indicating that only the Trak1 deletion mutants containing the conserved Hrs-binding region are capable of inhibiting trafficking, whereas the Trak1 deletion mutant lacking the Hrs-binding region had no effect. Similar effects of overexpressing wild-type and mutant Trak1 on the degradative trafficking of internalized TR-EGF were also seen at the 2-hour and 3-hour chase time points (data not shown). These data obtained from the TR-EGF trafficking assays are in agreement with the results of our 125I-EGF degradation assays (Fig. 6c), and suggest that Trak1 regulates EGF-induced EGFR trafficking from the early endosome to the lysosome in an Hrs-dependent manner.
Fig. 7. Trak1 overexpression inhibits trafficking of EGF-EGFR complexes from early endosomes to the lysosomal pathway.
(a) HeLa cells expressing GFP or GFP-tagged Trak1, Trak1Δhyrt, Trak1Δ1,Trak1Δ2, and Hrs were allowed to internalize TR-EGF for 10 minutes and then chased for 1 hour at 37°C. Cells were stained with a primary antibody against EEA1 and a CY5-conjugated secondary antibody. Arrowheads and asterisks indicate transfected and untransfected cells, respectively. Bar,10 µm. (b) HeLa cells were transiently transfected with GFP vector, GFP-tagged Hrs (Hrs) or the following GFP-tagged Trak1 constructs: Trak1 (WT), Trak1Δhyrt (Δh), Trak1Δ1 (Δ1), or Trak1Δ2 (Δ2). Untransfected HeLa cells were used as a control (CTL). The amount of remaining TR-EGF after 1-hour chase was quantified and expressed as a percentage of the initially internalized TR-EGF. Data represent mean ± s.e.m. from three independent experiments. The asterisks indicate a statistically significant difference (p < 0.05) from the GFP vector-transfected cells, whereas the pound sign indicates a statistically significant difference (p < 0.05) from the GFP-Trak1 WT-transfected cells.
Trak1 is essential for ligand-induced EGFR degradation
To provide further evidence supporting the role of Trak1 in regulation of EGFR trafficking, we examined the effect of siRNA-mediated knockdown of Trak1 expression on the uptake and degradation of 125I-EGF in HeLa cells, using the knockdown of Hrs as a positive control. For selective depletion of endogenous Trak1 or Hrs, HeLa cells were transfected with Trak1 siRNA-1, Trak1 siRNA-2, Hrs siRNA-1, or Hrs siRNA-2. Immunoblot analysis of transfected cell lysates confirmed the knockdown of endogenous Trak1 or Hrs (Fig. 8a). We found that depletion of Trak1 or Hrs had no statistically significant effect on 125I-EGF internalization (Fig. 8b). As shown in Fig. 8c, we observed a statistically significant (p < 0.001) decrease in 125I-EGF degradation in both Trak1 siRNA-1 (36.2 ± 5.2%, n = 3) and Trak1 siRNA-2 (32.1 ± 7.1%, n = 3) transfected HeLa cells compared to the control siRNA-transfected cells (64.1 ± 2.8%, n = 3) after the 1-hour chase. The effect of Trak1 depletion on 125I-EGF degradation is comparable to the effect exerted by Hrs depletion from treatment with Hrs siRNA-1 (31.9 ± 9.2%, n = 3) and Hrs siRNA-2 (35.5 ± 10.5%, n = 3). Similar effects of Trak1 and Hrs on 125I-EGF degradation were also seen at the 2-hour and 3-hour chase time points (data not shown). Together, these data provide strong evidence supporting a functional role for Trak1 in regulation of the trafficking of internalized EGF-EGFR complexes to the lysosome for degradation.
Fig. 8. Trak1 knockdown inhibits degradation, but not endocytosis of 125I-EGF.
(a) HeLa cells were transiently transfected with the indicated siRNA constructs. Whole cell lysates were subjected to SDSPAGE followed by immunoblotting with anti-Trak1 or anti-Hrs antibodies. Equal loading was confirmed by immunoblotting with anti-EEA1 antibody. (b) HeLa cells transfected with the indicated siRNAs were incubated with 125I-EGF for 10 minutes at 37°C. The internalized 125I-EGF is expressed as a percentage of the initially bound 125I-EGF. (c) HeLa cells transfected with the indicated siRNA constructs were allowed to internalized 125I-EGF for 10 minutes and then chased for 1 hour at 37°C. The degraded 125I-EGF is expressed as a percentage of the initially internalized 125I-EGF. Data represent mean ± s.e.m. from three independent experiments. The asterisks indicate a statistically significant difference (p < 0.05) from the control siRNA-transfected cells.
Discussion
Recent identification of a homozygous frameshift mutation in mouse Trak1 as the cause for a recessively transmitted form of hypertonia10 highlights the importance of understanding the cellular role of Trak1, a newly discovered protein of unknown function. In the current study, we found that Trak1 interacts with the endosomal sorting machinery component Hrs and provided evidence supporting a role for Trak1 as a novel regulator of endosome-to-lysosome trafficking.
The subcellular localization of Trak1 is poorly defined; previous studies reported a localization of Trak1 in the nucleus,7 cytosol,10 mitochondria,9; 11 and vesicular structures of unknown identity.7 To clarify the subcellular localization of Trak1, we generated a specific anti-Trak1 antibody and found that endogenous Trak1 is partially localized to mitochondria, in agreement with the reported interaction of Trak1 with the mitochondrial Miro proteins.11 Interestingly, our studies revealed that a population of endogenous Trak1 is associated with Hrs-and EEA1-positive early endosomes. Furthermore, deletion analysis showed that the localization of Trak1 to early endosomes depends on its interaction with Hrs. These data suggest that Trak1 is appropriately localized to influence Hrs-mediated endosomal sorting. In support of this view, our functional studies indicated that overexpression of Trak1 inhibits EGF-induced EGFR degradation by blocking endosome-to-lysosome trafficking of the EGFR. Moreover, Trak1 siRNA experiments showed that Trak1 is required for ligand-induced degradation of the internalized EGF-EGFR complexes, providing further evidence supporting a functional role for Trak1 in the regulation of EGFR endosomal trafficking.
Trak1 is a member of a small family of proteins characterized by the presence of the HAPN domain. Our previous work has shown that the other two members of the HAPN family, HAP1 and GRIF1, both interact with Hrs and regulate endosome-to-lysosome trafficking of internalized EGFRs.14; 15 Thus, the HAPN proteins seem to share a common function as regulators of endosomal trafficking. We and others have shown that these three proteins are differentially expressed; Trak1 is ubiquitously expressed in many tissues and cell types,7; 10 whereas HAP1 and GRIF1 are mainly expressed in the brain where they are highly enriched in neuronal cells.7; 14; 24; 25 Moreover, these HAPN proteins may have distinct functions. For example, HAP1 overexpression induces enlarged early endosomes15 and GRIF1 overexpression produces a perinuclear clustering of endosomes, 14 whereas Trak1 overexpression fails to induce either of these phenotypes. Trak1 and GRIF1 also associate with mitochondria, but HAP1 does not.11 HAP1 also associates with kinesin light chain,26 rather than the conventional kinesin heavy chains, which bind to both GRIF1 and Trak1.9 These similarities and differences among the HAPN proteins suggest that this family of proteins may have overlapping yet distinct functional roles.
In addition to regulating EGFR endosomal trafficking, the HAPN proteins may also regulate trafficking of a variety of cell surface receptors. All three members of the HAPN family have been shown to associate with GABAAR subunits.10; 24; 27 In support of this hypothesis, HAP1 has been implicated in the regulation of endosomal trafficking of GABAARs27 and the NGF receptor, TrkA.28 Moreover, the altered levels of GABAA receptors observed in Trak1 mutant mice10 suggests that Trak1 may also participate in regulation of GABAAR endosomal trafficking.
The link between the homozygous Trak1 truncation mutation and a recessively transmitted form of hypertonia10 suggests the Trak1 truncation mutation causes hypertonia through a “loss of function” mechanism. Based on our finding that Trak1 is a ubiquitously expressed regulator of endosome-to-lysosome trafficking in mammalian cells, one would expect that the Trak1 truncation mutation leads to dysregulation of endosome-to-lysosome trafficking in many cell types. The hypertonic phenotype of the Trak1 mutant mice suggests that, compared to other cell types, neurons are particularly vulnerable to defects in endosomal trafficking. Consistent with this notion, aberrant endosomal trafficking has recently been implicated in the pathogenesis of a number of neurological diseases. For example, mutations in endosomal fusion regulators Rab5 guanine nucleotide exchange factor alsin29 and Vps5430 cause motor neuron diseases in mammals. Moreover, mutations in the endosomal sorting regulators CHMP2B and Mahogunin cause frontotemporal dementia31 and spongiform neurodegeneration.32; 33 These lines of genetic evidence provide a direct link between the dysregulation of endosomal trafficking and neuronal dysfunction.
The mechanism by which the Trak1 truncation mutation causes hypertonia is unclear. Our analyses failed to find any difference between Trak1Δhyrt and Trak1 in their localization or function. Previous studies have reported a lack of effect of the Trak1 truncation mutation on the interaction with the GABAAR.10 Since the kinesin-9 and Hrs-binding domains14 are outside of the deleted C-terminal region, the Trak1Δhyrt mutation is not expected to affect the ability of Trak1 to bind kinesin or Hrs. In contrast, the Trak1Δhyrt mutation might disrupt the ability of Trak1 to bind the OGT enzyme because truncation of Trak1 at residue 824 deletes a portion of the OGT-binding domain (residues 639–859).8 Trak1 has been shown to undergo O-GlcNAc modification by OGT enzyme,8 although the functional consequence of this modification has not yet been examined. It is conceivable that the activity of Trak1 may be regulated by post-translational modifications, such as phosphorylation and O-GlcNAc modification34; 35 and that the Trak1Δhyrt mutation may alter this regulation, thereby leading to neuronal dysfunction. Future studies illuminating the mechanisms of Trak1 action and its regulation should advance our understanding of endosomal trafficking and its dysregulation in hypertonia and other neurological disorders.
Materials and Methods
Antibodies
An anti-Trak1 antibody was raised in rabbit against the synthetic peptide ILTSGILMGAKLPKQTSLR, corresponding to residues 935–953 of human Trak1. The antibody was affinity purified as previously described.36 Other primary antibodies used in this study include: anti-Hrs36; anti-EEA1 and anti-TIM23 (BD Transduction Laboratories); anti-GFP B2 and anti-GST (Santa Cruz Biotechnologies); anti-KDEL (Stressgen); anti-LAMP2 (H4B4; Developmental Studies Hybridoma Bank, Iowa City, IA); and the mouse monoclonal anti-HA (12CA5) antibody. Horseradish peroxidase-conjugated secondary antibodies and fluorescein isothiocyanate (FITC)-, CY5-, or Texas Red (TR)-conjugated secondary antibodies (Jackson Immunoresearch Laboratories) were used for immunoblotting and immunostaining, respectively.
Expression Constructs
Human full-length Trak1 cDNA (KIAA1042; GenBank accession number AB028965) was a gift from Dr. Takahiro Nagase (Kazusa DNA Institute, Japan). Conventional molecular biological techniques were used to generate the following expression constructs with N-terminal GFP (pGFP), HA (pHA), or GST (pGST) tags: Trak1 (residues 1–953), Trak1Δhyrt (residues 1–824), Trak1Δ1 (residues 1–419), Trak1Δ2 (residues 354–953). A schematic representation of the Trak1 constructs is shown in Fig. 4a and Fig 5a. The full-length Hrs expression construct has been described previously.15; 22; 36
Western Blot Analyses
Cell lysates were homogenized in 1% SDS and subjected to SDS-PAGE. Western blot analysis was carried out as described previously.14
In Vitro Binding Assays
His6-tagged Hrs, GST or various GST-Trak1 fusion proteins were individually expressed in Escherichia coli BL21 cells and purified as described previously. 15; 37 In vitro binding assays were performed as described previously15 by incubation of equal amounts of GST or GST-Trak1 fusion proteins immobilized on glutathione-agarose beads (Sigma) with soluble His6-tagged Hrs. Bound proteins were analyzed by SDS-PAGE and immunoblotting.
Cell Transfections and Immunoprecipitations
HeLa cells were transfected with the indicated plasmids using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Immunoprecipitations were carried out 24 hours post-transfection as described previously22 using whole cell lysates and the indicated primary antibodies. Immunocomplexes were then analyzed by SDS-PAGE and immunoblotting.
Immunofluorescence Confocal Microscopy
HeLa cells were grown on poly-L-lysine coated glass coverslips. Cells were fixed in 4% paraformaldehyde, stained with appropriate primary and secondary antibodies, and processed for indirect immunofluorescence microscopy as described previously.15 Analysis and acquisition were performed using a Zeiss LSM 510 confocal laser-scanning microscope. Images were exported in TIFF format using LSM-510 software (Carl Zeiss MicroImaging, Inc.) and processed using Adobe Photoshop CS (Adobe Systems, Inc.).
Quantitative Analysis of Colocalization
Quantification of the colocalization of Trak1 with various marker proteins (Hrs, EEA1, LAMP2, TIM23, and KDEL) was performed on unprocessed images of cells double-labeled for Trak1 and the indicated marker protein by using Metamorph Imaging System Software (Universal Imaging Corp.) as previously described.33; 38 Briefly, the average grayscale pixel intensity +1 standard deviation of a small region of the cell-free area was measured in the Trak1 and marker channels and defined as background. Each field contained several cells and single cells were manually selected by tracing the cell outlines. Background was subtracted from the cell images by setting the threshold of each channel to the value obtained for background. The percentage of overlap between Trak1 pixels and Hrs, EEA1, LAMP2, TIM23, or KDEL pixels was determined. For quantification of Trak1 colocalization with each of the marker proteins, 25 cells were randomly selected for analysis. Experiments were repeated at least three times, and the data were subjected to statistical analysis by ANOVA and Tukey’s post hoc test.
Small Interfering RNAs (siRNAs) Transfection
Two siRNAs (Dharmacon) were generated against the following human Trak1 mRNA sequences: 3′-UGAAAGAGUUGGCCAGAUGUU-5′ and 3′-GACGAAGUGUACUGCCUUAUU-5', called Trak1 siRNA-1 and siRNA-2, respectively. In addition, a control siRNA with no known mammalian homology (siCONTROL Non-Targeting siRNA #1, Dharmacon) was used as a negative control. HeLa cells were transfected with the indicated siRNA (100 nM) using the TransIT siQUEST (Mirus) reagent according to the manufacturer's instructions. 48 hours later, the cells were transfected a second time with the siRNA. Experiments were performed 48 hours after the final siRNA treatment. For silencing Hrs expression, we used two small hairpin RNAs (shRNAs) targeted against the human Hrs mRNA sequences: 3’-CCGGCCGCATGAAGAGTAACCACATCTCGAGATGTGGTTACTCTTCATGCGGTTTTTG-5’ and 3’-CCGGGCACGTCTTTCCAGAATTCAACTCGAGTTGAATTCTGGAAAGACGTGCTTTTTG-5’, called Hrs siRNA-1 and Hrs siRNA-2, respectively (MISSION shRNAs, Sigma). HeLa cells were transfected with the indicated shRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Experiments were performed 48 hours after transfection.
Endocytic Trafficking Assays
For measurement of TR-transferrin or TR-EGF endocytosis, HeLa cells transfected with indicated GFP-tagged Trak1 and control plasmids were incubated in serum-free media for 1 hour, and then treated with 100 µg/ml TR-transferrin (Invitrogen) at 37°C for 30 minutes, or with 3 µg/ml TR-EGF (Invitrogen) in the presence of 0.1% bovine serum albumin (BSA) at 37°C for 10 minutes. The cells were then processed for immunofluorescence microscopy.15 For measurement of TR-EGF trafficking after internalization, cells were washed three times with the media to remove extracellular TR-EGF and incubated for an additional 1, 2, or 3 hours at 37°C prior to fixation and processing for immunofluorescence microscopy.15 Quantification of the amount of intracellular TR-EGF was performed on unprocessed confocal images of 100 randomly selected transfected cells by using Metamorph Imaging System Software as previously described.33; 38 Background was subtracted from the cell images, and the integrated TR-EGF pixel intensity was determined. Experiments were repeated at least three times, and the data were subjected to statistical analysis by ANOVA and Tukey’s post hoc test.
125I-EGF Internalization and Degradation Assays
For measurement of 125I-EGF internalization, HeLa cells transfected with indicated Trak1 and control plasmids were serum starved for 2 hours, then incubated on ice with ~20 ng/ml 125I-EGF (MP Biochemicals) in binding buffer (1% BSA in serum-free DMEM). Cells were then washed with cold binding buffer and either lysed immediately to measure the initially bound 125I-EGF or transferred to 37°C for 10 minutes. After washing cells with acid wash (0.5 M NaCl, 0.2 M acetic acid, pH 2.8) on ice, the internalized 125I-EGF was measured as described39; 40 and expressed as a percentage of the initially bound 125I-EGF. For measurement of 125I-EGF degradation after internalization, transfected HeLa cells were incubated with 125I-EGF at 37°C for 10 minutes and then acid washed to remove unbound and surface bound 125I-EGF. Cells were then lysed to measure the internalized 125I-EGF or chased in serum-free DMEM containing 1.5 µg/ml EGF and 1% BSA at 37°C for 1, 2, or 3 hours. The amount of 125I-EGF remaining in the cells at the indicated chase time point was measured as described.39; 40 The amount of degraded 125I-EGF was determined by subtraction of the remaining 125I-EGF from the internalized 125I-EGF and was expressed as a percentage of the internalized 125I-EGF. All measurements of 125I-EGF levels were performed by determining the radioactivity (125I counts per minute) in a Wallac Wizard 1470 Automatic Gamma Counter. Data were obtained from at least three independent experiments and analyzed by ANOVA and Tukey’s post hoc test.
Acknowledgements
We thank Dr. Takahiro Nagase (Kazusa DNA Research Institute, Japan) for the gift of the KIAA1042 cDNA and Sharon Swanger (Emory University) for assistance in generating the Trak1 deletion constructs. This work was supported by grants from National Institutes of Health (NS050650, NS047575, and ES015813).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 2.Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- 3.Mullins C, Bonifacino JS. The molecular machinery for lysosome biogenesis. Bioessays. 2001;23:333–343. doi: 10.1002/bies.1048. [DOI] [PubMed] [Google Scholar]
- 4.Yan Q, Sun W, Kujala P, Lotfi Y, Vida TA, Bean AJ. CART: an Hrs/actinin-4/BERP/myosin V protein complex required for efficient receptor recycling. Mol Biol Cell. 2005;16:2470–2482. doi: 10.1091/mbc.E04-11-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. Embo J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickerson DP, Russell MR, Odorizzi G. A concentric circle model of multivesicular body cargo sorting. EMBO Rep. 2007;8:644–650. doi: 10.1038/sj.embor.7401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iyer SP, Akimoto Y, Hart GW. Identification and cloning of a novel family of coiled-coil domain proteins that interact with O-GlcNAc transferase. J Biol Chem. 2003;278:5399–5409. doi: 10.1074/jbc.M209384200. [DOI] [PubMed] [Google Scholar]
- 8.Iyer SP, Hart GW. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem. 2003;278:24608–24616. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- 9.Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem. 2005;280:14723–14732. doi: 10.1074/jbc.M409095200. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert SL, Zhang L, Forster ML, Iwase T, Soliven B, Donahue LR, Sweet HO, Bronson RT, Davisson MT, Wollmann RL, Lahn BT. Trak1 mutation disrupts GABA(A) receptor homeostasis in hypertonic mice. Nat Genet. 2006;38:245–250. doi: 10.1038/ng1715. [DOI] [PubMed] [Google Scholar]
- 11.Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 12.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- 13.Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci U S A. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirk E, Chin LS, Li L. GRIF1 binds Hrs and is a new regulator of endosomal trafficking. J Cell Sci. 2006;119:4689–4701. doi: 10.1242/jcs.03249. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Chin LS, Levey AI, Li L. Huntingtin-associated protein 1 interacts with hepatocyte growth factor-regulated tyrosine kinase substrate and functions in endosomal trafficking. J Biol Chem. 2002;277:28212–28221. doi: 10.1074/jbc.M111612200. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 17.Olzmann JA, Li L, Chudaev MV, Chen J, Perez FA, Palmiter RD, Chin LS. Parkin-mediated K63-linked polyubiquitination targets misfolded DJ-1 to aggresomes via binding to HDAC6. J Cell Biol. 2007;178:1025–1038. doi: 10.1083/jcb.200611128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morino C, Kato M, Yamamoto A, Mizuno E, Hayakawa A, Komada M, Kitamura N. A role for Hrs in endosomal sorting of ligand-stimulated and unstimulated epidermal growth factor receptor. Exp Cell Res. 2004;297:380–391. doi: 10.1016/j.yexcr.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 20.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 21.Mellman I. Membranes and sorting. Curr Opin Cell Biol. 1996;8:497–498. doi: 10.1016/s0955-0674(96)80026-3. [DOI] [PubMed] [Google Scholar]
- 22.Chin LS, Raynor MC, Wei X, Chen HQ, Li L. Hrs interacts with sorting nexin 1 and regulates degradation of epidermal growth factor receptor. J Biol Chem. 2001;276:7069–7078. doi: 10.1074/jbc.M004129200. [DOI] [PubMed] [Google Scholar]
- 23.Kanazawa C, Morita E, Yamada M, Ishii N, Miura S, Asao H, Yoshimori T, Sugamura K. Effects of deficiencies of STAMs and Hrs, mammalian class E Vps proteins, on receptor downregulation. Biochem Biophys Res Commun. 2003;309:848–856. doi: 10.1016/j.bbrc.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 24.Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem. 2002;277:30079–30090. doi: 10.1074/jbc.M200438200. [DOI] [PubMed] [Google Scholar]
- 25.Li XJ, Li SH, Sharp AH, Nucifora FC, Jr, Schilling G, Lanahan A, Worley P, Snyder SH, Ross CA. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995;378:398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- 26.McGuire JR, Rong J, Li SH, Li XJ. Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J Biol Chem. 2006;281:3552–3559. doi: 10.1074/jbc.M509806200. [DOI] [PubMed] [Google Scholar]
- 27.Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating {gamma}-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong J, McGuire JR, Fang ZH, Sheng G, Shin JY, Li SH, Li XJ. Regulation of intracellular trafficking of huntingtin-associated protein-1 is critical for TrkA protein levels and neurite outgrowth. J Neurosci. 2006;26:6019–6030. doi: 10.1523/JNEUROSCI.1251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, Hung WY, Ouahchi K, Yan J, Azim AC, Cole N, Gascon G, Yagmour A, Ben-Hamida M, Pericak-Vance M, Hentati F, Siddique T. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29:160–165. doi: 10.1038/ng1001-160. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt-John T, Drepper C, Mussmann A, Hahn P, Kuhlmann M, Thiel C, Hafner M, Lengeling A, Heimann P, Jones JM, Meisler MH, Jockusch H. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet. 2005;37:1213–1215. doi: 10.1038/ng1661. [DOI] [PubMed] [Google Scholar]
- 31.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, Nielsen JE, Hodges JR, Spillantini MG, Thusgaard T, Brandner S, Brun A, Rossor MN, Gade A, Johannsen P, Sorensen SA, Gydesen S, Fisher EM, Collinge J. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 32.He L, Lu XY, Jolly AF, Eldridge AG, Watson SJ, Jackson PK, Barsh GS, Gunn TM. Spongiform degeneration in mahoganoid mutant mice. Science. 2003;299:710–712. doi: 10.1126/science.1079694. [DOI] [PubMed] [Google Scholar]
- 33.Kim BY, Olzmann JA, Barsh GS, Chin LS, Li L. Spongiform neurodegeneration-associated E3 ligase Mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol Biol Cell. 2007;18:1129–1142. doi: 10.1091/mbc.E06-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Kwong J, Roundabush FL, Hutton Moore P, Montague M, Oldham W, Li Y, Chin LS, Li L. Hrs interacts with SNAP-25 and regulates Ca(2+)-dependent exocytosis. J Cell Sci. 2000;113(Pt 12):2273–2284. doi: 10.1242/jcs.113.12.2273. [DOI] [PubMed] [Google Scholar]
- 37.Chin LS, Nugent RD, Raynor MC, Vavalle JP, Li L. SNIP, a novel SNAP-25-interacting protein implicated in regulated exocytosis. J Biol Chem. 2000;275:1191–1200. doi: 10.1074/jbc.275.2.1191. [DOI] [PubMed] [Google Scholar]
- 38.Volpicelli LA, Lah JJ, Levey AI. Rab5-dependent trafficking of the m4 muscarinic acetylcholine receptor to the plasma membrane, early endosomes, and multivesicular bodies. J Biol Chem. 2001;276:47590–47598. doi: 10.1074/jbc.M106535200. [DOI] [PubMed] [Google Scholar]
- 39.Valiathan RR, Resh MD. Expression of human immunodeficiency virus type 1 gag modulates ligand-induced downregulation of EGF receptor. J Virol. 2004;78:12386–12394. doi: 10.1128/JVI.78.22.12386-12394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]