Abstract
Associations between Culex quinquefasciatus, Aedes albopictus and West Nile virus (WNV) activity, temperature, and rainfall in Harris County, Texas 2003–06 are discussed. Human cases were highly correlated to Cx. quinquefasciatus (r = 0.87) and Ae. albopictus (r = 0.78) pools, blue jays (r = 0.83), and Ae. albopictus collected (r = 0.71), but not Cx. quinquefasciatus collected (r = 0.45). Human cases were associated with temperature (r = 0.71), not rainfall (r = 0.29), whereas temperature correlated with Ae. albopictus and Cx. quinquefasciatus collections (r = 0.88 and 0.70, respectively) and Cx. quinquefasciatus pools (r = 0.75), but not Ae. albopictus pools (r = 0.55). Both species (collections and pools) and blue jays were weakly correlated (r ≤ 0.41) with rainfall, but blue jays were better correlated with Cx. quinquefasciatus pools (r = 0.87), compared with Ae. albopictus pools (r = 0.67), Ae. albopictus collections (r = 0.69), and Cx. quinquefasciatus collections (r = 0.46). Peak minimum infection rate for Cx. quinquefasciatus (4.55), and Ae. albopictus (4.41) was in August with highest human cases (17.87), blue jays (55.58), and temperature (29.01°C). Between both species, blood meal analysis indicated 68.18% of Cx. quinquefasciatus mammalian hosts were dog, while 22.72% were human, whereas Ae. albopictus had higher human (44.44%) but fewer dog hosts (22.22%). Ten bird species were identified as hosts for Cx. quinquefasciatus, with northern cardinal and blue jay representing 26.66% and 20.00%, respectively. No bird feeding activity was observed in Ae. albopictus. The earliest and latest human blood meal occurred in May (Ae. albopictus) and November (Cx. quinquefasciatus); 66.66% of human host identifications between both species occurred in October–November, after the seasonal human case peak. Based upon our data, WNV activity in both mosquito species warrants further investigation of their individual roles in WNV ecology within this region.
Keywords: West Nile virus, Culex quinquefasciatus, Aedes albopictus, gravid trap, minimum infection rate, Cyanocitta cristata, blue jay, blood meal analysis
INTRODUCTION
Since the discovery of West Nile virus (WNV) in Harris County (Houston), Texas in 2002, local studies have focused primarily upon Culex quinquefasciatus Say, considered the primary vector of WNV in the western Gulf Coast region, and this species’ particular role in WNV ecology (Lillibridge et al. 2004, Tesh et al. 2004). Little attention has been given to other mosquito species within the region that potentially could be involved in WNV transmission.
Overshadowed by the number of Cx. quinquefasciatus collected through routine gravid trapping in diverse habitats throughout Harris County, Aedes albopictus (Skuse) was in 2nd place in terms of producing positive WNV pools, from albeit comparatively small samples. On average, gravid traps collected 52.37 Cx. quinquefasciatus compared with 3.17, or approximately 17 times as many, Ae. albopictus on a per trapping period basis.
The findings presented herein were obtained during routine WNV surveillance using gravid traps for mosquito collection throughout Harris County and represent the 2 most commonly infected mosquito species observed, Cx. quinquefasciatus and Ae. albopictus, and associations between each species, WNV activity, and climatic data over the past 4 years. This information was gained through ongoing collaboration between the Harris County Public Health and Environmental Services, Mosquito Control Division (MCD), and the Department of Pathology, Center for Biodefense and Emerging Infectious Diseases, University of Texas Medical Branch at Galveston (UTMB-G) during the period of January 2003 through December 2006; mosquito blood meal analysis resulted from collaboration between MCD and the Center for Geographic Medicine, Division of Infectious Diseases, University of Alabama at Birmingham.
MATERIALS AND METHODS
The MCD gravid trapping program centers upon the Harris County Gravid Trap (HCGT), designed to MCD specifications and manufactured by John W. Hock Company (model 1712.55; John W. Hock Company, Gainesville, FL, http://www.johnwhockco.com), coupled with Vollrath® Models 52636 and 52660 black polypropylene bus boxes (The Vollrath Co., LLC, Sheboygan, WI, http://www.vollrathco.com) containing a 6.3 cm depth of 3.7–4.7 liters of Coastal bermudagrass (Cynodon dactylon (L.) Pers.) hay infusion, 10–14 days old. These traps were powered by 4 D-size heavy-duty alkaline batteries, connected in series-parallel, which were replaced after every 4–5 trapping periods.
Each week, up to 314 HCGT trapping periods (18–24 h each) were obtained by the MCD Surveillance Section (134 gravid traps set throughout county) and the UTMB-G Project Section (180 gravid traps set within Interstate Highway [IH] 610 loop surrounding Houston) by setting traps within mosquito control operational areas that subdivide Harris County. Upon retrieval of traps, all mosquito collections were transported, handled, and stored in a manner described by Lillibridge et al. (2004).
Mosquito pools from UTMB-G study areas within the IH 610 loop were transported to Galveston and processed individually for determination of WNV infection rates. Mosquito pools were homogenized individually in about 1.0 ml of phosphate-buffered saline, pH 7.4, containing 30% fetal bovine serum and antibiotics (penicillin, streptomycin, and amphotericin), using a Ten Broeck tissue grinder or a TissueLyser (Qiagen, Inc., Valencia, CA). The resultant suspension was centrifuged at ~13,600 × g (12,000 RPM) for 5 min; then 200 μl of the supernatant was inoculated into a culture of Vero E6 cells. After absorption for 1 h at 37°C, maintenance medium was added. Cultures were maintained in an incubator at 37°C and examined daily for evidence of viral cytopathic effect for 14 days. If the culture showed cytopathic effect, the culture medium was tested by VecTest® West Nile antigen-capture assay (Medical Analysis Systems, Microgenics Corporation, Fremont CA) for confirmation, following the manufacturer’s instructions. Dead birds including blue jays (Cyanocitta cristata L.) were collected, screened, stored, and tested in a manner consistent with Lillibridge et al. (2004).
All Cx. quinquefasciatus and Ae. albopictus collected throughout Harris County and tested by MCD over the 4-year period were contained in pools (≤50) and homogenized using a TissueLyser with approximately 1.7 ml of BA-1 (pH 7.4) containing steel beads, antibiotics, and centrifuged at ~9,400 × g (10,000 RPM) for 4–8 min (4°C). Throughout this period, all mosquito pools submitted were tested by enzyme-linked immunosorbent assay (ELISA) antigen-capture immunoassay to screen for the presence of both West Nile (100 μl supernatant) and St. Louis encephalitis (SLE; 50 μl supernatant) viruses in enzyme immunoassay (EIA) plates. SLE capture antibody 4A4C-4, diluted 1:4,000, and West Nile monoclonal capture antibody 3.91D, diluted 1:2,000, were used to sensitize the microtest plates. Flavivirus group specific, monoclonal detection antibody 6B6C-1 HRP, diluted 1:3,000 for SLE and 1:10,000 for West Nile, was used in assays. Both capture antibodies and the detection antibody were supplied by the Centers for Disease Control and Prevention, Division of Vector-Borne Infectious Diseases, Fort Collins, CO, until May 2004, when the West Nile capture antibody became commercially available. Antibody 3.91D was subsequently purchased from Chemicon International (Temecula, CA) during the latter half of 2004.
In 2003, test methods included ELISA, ELISA inhibition assay (to verify ELISA results), plaque assays in Vero cell cultures, VecTest WNV/SLE antigen capture, mice inoculations, and complement fixation (CF) tests for viral identification and confirmation. During inoculation of Vero 76 cells, 200 μl of supernatant was allowed 1 h absorption at 37°C. All plaque assays consisted of double-agar overlays, with neutral red dye incorporated in the 2nd overlay, which were conducted on West Nile and SLE EIA positive pools, with 200 μl of mosquito homogenate inoculated into one 25-cm2 flask of Vero cells for each positive-suspect pool. The initial overlay was done after a 1-h incubation of inoculated cultures. The 2nd overlay was conducted following a 4-day incubation period. Cultures showing plaque formation and/or cell lysis were harvested, with 125 μl of mosquito pool sample mixed with 125 μl of VecTest buffer and tested using VecTest. Select pools from areas with prior histories of viral activity were tested concurrently by ELISA and in cell cultures. Vero harvests of positive-suspect pools showing plaque formation or cell lysis were tested by VecTest and then inoculated into suckling mice. Pools positive in mice were tested by CF test for final confirmation. Mice inoculations were suspended in early August of 2003 because of laboratory facility construction.
The Rapid Analyte Measurement Platform (RAMP®; manufactured by Response Biomedical Corp., Burnaby, BC, Canada, and supplied in the United States by Adapco, Inc., Sanford, FL) was evaluated in the fall of 2003 as a rapid means of confirming West Nile isolates using 50 μl supernatant.
In 2004, activities mirrored those of 2003 except that mice inoculations were eliminated and the RAMP test was used for confirmation of West Nile isolates and only those mosquito pools that were ELISA positive-suspect were inoculated in tissue cultures. The CF test was retained as a means of confirming SLE isolates from tissue culture positive pools. In 2005, EIA inhibition assays and cell culture inoculations for West Nile were dropped because of the successful incorporation of the RAMP test to confirm ELISA WNV positives from original mosquito pool samples. Owing to the reliability of the RAMP test for confirmation of West Nile, Vero tissue culture inoculations were conducted solely on SLE EIA/inhibition assay positive-suspect pools. VecTest and/or complement fixation tests were conducted on positive and lysed Vero tissue culture harvests for virus identification and confirmation. The test timeline was restructured so that pools could be tested within the same week of collection. Prior to this, pools were collected one week but not tested until the following week once all samples had been identified and pooled. Under the new plan, trapping and pooling was conducted 3 or 4 times a week, and ELISA and RAMP testing was changed from conducting 1 large weekly test to testing up to 3 times a week. Consequently, we were able to get results within a week of collection (3 days), which, in turn, allowed for faster mosquito control operational response.
During 2005, mosquitoes with blood meals were pooled, placed within labeled vials, frozen (−20°C), and shipped periodically to the University of Alabama at Birmingham for blood meal analysis. Abdomens from blooded mosquitoes were homogenized in 1 ml of phosphate-buffered saline; homogenates were subjected to centrifugation at ~15,900 × g (13,000 RPM) at room temperature for 10 min, and DNA prepared from 225 μl of the supernatants using the DNeasy preparation kits (Qiagen), following the manufacturer’s protocol. The DNA was eluted into 50 μl of AE buffer (Qiagen) and used as a template in a modified version of a previously described polymerase chain reaction (PCR) based assay for the specific amplification of a portion of the cytochrome B gene encoded in the DNA present in avian derived blood meals (Lee et al. 2002). In brief, 2.5 μl of the template DNA was added to a reaction mixture containing 60 mM Tris-HCl (pH 8.5),15 mM ammonium sulfate, 1.5 mM MgCl2, 200 μM of each deoxyribonucleotide triphosphate (dNTP), 0.5 μM of each primer, and 2.5 units of Taq1 polymerase. The primers used in the reaction were as follows: 5′-CCCCTCAGAATGATATTTGTCCTCA-3′ and 5′-CCATCCAACATCTCAGCATGATGAAA-3′. The final volume of the reaction was 50 μl. Cycling conditions consisted of an initial incubation at 95°C for 3.5 min, followed by 40 cycles of 30 sec at 95°C, 50 sec at 60°C, and 40 sec at 72°C. The reaction completed by final extension at 72°C for 6 min. One microliter of this reaction was then employed in a nested PCR reaction containing 60 mM Tris-HCl (pH 8.5), 15 mM ammonium sulfate, 1.5 mM MgCl2, 200 μM of each dNTP, 0.5 μM of each primer, and 1.25 units of Taq1 polymerase, in a total volume of 50 μl. The primers used in the nested PCR reaction were as follows: 5′-TCWRCHT GATGAAACTTCGG-3′ and 5′-ACRAA RGCVGTKGCYATDAG-3′ where W = A or T; R = A or G; H = A, C, or T; V = A, C, or G; K = G or T; Y = C or T; and D = A, G, or T. Cycling conditions consisted of an initial step of 95°C for 3 min, followed by 40 cycles of 30 sec at 95°C, 1 min at 55°C, and 1 min at 72°C. The reaction completed by final extension at 72°C for 7 min. The amplification products were analyzed by agarose gel electrophoresis.
Samples that produced no amplicons in the avian specific PCR assay were then retested in a vertebrate-specific assay, which was a slight modification of previously published methods (Cupp et al. 2004). Amplifications were carried out in a total volume of 50 μl containing 60 mM Tris HCl (pH 8.5), 15 mM ammonium sulfate, 3.5 mM MgCl2, 200 μM of each dNTP, 0.5 μM of each primer, 2.5 units of Taq1 polymerase, and 2.5 μl of template DNA. The primers used in the reaction were as follows: 5′-CCCCTCAGAATGATATTTGTCCTCA - 3 ′ and 5 ′-GCHGAYACHWVHHYHGCHTTYTCHTC-3′, where W = A or T; H = A, C, or T; Y = C or T; V = A, C, or G; M = A or C; K = G or T; and R = A or G. Cycling conditions consisted of an initial step of 94°C for 2 min, followed by 55 cycles comprising 45 sec at 94°C, 50 sec at 50°C, and 1 min at 72°C. The reaction was completed by final extension at 72°C for 7 min. Products were analyzed by agarose gel electrophoresis as described above.
Amplicons from reactions producing products were purified using the Qiaquick PCR purification kit (Qiagen) and their DNA sequence directly determined. The origin of the blood meal was then determined by comparison of the DNA sequence with the sequences present in the Genbank DNA sequence bank. A sequence match of greater than 95% to 1 in the Genbank was considered as match the target species.
Field data and pooling information were entered into Microsoft®Access 2000 databases for archiving and retrieval, whereas database queries of trapping and laboratory results were entered into JMP™ v. 5.0 (SAS Institute, Inc., Cary, NC) and SigmaPlot® 2002 (Systat Software, Inc., Richmond CA) for analysis of log(Y + 1) transformed data and graphing purposes. The estimated minimum infection rate (MIR) presented by month for both mosquito species was calculated using the standard formula cited in Condotta et al. (2004), which follows:
so that laboratory findings from MCD (variable pool sizes) and UTMB-G (individually pooled) during the sampling period covered could be reported together. Since MIR generally represents a rate of infection for a particular mosquito species within a given area over time based upon a single infected specimen, regardless of pool size, this formula was used to estimate monthly rates. For both species, transformed data were subjected to pairwise correlations within a multivariate platform to generate Pearson product-moment correlations, and significance probabilities for each pair of variables using monthly data over 4 years to identify associations between 6 variables (mean mosquitoes collected, confirmed mosquito pools, confirmed blue jays, confirmed human cases [based upon month of onset], rainfall, and temperature). Transformed data were also subjected to analysis of variance to determine monthly differences for each variable throughout the period, and Tukey-Kramer honestly significant difference tests for means separation. For graphic interpretation of both mosquito species, monthly mean number of mosquitoes collected and confirmed pools were plotted against monthly mean human cases, confirmed blue jays, rainfall, and temperature. Weather data were obtained from William Hobby Airport (Houston) and was representative of the portion of Harris County that contained >70% of all positive mosquito pools from 2003 to 2006. Results of blood meal analyses were presented in chronological order and percentage of total positive mammalian and avian host identifications made for both mosquito species.
RESULTS
Monthly human WNV cases were highly correlated to positive Cx. quinquefasciatus (r = 0.87) and Ae. albopictus (r = 0.78) pools, blue jays (r = 0.83), and number of Ae. albopictus collected (r = 0.71), but weakly correlated with the number of Cx. quinquefasciatus collected (r = 0.45) (Tables 1 and 2). Human WNV cases were also strongly associated with monthly temperature (r = 0.71), compared with rainfall (r = 0.29) (Tables 1 and 2). Monthly temperature was highly correlated with Ae. albopictus and Cx. quinquefasciatus collections (r = 0.88 and 0.70, respectively), and positive Cx. quinquefasciatus pools (r = 0.75), but not positive Ae. albopictus pools (r = 0.55) (Tables 1 and 2). Mosquitoes collected and positive pools between both species, and positive blue jays were rather weakly correlated (r ≤ 0.41) with monthly rainfall (Tables 1 and 2). Positive blue jays were strongly correlated with positive Cx. quinquefasciatus pools (r = 0.87), compared with Ae. albopictus pools (r = 0.67), monthly Ae. albopictus collections (r = 0.69), and Cx. quinquefasciatus collections (r = 0.46) (Tables 1 and 2).
Table 1.
Pairwise correlations between gravid trap collections of Culex quinquefasciatus, confirmed West Nile virus activity, temperature, and rainfall in Harris County, Texas from 2003 to 2006.1
| Variable | Variable | Correlation | Count | Significant probability |
|---|---|---|---|---|
| No. confirmed blue jays | No. mosquitoes collected | 0.4635 | 48 | 0.0009 |
| No. confirmed + pools | No. mosquitoes collected | 0.5417 | 48 | 0.0001 |
| No. confirmed + pools | No. confirmed blue jays | 0.8708 | 48 | 0.0000 |
| Rainfall (cm) | No. mosquitoes collected | 0.2589 | 48 | 0.0756 |
| Rainfall (cm) | No. confirmed blue jays | 0.3254 | 48 | 0.0240 |
| Rainfall (cm) | No. confirmed + pools | 0.4067 | 48 | 0.0041 |
| Temperature (°C) | No. mosquitoes collected | 0.7070 | 48 | 0.0000 |
| Temperature (°C) | No. confirmed blue jays | 0.7409 | 48 | 0.0000 |
| Temperature (°C) | No. confirmed + pools | 0.7564 | 48 | 0.0000 |
| Human cases | No. mosquitoes collected | 0.4587 | 48 | 0.0010 |
| Human cases | No. confirmed blue jays | 0.8357 | 48 | 0.0000 |
| Human cases | No. confirmed + pools | 0.8704 | 48 | 0.0000 |
| Human cases | Rainfall (cm) | 0.2985 | 48 | 0.0394 |
| Human cases | Temperature (°C) | 0.7114 | 48 | 0.0000 |
Based upon analysis of log(Y + 1) transformed monthly data for all variables; for each Pearson product-moment correlation (r) listed, significant probability refers to likelihood of gaining a correlation with greater absolute value should no relationship exist between specific variables.
Table 2.
Pairwise correlations between gravid trap collections of Aedes albopictus, confirmed West Nile virus activity, temperature, and rainfall in Harris County, Texas from 2003 to 2006.1
| Variable | Variable | Correlation | Count | Significant probability |
|---|---|---|---|---|
| No. confirmed blue jays | No. mosquitoes collected | 0.6959 | 48 | 0.0000 |
| No. confirmed + pools | No. mosquitoes collected | 0.6232 | 48 | 0.0000 |
| No. confirmed + pools | No. confirmed blue jays | 0.6791 | 48 | 0.0000 |
| Rainfall (cm) | No. mosquitoes collected | 0.3007 | 48 | 0.0378 |
| Rainfall (cm) | No. confirmed blue jays | 0.3254 | 48 | 0.0240 |
| Rainfall (cm) | No. confirmed + pools | 0.3115 | 48 | 0.0311 |
| Temperature (°C) | No. mosquitoes collected | 0.8850 | 48 | 0.0000 |
| Temperature (°C) | No. confirmed blue jays | 0.7409 | 48 | 0.0000 |
| Temperature (°C) | No. confirmed + pools | 0.5546 | 48 | 0.0000 |
| Human cases | No. mosquitoes collected | 0.7155 | 48 | 0.0000 |
| Human cases | No. confirmed blue jays | 0.8357 | 48 | 0.0000 |
| Human cases | No. confirmed + pools | 0.7819 | 48 | 0.0000 |
| Human cases | Rainfall (cm) | 0.2985 | 48 | 0.0394 |
| Human cases | Temperature (°C) | 0.7114 | 48 | 0.0000 |
Based upon analysis of log (Y + 1) transformed monthly data for all variables; for each Pearson product-moment correlation (r) listed, significant probability refers to likelihood of gaining a correlation with greater absolute value should no relationship exist between specific variables.
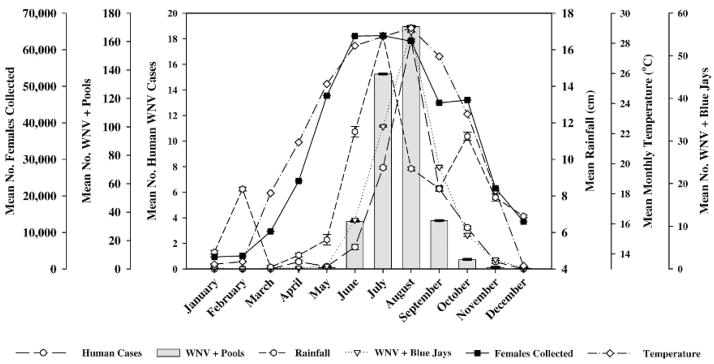
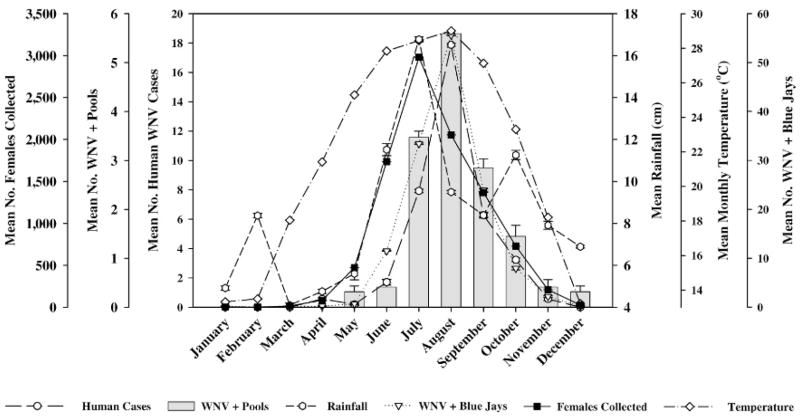
Highly significant monthly differences (P < 0.05) were observed in the number of Cx. quinquefasciatus trapped; during June through August, over 62,000 mosquitoes were collected compared with January, with only 3,282 collected (Table 3 and Fig. 1). Monthly collections of Ae. albopictus peaked in July with 2,981 mosquitoes trapped, which was not significantly different (P ≥ 0.05) from June (1,736), August (2,054), and September (1,367) and smaller collections in May (472) and October (728) (Table 3 and Fig. 2).
Table 3.
Mean (±SE) monthly collections per gravid trap and estimated infection rates of Culex quinquefasciatus and Aedes albopictus observed from January 2003 through December 2005 in Harris County (Houston), Texas.
| Specimens collected1 |
Minimum infection rate2 |
|||
|---|---|---|---|---|
| Month | Cx. quinquefasciatus | Ae. albopictus | Cx. quinquefasciatus | Ae. albopictus |
| January | 3,282.85 (0.53) B | 3.00 (0.27) F | 0.00 D | 0.00 A |
| February | 3,578.52 (0.43) AB | 1.21 (0.16) F | 0.00 D | 0.00 A |
| March | 10,259.17 (0.33) AB | 15.22 (0.40) EF | 0.00 D | 0.00 A |
| April | 24,068.77 (0.23) AB | 82.94 (0.18) CDE | 0.00 D | 0.00 A |
| May | 47,398.29 (0.21) AB | 472.20 (0.28) ABC | 0.07 (0.03) D | 0.37 (0.13) A |
| June | 63,725.04 (0.13) A | 1,736.57 (0.23) AB | 1.06 (0.08) BC | 0.16 (0.06) A |
| July | 63,851.25 (0.13) A | 2,981.80 (0.09) A | 4.06 (0.03) A | 1.92 (0.07) A |
| August | 62,300.54 (0.08) A | 2,054.50 (0.21) AB | 4.55 (0.03) A | 4.41 (0.24) A |
| September | 45,444.53 (0.04) AB | 1,367.59 (0.12) AB | 1.30 (0.08) B | 3.17 (0.14) A |
| October | 46,204.19 (0.10) AB | 728.04 (0.15) ABC | 0.31 (0.04) CD | 1.84 (0.26) A |
| November | 22,087.22 (0.15) AB | 208.30 (0.20) BCD | 0.09 (0.01) D | 0.57 (0.19) A |
| December | 12,950.06 (0.16) AB | 26.15 (0.15) DEF | 0.06 (0.02) D | 2.13 (0.49) A |
Reported as back-transformed means after analysis of variance for each variable with n = 48, and α = 0.05; F ratio (P > F) for collections and minimum infection rate were 3.33 (0.003), 40.13 (<0.0001) for Cx. quinquefasciatus and 23.85 (<0.0001), 1.90 (0.07) for Ae. albopictus. Means within columns for a specific variable followed by the same uppercase letter are not significantly different from one another based upon Tukey-Kramer honestly significant difference tests.
The number of positive mosquito pools/number of mosquitoes tested × 1,000, based upon the premise that a single positive specimen was contained within pools of up to 50 specimens total.
Fig. 1.
Overlay of female Culex quinquefasciatus collected using gravid traps, West Nile virus (WNV) activity, average temperature, and rainfall by month from January 2003 through December 2006. Based upon log(Y + 1) transformed monthly data; plotted means reflect back-transformation to original scale, whereas standard errors remain on transformed scale, as suggested by Steel and Torrie (1980).
Fig. 2.
Overlay of female Aedes albopictus collected using gravid traps, West Nile virus (WNV) activity, average temperature, and rainfall by month from January 2003 through December 2006. Based upon log(Y + 1) transformed monthly data; plotted means reflect back-transformation to original scale, whereas standard errors remain on transformed scale, as suggested by Steel and Torrie (1980).
Peak monthly MIRs for Cx. quinquefasciatus were observed in July (4.06) and August (4.55), which were significantly different from June and September (1.06, and 1.30, respectively), and remaining months with MIR ≤ 0.31; no significant monthly differences were detected in terms of MIR for Ae. albopictus (Table 3, Figs. 1 and 2).
The highest number of monthly human WNV cases was in August (17.87), which was not significantly different (P ≥ 0.05) from the number observed in July (7.92) and September (6.27); human WNV cases were observed April through November, with no human cases December through March (Table 4, Figs. 1 and 2). Similarly, peak activity in positive blue jays also occurred in August (55.58), which was not significantly different from June (11.48), July (33.38), and September (23.93); with the exception of February, monthly WNV activity in blue jays was observed (Table 4, Figs. 1 and 2).
Table 4.
Mean (±SE) monthly West Nile virus (WNV) confirmed human cases, confirmed blue jays, temperatures, and rainfall observed from January 2003 through December 2006 in Harris County (Houston), Texas.1
| Month | Human cases2 | Blue jays3 | Temperature (°C)4 | Rainfall (cm)4 |
|---|---|---|---|---|
| January | 0.00 E | 0.18 (0.07) E | 13.31 (0.03) E | 4.91 (0.10) A |
| February | 0.00 E | 0.00 E | 13.49 (0.02) E | 8.37 (0.10) A |
| March | 0.00 E | 0.18 (0.07) E | 18.04 (0.01) D | 4.09 (0.10) A |
| April | 0.56 (0.11) DE | 0.18 (0.07) E | 21.42 (0.01) CD | 4.75 (0.10) A |
| May | 0.18 (0.07) E | 0.68 (0.14) DE | 25.29 (0.00) ABC | 5.59 (0.29) A |
| June | 1.71 (0.19) CDE | 11.48 (0.09) ABC | 27.84 (0.00) A | 11.51 (0.28) A |
| July | 7.92 (0.11) AB | 33.38 (0.12) AB | 28.44 (0.00) A | 16.77 (0.11) A |
| August | 17.87 (0.09) A | 55.58 (0.12) A | 29.01 (0.00) A | 9.49 (0.07) A |
| September | 6.27 (0.13) ABC | 23.93 (0.24) AB | 27.13 (0.01) AB | 8.39 (0.14) A |
| October | 3.22 (0.12) BCD | 7.92 (0.30) BCD | 23.30 (0.01) BC | 11.25 (0.22) A |
| November | 0.56 (0.11) DE | 2.15 (0.21) CDE | 18.21 (0.00) D | 7.90 (0.19) A |
| December | 0.00 E | 0.18 (0.07) E | 13.21 (0.00) E | 6.88 (0.06) A |
Reported as back-transformed means after analysis of variance for each variable with n = 48, and α = 0.05; F ratio (P > F) for human cases, blue jays, temperature, and rainfall were 18.12 (<0.0001), 18.53 (<0.0001), 76.62 (<0.0001), and 0.90 (0.54), respectively. Means within columns for a specific variable followed by the same uppercase letter are not significantly different from one another based upon Tukey-Kramer honestly significant difference tests.
Based upon 174 WNV human cases with estimated dates of onset within Harris County.
WNV-confirmed blue jays obtained from dead bird collections throughout Harris County out of 709 total.
Meteorological data (2003–06) from William Hobby Airport in southeastern Houston.
Highest monthly temperature was also in August (29.01°C), which was not significantly different from temperatures observed in May (25.29°C), June (27.84°C), July (28.44°C), and September (27.13°C); significantly lower temperatures occurred during December (13.21°C), January (13.31°C), and February (13.49°C) (Table 4, P Figs. 1 and 2). No significant differences ( ≥ 0.05) were observed in monthly rainfall during the period, but amounts ranged from 4.09 cm in March to 16.77 cm in July (Table 4, Figs. 1 and 2).
Between both species collected in 2005, mammalian hosts identified through blood meal analysis consisted of dog (54.83%), human (29.03%), and rat (9.67%); 68.18% of Cx. quinquefasciatus mammalian hosts were dog, while 22.72% were human (Table 5). One multiple blood meal from Cx. quinquefasciatus was identified as being from both wild turkey and dog (Table 5). Although sample sizes were small, a higher percentage of human hosts was observed in Ae. albopictus (44.44%), but fewer dog hosts (22.22%) were identified, and comparable in number to rat hosts (22.22%) (Table 5).
Table 5.
Number and percentage of positive mammalian blood meal identifications made between Culex quinquefasciatus and Aedes albopictus collected from gravid traps within Harris County’s Interstate Highway 610 inner loop during 2005.1
| Number (%)
|
|||
|---|---|---|---|
| Hosts identified | Cx. quinquefasciatus | Ae. albopictus | Number (%) both species |
| Dog | 15 (68.18) | 2 (22.22) | 17 (54.83) |
| Human | 5 (22.72) | 4 (44.44) | 9 (29.03) |
| Rat | 1 (4.54) | 2 (22.22) | 3 (9.67) |
| Mixed (wild turkey/dog) | 1 (4.54) | — | 1 (3.22) |
| Pig | — | 1 (11.11) | 1 (3.22) |
| Total mammalian | 22 (99.98) | 9 (99.99) | 31 (99.97) |
Number of positive host identifications made out of 121 Cx. quinquefasciatus and 26 Ae. albopictus submitted for blood meal analysis.
Ten bird species, across 8 families, were identified as hosts for Cx. quinquefasciatus, with northern cardinal (8) and blue jay (6) having the highest host percentages (26.66% and 20.00%, respectively) out of 30 avian host identifications; however, no bird feeding activity was observed in 26 Ae. albopictus submitted (Table 6).
Table 6.
Number and percentage of positive avian blood meal identifications made between Culex quinquefasciatus and Aedes albopictus collected from gravid traps within Harris County’s Interstate Highway 610 inner loop during 2005.1
| Number (%)
|
|||
|---|---|---|---|
| Hosts identified | Cx. quinquefasciatus | Ae. albopictus | Number (%) both species |
| Northern cardinal | 8 (26.66) | — | 8 (26.66) |
| Blue jay | 6 (20.00) | — | 6 (20.00) |
| Common grackle | 4 (13.33) | — | 4 (13.33) |
| Chicken | 3 (10.00) | — | 3 (10.00) |
| House sparrow | 3 (10.00) | — | 3 (10.00) |
| Wild turkey | 2 (6.66) | — | 2 (6.66) |
| Boat-tailed grackle | 1 (3.33) | — | 1 (3.33) |
| Cooper’s hawk | 1 (3.33) | — | 1 (3.33) |
| Eastern screech-owl | 1 (3.33) | — | 1 (3.33) |
| Mourning dove | 1 (3.33) | — | 1 (3.33) |
| Total avian | 30 (99.97) | — | 30 (99.97) |
Number of positive host identifications made out of 121 Cx. quinquefasciatus and 26 Ae. albopictus submitted for blood meal analysis.
The earliest human blood meal occurred in May (Ae. albopictus), and the latest in November (Cx. quinquefasciatus) (Table 7). During the months of peak human WNV cases (July–September), only 1 human blood meal was identified, and belonged to Cx. quinquefasciatus; 66.66% of human host identifications between both species occurred in October–November, after the seasonal peak in human WNV cases, with an equal number of both species obtaining human blood meals (Table 7). During July–September, a majority of Cx. quinquefasciatus sampled were feeding upon birds (61.90%), and to a lesser extent, dogs (33.33%), whereas in October, both species acquired human and dog blood meals (Table 7). Of 6 Cx. quinquefasciatus blood meals identified as specifically from blue jays, 50% were obtained during this same period, coinciding with peak blue jay mortality.
Table 7.
Vertebrate hosts identified by month through blood meal analysis of Culex quinquefasciatus and Aedes albopictus collected from gravid traps located within Harris County’s Interstate Highway 610 inner loop during 2005.
| Month | Week | Mosquito species | Sequence match (species) | Family |
|---|---|---|---|---|
| May | 20 | aeab | Human (Homo sapiens) | Hominidae |
| May | 20 | aeab | Norway rat (Rattus norvegicus) | Muridae |
| May | 20 | cxqf | Northern cardinal (Cardinalis cardinalis) | Cardinalidae |
| May | 20 | cxqf | Common grackle (Quiscalus quiscula) | Icteridae |
| May | 21 | cxqf | Human (H. sapiens) | Hominidae |
| June | 22 | cxqf | Chicken (Gallus gallus) | Phasianidae |
| June | 23 | cxqf | Dog (Canis lupus)1 | Canidae |
| June | 23 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| June | 23 | cxqf | Blue jay (Cyanocitta cristata) | Corvidae |
| June | 24 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| June | 26 | cxqf | Dog (C. lupus) | Canidae |
| June | 26 | cxqf | Eastern Wood Rat (Neotoma floridana) | Muridae |
| June | 26 | cxqf | Boat-tailed grackle (Q. major) | Icteridae |
| June | 26 | cxqf | Mourning dove (Zenaida macroura) | Columbidae |
| July | 27 | cxqf | Dog (C. lupus) | Canidae |
| July | 27 | cxqf | House sparrow (Passer domesticus) | Passeridae |
| July | 27 | cxqf | Common grackle (Q. quiscula) | Icteridae |
| July | 28 | cxqf | Dog (C. lupus) | Canidae |
| July | 28 | cxqf | Dog (C. lupus) | Canidae |
| July | 28 | cxqf | Wild turkey (Meleagris gallopavo) | Phasianidae |
| July | 29 | aeab | Norway Rat (R. norvegicus) | Muridae |
| July | 29 | cxqf | Dog (C. lupus) | Canidae |
| July | 30 | cxqf | Blue jay (C. cristata) | Corvidae |
| July | 30 | cxqf | Chicken (G. gallus) | Phasianidae |
| August | 31 | cxqf | Dog (C. lupus) | Canidae |
| August | 32 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| August | 32 | cxqf | Blue jay (C. cristata) | Corvidae |
| August | 32 | cxqf | Blue jay (C. cristata) | Corvidae |
| August | 32 | cxqf | Wild turkey/dog (mixed) (M. gallopavo, C. lupus) | Phasianidae/Canidae |
| August | 33 | aeab | Pig (Sus scrofa) | Suidae |
| August | 34 | cxqf | Human (H. sapiens) | Hominidae |
| August | 35 | cxqf | Dog (C. lupus) | Canidae |
| August | 35 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| September | 36 | cxqf | Dog (C. lupus) | Canidae |
| September | 36 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| September | 36 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
| September | 39 | cxqf | Cooper’s hawk (Accipiter cooperii) | Accipitridae |
| October | 40 | aeab | Dog (C. lupus) | Canidae |
| October | 40 | aeab | Human (H. sapiens) | Hominidae |
| October | 40 | aeab | Human (H. sapiens) | Hominidae |
| October | 40 | cxqf | Dog (C. lupus) | Canidae |
| October | 40 | cxqf | Blue jay (C. cristata) | Corvidae |
| October | 40 | cxqf | Blue jay (C. cristata) | Corvidae |
| October | 41 | cxqf | Human (H. sapiens) | Hominidae |
| October | 41 | cxqf | House sparrow (P. domesticus) | Passeridae |
| October | 42 | cxqf | Common grackle (Q. quiscula) | Icteridae |
| October | 43 | aeab | Human (H. sapiens) | Hominidae |
| October | 43 | cxqf | Human (H. sapiens) | Hominidae |
| November | 44 | cxqf | House sparrow (P. domesticus) | Passeridae |
| November | 45 | aeab | Dog (C. lupus) | Canidae |
| November | 45 | cxqf | Dog (C. lupus) | Canidae |
| November | 45 | cxqf | Dog (C. lupus) | Canidae |
| November | 45 | cxqf | Eastern screech-owl (Megascops asio) | Strigidae |
| November | 46 | cxqf | Dog (C. lupus) | Canidae |
| November | 46 | cxqf | Chicken (G. gallus) | Phasianidae |
| November | 47 | cxqf | Dog (C. lupus) | Canidae |
| November | 47 | cxqf | Dog (C. lupus) | Canidae |
| November | 47 | cxqf | Human (H. sapiens) | Hominidae |
| November | 48 | cxqf | Wild turkey (M. gallopavo) | Phasianidae |
| December | 50 | cxqf | Common grackle (Q. quiscula) | Icteridae |
| December | 51 | cxqf | Northern cardinal (C. cardinalis) | Cardinalidae |
Canis lupus familiaris.
West Nile virus human cases occurred from April through November, whereas positive blue jays were obtained monthly, with the exception of February, during which no birds were collected 2003–06 (Table 4). The majority of WNV human cases occurred in July, August, and September, mirroring the highest level of blue jay mortality, and environmental temperatures in excess of 28.19°C. Temperatures from May–September were in excess of that required for extrinsic virus amplification in both species (Dohm et al. 2002; Sardelis et al. 2002; Girard et al. 2004).
December, January, and February temperatures were lower than 14°C, a limiting temperature that Reisen et al. (2006b) suggested as minimum for infection of Cx. quinquefasciatus with WNV, although year-round virus activity has been reported in this species (Tesh et al. 2004). Mean peak activity in terms of human cases (17.87), and positive blue jays (55.58), coincided with a mean August temperature of 29.01°C (Table 4). The highest mosquito production occurred in July for Cx. quinquefasciatus (>63,000) and for Ae. albopictus (>2,900), whereas maximum observed infection rates for both species, 4.55 and 4.41, occurred in August (Table 3).
The occurrence of confirmed human cases, positive blue jays, and climatic conditions delineated a general virus activity pattern in Figs. 1 and 2, whereas collection data and number of positive pools for both species superposed over the general pattern yielded some unexpected outcomes, namely, the temporal differences of both species during peak virus activity.
DISCUSSION
The historical prominence of Cx. quinquefasciatus within Harris County as a primary vector for WNV has been previously well established, and our data reflect full agreement with previous local studies (Lillibridge et al. 2004). Although WNV activity in Cx. quinquefasciatus throughout the year has been reported within the western Gulf Coast region, our data suggest that WNV remains at extremely low, or undetectable, levels January–April (Tesh et al. 2004).
Currently, Ae. albopictus is commonly referred to as a secondary, or bridge vector, and it is simply not collected in numbers comparable with that of Cx. quinquefasciatus in HCGT; however, our data suggest that Ae. albopictus may potentially be more involved in WNV transmission, in the western Gulf Coast region, than previously thought. In reference to Fig. 2, Barnett (1960) observed similar temporal relationships between Cx. tritaeniorhynchus Giles populations and human cases of Japanese encephalitis in Tokyo, Japan, during the 1950 epidemic.
Barnett (1960) and Harwood and James (1979) stated that several criteria exist when implicating a specific arthropod with transmission of a pathogen, namely, 1) association (feeding upon the host), 2) specific connection (spatial and/or temporal between arthropod and clinical/subclinical host infection), 3) consistency (naturally harbors pathogen), 4) transmission (pathogen transfer under controlled conditions), and 5) biological gradient (high/low arthropod population matches high/low host disease). Four of these criteria were highlighted by DeFoliart et al. (1986) in their description of the importance of localized ecological conditions, human activity, vector bionomics, and useful, real-world application of research findings toward reduction of mosquito-borne arboviruses. Our data suggest that Cx. quinquefasciatus and Ae. albopictus meet these criteria under our localized ecological conditions and support potential involvement Ae. albopictus may have in transmission of WNV within Harris County.
Host-feeding studies indicate that Ae. albopictus is quite opportunistic and will feed upon a broad range of vertebrate hosts (Sullivan et al. 1971, Savage et al. 1993, Niebylski et al. 1994, Ponlawat and Harrington 2005, Richards et al. 2006). In all 5 studies, blood meals from humans and dogs were commonly detected; however, passeriform birds were also detected to a much lesser extent within 3 studies (Savage et al. 1993, Niebylski et al. 1994, Richards et al. 2006).
The opportunistic nature of Cx. quinquefasciatus and Ae. albopictus we observed through blood meal analysis was in agreement with previous studies, especially in that humans and dogs were fed upon by both species (Niebylski and Meek 1992, Zinser et al. 2004). Recently, WNV activity was detected through hemagglutination-inhibition tests (against WNV antigen) in stray dogs collected from throughout Harris County, and 72 of 86 (83.72%) blood samples obtained during October–November 2006 ranged in titer from 1:80 to 1:2,560 (Harris County Public Health and Environmental Services and UTMB-G, unpublished data). This percentage is considerably higher than previously obtained in a study by Lillibridge et al. (2004) that reported 56.5% of stray dogs sampled from the Houston metropolitan area, shortly after WNV establishment in 2002, were WNV positive.
Owing to their opportunistic feeding and primarily ground-level appetitive flight habits, Ae. albopictus may feed upon passerine or other bird species that routinely forage or rest within vegetative ground cover where these mosquitoes are predominately found. Richards et al. (2006) indicated that Ae. albopictus had fed upon a northern cardinal, Cardinalis cardinalis L., a passeriform species that has routinely tested positive for WNV through serological testing of blood samples collected from mist-netted birds in Harris County from 2002 to 2005. During this period, 87 of 428 (20.3%) cardinals tested positive, compared with 81 of 479 (16.91%) blue jays during the same period (MCD and UTMB-G, unpublished data).
Blue jays have been termed regionally effective indicator species in terms of disease surveillance, owing in part to naturally high susceptibility to WNV, development of viremia as high as 12.1 log10 plaque-forming units (PFU)/ml 3 days after mosquito bite, and mortality in 4–5 days (Komar et al. 2003, Gibbs et al. 2005, Reisen et al. 2006a). In our study, the number of positive blue jays and pools for both mosquito species correlated well with the number of human cases, considering that Ae. albopictus was encountered far less than Cx. quinquefasciatus.
With the exception of salt marsh and ephemeral floodwater mosquito species that periodically appear after tropical or heavy storm activity, the most common nuisance pest in Harris County is Ae. albopictus, associated within a wide variety of peridomestic habitats, oftentimes unintentionally generated by homeowners who unknowingly infests their neighbors’ yards as a result of the mosquito’s limited flight range, up to 525 m (Niebylski and Craig 1994).
Based upon mean monthly onset from 2003 to 2006, human cases appeared in April and continued through November, with peak activity during August, which coincides with peak estimated MIR for both species. Temporally, onset of human cases and MIR overlap well with surveillance of both mosquito species. Based upon the modified description in Harwood and James (1979), peak mosquito abundance should, however, be slightly offset earlier in time from human case (and MIR) peaks, allowing for temperature-dependent amplification of virus within a population of a particular mosquito species (extrinsic incubation period [EIP]); temporal measurement from peak mosquito abundance to peak human cases should reflect the length of time required for one (host) to receive an infective bite and develop clinical symptoms (intrinsic incubation period).
The EIP for both species has been determined under laboratory conditions; Cx. quinquefasciatus has an EIP of 8 days at 28°C (Girard et al. 2004), whereas Ae. albopictus has an EIP of 10 days at 26°C (Sardelis et al. 2002). According to Heymann (2004), the intrinsic incubation period for West Nile fever in humans is 3–12 days upon receiving an infective bite. Data presented in Fig. 1 illustrate seasonal abundance of Cx. quinquefasciatus that remains consistently high June–July up to peak human cases in August, whereas only 1 month separates seasonal peak abundance of Ae. albopictus from peak human cases in Fig. 2. The upward population trend of Ae. albopictus, from early buildup in May through the seasonal peak in July, closely follows monthly rainfall, although weak correlation was evident between numbers of Ae. albopictus and rainfall year round, whereas the downward trend of human cases also coincides with a population decrease in Ae. albopictus, as opposed to the gradual population decrease through time with Cx. quinquefasciatus.
Recent climatic trends within the western Gulf Coast region may induce biological changes within Ae. albopictus as well; Pumpuni et al. (1992) suggested that at temperatures of 29°C, the incidence of diapause in Ae. albopictus was significantly reduced, whereas Lounibos et al. (2003) suggested that variable diapause in Florida Ae. albopictus could exist as far north as Jacksonville (30°N latitude). Harris County (Houston) is located at 29°N latitude, which provides one possible explanation why Ae. albopictus was still collected during December–January in Harris County, but this needs further verification. Alternatively, if a change in photoperiodic diapause was not a factor, the mosquito population witnessed during December–January, albeit low, may have been due to reflooded older, nondiapausal eggs deposited by earlier summer females. Even if Ae. albopictus is still active during the warmer winter months, changes in human habits come into play; reduced time outdoors due to shorter days and seasonal trends limits potential exposure to the insects. Our data also suggests that monthly temperature was more highly correlated to monthly mosquitoes collected, positive mosquito pools, and positive blue jays, than rainfall. This is inconsistent with Takeda et al. (2003), who reported that in terms of arbovirus activity, precipitation was more highly correlated than temperature, but this may be due to regional climatic and species differences.
During 2003–06, the average MIR and number of WNV positive pools per year for Ae. albopictus were 3.19 and 18.75, compared with Cx. quinquefasciatus with 1.61 and 419.50, respectively. The HCGT did not collect remarkable numbers of Ae. albopictus comparable with Cx. quinquefasciatus but did collect enough positive samples to elevate suspicion.
Numerous studies have shown that Ae. albopictus could potentially serve as an important vector of a wide variety of arboviruses, including WNV, under laboratory settings (Shroyer 1986, Mitchell 1991). Aedes albopictus were determined to be highly susceptible to infection, develop disseminated infections, and transmit WNV by bite to chickens in laboratory studies by Turell et al. (2001) using viremia titers of 105 and 107 PFU/ml, at levels they considered to be naturally occurring, whereas Tiawsirisup et al. (2005a) found that Ae. albopictus was more efficient as a vector of WNV than Cx. pipiens L. when fed blood meals from chickens with viremia titers > 107.0 mean culture infectious doses (CID50s)/ml, although Tiawsirisup et al. (2005b) reported in a separate study that Ae. albopictus was not infected by feeding upon cottontail rabbits, Sylvilagus floridanus (Allen), with titers of > 105.0 CID50s/ml. Using North American strains of Ae. albopictus, Sardelis et al. (2002) determined that the Texas strains originating from Chambers and Liberty counties, which are adjacent to and east of Harris County (Houston), were the most competent vectors of WNV, with 92% or greater transmission rates, 13 days after exposure to chickens with viremias of 105.7–6.8 PFU/ml. While these studies indicate transmission of WNV under controlled conditions, actual transmission rates under field conditions may be considerably less than expected infection rates, as was the case with Cx. nigripalpus Theobald in Florida during 2001 (Rutledge et al. 2003).
Several attempts by UTMB-G to determine titer of WNV within small samples of Ae. albopictus collected from Harris County have so far resulted in low levels of WNV within individuals of this species (H. Guzman and R. Tesh, unpublished data). Colton et al. (2005) indicated Ae. albopictus had greater mean levels of infectious virus within saliva samples compared with Cx. quinquefasciatus after intrathoracic inoculation of WNV under laboratory conditions, but this has yet to be proven from field-caught specimens in the western Gulf Coast region. Although incredible numbers of Cx. quinquefasciatus are collected and tested annually by MCD and UTMB-G, individual virus titer will range from high to undetectable levels as well. Reassessment of Ae. albopictus role in WNV transmission within this region will depend upon increasing the number of mosquitoes collected and tested, which has remained extremely low compared with Cx. quinquefasciatus.
In an effort to further expand existing knowledge of WNV ecology, mosquito bionomics, and control methodologies in Harris County, MCD and UTMB-G are currently engaged in collaborations that will hopefully lead to increased understanding of the individual roles these mosquito species have in the transmission of WNV in Harris County.
Acknowledgments
We wish to extend thanks to the dedicated permanent and seasonal personnel at MCD and UTMB-G during performance of this intensive project. This work was supported in part by National Institutes of Health contract RO1-AI25489 and grants from the Centers for Disease Control and Prevention (Project R01 CI000226) and the National Institute of Allergy and Infectious Diseases (Project R01 AI049724).
Footnotes
Mention of commercial products does not imply a recommendation for use or sale by Harris County Public Health and Environmental Services or the Mosquito Control Division, or the University of Texas Medical Branch
REFERENCES CITED
- Barnett HC. The incrimination of arthropods as vectors of disease. Proc 11th Inter Cong Entomol. 1960;2:341–345. [Google Scholar]
- Colton L, Biggerstaff BJ, Johnson A, Nasci RS. Quantification of West Nile virus in vector mosquito saliva. J Am Mosq Control Assoc. 2005;21:49–53. doi: 10.2987/8756-971X(2005)21[49:QOWNVI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Condotta SA, Hunter FF, Bidochka MJ. West Nile virus infection rates in pooled and individual mosquito samples. Vector-Borne Zoonotic Dis. 2004;4:198–208. doi: 10.1089/vbz.2004.4.198. [DOI] [PubMed] [Google Scholar]
- Cupp EW, Zhang D, Yue X, Cupp MS, Guyer C, Korves T, Unnasch TR. Identification of reptilian and amphibian bloodmeals from mosquitoes in an eastern equine encephalomyelitis virus focus in central Alabama. Am J Trop Med Hyg. 2004;71:272–276. [PMC free article] [PubMed] [Google Scholar]
- DeFoliart GR, Watts DM, Grimstad PR. Changing patterns in mosquito-borne arboviruses. J Am Mosq Control Assoc. 1986;2:437–455. [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2002;39:221–225. doi: 10.1603/0022-2585-39.1.221. [DOI] [PubMed] [Google Scholar]
- Gibbs SEJ, Ellis AE, Mead DG, Allison AB, Moulton JK, Howerth EW, Stallknecht DE. West Nile virus detection in the organs of naturally infected blue jays (Cyanocitta cristata) J Wildlife Dis. 2005;41:354–362. doi: 10.7589/0090-3558-41.2.354. [DOI] [PubMed] [Google Scholar]
- Girard YA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector-Borne Zoonotic Dis. 2004;4:109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- Harwood RF, James MT. Entomology in human and animal health. 7. New York: Macmillan; 1979. [Google Scholar]
- Heymann DL, editor. Control of communicable diseases manual. 18. Washington, DC: American Public Health Association; 2004. [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hassan H, Hill G, Cupp EW, Higazi TB, Mitchell CJ, Godsey MS, Unnasch TR. Identification of mosquito avian-derived blood meals by polymerase chain reaction-heteroduplex analysis. Am J Trop Med Hyg. 2002;66:599–604. doi: 10.4269/ajtmh.2002.66.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillibridge KM, Parsons R, Randle Y, Travassos Da Rosa APA, Guzman H, Siirin M, Wuithiranyagool T, Hailey C, Higgs S, Bala AA, Pascua R, Meyer T, Vanlandingham DL, Tesh RB. The 2002 introduction of West Nile virus into Harris County, Texas, an area historically endemic for St. Louis encephalitis. Am J Trop Med Hyg. 2004;70:676–681. [PubMed] [Google Scholar]
- Lounibos LP, Escher RL, Lourenco-De-Oliveira R. Asymmetric evolution of photoperiodic diapause in temperate and tropical invasive populations of Aedes albopictus (Diptera: Culicidae) Ann Entomol Soc Am. 2003;96:512–518. [Google Scholar]
- Mitchell CJ. Vector competence of North and South American strains of Aedes albopictus for certain arboviruses: a review. J Am Mosq Control Assoc. 1991;7:446–451. [PubMed] [Google Scholar]
- Niebylski ML, Craig GB., Jr Dispersal and survival of Aedes albopictus at a scrap tire yard in Missouri. J Am Mosq Control Assoc. 1994;10:339–343. [PubMed] [Google Scholar]
- Niebylski ML, Meek CL. Blood-feeding of Culex mosquitoes in an urban environment. J Am Mosq Control Assoc. 1992;8:173–177. [PubMed] [Google Scholar]
- Niebylski ML, Savage HM, Nasci RS, Craig GB., Jr Blood hosts of Aedes albopictus in the United States. J Am Mosq Control Assoc. 1994;10:447–450. [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- Pumpuni CB, Knepler J, Craig GB., Jr Influence of temperature and larval nutrition on the diapause inducing photoperiod of Aedes albopictus. J Am Mosq Control Assoc. 1992;8:223–227. [PubMed] [Google Scholar]
- Reisen WK, Barker CM, Carney R, Lothrop HD, Wheeler SS, Wilson JL, Madon MB, Takahashi R, Carroll B, Garcia S, Fang Y, Shafii M, Kahl N, Ashtari S, Kramer V, Glaser C, Jean C. Role of corvids in epidemiology of West Nile virus in southern California. J Med Entomol. 2006a;43:356–367. doi: 10.1603/0022-2585(2006)043[0356:rocieo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006b;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:hpoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge CR, Day JF, Lord CC, Stark LM, Tabachnick WJ. West Nile virus infection rates in Culex nigripalpus (Diptera: Culicidae) do not reflect transmission rates in Florida. J Med Entomol. 2003;40:253–258. doi: 10.1603/0022-2585-40.3.253. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, O’Guinn ML, Andre RG, Roberts DR. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J Am Mosq Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- Savage HM, Niebylski ML, Smith GC, Mitchell CJ, Craig GB., Jr Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J Med Entomol. 1993;30:27–34. doi: 10.1093/jmedent/30.1.27. [DOI] [PubMed] [Google Scholar]
- Shroyer DA. Aedes albopictus and arboviruses: a concise review of the literature. J Am Mosq Control Assoc. 1986;2:424–428. [PubMed] [Google Scholar]
- Steel RGD, Torrie JH. Principles and procedures of statistics: a biometrical approach. 2. New York: McGraw-Hill; 1980. [Google Scholar]
- Sullivan MF, Gould DJ, Maneechai S. Observations on the host range and feeding preferences of Aedes albopictus (Skuse) J Med Entomol. 1971;8:713–716. doi: 10.1093/jmedent/8.6.713. [DOI] [PubMed] [Google Scholar]
- Takeda T, Whitehouse CA, Brewer M, Gettman AD, Mather TN. Arbovirus surveillance in Rhode Island: assessing potential ecologic and climatic correlates. J Am Mosq Control Assoc. 2003;19:179–189. [PubMed] [Google Scholar]
- Tesh RB, Parsons R, Siirin M, Randle Y, Sargent C, Guzman H, Wuithiranyagool T, Higgs S, Vanlandingham DL, Bala AA, Haas K, Zerinque B. Year-round West Nile virus activity, Gulf Coast region, Texas and Louisiana. Emerg Infect Dis. 2004;10:1649–1652. doi: 10.3201/eid1009.040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiawsirisup S, Platt KB, Evans RB, Rowley WA. A comparison of West Nile virus transmission by Ochlerotatus trivittatus (Coq.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector-Borne Zoonotic Dis. 2005a;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S, Platt KB, Tucker BJ, Rowley WA. Eastern cottontail rabbits (Sylvilagus floridanus) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector-Borne Zoonotic Dis. 2005b;5:342–350. doi: 10.1089/vbz.2005.5.342. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Zinser M, Ramberg F, Willott E. Culex quinquefasciatus (Diptera: Culicidae) as a potential West Nile virus vector in Tucson, Arizona: blood meal analysis indicates feeding on both humans and birds. J Insect Sci. 2004;4:20. doi: 10.1093/jis/4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]