Abstract
Myeloid-derived suppressor cells (MDSC) are a heterogeneous group of cells that play a critical role in tumor associated immune suppression. In an attempt to identify a specific subset of MDSC primarily responsible for immunosuppressive features of these cells, 10 different tumor models were investigated. All models showed variable but significant increase in the population of MDSC. Variability of MDSC expansion in vivo matched closely the effect of tumor-cell condition media (TCCM) in vitro. MDSC consists of two major subsets of Ly6G+Ly6Clow granulocytic and Ly6G-Ly6Chigh monocytic cells. Granulocytic MDSC have increased level of reactive oxygen species (ROS) and undetectable level of nitric oxide (NO) whereas monocytic MDSC had increased level of NO but undetectable levels of ROS. However, their suppressive activity per cell basis was comparable. Almost all tumor models demonstrated a preferential expansion of granulocytic subset of MDSC. We performed a phenotypical and functional analysis of several surface molecules previously suggested to be involved in MDSC mediated suppression of T cells: CD115, CD124, CD80, PD-L1, and PD-L2. Although substantial proportion of MDSC expressed those molecules no differences in the level of their expression or the proportion, positive cells were found between MDSC and cells from tumor-free mice that lack immune suppressive activity. The level of MDSC mediated T-cell suppression did not depend on the expression of these molecules. This data indicates that suppressive features of MDSC is caused not by expansion of a specific subset but more likely represents a functional state of these cells.
Keywords: rodent, myeloid-derived suppressor cells, tumor immunity
Introduction
Myeloid derived suppressor cells (MDSC) are a group of myeloid cells comprised of precursors of macrophages, granulocytes, dendritic cells (DC) and myeloid cells at earlier stages of differentiation (rev. (1-4)). In mice these cells are broadly defined as Gr-1+CD11b+ cells. They have a very rapid turnover and accumulate in large numbers in lymphoid tissues of tumor-bearing mice as well as in mice with infectious diseases, sepsis, and trauma (5-10). MDSC have been also described in cancer patients (11-15). In a recent study circulating MDSC were found to be significantly increased in cancer patients of all stages relative to healthy volunteers. A significant correlation between circulating MDSC and clinical cancer stage was also observed. Among stage IV patients, those with extensive metastatic tumor burden had the highest percent and absolute number of MDSC (16). The main feature of these cells is their ability to suppress T-cell responses in antigen-specific or a non-specific manner depending on the condition of T-cell activation (2, 4). These cells are now considered as one of the major factors responsible for tumor associated immune defects and are an attractive target for therapeutic intervention. In naïve tumor-free mice Gr-1+CD11b+ immature myeloid cells (IMC) are present in bone marrow and in small numbers in spleens. They contain precursors of mature myeloid cells and quickly differentiate in vitro or after adoptive transfer in vivo (17). Importantly in contrast to MDSC these cells lack immunosuppressive activity (18-21).
The heterogeneous nature of these cells prompted a search for more narrowly defined specific populations that are primarily responsible for the observed effect of MDSC. Identification of such population would help not only better understand the biology of tumor-induced immune suppression, but also would provide better targets for therapeutic intervention. A number of different surface molecules (CD80, M-CSF receptor, IL-4Rα, PD-L1, PD-L2, B7-H4) were suggested to define different immune suppressive myeloid cells (22-27). However, their specificity for MDSC remains unknown. It is not clear whether there is an expansion of specific subsets within the population of MDSC as compared to their normal non-immunosuppressive counterpart IMC. Such an expansion would be a first sign of specific role of individual subgroup of MDSC in cancer. It is not clear whether individual subsets are different in their ability to inhibit T-cell responses. Most of the studies of MDSC were restricted to one or two tumor models and often conflicting results reported regarding phenotype of these cells. This raises a question about the tumor specificity of the observations. In this study we tried to address those questions by evaluating side by side in vivo and in vitro ten different models of lung, breast, colon cancer, melanoma, and sarcoma developed in three different strains of mice. We performed a phenotypic and functional analysis of different subsets of MDSC with the goal to identify a subset of MDSC directly responsible for immune suppression attributed to these cells.
Materials and Methods
Mice, reagents, and tumor models
Female BALB/c, C57BL/6, and FVB/N mice aged 6-8 wk were obtained from the National Cancer Institute (Frederick, MD). Mice were kept in pathogen free conditions and handled in accordance with the requirements of the Guideline for Animal Experiments. The following subcutaneous tumor models were used. In C57BL/6 mice: EL4 thymoma (obtained from American Type Culture Collection (ATCC), Manassas, VA), Lewis Lung Carcinoma (LLC), B16F10 melanoma (provided by E. Celis, H. Lee Moffitt Cancer Center, Tampa, FL), MC38 colon carcinoma (provided by I. Turkova, University of Pittsburgh, Pittsburgh, PA), and C3 sarcoma (provided by W. Kast, University of Southern California, Los Angeles, CA). In BALB/c mice: DA3 mammary carcinoma (provided by D. Lopez, University of Miami, FL), 4T1 mammary carcinoma (provided by S. Ostrand-Rosenberg, University of Maryland, Baltimore, MD), CT26 colon carcinoma (ATCC), and MethA sarcoma (provided by L. J. Old, Ludwig Institute for Cancer Research, New York, NY). In FVB/N mice: ANV mammary carcinoma (provided by K. Knutson, Mayo Clinic, Rochester, MN). The number of tumor cells injected was different for each model and was selected based on the ability to form tumor with 1.5 cm in diameter within 2-3 weeks of injection. To generate tumor cells conditioned medium (TCCM), sub-confluent cells were kept in RPMI medium with a reduced (3%) serum concentration for 48 hr. After that time supernatants were collected, aliquoted and kept at -80°C until further use. Rabbit polyclonal anti-nitrotyrosine antibody was purchased from Upstate Cell Signaling (Millipore Corp, Billerica, MA) and goat anti-rabbit IgG (H+L)-Alexa Fluor 647 was obtained from Invitrogen.
RPMI 1640, DMEM, FBS, 2-ME, recombinant murine GM-CSF and antibiotics were obtained from Invitrogen Life Technologies (Carlsbad, CA). IL-4 from R&D Systems (Minneapolis, MN). The antibodies used for flow cytometry: anti-Gr-1 (clone RB6-8C5), anti-Ly6G, anti-Ly6C, anti-CD11b, anti-I-Ab, anti-I-Ad, anti-H2-Kb, anti-H2-Kd, anti-CD80, anti-CD86, anti-CD124 (IL-4Rα), and isotype control antibodies, were obtained from BD PharMingen (San Diego, CA), anti-PD-L1, anti-PD-L2, and anti-CD115 antibodies were purchased from eBioscience (San Diego, CA). Neutralizing anti-PD-L1 antibody was kindly provided by Dr. L.Chen (John Hopkins University, Baltimore, MD). OVA-derived (H-2Kb, SIINFEKL) and control (H-2Kb, RAHYNIVTF) peptides were obtained from QCB.
Flow cytometry
Spleens were harvested under sterile conditions. Single-cell suspensions were prepared, and red cells were removed using ACK lysing buffer. Two million splenocytes were incubated for 30 min on ice in staining media (1% FBS in PBS) with the relevant Abs and then washed with PBS. For intracellular staining of nitrotyrosine, cells were labeled with anti-CD11b-APC, anti-Ly6C-FITC, and anti-Ly6G-PE, fixed and permeabilized with Cytofix/Cytoperm Buffer (BD Biosciences) and washed with a 1× PermWash solution (BD Biosciences). The cells were incubated with rabbit polyclonal anti-nitrotyrosine antibody for 1 hr on ice. After washing, the cells were incubated with the secondary detection reagents, goat anti-rabbit IgG (H+L)-Alexa Fluor 647 for 45 min on ice. After washing, the samples were analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and were analyzed using FlowJo software (Tree Star, Ashland, OR).
Generation of cells from bone marrow progenitors
Bone marrow (BM) cells were obtained from the femurs and tibias. One million BM cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 10 ng/ml GM-CSF, 10 ng/ml IL-4, and 50 uM 2-ME, alone or in the presence of 30% v/v control (from 3T3 fibroblasts) or TCCM. The cultures were maintained at 37°C in 5% CO2-humidified atmosphere in 24-well plates. On day 3 of culture, floating cells were gently removed, and fresh medium with cytokines and TCCM was replaced. Cells were collected on day 5 and analyzed by flow cytometry.
Isolation of cells and functional assays
MDSC and their subsets were isolated from spleens of tumor-bearing or control mice using cell sorting on FACSAria cell sorter (Becton Dickinson). The purity of cell populations was >99%. As responder cells we used total spleen cells from OT-1 mice. CD8+ T cells from these mice have a TCR that recognize OVA-derived peptide SIINFEKL. The number of IFN-γ producing cells in response to stimulation to the specific or control peptides (10 μg/ml) was evaluated in an ELISPOT assay and performed as described earlier (28). The numbers of spots were counted in triplicates and calculated using an automatic ELISPOT counter (Cellular Technology, Ltd). Cell proliferation induced by antigen specific or CD3 (0.5 μg/ml)/CD28 (5 μg/ml) stimulation was evaluated using 3H-thymidine incorporation as described previously (29). For experiments which examined the effect of NO, arginase, or ROS inhibitor, L-NMMA (0.5 mM; Calbiochem, San Diego, CA), nor-NOHA (0.5 mM; Calbiochem, San Diego, CA), or catalase (1000 U/mL; Sigma-Aldrich) were added at the beginning of the culture.
ROS production
The oxidation-sensitive dye hydroethidine (HE) was used for the measurement of ROS production by MDSC. Cells were incubated at room temperature in DMEM in the presence of 1 μM HE with or without 300 nM PMA for 30 min, washed with PBS, and then labeled with anti-CD11b-APC, anti-Ly6C-FITC, and anti-Ly6G-PE. After incubation on ice for 20 min, cells were washed with PBS and analyzed using flow cytometry.
Arginase activity
Arginase activity was measured in cell lysates, as previously described (30). Briefly, cells were lysed for 30 min with 100 μl of 0.1% Triton X-100. Subsequently, 100 μl of 25 mM Tris-HCl and 10 μl of 10 mM MnCl2 were added, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was conducted by incubating the lysate with 100 μl of 0.5 M L-arginine (pH 9.7) at 37°C for 120 min. The reaction was stopped with 900 μl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). Urea concentration was measured at 540 nm after addition of 40 μl of α-isonitrosopropiophenone (dissolved in 100% ethanol), followed by heating at 95°C for 30 min.
NO production
Equal volumes of culture supernatants (100 μl) were mixed with Greiss reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-naphthyl-ethylenediamine dihydrochloride in double-distilled water). After a 10 min incubation at room temperature, the absorbance at 550 nm was measured using microplate plate reader (Bio-Rad). Nitrite concentrations were determined by comparing the absorbance values for the test samples to a standard curve generated by serial dilution of 0.25 mM sodium nitrite.
Statistical analysis
The statistical significance between values was determined by Student’s t-test. All data were expressed as the mean ± SD. Probability values 0.05 or less were considered significant.
Results
The expansion of MDSC in different tumor models
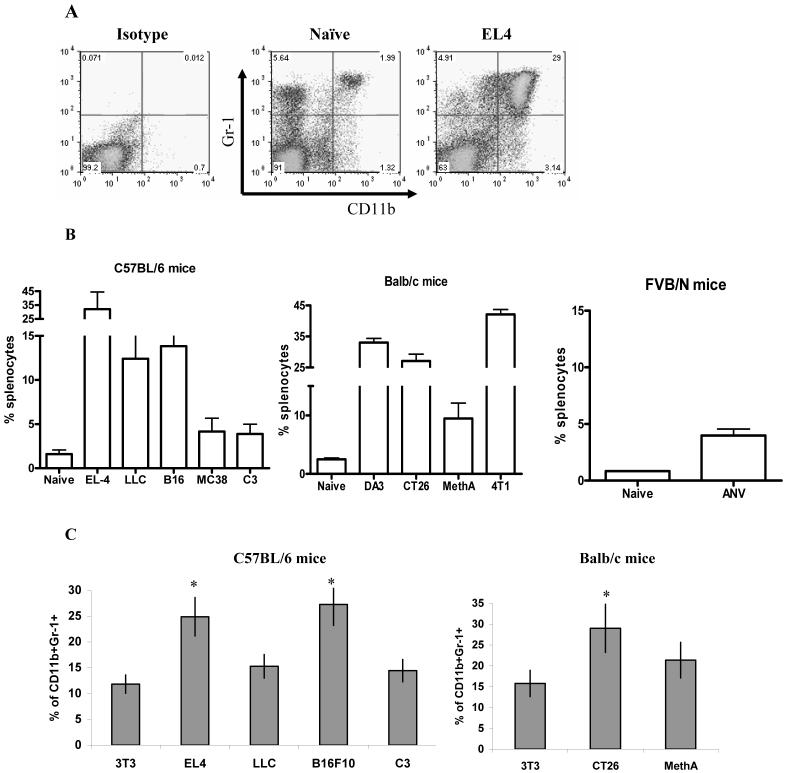
To address the main question of the study ten different tumor models on three different mouse strains were used. All mice were injected s.c. with a different number of tumor cells to provide for the development of similar size (1.5 cm in diameter) tumors within 3 weeks of inoculation. These conditions were selected because they were routinely used in experiments with MDSC mediated immune suppression. Spleens from naïve tumor-free mice contained less than 2.5% of Gr-1+CD11b+ cells (Fig. 1A,B). The total number of splenocytes was increased in all tumor models from 1.5 to 3 fold (data not shown). A significant increase in the proportion of Gr-1+CD11b+ MDSC in spleens was observed in all 10 tumor models (Fig. 1B). There was variability in MDSC frequency between different tumor models. Some tumor models were associated with a substantial expansion (>15%) of MDSC (EL-4, DA3, 4T1, and CT26), some had limited (<5%) expansion (MC38, C3, ANV) and some had intermediate (10-15%) level of MDSC (LLC, B16, MethA) (Fig. 1B). We asked whether this variability was mediated by tumor-derived factors or was attributed to specific mouse strains. Bone marrow cells from naïve C57BL/6 or BALB/c mice were cultured for 5 days with GM-CSF and IL-4 in the presence of conditioned medium from control 3T3 fibroblasts or different tumor cell lines. The proportion of Gr-1+CD11b+ cells was evaluated after 5 days. There was a high level of concordance between the effect of TCCM on MDSC production in vitro and the frequency of MDSC in tumor-bearing mice. TCCM from LLC, C3, and MethA sarcoma cell lines produced very little change in the frequency of MDSC in vitro (Fig. 1C). These three models were characterized by a relatively small expansion of MDSC in vivo (Fig. 1B). In contrast, TCCM from EL-4, B16, and CT26 induced a significantly higher generation of Gr-1+CD11b+ cells in vitro (Fig. 1C). These three models also showed a high proportion of MDSC in spleens (Fig. 1B).
Figure 1. Accumulation of Gr-1+CD11b+ cells in spleens of tumor-bearing mice.
Splenocytes from naïve or tumor-bearing mice (3 weeks after tumor inoculation, tumor size 1.5 cm in diameter) were stained with anti-CD11b and anti-Gr-1 antibodies. A. Typical example of flow cytometry analysis. B. The percentage of Gr-1+CD11b+ cells in spleen from naïve or tumor-bearing mice on the C57BL/6, BALB/c, or FVB/N backgrounds as indicated. Each group included from 3 to 8 mice. Mean and standard deviation are shown. Differences between proportion of Gr-1+CD11b+ cells in spleens from naïve and tumor-bearing mice were statistically significant for all tumor models (p<0.05). C. Bone marrow cells from naïve mice were cultured for 5 d with 10 ng/ml GM-CSF and IL-4 in the presence of control (3T3) or conditioned media from indicated tumor cell lines. Cells were collected, labeled with anti-Gr-1 and anti-CD11b Abs, and analyzed by flow cytometry. * - statistically significant differences from control (3T3) level (p<0.05).
MDSC subsets are differentially regulated in tumor-bearing mice
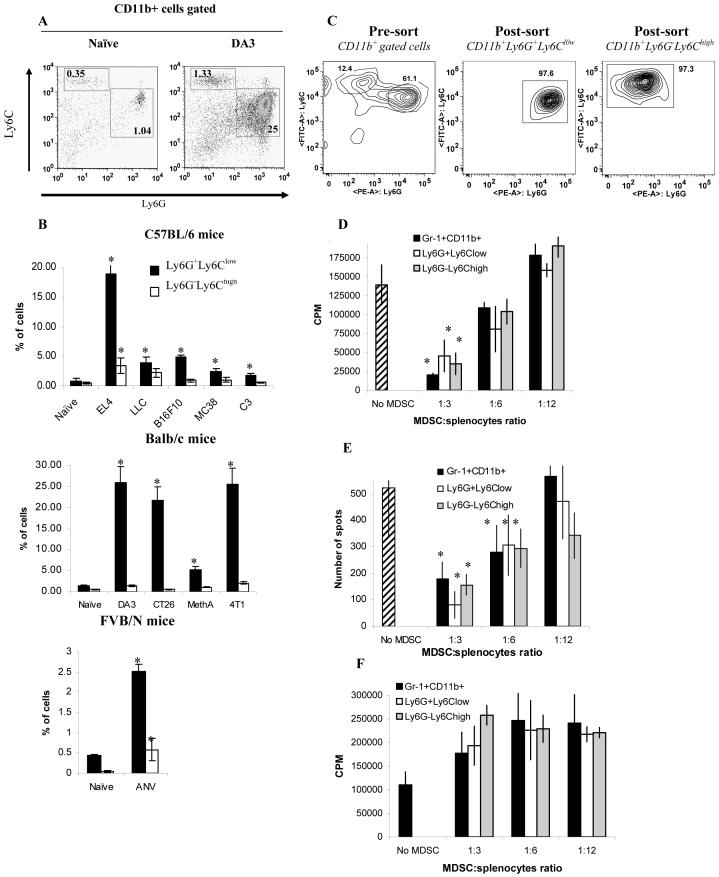
Myeloid differentiation antigen Gr-1 consists of two epitopes recognized by anti-Ly-6G and anti-Ly6C antibodies. Accordingly, a population of CD11b+Gr-1+ MDSC consists of two major subsets: cells with granulocytic phenotype that express Ly-6G marker and cells with monocytic phenotype expressing Ly6C marker. Recent reports indicated that these two populations might have different functions in infectious and autoimmune diseases and cancer (10, 31-33). We asked how these populations are changed in spleens of tumor-bearing mice by analyzing the frequency of CD11b+Ly6G+Ly6Clow granulocytic and CD11b+Ly6G-Ly6Chigh monocytic populations (Fig. 2A). In naïve mice the ratio between these two populations was 3:1. In tumor-bearing mice the population of granulocytic CD11b+Ly6G+Ly6Clow MDSC was consistently increased in all tumor models, whereas the frequency of monocytic CD11b+Ly6G-Ly6Chigh MDSC was significantly increased in only few models (EL-4, LLC, and ANV). Moreover, in mice bearing EL-4 or ANV tumors granulocytic population of MDSC was increased substantially more than the monocytic cells. In only one model (LLC) these two populations were increased equally (Fig. 2B). Thus, granulocytic subset of MDSC was a predominant population of MDSC expanded in tumor-bearing mice.
Figure 2. The presence and functional activity of granulocytic and monocytic subsets of MDSC in tumor-bearing mice.
Splenocytes from naïve and tumor-bearing mice were stained with CD11b, Ly6G and ly6C antibodies. A. Typical example of flow cytometry analysis. B. The percentage of Ly6G+Ly6Clow or Ly6G-Ly6Chigh cells in spleen from naïve or tumor-bearing mice. * - statistically significant differences between naïve and tumor-bearing mice (p<0.05).
Gr-1+CD11b+ total MDSC, CD11b+Ly6G+Ly6Clow granulocytic, and CD11b+Ly6G-Ly6Chigh monocytic MDSC were sorted from spleens of EL-4 tumor-bearing mice using FACSAria cell sorter. C. An example of cell sort of granulocytic and monocytic subsets of MDSC. D, E. Sorted MDSC subsets were cultured at different ratios with 2×105 splenocytes from OT-1 mice in the presence of control or specific peptides. Cell proliferation (D) and IFN-γ production (E) were measured using 3H-thymidine uptake and ELISPOT assay as described in Material in Methods. Each experiment was performed in triplicates. Three experiments with similar results were performed. The values of T-cell activity in the presence of control peptide were subtracted from the values obtained in the presence of specific peptide. F. Splenocytes from C57BL/6 mice were cultured with anti-CD3/CD28 antibody in the presence of different ratios of MDSC subsets. Cell proliferation was measured in triplicates and Mean ± SD are shown.
The function of MDSC subsets was evaluated in the model of EL-4 tumor-bearing mice since this model was characterized by an increase in both cell populations. Gr-1+CD11b+, CD11b+Ly6G+Ly6Clow, and CD11b+Ly6G-Ly6Chigh cells were sorted from spleens of tumor-bearing mice (Fig. 2C). These cells were added at different ratios to OT-1 splenocytes in the presence of specific (SIINFEKL) or control peptides and T-cell proliferation or IFN-γ production was evaluated. At 1:3 ratio all MDSC population significantly suppressed CD8+ T-cell proliferation and IFN-γ production, whereas at 1:12 ratio no suppressive activity was observed (Fig. 2 D,E).Slight trend in increased T-cell proliferation could be seen at 1:12 ratio. However, it did not reach statistical significance. T-cell proliferation was similar in the presence of all MDSC populations (Fig. 2D). When the ability of CD8+ T cells to produce IFN-γ was evaluated, granulocytic MDSC demonstrated more profound (although not statistically significant) suppressive activity per cell basis than monocytic or total population of MDSC (Fig. 2E). However, this effect was observed only at the highest MDSC:splenocytes ratio (1:3) and was not seen at lower MDSC concentrations. Neither of MDSC populations was able to inhibit T-cell proliferation induced by anti-CD3/CD28 antibodies (Fig. 2F). Moreover, slight increase in T-cell proliferation, albeit not statistically significant, was observed in the presence of all MDSC subsets tested in these experiments.
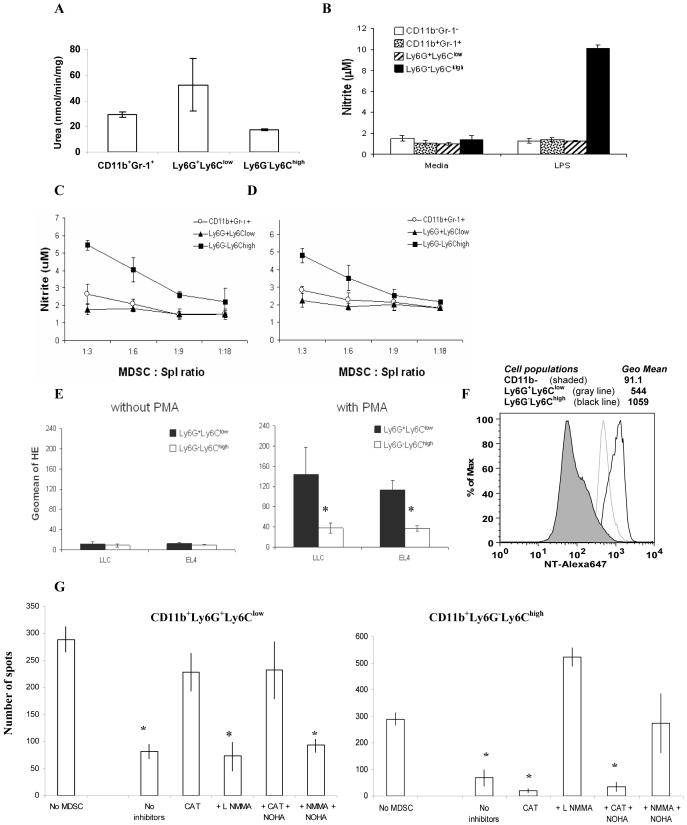
To evaluate the possible mechanism of suppressive activity of different MDSC subsets, Gr-1+CD11b+, CD11b+Ly6G+Ly6Clow, and CD11b+Ly6G-Ly6Chigh cells were sorted from spleens of tumor-bearing mice and the level of arginase activity, NO, and ROS production was evaluated. No statistically significant differences were observed in the level of arginase activity between those cell populations (Fig. 3A). Major differences between cell populations were observed in the ability of cells to produce NO and ROS. In response to stimulation with LPS (Fig. 3B) or in the presence of OT-1 T cells stimulated with specific peptide or anti-CD3/CD28 antibody (Fig. 3C,D) monocytic CD11b+Ly6G-Ly6Chigh MDSC produced significantly higher level of NO than granulocytic MDSC or the total population of MDSC. In contrast, CD11b+Ly6G+Ly6Clow granulocytic MDSC had significantly higher levels of ROS production than CD11b+Ly6G- Ly6Chigh monocytic MDSC (Fig. 3E). MDSC production of peroxynitrates was previously implicated in a number of studies in MDSC mediated T-cell suppression (34, 35). We asked whether MDSC subsets differed in their ability to produce peroxynitrite (as was evaluated by the level of nitrotyrosine (NT) in the cells). Both granulocytic and monocytic subsets of MDSC expressed dramatically (6-10-fold) higher levels of NT than CD11b negative cells (Fig. 3F). CD11b+Ly6G-Ly6Chigh monocytic subset of MDSC expressed two-fold higher level of NT than CD11b+Ly6G+Ly6Clow granulocytic MDSC (Fig. 3F). To investigate whether those differences would affect the ability of MDSC to increase NT level in T cells, OT-1 splenocytes were cultured at 3:1 ratio with sorted subsets of MDSC in the presence of specific peptide. The proportion of NT positive cells among CD8+ T cells was evaluated by flow cytometry after 24 hr incubation. Monocytic subset of MDSC induced substantially higher level of NT+ CD8+ cells than granulocytic subset (Fig. 3G). Consistent with previous observations Gr-1+CD11b+ cells from naïve mice did not affect the level of NT in CD8+ T cells (Fig. 3G).
Figure 3. The mechanisms of suppressive activity of different MDSC subsets.
Gr-1+CD11b+ total MDSC, CD11b+Ly6G+Ly6Clow granulocytic, and CD11b+Ly6G-Ly6Chigh monocytic MDSC were sorted from spleens of EL-4 tumor-bearing mice using FACSAria cell sorter. A. Arginase activity of different cell populations was measured in triplicates as described in Material and Methods. Mean ± SD are shown. B. Sorted populations of MDSC were stimulated with 1 μg/ml LPS for 24-hr, supernatants were collected and nitrite concentration was measured as described in Material and Methods. Experiments were performed in triplicates. C. OT-1 splenocytes (105 cells per well) were stimulated in triplicates for 48 hr with specific peptide in the presence of different ratios of sorted indicated subsets of MDSC and NO level was measured in supernatants. Mean ± SD are shown. D. Splenocytes from naïve C57BL/6 mice were stimulated from 48 hr with anti-CD3/CD28 antibodies in the presence of different ratios of indicated MDSC subsets. NO level was measured in supernatants as described in Material and Methods. Mean ± SD are shown. E. The level of ROS in MDSC subsets was measured using HE staining and flow cytometry as described in Material and Methods. * - statistically significant differences between groups (p<0.05). F. Expression of nitrotyrosine (NT) in MDSC subsets. Splenocytes from EL4 tumor bearing mice were stained with CD11b, Ly6G, Ly6C, and intracellular NT. Expression of NT was evaluated in CD11b- cells (shade area), CD11b+Ly6G+Ly6Clow (gray line), CD11b+Ly6G-Ly6Chigh (black line). G. Indicated populations of MDSC were sorted from spleens of naive or tumor-bearing mice and incubated in the presence of 10 μg/ml specific peptide SIINFEKL at 1:3 ratio with 106 splenocytes from OT-1 mice. After 24 hr cells were collected and labeled with anti-CD8 and anti-NT antibodies. CD8+ cells were gated and the proportion of NT positive cells was calculated. I. Suppressive activity of indicated MDSC subsets was evaluated as described in Fig. 3B. Sorted granulocytic and monocytic subsets of MDSC were cultured at 1:4 ratio with OT-1 splenocytes in the presence of specific or control peptide. L-NMMA (0.5 mM), nor-NOHA (0.5 mM), or catalase (1000 U/mL) were added at the beginning of the culture. The number of IFN-γ producing cells was evaluated in ELISPOT assay performed as described earlier (28). The numbers of spots were counted in triplicates and calculated using an automatic ELISPOT counter (Cellular Technology, Ltd). The values of IFN-γ production in the presence of control peptide were subtracted from the values obtained in the presence of specific peptide. *- statistically significant difference from splenocytes cultured without MDSC (p<0.05).
Inhibitor of hydroxyl peroxide, catalase completely blocked suppressive activity of granulocytic MDSC subset but had no effect of monocytic MDSC (Fig. 3I). In contrast, combination of iNOS inhibitor LMMA with arginase inhibitor Nor-NOHA blocked suppressive activity of monocytic MDSC but not granulocytic MDSC (Fig. 3I).
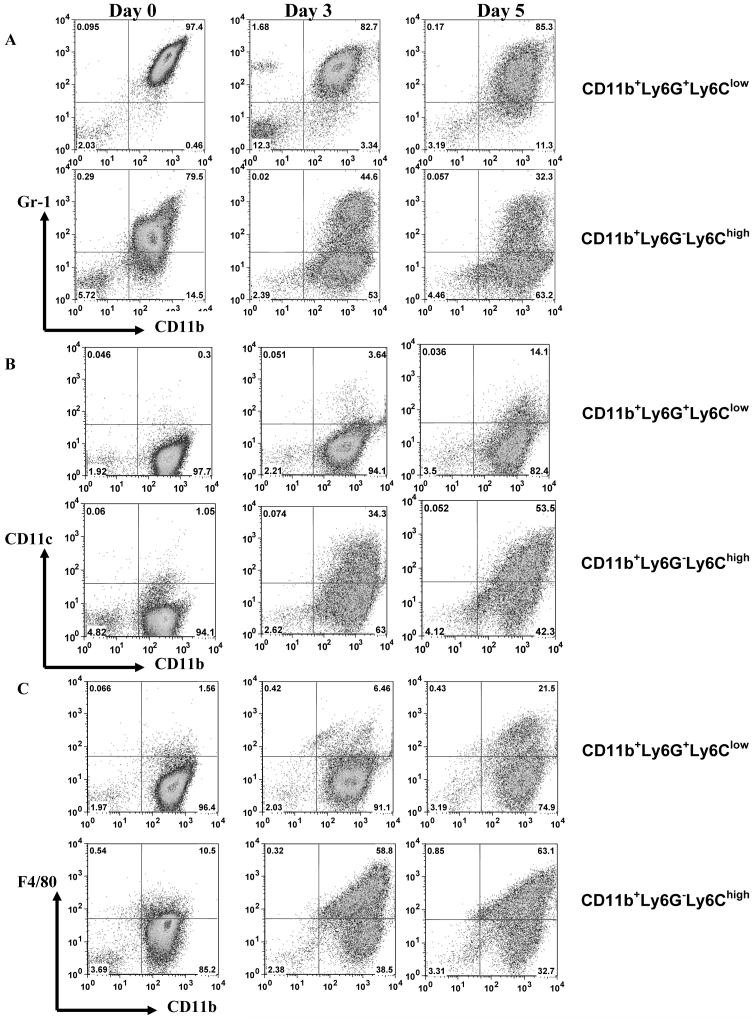
We asked whether there was a difference in the ability of MDSC subsets to differentiate in vitro. CD11b+Ly6G+Ly6Clow granulocytic and CD11b+Ly6G-Ly6Chigh monocytic MDSC were sorted as described above and cultured in the presence of GM-CSF for 3 and 5 days. During culture monocytic MDSC did not acquire markers of granulocytic MDSC and vice versa (data not shown). However, major differences were found in the ability of these subsets to acquire markers of mature myeloid cells. Granulocytic MDSC retained immature Gr-1+ CD11b+phenotype during 5-day culture, whereas monocytic MDSC rapidly differentiated. By day 3 less than half of the cells retained immature phenotype and by day 5 only 1/3 of cells were Gr-1+CD11b+ cells (Fig. 4A). Little bit more than 10% of granulocytic MDSC differentiated into CD11c+ DCs, whereas more than 50% of cells differentiated from monocytic MDSC were CD11c+ cells (Fig. 4B). About 20% of granulocytic MDSC acquired F4/80 marker specific for macrophages comparing with more than 60% of cells differentiated from monocytic MDSC (Fig. 4C). Please note that total proportion of CD11c+, F4/80+, and Gr-1+ cells is higher than 100% because some of the cells express several of these markers. This may reflect different stages of myeloid cell differentiation.
Figure 4. Differentiation of MDSC subsets in vitro.
CD11b+Ly6G+Ly6Clow granulocytic MDSC and CD11b+Ly6G-Ly6Chigh monocytic MDSC were sorted from spleens of EL-4 tumor-bearing mice as described in Fig. 2. Cells were culutured in the presence of 10 ng/ml GM-CSF for 3 and 5 days and phenotype of cells was evaluated as indicated. A. Staining with anti-Gr-1 and Cd11b antibodies, B. Staining with anti-CD11c and Cd11b antibodies, C. Staining with anti-F4/80 and CD11b antibodies.
Potential role of B7-family molecules in MDSC mediated T-cell suppression in cancer
Previous studies have demonstrated that Gr-1+CD11b+ IMC isolated from spleens of naïve mice lack suppressive activity against T cells, whereas cells with similar phenotype (Gr-1+CD11b+) from tumor-bearing mice showed potent suppressive activity. Therefore, if MDSC subset expressing specific surface marker is truly responsible for the MDSC function, then its proportion among MDSC should be substantially higher than among IMC or this cell population should have higher suppressive activity on a per cell basis. Based on this assumption we investigated the role of various molecules in MDSC function.
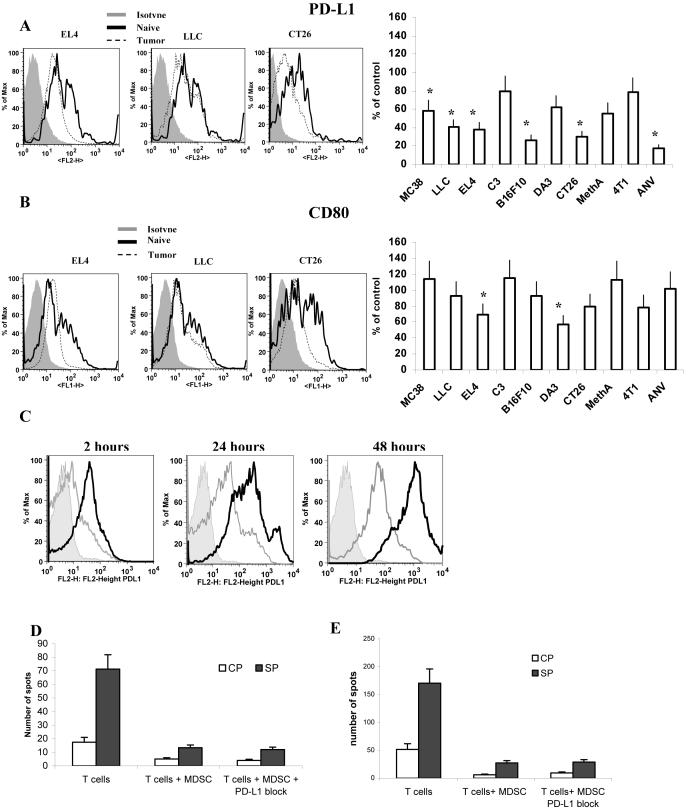
Inhibitory PD-L1 and PD-L2 receptors of B7 family are expressed on the variety of myeloid cells and were shown to be directly involved in suppression of immune responses (36, 37). Another member of this family B7.1 (CD80) was recently implicated in MDSC mediated immune suppression in ovarian carcinoma (38). We evaluated the level of the expression of these molecules on MDSC. Most of IMC and MDSC expressed PD-L1 and CD80 but not PD-L2 (data not shown). We compared the percentage of positive cells and the level of expression of these molecules between IMC from naïve tumor-free mice and MDSC from tumor-bearing mice. No significant differences were found in the percentage of PD-L1 or CD80 positive cells within the populations of Gr-1+CD11b+ MDSC and Gr-1+CD11b+ IMC (data not shown). No increase in the level of expression of either PD-L1 or CD80 was found in MDSC compared with IMC. Moreover, in six out of ten tested tumor models MDSC had significantly lower levels of PD-L1 expression than IMC (Fig. 5A). Similar results were obtained after the analysis of CD80 expression. In two models the levels of CD80 expression on MDSC were significantly lower than those at IMC (Fig. 5B). Lack of increased expression of these molecules in MDSC may suggest that PD-L1, PD-L2, and CD80 may have a limited role in MDSC mediated immune suppression in cancer.
Figure 5. PD-L1 and CD80 expression on MDSC from tumor-bearing mice.
Splenocytes from naïve or tumor-bearing mice were stained with anti-Gr-1-APC, anti-CD11b-PE-Cy7 and anti-PD-L1-PE (A) or anti-CD80-FITC (B) antibodies. Expression of PD-L1 or CD80 within the population of Gr-1+ CD11b+ MDSC was measured and calculated as percentage of change from the level of control mice with matched haplotype. Each tumor model included at least 4 mice. * - statistically significant differences from control (p<0.05). C. Gr-1+CD11b+ MDSC sorted from spleens of EL-4 tumor-bearing mice were cultured for 2, 24, or 48 h with 1 μg/ml of control IgG or specific anti-PD-L1 antibody (kind gift from Dr. L. Chen, John Hopkins University) in complete medium. Cells were stained with either isotype control (shaded area), or anti-PD-L1 antibody. PD-L1 in cells pre-treated with control IgG is shown as a black line, pre-treated with anti-PD-L1 antibody as a grey line. D. MDSC isolated from spleens of EL-4 tumor-bearing mice were pre-treated for 30 min on ice with control IgG or anti-PD-L1 antibody. Excess of antibody was washed and MDSC were cultured at 1:4 ratio with OT-1 splenocytes in the presence of control (CP) or specific (SP) peptides. IFN-γ production was evaluated in quadruplicates after 36 hr of culture suing ELISPOT assay. Mean ± SD of the number of spots per 5×104 splenocytes is shown. E. CD8+ T-cell tolerance was induced in mice as described previously (18, 35). OT-1 T cells (5×106) were injected i.v. into naïve C57BL/6 recipients. Two days later 3×106 MDSC isolated from EL-4 tumor-bearing mice were injected i.v. followed by immunization with 100 μg of specific (SIINFEKL) peptide in IFA. Control IgG or anti-PD-L1 antibody (100 μg/mouse) were injected i.p. three times: 2 days prior to cell transfer and immunization, 2 days after immunization of mice and 3 days later. Ten days after immunization mice were sacrificed and lymph node cells were re-stimulated with control (CP) or specific (SP) peptides and the number of IFN-γ producing cells was evaluated in quadruplicates in ELISPOT assay. Mean ± SD of the number of spots per 2×105 lymph node cells are shown.
To directly test the functional role of PD-L1 in MDSC mediated T-cell suppression, expression of PD-L1 on MDSC surface was blocked in vitro using anti-PD-L1 antibody (Fig. 5C). MDSC were then added at 1:4 ratio to OT-1 splenocytes and incubated with control or specific peptides for 36 hr. The response of CD8+ T cells was measured in IFN-γ ELISPOT assay. Block of PD-L1 expression did not eliminate suppressive activity of MDSC (Fig. 5D). In another set of experiments, CD8+ T-cell tolerance was induced in mice after an adoptive transfer of OT-1 T cells and MDSC as described previously (18, 35). Anti-PD-L1 antibody was injected i.p. three times: 2 days prior to cell transfer and immunization, 2 days after immunization of mice and 3 days later. Treatment of mice with anti-PD-L1 antibody was not able to eliminate or reduce MDSC induced T-cell tolerance (Fig. 5E).
Potential role of M-CSF and IL-4 receptors in MDSC mediated T-cell suppression in cancer
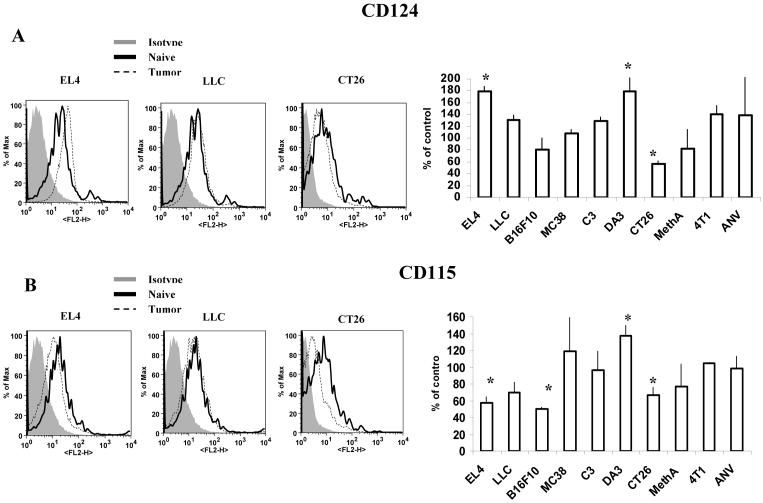
It has been previously reported that CD11b+ cells expressing M-CSF receptor (CD115) or IL-4 receptor α (CD124) had potent immunosuppressive activity (22, 23). In our study most of MDSC expressed both these receptors (Fig. 6A,B and data not shown). However, in only two models (EL-4 and DA3) the level of IL-4R (CD124) in MDSC was significantly higher than in IMC (Fig. 6A). Similar selectivity was observed in the expression of CD115. In only two of the tested models (DA3 and MC38) the level of CD115 in MDSC was significantly higher than in IMC, whereas in three models (EL-4, B16, and CT26) it was substantially lower (Fig. 6B).
Figure 6. Expression of CD124 (IL-4Rα) and CD115 (M-CSFR) on MDSC from tumor-bearing mice.
Splenocytes from naïve or tumor-bearing mice were stained with anti-Gr-1-APC, anti-CD11b-PE-Cy7 and anti-CD124-PE (A) or anti-CD115-PE (B) antibodies. Expression of CD124 or CD115 within the population of Gr-1+CD11b+ MDSC was measured and calculated as percentage of change from the level of control mice with matched haplotype. Each tumor model included at least 4 mice. * - significant differences from control (p<0.05).
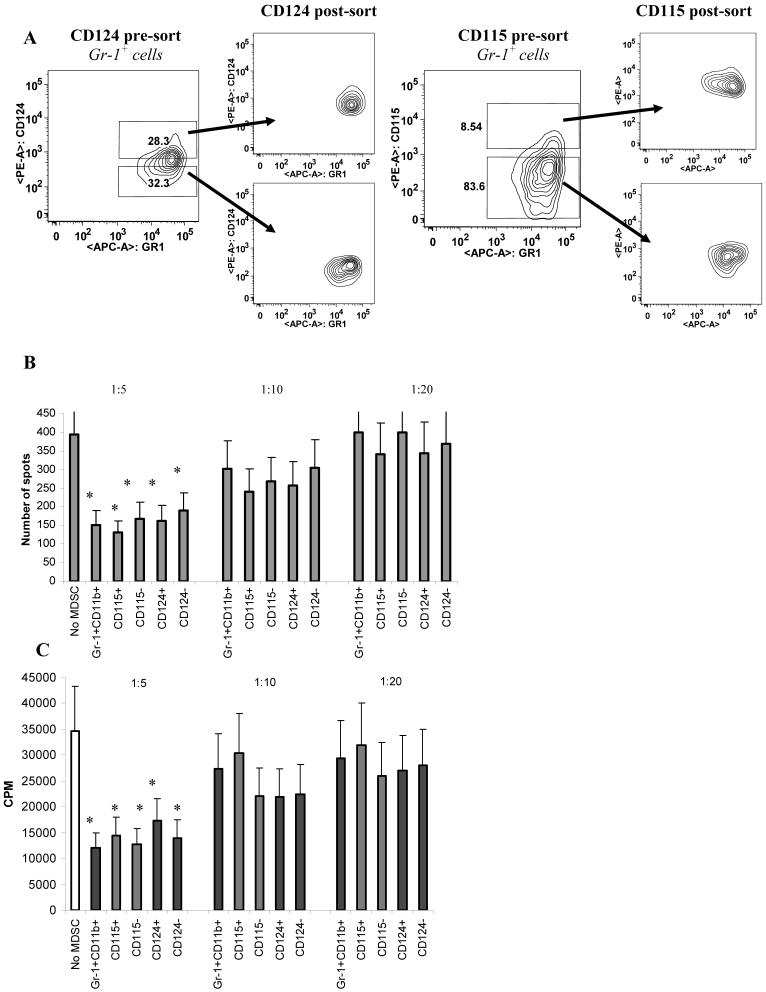
To compare immune suppressive activity of these cells, five populations were sorted from spleens of EL-4 tumor-bearing mice: total population of Gr-1+CD11b+ MDSC; Gr-1+CD11b+CD115+; Gr-1+CD11b+CD115-; Gr-1+CD11b+CD124+; and Gr-1+CD11b+CD124- (Fig. 7A). Cells were then added at different ratios to OT-1 splenocytes and incubated in the presence of OT-1 specific or control peptides. CD8+ T-cell response was evaluated in IFN-γ ELIPOST assay and proliferation assay. At 1:5 ratio all five populations significantly inhibited CD8+ T cells response (Fig. 7B,C). At 1:10 and 1:20 ratio suppressive effect was undetectable in all populations. No differences in the level of suppressive activity were detected between all these populations. Thus, at least in EL-4 tumor model expression of CD115 or IL-4Rα did not render MDSC more or less immune suppressive.
Figure 7. Functional activity of different populations of MDSC.
Five populations of cells were sorted from spleens of EL-4 tumor-bearing mice: Gr-1+CD11b+; Gr-1+CD11b+CD115+; Gr-1+CD11b+CD115-; Gr-1+CD11b+CD124+; Gr-1+CD11b+CD124-. A. Typical example of sorting gates. B. Myeloid cells were added at indicated ratios to OT-1 splenocytes and cultured for 36 hr in the presence of control or specific peptides. IFN-γ production was measured in quadruplicates in ELISPOT assay. The number of spots per 105 splenocytes are shown. The experimental values obtained in the presence of control peptide were subtracted from the values obtained in the presence of specific peptide. * - statistically significant differences in the number of spots from spleens cultured without MDSC. C. Experiments were set up as described in Fig. 7B. Cell proliferation was measured after 4 days of culture using 3H-thymidine assay.
Discussion
In recent years an important role of MDSC in tumor-associated immune suppression has been established in a large number of studies (rev. in (2, 4, 39). Besides cancer, an increased production of MDSC was also reported in a number of pathological conditions including traumatic stress, bacterial and parasitic infections (24, 40, 41). It became apparent that MDSC does not represent single cell population but in fact are comprised of immature myeloid cells at different stages of cell differentiation (12, 42-45). The characterization of specific subset of these cells, primarily responsible for tumor-associated immune suppression, would be very important for understanding the biology of these cells and mechanisms of tumor escape. To address this question we used 10 different tumor models on three different mouse strains. The purpose of using a broad array of different types of tumor was to establish common mechanisms of MDSC expansion in cancer. MDSC are produced in a response to a variety of tumor-derived factors. These factors differ from one tumor model to another. This may explain the often contradictory results regarding the phenotype of these cells reported in different studies. We found that although the level of MDSC was significantly elevated in all tumor models the extent of MDSC expansion varied between different tumor models. Sarcomas demonstrated the lowest level of MDSC expansion whereas breast carcinoma, thymoma, and colon carcinoma the highest. Our in vitro data suggest that the level of MDSC expansion was determined by the nature of soluble factors produced by tumors. Currently, the number of factors affecting myeloid cell expansion in cancer has been proposed. They include COX-2 and prostaglandins (46-48), SCF (49), GM-CSF (50), VEGF (51), CXCR2 ligands (10) and others. Detailed characterization of the factors responsible for MDSC accumulation was outside the scope of this investigation. However, the described differences in the level of MDSC between different tumor models could be exploited for identification of the specific tumor-derived factors mediating this phenomenon. It is not clear at this time whether these findings are directly attributed to cancer patients. Previous and recent studies have demonstrated a substantial expansion of MDSC in cancer patients that correlated with the stage of the disease (11, 13, 14, 16). However, a direct comparison between different types of cancer has not been performed yet.
It is known that population of Gr-1+CD11b+ cells are comprised of two subsets: cells with granulocytic phenotype that express Ly-6G marker and cells with monocytic phenotype expressing Ly6C marker. Recent reports indicated that these two populations might have different functions in infectious and autoimmune diseases and cancer (10, 31-33). Our data demonstrated expansion of only one subset Ly6G+Ly6ClowCD11b+ granulocytic MDSC in most of the tumor models. This result would seem to contradict with a recently reported study that demonstrated both populations were equally increased in mice bearing T-cell lymphoma and thymoma (33). However, this case underscores the importance of analysis of the wide range of tumors in order to draw a conclusion regarding the potential role of MDSC subsets in cancer. In our study EL-4 tumor model was one of the notable exceptions showing that its monocytic subset of MDSC was substantially expanded. This was precisely the type of tumor used in a previous study (33). Apparently, various tumor-derived factors produced by different types of tumor cells define the expansion of MDSC subsets. The exact nature of these factors needs to be determined.
Our data demonstrated that these two populations employed different mechanisms of T-cell suppression. Granulocytic subset of MDSC expressed a high level of ROS and very little NO, whereas monocytic subset had very little ROS but a high level of NO. Accordingly immune suppressive activity of granulocytic MDSC was blocked by ROS inhibitor catalase, whereas activity of monocytic subset of MDSC by iNOS inhibitor LMMA. Interestingly despite that different mechanism of action, both populations suppressed antigen-specific T-cell function equally. This may explain why MDSC mediated T-cell suppression is observed in all tested tumor models regardless of the type of MDSC subset that preferentially expanded. Both populations of MDSC had increased level of peroxynitrite. This is not surprising since peroxynitrite is a product of interaction between NO and superoxide. Therefore, either increased level of ROS or NO would result in increased peroxynitrite level. Observed differences in the levels of peroxynitrite between monocytic and granulocytic subsets apparently were not sufficient to significantly affect their T-cell suppressive activity. More studies are needed to clarify exact role of specific mechanisms mediating immune suppression by these MDSC subsets.
We have investigated the potential role of a number of different surface molecules implicated in immune suppressive activity of MDSC. In order to determine that specific population of MDSC has a specific role in T-cell suppression we used two basic principles. This population should be either preferentially expanded in tumor-bearing mice or has higher a suppression activity per cell basis. As a baseline we used Gr-1+CD11b+ IMC isolated from spleens of naïve tumor-free mice. It is known that these cells are not able to induce T-cell suppression (18-21). Members of the B7 family inhibitory molecules like PD-L1 and PD-L2 and receptors of B7 family CD80 are expressed on a variety of myeloid cells and were shown to be directly involved in the suppression of immune responses (36, 37). Although the total number of MDSC expressing these molecules was increased in tumor-bearing mice, there was no preferential accumulation of the cells expressing PD-L1, PD-L2, or CD80 in any of tested tumor model. Moreover in most of the models we observed a significant decrease in the level of expression of these molecules comparing with control IMC. Direct experiments with block of PD-L1 in vitro or in vivo did not prevent an inhibitory effect of MDSC. These data indicate that these molecules may not necessarily be directly involved in T-cell suppression by MDSC.
CD11b+ cells expressing M-CSF receptor (CD115) or IL-4 receptor α (CD124) have been shown to have immunosuppressive activity (22, 23). We found higher levels of CD115 and CD124 expression in only two tumor models (DA3 breast carcinoma for both receptors, EL-4 thymoma for CD124, and MC38 colon carcinoma for CD115). After sorting of these cells we found that IL-4Rα and M-CSFR positive MDSC suppressed antigen-specific T-cell responses at the same level as non-separated MDSC.
Our data support previous observation that MDSC expressing M-CSFR or IL-4Rα have potent immune-suppressive activity (22, 23). However, they also indicate that the role of these molecules apparently is restricted to only few tumor models and at least in EL-4 tumor-bearing mice these cells are equally potent in suppressing T cells.
Our data may clarify the nature of immune suppressive activity of MDSC in cancer. These cells represent not a defined subset of cells but rather a group of phenotypic heterogeneous myeloid cells that are bound together by common biological activity rather than specific phenotypic characteristics. It is possible that immune suppression activity of MDSC is not restricted to one or several subsets of these cells, but rather attributed to entire population of these cells that reflect the biology of these cells in cancer.
Acknowledgement
We thank Drs. W. Kast, D. Lopez, L. J. Old, E. Celis, I. Turkova, and K. Knutson for providing us with tumor cell lines. We thank Dr. L. Chen for providing us with neutralizing anti-PD-L1 antibody.
Footnotes
This work was supported by NIH grant CA 84488 to DIG. This work has been supported in part by the Analytic Microscopy and Flow Cytometry Core Facility at the H. Lee Moffitt Cancer Center.
Publisher's Disclaimer: “This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.”
References
- 1.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusmartsev S, Gabrilovich DI. Role Of Immature Myeloid Cells in Mechanisms of Immune Evasion In Cancer. Cancer Immunol Immunother. 2006;55:237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. [DOI] [PubMed] [Google Scholar]
- 5.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomez-Garcia L, Lopez-Marin LM, Saavedra R, Reyes JL, Rodriguez-Sosa M, Terrazas LI. Intact glycans from cestode antigens are involved in innate activation of myeloid suppressor cells. Parasite immunology. 2005;27:395–405. doi: 10.1111/j.1365-3024.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 7.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, Pikarsky E, Shapira L, Baniyash M. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL, Thomas R, Hill GR. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 9.Paraiso KH, Ghansah T, Costello A, Engelman RW, Kerr WG. Induced SHIP deficiency expands myeloid regulatory cells and abrogates graft-versus-host disease. J Immunol. 2007;178:2893–2900. doi: 10.4049/jimmunol.178.5.2893. [DOI] [PubMed] [Google Scholar]
- 10.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008 doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 11.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 12.Almand B, Clark JI, Nikitina E, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients. A mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 13.Mirza N, Fishman M, Fricke I, Dunn M, Neuger A, Frost T, Lush R, Antonia S, Gabrilovich D. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricke I, Mirza N, Dupont J, Lockhart G, Jackson A, Lee J-H, Sosman JA, Gabrilovich DI. Treatment of cancer patients with VEGF-Trap overcomes defects in DC differentiation but is insufficient to improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 15.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 18.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol. 2005;175:4583–4592. doi: 10.4049/jimmunol.175.7.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 20.Zhou R, He PL, Ren YX, Wang WH, Zhou RY, Wan H, Ono S, Fujiwara H, Zuo JP. Myeloid suppressor cell-associated immune dysfunction in CSA1M fibrosarcoma tumor-bearing mice. Cancer science. 2007;98:882–889. doi: 10.1111/j.1349-7006.2007.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto Y, Ishigaki H, Ishida H, Itoh Y, Noda Y, Ogasawara K. Analysis of splenic Gr-1(int) immature myeloid cells in tumor-bearing mice. Microbiology and immunology. 2008;52:47–53. doi: 10.1111/j.1348-0421.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 23.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atochina O, Daly-Angel T, Piskorska D, Harn D. A shistosome expressed immunomodulatory glycoconjugate expand peritoneal Gr1+ macrophages that suppress naïve CD4+ T cell proliferation via an interferon-gamma and nitric oxide dependent mechanism. J Immunol. 2001;167:4293–4302. doi: 10.4049/jimmunol.167.8.4293. [DOI] [PubMed] [Google Scholar]
- 25.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 27.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–260. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 28.Gabrilovich DI, Velders M, Sotomayor E, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 29.Kusmartsev S, Gabrilovich D. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 30.Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods. 1994;174:231–235. doi: 10.1016/0022-1759(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 31.Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol. 2007;81:1205–1212. doi: 10.1189/jlb.1006640. [DOI] [PubMed] [Google Scholar]
- 32.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6Chi Suppressive Monocytes in Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 33.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood. 2008 doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 34.Bronte V, Casic T, Gri G, Gallana K, Borsellino G, Marrigo I, Battistini L, Iafrate M, Prayer-Galletti U, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber D, Schneck J, Gabrilovich D. Altered recognition of antigen is a novel mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome biology. 2005;6:223. doi: 10.1186/gb-2005-6-6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang R, Cai Z, Zhang Y, Yutzy W. H. t., Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 39.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 41.Mencacci A, Montagnoli C, Bacci A, Cenci E, Pitzurra L, Spreca A, Kopf M, Sharpe A, Romani L. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunol. 2002;169:3180–3190. doi: 10.4049/jimmunol.169.6.3180. [DOI] [PubMed] [Google Scholar]
- 42.Pandit R, Lathers D, Beal N, Garrity T, Young M. CD34+ immune suppressive cells in the peripheral blood of patients with head and neck cancer. Ann Otol Rhinol Laryngol. 2000;109:749–754. doi: 10.1177/000348940010900809. [DOI] [PubMed] [Google Scholar]
- 43.Bronte V, Serafini P, Appoloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J. Immunoth. 2001;24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–2145. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 45.Gabrilovich DI. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7:140–151. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 48.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 49.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid derived suppressor cell development by blockade of SCF function. Blood. 2007 doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-Dose GM-CSF-Producing Vaccines Impair The Immune Response Through The Recruitment Of Myeloid Suppressor Cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]