Abstract
The mediodorsal nucleus of the human thalamus is in a crucial position that allows it to establish connections with diverse cerebral structures, particularly the prefrontal cortex. The present review examines existing neurobiologic studies of the brains of people with and without schizophrenia that indicate a possible involvement of the mediodorsal nucleus in this psychiatric disorder. Studies at synaptic and cellular levels of the neurobiology of the mediodorsal nucleus, together with a better anatomic understanding of this diencephalic structure owing to neuroimaging studies, should help to establish a more deep and solid pathophysiologic model of schizophrenia.
Medical subject headings: mediodorsal thalamic nucleus, schizophrenia
Abstract
Le noyau dorsomédian du thalamus humain se trouve à un endroit crucial qui lui permet d'établir des liens avec diverses structures cérébrales, et en particulier le cortex préfrontal. La présente synthèse porte sur des études neurobiologiques existantes du cerveau de personnes atteintes ou non de schizophrénie qui indiquent que le noyau médiodorsal peut jouer un rôle dans ce trouble psychiatrique. Des études aux niveaux synaptique et cellulaire portant sur la neurobiologie du noyau dorsomédian, ainsi qu'une meilleure compréhension anatomique de cette structure du diencéphale découlant d'études de neuro-imagerie, devraient aider à établir un modèle pathophysiologique plus profond et solide de la schizophrénie.
Neuromorphology of the mediodorsal nucleus of the thalamus
The anatomic location of the thalamus in humans is crucial to its principal function: interconnecting different cerebral structures, particularly to the cerebral cortex. The thalamus sends and receives projections from multiple regions in the cortex and the brainstem, and it has traditionally been assigned a fundamental role in the process of filtering nervous information.1
The thalamus comprises a large number of neuronal groups involved in a wide range of cognitive, sensorimotor and limbic functions. The external medullary lamina, a layer of myelinic axons, covers the lateral face of the thalamus, and the medial thalamic surface abuts on the third ventricle.2 The classic separation between the dorsal and ventral thalamus is determined in part by the fusion between the thalamic fascicle and the external medullary lamina and between the thalamic reticular nucleus and the zona incerta.2
The main cell groups of the dorsal thalamus are limited by the internal medullary lamina.2 The mediodorsal nucleus (MD) is part of the medial nuclear group (Fig. 1). In humans, as in most other mammals, this nucleus is generally divided into 3 subnuclei. Thus according to Jones' extensive review on the thalamus,2 the MD can be parcellated in a magnocellular or medial region (pars fibrosus) composed of large cells; a parvocellular or central and posterior region (pars fasciculosis) consisting of smaller neurons; and a multiform or lateral region (subnucleus caudalis) with cells of mixed sizes, including a paralaminar area or nucleus that would probably be better placed in the central lateral intralaminar nucleus.2,3 However, other authors have claimed that this subnuclear division of the MD is not clear and have suggested that the subdivisions are based on myeloarchitectonic differences,4–6 which implies the MD is a single entity.7
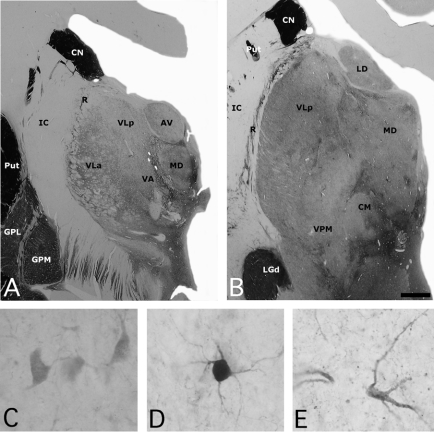
Fig. 1: (A, B) Photomicrographs of 2 sections of the human thalamus stained for acetylcholinesterase (AChE), illustrating the anatomic location of the mediodorsal nucleus (MD). High-power views of some neurons stained for (C) calbindin, (D) parvalbumin and (E) calretinin neurons in the human MD. Scale: 3 mm (A, B) and 25 μm (C, D, E). AV = anteroventral nucleus; CM = central medial nucleus; CN = caudate nucleus; GPL = globus pallidus lateral segment; GPM = globus pallidus medial segment; IC = internal capsule; LD = lateral dorsal nucleus; LGd = lateral eniculate nucleus; Put = putamen; R = reticular nucleus; VA = ventral anterior nucleus; VLa = ventral lateral anterior nucleus; VLp = central lateral posterior nucleus; VPM = ventral posterior medial nucleus.
Connections
Afferent projections to the magnocellular, parvocellular and multiform regions of the MD
Olfactory impulses from the entorhinal cortex, the prepiriform cortex and adjacent regions such as the olfactory tubercle reach the MD magnocellular subdivision, indicating the existence of a route that carries olfactory impulses through the thalamic MD to neocortical regions.2,8 The same MD subdivision also receives projections from the amygdala.2 The prepiriform and entorhinal cortices and the amygdala project to the same cerebral areas as the magnocellular subdivision of the MD, thus forming a circuit that is not found in the other 2 subdivisions (Fig. 2).2 Other GABAergic projections from the ventral pallidum, globus pallidus and pars reticulata of the substantia nigra reach all 3 subdivisions of the MD.2,9–13
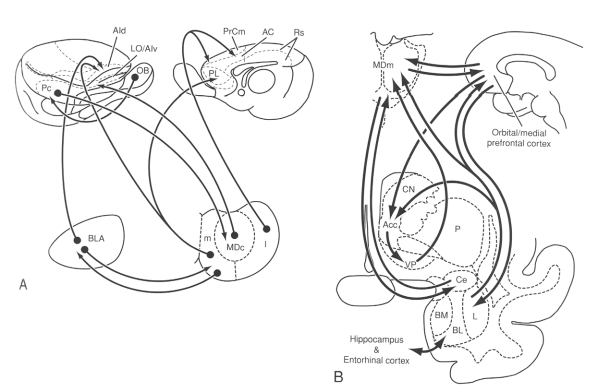
Fig. 2: Schematic drawings illustrating the connections of the different subdivisions of the mediodorsal nucleus (MD) with the amygdala and the cerebral cortex (A) in the rat and (B) monkey. Reprinted with permission from Jones EG. The thalamus. 2nd ed. New York: Cambridge University Press; 2007. p. 1189.2 AC = anterior cingulate area; Acc = nucleus accumbens; AId = dorsal agranular insular area; AIv = ventral agranular insular area; BL/BLA = basolateral nucleus of the amygdala; BM = basomedial nucleus of the amygdala; Ce = central nucleus of the amygdala; CN = caudate nucleus; I = lateral segment of the mediodorsal nucleus; L = lateral nucleus of the amygdala; LO = lateral orbital area; m = medial segment of the mediodorsal nucleus; MDc = central part of the mediodorsal thalamic nucleus; MDm = medial part of the mediodorsal thalamic nucleus; OB = olfactory bulb; P = putamen; Pc = prepiriform cortex; PL = prelimbic area; PrCm = medial precentral area; Rs = retrosplenial area; VP = ventral pallidum.
The parvocellular and multiform subdivisions receive abundant projections from the brainstem. The projections to the parvocellular subdivision from the superior colliculus, pars reticulata of the substantia nigra, medial vestibular nucleus, dorsal tegmental nucleus and other tegmental regions have received little attention, which is surprising since projections from this subdivision occupy a large cortical area.2,9,11
Nonetheless, the cortical projections, particularly in zones receiving projections from the different MD subdivisions, other thalamic nuclei and the hypothalamus, are very important in controlling MD function. This thalamic nucleus, similar to most thalamic nuclei, can be considered a subcortical step in corticocortical communication.14,15
Efferent projections from the magnocellular, parvocellular and multiform regions of the MD
The comments that follow focus on the cortical connections (Fig. 2) and the connections with the basal ganglia. Studies involving nonhuman primates have indicated that the patterns of innervation between the different MD subdivisions and the prefrontal cortex are highly organized and bidirectional.2,11,13,16,17 Thus projections from the magnocellular subdivision are directed to the orbital region; the projections from the parvocellular region to the dorsolateral region and those from the multiform subdivision of the MD are directed to the frontal ocular area.2 These connections with the prefrontal cortex are the main source of efferent MD connections.18,19 However, studies have shown that the MD also sends projections to other cortical regions in macaques, including the cingular, insular, parietal, premotor, inferior premotor and supplementary premotor cortices and the supplementary motor area.20–26
Studies in rats have also indicated that the MD has connections with the caudate nucleus,27,28 although some authors do not agree.29 These projections are also controversial in cats30–32 and primates.33
Chemical anatomy of the MD
Histochemistry and immunohistochemistry
Acetylcholinesterase enzyme
Acetylcholinesterase enzyme (AChE) staining in the MD is not homogeneous;34 it is weaker at anterior than at posterior levels. Also, the posterolateral zone adjacent to the internal medullary lamina is more intensely stained than the other areas of this thalamic nucleus. In addition, there are irregular patches of AChE staining throughout the MD.
Nissl staining shows that the heterogeneously distributed patches of AChE in the MD can be correlated with different cell populations. The magnocellular medial region, which is composed of large cells, stains very strongly with AChE.2 The ventral region, which has different sizes of cells, corresponds to the multiform subdivision and shows less AChE staining than the magnocellular subdivision. Lastly, the dorsolateral region, which is composed of small cells, corresponds to the parvocellular subdivision and shows stronger AChE staining than the other 2 subdivisions.2
Intracellular calcium-binding proteins: calbindin, parvalbumin and calretinin
Calbindin (CB), parvalbumin (PV) and calretinin (CR) are implicated in intracellular storage and transport and in the regulation of various enzymatic systems.34,35 They have been used as neurochemical markers to delineate the functional territories in different regions of the human brain, including the thalamus (Fig. 1).
Calcium-binding proteins make excellent markers for the study of cellular morphology and the different nuclear subdivisions of the MD.35 In fact, it has been proposed that the thalamic distribution of CB-and PV-immunoreactive neurons may be useful in the identification of different anatomic and functional types of thalamocortical projection neurons.36 Thus CB-immunoreactive neurons would project diffusely to the superficial cortical layers and be involved in multiple aspects of sensorial experience, and PV-immunoreactive neurons would project in an orderly manner to deeper cortical layers and be involved in perception processes.36 Moreover, CR-immunoreactive neurons would be interneurons or projection neurons and would be implicated in modulating motivational and emotional states.36
Three studies involving humans have described the distribution of CB-, PV-and CR-immunopositive neurons in the MD.35,37,38 All 3 report different findings, perhaps because they used different antibodies or fixation processes.34,35 Münkle and colleagues35 concluded that MD neurons were immunoreactive for CB, PV and CR but showed different degrees of staining intensity: PV-positive neurons stained heavily, CB-positive neurons stained with medium intensity and CR-positive neurons stained with the weakest intensity.35 Their results differed from those of Morel and colleagues,37 who concluded that only weakly labelled PV-positive cells appeared in the MD. Finally, the study by Fortin and colleagues,38 which was centred only on the distribution of CR-immunoreactive neurons, concluded that the MD contained neurons of different sizes that were uniformly distributed throughout the entire nucleus. The moderately CR-positive cells, which are of medium and large size, were the more abundantly observed cells in the MD.
Substance P and enkephalin
Throughout its anteroposterior extension, the MD contains numerous fibres positive for substance P and met-enkephalin.
Substance P mediates biological actions through a G protein that bonds to the tachykinin receptor.34 In primates, the distribution of substance P is more dense and widespread than that of other peptides such as somatostatin, neuropeptide Y or cholecystokinin.39 In both human and nonhuman primates, the thalamic nuclei with the most substance P– positive innervation are the anterior, medial and intralaminar nuclei; the epithalamus; and the reticular nucleus. This observation concurs with evidence reported in a radioimmunoanalysis study, which found that the medial regions of the human thalamus present greater levels of substance P than the other thalamic regions.40 This peptide has been assigned an important role in pain, anxiety and depressive disorders.34
On the other hand, met-enkephalin has been described as an endogenous opioid and μ-opioid receptor agonist implicated in the suppression of the affective quality of painful stimuli, in the regulation of different emotional aspects of memory and in the regulation of behaviour and thermal pain perception.41,42 The distribution of met-enkephalin in the human thalamus is virtually identical to that of substance P, which suggests the possibility that both neuropeptides collocate in the same fibres.40
Dopamine
Studies involving different in-situ hybridization, immunohistochemical and neuroimaging techniques have reported the presence of dopamine in the human thalamus.43–46 However, these reports are contradictory. Different authors, including Jones,2 have reported that dopaminergic innervation in the thalamus is not substantial and is restricted mainly to epithalamic regions.2 More recent papers have noted the contrary: in both human and nonhuman primates, the MD is one of the thalamic nuclei with the heaviest dopaminergic innervation, showing that the MD has a moderate density of dopamine D2–like receptors.46–48 These studies have noted that the MD innervation pattern is heterogeneous and quite dense in the medioventral zone and that density gradually decreases along the medioventral to laterodorsal axis.46 The dopaminergic fibres proceed from a thalamic innervation system with multiple origins in the hypothalamus, periaqueductal grey matter, ventral mesencephalon and parabrachial lateral nucleus.46,47 Interestingly, as we have noted above, the MD has numerous connections with the prefrontal cortex — a region that is profoundly affected in schizophrenia — so it is possible that it is influenced indirectly by the action of dopamine in the MD and directly by dopaminergic terminations within the MD.
Serotonin
The serotonergic fibres that reach the thalamus come from the nuclei of the mesencephalic raphe.49–54 The density of the serotonergic innervation in the human thalamus is relatively high,2 with the anterior nuclei (anteroventral, anteromedial and anterodorsal) presenting the lowest levels of serotonin (5-HT).55 The levels of this neurotransmitter rise in the more posterior regions where the intralaminar nuclei are located.55 The pattern of serotonergic distribution also extends to the lateral ventral nucleus and the MD.55 These reports confirm the results of neuroimaging and postmortem human brain material studies that found that the latter thalamic nucleus has high concentrations of 5-HT as well as a high density of serotonergic receptors, high levels of 5-HT1B receptor messenger RNA expression and 5-HT7 receptors. The MD is also one of the principal sites where selective serotonin reuptake inhibitors (SSRIs) accumulate.56 Moreover, numerous reports have described maniac conditions of patients with damage around and within the MD, suggesting that the neuronal activity of this nucleus is involved in affective behaviour.56 Overall, these data suggest that the MD is implicated in the pathophysiology of affective disorders and in the therapeutic actions of SSRIs.
Mediodorsal nucleus and cognitive functions
In the performance of tasks in which short-term memory is evaluated, there is an increase in the glucose metabolism in the MD, which suggests that this thalamic structure could be involved in the first phases of information processing for memory and learning.2
Studies of lesions in the MD in nonhuman primates have evaluated the involvement of this nucleus in different cognitive processes or domains and in behaviour. For instance, lesions in the MD produced a deficit in association and visual object recognition as well as in processes associating stimulus and reward.2
In humans, lesions in the medial part of the thalamus have been associated with alterations in memory.2 However, the role of the MD in amnesia has not been defined clearly and is controversial. The first neuropathologic study by Victor and colleagues57 noted damage in the MD among all patients with diagnosed Korsakoff amnesia, although the authors also noted damage in other regions of the patients' brains.57,58 Conversely, other studies have proposed that Korsakoff amnesia occurs without any alteration in the MD.59 More recently, Graff-Radford and colleagues60 have noted damage to the MD among patients who did not have amnesia, whereas still other researchers have argued the contrary,61 generating doubts about the true role of the MD in diencephalic amnesia.58
Different studies involving alcoholic patients have found a correlation between the presence of a lesion in the anterior nuclei of the thalamus and amnesia. This finding is consistent with the well known involvement of the hippocampus– fornix– mammillary body circuit in memory because these thalamic nuclei are the main target of the projections from the mammillary bodies.62 These observations do not exclude the role of lesions in the MD in amnesia, particularly among patients with some additional cerebral dysfunction.63
The MD is part of the cerebral circuits underlying the neuronal network for executive control — one of the cognitive domains that is impaired in schizophrenia.64 This network makes it possible to exercise voluntary control on processing in situations that require the initiation, planning, sequencing and monitoring of complex behaviours directed toward a particular goal and in the inhibition of automatic responses and the development of strategies or conflict resolution among stimuli.65
Cerebral cognitive circuits
Three types of cerebral circuits have been identified: the dorsolateral prefrontal, orbitofrontal lateral and anterior cingulate circuits.
Dorsolateral prefrontal circuit
The corticofugal fibres reach the dorsolateral region of the caudate nucleus, which also receives projections from the posterior parietal cortex and the premotor cortical region. The connective circuit continues with the innervation of the dorsolateral region of the globus pallidus and the rostral zone of the pars reticulata of the substantia nigra, continuing into the parvocellular region of the MD and the ventral anterior nucleus of the thalamus. The circuit is closed with the efferent thalamocortical projections toward the dorsolateral prefrontal cortex. Lesions in this circuit produce alterations in several higher cognitive functions such as selecting objectives, planning and sequencing the generation of an answer set, and memory of verbal and spatial tasks.66,67
Orbitofrontal lateral circuit
The cortical projections reach the ventromedial region of the caudate nucleus, which also receives projections from other associative cortical areas such as the superior and inferior temporal gyri and brainstem regions (reticular formation). The projections continue into mediodorsal regions of the medial globus pallidus and rostromedial areas of the pars reticulata of the substantia nigra, ending in the magnocellular division of the MD and in the ventral anterior nucleus of the thalamus. The circuit closes with the thalamocortical projections toward the lateral orbitofrontal cortex. This circuit is implicated in the inhibition of inappropriate behavioural responses and in the evaluation of risk.65,66,68
Anterior cingulate circuit
This cerebral circuit connects with the accumbens nucleus and the olfactory tubercle in the ventral striatum, which receive projections from the anterior temporal pole, entorhinal cortex, hippocampus and amygdala. The circuit continues with the innervation of the ventral pallidum and the rostrodorsal regions of the pars reticulata of the substantia nigra and the MD, and it ends in the anterior cingulate cortical zone. The circuit is involved in monitoring behaviour and correcting errors.65
Mediodorsal nucleus and schizophrenia
Several lines of investigation have focused on the study of prefrontal and temporal cortical dysfunction in schizophrenia, suggesting that these cortical alterations underlie the cognitive abnormalities seen in the disease. However, more recent attention has focused on the thalamus, the brain's central sensory switchboard, implicating this structure in the pathophysiology of schizophrenia.
Regarding the role of the MD in schizophrenia, the fact that this structure is a key in the communication between distinct associative cortical areas indicates that alterations in this thalamic nucleus could lead to dysfunctions in corticosubcortical and corticocortical connections, which supports the schizophrenic disconnection hypothesis.69
From the anatomic point of view, one of the most repeated findings in neuroimaging studies of patients with schizophrenia is the dilation of the lateral and third ventricles,70 suggesting that ventricular size increases as the disease progresses.71 Nevertheless, studies of the possible variations in thalamic volume in patients with schizophrenia have yielded different results, probably owing to the difficulty of identifying the distinct nuclei on magnetic resonance images.72 There are studies that find no difference in thalamic volume between patients with schizophrenia and healthy controls,72–74 whereas other studies have found statistically significant differences.75–81 The main finding in one of the most comprehensive meta-analyses on the size of the thalamus in schizophrenia was that the size of the thalamus relative to total brain size was smaller among patients with schizophrenia than among healthy controls.82 However, this finding should be tempered by the evidence that the effect sizes were quite variable across studies.
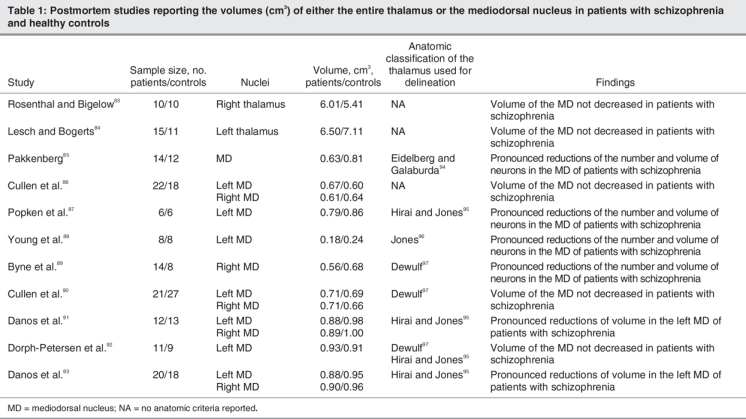
There are analyses that have implicated the MD in schizophrenia, and they have used neuroimaging techniques73,77–79,81 as well as postmortem material (Fig. 3 and Table 1).83–97 These reports have noted the existence of anatomic abnormalities in the brains of patients with schizophrenia, although the data, particularly from the postmortem studies, present contradictory results. Some papers have suggested statistically significant reductions either in the volume and cell number98–100 or in the volume only,101 whereas other studies have not found these decreases in the MD.86,90,102 The disparity in these results may be explained by the difficulties in establishing the neuroanatomical limits of the MD, the study of only one of the cerebral hemispheres, the size of the samples and age of the participants, and the characteristics of patients in the study and the control groups.101
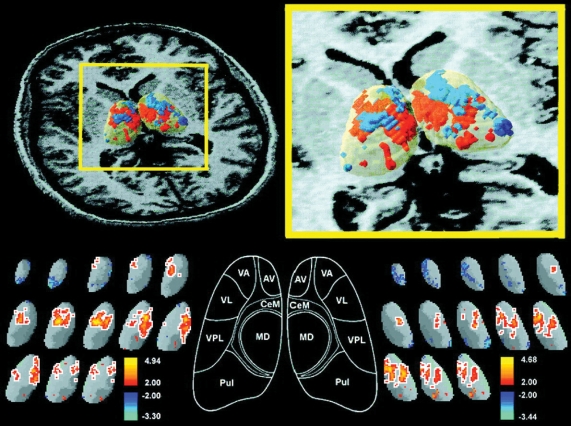
Fig. 3: Three-dimensional significance probability mapping of the thalamus in healthy controls and patients with schizophrenia using positron emission tomography (PET) superimposed on magnetic resonance imaging (MRI) scans. Note the significantly decreased glucose metabolic rate, located bilaterally in the region of the mediodorsal nucleus (MD) in patients with schizophrenia. For more details about methodologic approaches, see reference.73 Reprinted with permission from the American Journal of Psychiatry,73 © 1999 American Psychiatric Association. AV = anteroventral nucleus; CeM = central median nucleus; MD = mediodorsal nucleus; Pul = pulvinar nucleus; VA = ventral anterior nucleus; VL = ventral lateral nucleus; VPL = ventral posterior lateral nucleus.
Table 1
The relation between dopamine and schizophrenia has been the subject of many investigations over the last 50 years, and current opinion is that dopaminergic neurotransmission is impaired in the thalamus in schizophrenia.103,104 The first formulation of the dopaminergic hypothesis in schizophrenia proposed that the symptoms characterizing the disease were caused by the hyperactive transmission of catecholamine, although no explanation about the appearance of the negative and cognitive symptoms was given. This hypothesis was based on the effects of antipsychotic drugs, all of which were antagonists of dopaminergic D2 receptors, which are mostly expressed in the striatum.105
The current hypothesis on the relation between dopamine and schizophrenia is a reformulation of the former theory. The hypothesis proposes that an imbalance exists between subcortical excess and an acute cortical deficit of dopamine at certain levels, specifically in the prefrontal cortex,103,106 a region that is the main nodal point in the cortical relations with the MD.18,19 However, in relation to the cognitive symptoms, the only conclusion to date is that, although it is possible that the dopamine in the prefrontal cortex is important for cognition, it cannot be claimed that a deficit of this neurotransmitter will cause the full cognitive deterioration characteristic of schizophrenia.103 Regarding these cognitive symptoms, some authors have noted that the MD shows a decrease in dopamine D2 receptors at subcortical levels;107 this decrease could contribute to an improper sensory gating function in schizophrenia, resulting in difficulties focusing on relevant information (i.e., thought disorder, confusion and hypersensitivity to stimuli).107
The relation between schizophrenia and 5-HT was first proposed based on the efficacy of second-generation antipsychotic drugs developed in the early 1990s that interact with serotonergic receptors like 5-HT2A.103 The blockade using receptor-specific antagonists of the 5-HT2A receptors, together with the D2 receptors, increases the level of dopamine in the prefrontal cortex.108–110 The modulation of prepulse inhibition by agonists of the 5-HT1B receptor and the inverse agonist activity of some antipsychotics suggest that the 5-HT1B receptor antagonist may have antipsychotic properties. In addition, the 5-HT1B heteroreceptors are localized in ventral striatopallidal regions, projections from the MD and the bed nucleus of the stria terminalis, all of which are circuits implicated in the pathophysiology of several psychiatric disorders, including depression, obsessive–compulsive disorder and schizophrenia.111
Future directions
We believe that factors associated with the synaptic and cellular neurobiology of subcortical structures will be at the forefront of basic neurobiologic research into schizophrenia. Further study in these areas could, first, give us important data directed toward establishing more precise correlations with the different clinical aspects of the disease and, second, allow a more targeted and effective treatment at the synaptic or cellular level.
The contradictory results from diverse research groups regarding MD volume and the number of cells in the brains of both healthy controls and patients with diagnosed schizophrenia make it necessary to continue working on this question to resolve the controversy and provide accurate neuromorphologic information.
Research similar to that undertaken by Lewis and colleagues112 to our further our understanding of the cortical interneurons of the dorsolateral prefrontal cortex is required to analyze the interneurons in the thalamus. Lewis and colleagues found a decrease in glutamic acid decarboxylase (GAD)–67 expression in cortical interneurons using in-situ hybridization techniques. These interneurons may play a very important role in synchronizing activity with pyramidal cells during task memorization, a process that is clearly affected in schizophrenia.112
We believe that thalamic interneurons may play a fundamental role in information processing in the subcortical structures, integrating and processing the information flow toward the cerebral cortex and organizing complex microcircuits that allow thalamosubcortical and corticothalamic transmission.113,114
Another area that requires further research is the analysis of the glutamatergic or GABAergic neurotransmission in the thalamus. For example, such research could include the analysis of the expression and distribution of the different subunits as well as synaptic proteins related to the alpha-amino- 3-hydroxy- 5-methyl- 4- isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA) and γ-aminobutyric acid (GABA) receptors in the distinct thalamic nuclei in both healthy controls and patients with schizophrenia. Ibrahim and colleagues115 observed lower levels of ionotropic glutamate receptor expression (primarily involving the NMDA receptor) in the thalamus of patients with schizophrenia, although these results are restricted to limbic nuclei.115 Regarding the metabotropic glutamate receptor expression in the thalamus of schizophrenic patients, Richardson-Burns116 and colleagues described a normal expression of these receptors, although they suggested that their results did not mean that these receptors are not involved in the pathophysiology of schizophrenia. Although these studies have given us some valuable information about neurobiologic aspects that are affected at the cellular, synaptic and molecular levels in schizophrenia, more synaptic research is needed.
Finally, we believe that, despite the great advances in the study of schizophrenia that have resulted from different neuroimaging techniques, it is still necessary to have a better neuroanatomical analysis of the relations between cognitive functions and the underlying anatomic structures.
Acknowledgments
We wish to thank Carol Fox Warren for her help with the English version of this manuscript. We also wish to thank Dr. Silvano de las Heras and Dr. Lucía Prensa for their advice. This study has been supported by grants from the Spanish Ministerio de Educación y Ciencia (BFI2003-02909 and BFU2006-01189).
Footnotes
Contributors: Both Drs. Alelú-Paz and Giménez-Amaya contributed equally to the review design, data acquisition and analysis, and writing and review of the article, and both authors approved it for publication.
Competing interests: None declared.
Correspondence to: Dr. J.M. Giménez-Amaya, Departamento de Anatomía, Histología y Neurociencia, Facultad de Medicina, Universidad Autónoma de Madrid, C/ Arzobispo Morcillo s/n. 28029 Madrid, Spain; fax 34 91 497 5338; josemanuel.gimenezamaya@uam.es
References
- 1.Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry 1997;42:27-33. [DOI] [PubMed]
- 2.Jones EG. The thalamus. 2nd ed. New York: Cambridge University Press; 2007.
- 3.Olszewski J. The thalamus of the Macaca mulatta. An atlas for use with the stereotaxic instrument. New York: Karger; 1952.
- 4.Vogt C. La myélocytoarchitecture du thalamus du cercopithèque. J Psychol Neurol 1909;12:285-324.
- 5.Hassler R. Anatomy of the thalamus. In: Schaltenbrand G, Bailey P, editors. Introduction to stereotaxic operations with an atlas of the human brain. Stuttgart: Thieme; 1959. p. 230-90.
- 6.Hassler R, Mundinger F, Riechert T. Stereotaxis in Parkinson syndrome. With an atlas of the basal ganglio in parkinsonism. Berlin: Springer; 1979.
- 7.Van Buren JM, Borke RC. Variations and connections of the human thalamus. Berlin: Springer; 1972.
- 8.Yarita H, Lino M, Tanabe T, et al. A transthalamic olfactory pathway to orbitofrontal cortex in the monkey. J Neurophysiol 1980;43:69-85. [DOI] [PubMed]
- 9.Velayos JL, Reinoso-Suarez F. Topographic organization of the brainstem afferents to the mediodorsal thalamic nucleus. J Comp Neurol 1982;206:17-27. [DOI] [PubMed]
- 10.Aggleton JP, Mishkin M. Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol 1984;222:56-68. [DOI] [PubMed]
- 11.Russchen FT, Amaral DG, Price JL. The afferent input to the magnocellular division of the mediodorsal thalamic nucleus in the monkey, Macaca fascicularis. J Comp Neurol 1987;256:175-210. [DOI] [PubMed]
- 12.Hreib KK, Rosene DL, Moss MB. Basal forebrain efferents to the medial dorsal thalamic nucleus in the rhesus monkey. J Comp Neurol 1988;277:365-90. [DOI] [PubMed]
- 13.Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 1988;277:195-213. [DOI] [PubMed]
- 14.Velayos JL, Reinoso-Suarez F. Prosencephalic afferents to the mediodorsal thalamic nucleus. J Comp Neurol 1985;242:161-81. [DOI] [PubMed]
- 15.Reinoso-Suarez F. Connectional patterns in parietotemporo-occipital association cortex of the feline cerebral cortex. In: Reinoso-Suarez F, Ajmone-Marsan C, editors. Cortical integration: basic, archicortical, and cortical association levels of neural integration. New York: Raven Press; 1984. p. 255-79.
- 16.Arikuni T, Sakai M, Kubota K. Columnar aggregation of prefrontal and anterior cingulate cortical cells projecting to the thalamic mediodorsal nucleus in the monkey. J Comp Neurol 1983;220:116-25. [DOI] [PubMed]
- 17.Siwek DF, Pandya DN. Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. J Comp Neurol 1991;312:509-24. [DOI] [PubMed]
- 18.Nauta WJH. Some efferent connections of the prefrontal cortex in the monkeys. In: Warren JM, Akert K, editors. The frontal granular cortex and behavior. New York: McGraw-Hill; 1964. p. 397-407.
- 19.Akert K, Hartmann-von Monakow K. Relationships of precentral premotor and prefrontal cortex to the mediodorsal and intralaminar nuclei of the monkey thalamus. Acta Neurobiol Exp (Wars) 1980;40:7-25. [PubMed]
- 20.Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 1979;204:205-7. [DOI] [PubMed]
- 21.Baleydier C, Mauguiere F. The duality of the cingulate gyrus in monkey. Neuroanatomical study and functional hypothesis. Brain 1980;103:525-54. [DOI] [PubMed]
- 22.Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 1985;242:535-60. [DOI] [PubMed]
- 23.Roberts TS, Akert K. Insular and opercular cortex and its thalamic projection in Macaca mulatta. Schweiz Arch Neurol Neurochir Psychiatr 1963;92:1-43. [PubMed]
- 24.Kasdon DL, Jacobson S. The thalamic afferents to the inferior parietal lobule of the rhesus monkey. J Comp Neurol 1978;177:685-706. [DOI] [PubMed]
- 25.Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 1984;4:539-60. [DOI] [PMC free article] [PubMed]
- 26.Rouiller EM, Tanne J, Moret V, et al. Origin of thalamic inputs to the primary, premotor, and supplementary motor cortical areas and to area 46 in macaque monkeys: a multiple retrograde tracing study. J Comp Neurol 1999;409:131-52. [DOI] [PubMed]
- 27.Deschênes M, Bourassa J, Parent A. Two different types of thalamic fibers innervate the rat striatum. Brain Res 1995;701:288-92. [DOI] [PubMed]
- 28.Erro ME, Lanciego JL, Giménez-Amaya JM. A re-examination of the striatal input from the mediodorsal thalamic nucleus in the rat. Eur J Anat 2002;4:95-101.
- 29.van der Kooy D. The organization of the thalamic, nigral and raphe cells projecting to the medial vs lateral caudate-putamen in rat. A fluorescent retrograde double labeling study. Brain Res 1979;169:381-7. [DOI] [PubMed]
- 30.de las Heras S, Mengual E, Velayos JL, et al. Re-examination of topographic distribution of thalamic neurons projecting to the caudate nucleus. A retrograde labeling study in the cat. Neurosci Res 1998;31:283-93. [DOI] [PubMed]
- 31.de las Heras S, Mengual E, Gimenez-Amaya JM. Double retrograde tracer study of the thalamostriatal projections to the cat caudate nucleus. Synapse 1999;32:80-92. [DOI] [PubMed]
- 32.Mengual E, de las Heras S, Erro E, et al. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat 1999;16:187-200. [DOI] [PubMed]
- 33.Gimenez-Amaya JM, McFarland NR, de las Heras S, et al. Organization of thalamic projections to the ventral striatum in the primate. J Comp Neurol 1995;354:127-49. [DOI] [PubMed]
- 34.Alelú-Paz R, Giménez-Amaya JM. Chemical parcellation of the anterior thalamic nuclei in the human brain. J Neural Transm 2007;114:969-81. [DOI] [PubMed]
- 35.Münkle MC, Waldvogel HJ, Faull RL. The distribution of calbindin, calretinin and parvalbumin immunoreactivity in the human thalamus. J Chem Neuroanat 2000;19:155-73. [DOI] [PubMed]
- 36.Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience 1998;85:331-45. [DOI] [PubMed]
- 37.Morel A, Magnin M, Jeanmonod D. Multiarchitectonic and stereotactic atlas of the human thalamus [published erratum in J Comp Neurol 1998;391:545]. J Comp Neurol 1997;387:588-630. [DOI] [PubMed]
- 38.Fortin M, Asselin MC, Gould PV, et al. Calretinin-immunoreactive neurons in the human thalamus. Neuroscience 1998;84:537-48. [DOI] [PubMed]
- 39.Molinari M, Hendry SHC, Jones EG. Distributions of certain neuropeptides in the primate thalamus. Brain Res 1987;426:270-89. [DOI] [PubMed]
- 40.Hirai T, Jones EG. Distribution of tachykinin-and enkephalin-immunoreactive fibers in the human thalamus. Brain Res Brain Res Rev 1989;14:35-52. [DOI] [PubMed]
- 41.Schmauss C, Yaksh TL. In vivo studies on spinal opiate receptor systems mediating antinociception. II. J Pharmacol Exp Ther 1984;228:1-12. [PubMed]
- 42.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 2001;293:311-5. [DOI] [PubMed]
- 43.Oke AF, Adams RN. Elevated thalamic dopamine: possible link to sensory dysfunctions in schizophrenia. Schizophr Bull 1987;13:589-604. [DOI] [PubMed]
- 44.Okubo Y, Olsson H, Ito H, et al. PET mapping of extrastriatal D2-like dopamine receptors in the human brain using an anatomic standardization technique and [11C]FLB 457. Neuroimage 1999;10:666-74. [DOI] [PubMed]
- 45.Rieck RW, Ansari MS, Whetsell WO Jr, et al. Distribution of dopamine D2-like receptors in the human thalamus: autoradiographic and PET studies. Neuropsychopharmacology 2004;29:362-72. [DOI] [PubMed]
- 46.García-Cabezas MA, Rico B, Sánchez-González MA, et al. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage 2007;34:965-84. [DOI] [PubMed]
- 47.Sánchez-González MA, García-Cabezas MA, Rico B, et al. The primate thalamus is a key target for brain dopamine. J Neurosci 2005;25:6076-83. [DOI] [PMC free article] [PubMed]
- 48.Melchitzky DS, Lewis DA. Dopamine transporter-immunoreactive axons in the mediodorsal thalamic nucleus of the macaque monkey. Neuroscience 2001;103:1033-42. [DOI] [PubMed]
- 49.Conrad LCA, Leonard CM, Pfaff DW. Connections of the median and dorsal raphe nuclei in the rat: an autoradiographic and degeneration study. J Comp Neurol 1974;156:179-205. [DOI] [PubMed]
- 50.Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J Comp Neurol 1978;179:641-67. [DOI] [PubMed]
- 51.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 1981;6:557-618. [DOI] [PubMed]
- 52.Cropper EC, Eisenman JS, Azmitia EC. An immunocytochemical study of the serotonergic innervation of the thalamus of the rat. J Comp Neurol 1984;224:38-50. [DOI] [PubMed]
- 53.Morrison JH, Foote SL. Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in Old and New World monkeys. J Comp Neurol 1986;243:117-38. [DOI] [PubMed]
- 54.Westlund KN, Sorkin LS, Ferrington DG, et al. Serotoninergic and noradrenergic projections to the ventral posterolateral nucleus of the monkey thalamus. J Comp Neurol 1990;295:197-207. [DOI] [PubMed]
- 55.Oke AF, Carver LA, Gouvion CM, et al. Three-dimensional mapping of norepinephrine and serotonin in human thalamus. Brain Res 1997;763:69-78. [DOI] [PubMed]
- 56.Smith DF. Neuroimaging of serotonin uptake sites and antidepressant binding sites in the thalamus of humans and “higher” animals. Eur Neuropsychopharmacol 1999;9:537-44. [DOI] [PubMed]
- 57.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. Philadelphia: Davis; 1971. [PubMed]
- 58.Ridley RM, Baker HF, Cummings RM, et al. Mild topographical memory impairment following crossed unilateral lesions of the mediodorsal thalamic nucleus and the inferotemporal cortex. Behav Neurosci 2005;119:518-25. [DOI] [PubMed]
- 59.Mair WGP, Warrington EK, Weiskrartz L. Memory disorder in Korsakoff's psychosis: a neuropathological and neuropsychological investigation of two cases. Brain 1979;102:749-83. [DOI] [PubMed]
- 60.Graff-Radford NR, Tranel D, Van Hoesen GW, et al. Diencephalic amnesia. Brain 1990;113:1-25. [DOI] [PubMed]
- 61.Squire LR, Moore RY. Dorsal thalamic lesion in a noted case of human memory dysfunction. Ann Neurol 1979;6:503-6. [DOI] [PubMed]
- 62.Harding A, Halliday G, Caine D, et al. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 2000;123:141-54. [DOI] [PubMed]
- 63.Markowitsch HJ. Thalamic mediodorsal nucleus and memory: a critical evaluation of studies in animals and man. Neurosci Biobehav Rev 1982;6:351-80. [DOI] [PubMed]
- 64.Antonova E, Sharma T, Morris R, et al. The relationship between structure and neurocognition in schizophrenia: a selective review. Schizophr Res 2004;70:117-45. [DOI] [PubMed]
- 65.Royall DR, Lauterbach EC, Cummings J, et al. Executive control function: a review of its promise and challenges for clinical research. J Neuropsychiatry Clin Neurosci 2002;14:377-405. [DOI] [PubMed]
- 66.Cummings JL. Anatomic and behavioral aspects of frontalsubcortical circuits. In: Grafman J, Holyoak KJ, Boller F, editors. Structure and functions of the human prefrontal cortex. New York: Ann NY Acad Sci; 1995. p. 1-13.
- 67.Fuster JM. The prefrontal cortex. 2nd ed. New York: Raven; 1980.
- 68.Rogers RD, Owen AM, Middleton HC, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 1999;19:9029-38. [DOI] [PMC free article] [PubMed]
- 69.Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome? Clin Neurosci 1995;3:89-97. [PubMed]
- 70.Shenton ME, Dickey CC, Frumin M, et al. A review of MRI findings in schizophrenia. Schizophr Res 2001;49:1-52. [DOI] [PMC free article] [PubMed]
- 71.Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry 2001;49:487-99. [DOI] [PubMed]
- 72.Deicken RF, Eliaz Y, Chosiad L, et al. Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res 2002;58:135-44. [DOI] [PubMed]
- 73.Hazlett EA, Buchsbaum MS, Byne W, et al. Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156: 1190-9. [DOI] [PubMed]
- 74.Preuss UW, Zetzsche T, Jäger M, et al. Thalamic volume in first-episode and chronic schizophrenic subjects: a volumetric MRI study. Schizophr Res 2005;73:91-101. [DOI] [PubMed]
- 75.Andreasen NC, Arndt S, Swayze V, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994;266:294-8. [DOI] [PubMed]
- 76.Gur RE, Maany V, Mozley PD, et al. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998;155:1711-7. [DOI] [PubMed]
- 77.Staal WG, Hulshoff-Pol HE, Schnack H, et al. Partial volume decrease of the thalamus in relatives of patients with schizophrenia. Am J Psychiatry 1998;155:1784-6. [DOI] [PubMed]
- 78.Byne W, Buchsbaum MS, Kemether E, et al. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001;58:133-40. [DOI] [PubMed]
- 79.Kemether EM, Buchsbaum MS, Byne W, et al. Magnetic resonance imaging of mediodorsal, pulvinar, and centromedian nuclei of the thalamus in patients with schizophrenia. Arch Gen Psychiatry 2003;60:983-91. [DOI] [PubMed]
- 80.Csernansky JG, Schindler MK, Splinter NR, et al. Abnormalities of thalamic volume and shape in schizophrenia. Am J Psychiatry 2004;161:896-902. [DOI] [PubMed]
- 81.Mitelman SA, Byne W, Kemether EM, et al. Correlations between volumes of the pulvinar, centromedian, and mediodorsal nuclei and cortical Brodmann's areas in schizophrenia. Neurosci Lett 2006;392:16-21. [DOI] [PubMed]
- 82.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 2001;49:28-38. [DOI] [PubMed]
- 83.Rosenthal R, Bigelow LB. Quantitative brain measurements in chronic schizophrenia. Br J Psychiatry 1972;121:259-64. [DOI] [PubMed]
- 84.Lesch A, Bogerts B. The diencephalon in schizophrenia: evidence for reduced thickness of the periventricular grey matter. Eur Arch Psychiatry Neurol Sci 1984;234:212-9. [DOI] [PubMed]
- 85.Pakkenberg B. The volume of the mediodorsal thalamic nucleus in treated and untreated schizophrenics. Schizophr Res 1992;7:95-100. [DOI] [PubMed]
- 86.Cullen TJ, Walker MA, Roberts H, et al. The mediodorsal nucleus of the thalamus in schizophrenia: a post-mortem study. In: Abstracts of the Tenth Biennial Winter Workshop on Schizophrenia; 2000 Feb 5–11; Davos, Switzerland. Schizophr Res 2000;41:5.
- 87.Popken GJ, Bunney WE Jr, Potkin SG, et al. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A 2000;97:9276-80. [DOI] [PMC free article] [PubMed]
- 88.Young KA, Manaye KF, Liang C, et al. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 2000;47:944-53. [DOI] [PubMed]
- 89.Byne W, Buchsbaum MS, Mattiace LA, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002;159:59-65. [DOI] [PubMed]
- 90.Cullen TJ, Walker MA, Parkinson N, et al. A postmortem study of the mediodorsal nucleus of the thalamus in schizophrenia. Schizophr Res 2003;60:157-66. [DOI] [PubMed]
- 91.Danos P, Baumann B, Krämer A, et al. Volumes of association thalamic nuclei in schizophrenia: a postmortem study. Schizophr Res 2003;60:141-55. [DOI] [PubMed]
- 92.Dorph-Petersen KA, Pierri JN, Sun Z, et al. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol 2004;472:449-62. [DOI] [PubMed]
- 93.Danos P, Schmidt A, Baumann B, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res 2005;140:281-9. [DOI] [PubMed]
- 94.Eidelberg D, Galaburda AM. Symmetry and asymmetry in the human posterior thalamus. I. Cytoarchitectonic analysis in normal persons. Arch Neurol 1982;39:325-32. [DOI] [PubMed]
- 95.Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 1989;14:1-34. [DOI] [PubMed]
- 96.Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997;23:483-501. [DOI] [PubMed]
- 97.Dewulf A. Anatomy of the normal human thalamus: topometry and standardized nomenclature. Amsterdam: Elsevier; 1971.
- 98.Popken GJ, Bunney WE Jr, Potkin SG, et al. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A 2000;97:9276-80. [DOI] [PMC free article] [PubMed]
- 99.Young KA, Manaye KF, Liang CL, et al. Reduced number of mediodorsal and anterior thalamic neurons in schizophrenia. Biol Psychiatry 2000;47:944-53. [DOI] [PubMed]
- 100.Byne W, Buchsbaum MS, Mattiace LA, et al. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002;159:59-65. [DOI] [PubMed]
- 101.Danos P, Schmidt A, Baumann B, et al. Volume and neuron number of the mediodorsal thalamic nucleus in schizophrenia: a replication study. Psychiatry Res 2005;140:281-9. [DOI] [PubMed]
- 102.Dorph-Petersen KA, Pierri JN, Sun Z, et al. Stereological analysis of the mediodorsal thalamic nucleus in schizophrenia: volume, neuron number, and cell types. J Comp Neurol 2004;472:449-62. [DOI] [PubMed]
- 103.Javitt DC, Laurelle M. Neurochemical theories. In: Lieberman JA, Stroup TS, Perkins DO, editors. Textbook of schizophrenia. Washington: American Psychiatric Publishing; 2006. p. 85-116.
- 104.Clinton SM, Ibrahim HM, Frey KA, et al. Dopaminergic abnormalities in select thalamic nuclei in schizophrenia: involvement of the intracellular signal integrating proteins calcyon and spinophilin. Am J Psychiatry 2005;162:1859-71. [DOI] [PubMed]
- 105.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. J Neuropsychiatry Clin Neurosci 1996;8:223-6. [DOI] [PubMed]
- 106.Davis KL, Kahn RS, Ko G, et al. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991;148:1474-86. [DOI] [PubMed]
- 107.Takahashi H, Higuchi M, Suhara T. The role of extrastriatal dopamine D2 receptors in schizophrenia. Biol Psychiatry 2006;59:919-28. [DOI] [PubMed]
- 108.Gessa GL, Devoto P, Diana M, et al. Dissociation of haloperidol, clozapine, and olanzapine effects on electrical activity of mesocortical dopamine neurons and dopamine release in the prefrontal cortex. Neuropsychopharmacology 2000;22:642-9. [DOI] [PubMed]
- 109.Ichikawa J, Ishii H, Bonaccorso S, et al. 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 2001;76:1521-31. [DOI] [PubMed]
- 110.Melis M, Diana M, Gessa GL. Clozapine potently stimulates mesocortical dopamine neurons. Eur J Pharmacol 1999;366:R11-3. [DOI] [PubMed]
- 111.Micallef J, Blin O. Neurobiology and clinical pharmacology of obsessive-compulsive disorder. Clin Neuropharmacol 2001;24:191-207. [DOI] [PubMed]
- 112.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 2005;6:312-24. [DOI] [PubMed]
- 113.Bernacer J, Prensa L, Gimenez-Amaya JM. Morphological features, distribution and compartmental organization of the nicotinamide adenine dinucleotide phosphate reduced-diaphorase interneurons in the human striatum. J Comp Neurol 2005;489:311-27. [DOI] [PubMed]
- 114.Bernácer J, Prensa L, Giménez-Amaya JM. Cholinergic interneurons are differentially distributed in the human striatum. PLoS ONE 2007;2:e1174 10.1371/journal.pone.0001174. [DOI] [PMC free article] [PubMed]
- 115.Ibrahim HM, Hogg AJ Jr, Healy DJ. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry 2000;157:1811-23. [DOI] [PubMed]
- 116.Richardson-Burns SM, Haroutunian V, Davies KL, et al. Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol Psychiatry 2000;47:22-8. [DOI] [PubMed]