Abstract
The chemical stability of tretinoin (RA) and isotretinoin (13RA) in ethanol and dermatological cream preparations exposed to solar simulated light (SSL), UVA, and visible light has been studied. Photostability was monitored by an HPLC method that allowed simultaneous analysis of RA and 13RA, thus allowing photodegradation due to isomerization to other retinoids and photolysis to non-retinoid products to be monitored. Both retinoids undergo both isomerization and photolysis following SSL, UVA and visible light exposure but RA is more sensitive to photodegradation than 13RA. Degradation of both retinoids by photolysis is considerably greater in cream formulations than in ethanol and the photodegradation follows second order kinetics. Rate constants and half-lives for degradation of RA and 13RA in ethanol solution and cream preparations subjected to different light sources are reported. The UVA component of SSL is the major contributor to photodegradation. Since UVA penetrates deeply into skin, our results suggest that photodegradation of RA may contribute to the photosensitivity associated with RA therapy. Our studies suggest that development of improved formulations and the use of effective UVA sunscreens may reduce the side effects of RA therapy.
Keywords: Retinoic acid, Photostability, UVA, Photoisomerization, Photolysis
1. Introduction
Retinoids are involved in several biological processes, particularly proliferation and differentiation (Fisher and Voorhees 1996). Three major isomers shown to be biologically active in human tissues include all-trans retinoic acid or tretinoin (RA), 13-cis retinoic acid or isotretinoin (13RA) and 9-cis retinoic acid (9RA) or alitretinoin (Lovat et al. 1997). Both RA and 13 RA are widely used in treatment of various dermatological conditions that include acne vulgaris, psoriasis, photodamaged skin and other skin disorders (Layton and Cunliffe 1992; Orfanos et al. 1997; Chivot 2005; Kang et al. 2005). Retinoids have been shown to undergo degradation when exposed to light generating RA isomers of which 13RA and 9RA are the most prevalent (Curley and Fowble 1988; Lucero et al. 1994; Brisaert et al. 1995; Cahnmann 1995; Brisaert and Plaizier-Vercammen 2000; Ioele et al. 2005). Light exposure also leads to degradation to non-retinoid products (Curley and Fowble 1988). The degradation of RA and 13RA in both the solid state and solution has been studied previously (Tan et al. 1992; Tan et al. 1993). The chemical stability of tretinoin in dermatological preparations has been investigated by Brisaert and co workers who have observed that RA photodegradation in lotions is rapid with the most harmful wavelength for degradation at 420 nm and not the wavelength of maximum absorption at 350 nm (Brisaert et al. 1995; Brisaert and Plaizier-Vercammen 2000). For 13RA, hydoxypropyl-β-cyclodextrin delays photodegradation and minimizes isomerization (Yap et al. 2005) and inclusion of RA in liposomes has been reported to protect against photodegradation (Brisaert et al. 2001). The chemical stability of RA in ethanol and niosomes under UV and artificial light conditions has been studied and incorporation of RA in niosomes led to reduce the rates of the photodegradation (Manconi et al. 2003). The photodegradation process of RA and 13RA in ethanol and liposomes has been studied by UV spectrophotometry and RA was found to rapidly isomerizes in ethanol to 13 RA and 9RA with first-order kinetics (Ioele et al. 2005). Other studies also have reported RA degradation to follow first-order kinetics (Brisaert and Plaizier-Vercammen 2000; Ioele et al. 2005) while studies reporting degradation to follow zero-order kinetics have appeared (Manconi et al. 2003).
We report here studies of the photodegradation of RA and 13RA using a recently developed analytical method (Tashtoush et al. 2006) that allow the distinction between degradation occurring by isomerization to other RA isomers from degradation occurring by photolysis to non-retinoid products. Our results show that the rates of photolysis are greater in cream formulations than in ethanol solution, that photodegradation of RA in ethanol solution and cream formulations follows second-order kinetics, and that the UVA portion of solar light accounts for a major portion of RA and 13RA photodegradation.
2. Experimental methods
2.1. Chemicals and reagents
Tretinoin, isotretinoin and trifluoroacetic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Acetonitrile and ethanol (HPLC grade) were purchased from EMD Chemicals Inc. (Gibbstown, NJ, USA). Brij-58, glyceryl monostearate, cetostearyl alcohol, white petrolatum, sorbic acid, butylated hydroxytoluene, simethicone, sorbitol 70% solution, propylene glycol and polyethylene glycol were purchased from Spectrum Chemical Mfg. Corp. (Gardena, CA, USA). Double distilled de-ionized water was used.
2.2. HPLC instrumentation and conditions
An HPLC system consisting of Varian Pro-star solvent delivery system model 230 (Varian Chromatography systems, CA, USA), connected to a UV/Visible Spectroflow 757 absorbance detector (ABI, NJ, USA) and HP 3395 integrator (Hewlett Packard, USA) was used for all experiments. The injector was fitted with a loop of 50µl. Reversed-phase chromatographic separation was performed using a Nucleosil 5µ C18 100A, 250 × 4.6 mm column (Phenomenex, CA, USA). The detection wavelength was set to 342nm. The mobile phase used was composed of 0.01% trifluoroacetic acid (TFA) and acetonitrile (15:85 v/v %) at a flow rate of 1.0 ml/min. The mobile phase was filtered through a 0.45µ membrane filter (Advantec MFS Inc., CA, USA).
2.3. Cream preparations
Tretinoin (0.025%) and isotretinoin (0.025%) creams contained the following components: water, propylene glycol, sorbitol, sorbic acid, butylated hydroxytoluene, simethicone, white petrolatum, cetostearyl alcohol, Brij-58, glyceryl monostearate, polyethylene glycol and tretinoin or isotretinoin. The ratio of oil phase to aqueous phase was (27.5:72.5% w/w). The water phase was mixed and dissolved in one container at 65–75°C and the oil phase was melted and mixed in another container at 65–75°C using a water bath. The oil phase was then added to the water phase and mixed until a cream was formed using an IKA mixer model RW 20DZM (IKA-works Inc. NC, USA). The cream was cooled to room temperature while stirring.
2.4. Standard stock solutions
Stock solutions containing 1mM of tretinoin or isotretinoin were prepared in ethanol. To compare with cream preparations, solutions containing 0.025% tretinoin and 0.025% isotretinoin were prepared in ethanol for photostability studies.
2.5. Irradiation conditions
A kilowatt (KW) large area light source solar simulator, model 91293, from Oriel Corporation (Stratford, Connecticut) was used, equipped with 1000W Xenon lamp power supply, model 68920, and a VIS-IR band pass blocking filter plus either an atmospheric attenuation filter (output 290–400 nm plus residual 650–800 nm, for SSL) or UVB and UVC blocking filter (output 320–400 nm plus residual 650–800 nm, for UVA), respectively. The output was quantified using a dosimeter from international light Inc. (Newburyport, MA), model IL 1700, with an SED240 detector for UVB (range 265–310 nm, peak 285 nm), or a SED033 detector for UVA (range 315–390 nm, peak 365 nm) at a distance of 36.5cm from the source, which was used for all experiments. At 365nm from the source, the SSL dose was 7.63 mJ/cm2/sec UVA and 0.40 mJ/ cm2/sec UVB radiation. Using a UVB/UVC blocking filter, the dose at 365nm from the source was 5.39 mJ/ cm2/sec UVA radiation with residual UVB dose of 3.16 µJ/cm2/sec.
For irradiation with visible light, Luzchem expo Panels composed of 5 Sylvania 8-W cool white light tubes was used to deliver visible light at an irradiance of 2.34 mJ/cm2/sec. The irradiance in the visible region (400–700nm) was determined using a digital light meter, Model SLM-110 from (A.W. Sperry Instruments Inc., NY, USA), at 20 cm from the light source. For photostability studies in ethanol, a 1.0 ml solution containing 0.025% tretinoin or isotretinoin in ethanol was placed into 1.5 ml Eppendorf centrifuge tubes. At the appropriate times of irradiation, a tube was placed into an amber container covered with aluminum foil until the time of analysis. For photostability studies in cream formulations, a 1gram sample of tretinoin or isotretinoin was spread uniformly over the cover of a 35mm tissue culture dish. At the appropriate times of irradiation, the sample covered with aluminum foil until time of analysis.
2.6. Sample preparation
A 0.5 gm sample of cream formulations was taken from the dish and weighed into a 50 ml conical centrifuge tube using a Mettler balance model PB-303S (Mettler-Toledo, Switzerland). The sample was dissolved into 30 ml acetonitrile by vortex mixing for 3 minutes then 1.5 ml was transferred to an Eppendorf centrifuge tube and subjected to centrifugation at 10,000rpm for 5 minutes using an Eppendorf microcentrifuge (Brinkmann Inst Inc., NY, USA). An aliquot of 50µl of the supernatant was injected directly into the HPLC. For photostability studies in ethanol, 0.5ml was withdrawn from the centrifuge tube and mixed with 30 ml ethanol, then injected directly into HPLC.
3. Results and discussion
3.1. Photostability of retinoic acids in ethanol solution
Studies of RA and 13RA in ethanol solution following exposure to SSL demonstrated that RA conversion to other products was very rapid as 80% of the original RA was gone by 2 min of exposure (Fig. 1A). A major product was 13RA as 40% of the RA originally present was rapidly converted to this product after which a slow loss of both RA and 13RA was observed. The chromatographic conditions used in this study were able to detect 9RA and other RA isomers and no significant amount of these RA isomers were detected. Compared to RA, the loss of 13RA was slower as 40% of 13RA still remained after 5 min and only 20% of the 13RA originally present was recovered as RA and a slow loss of RA was observed with increasing times of irradiation (Fig. 1B). These results demonstrate that two types of reactions were occurring for both RA and 13RA, photoisomerization to other RA isomers and photolysis to non-retinoid degradation products. Similar rates of photodegradation for RA and 13RA were observed when only the UVA portion of SSL was used as an irradiation source (Fig. 1C, D). Following exposure to visible light, rates of photodegradation of both RA and 13RA were slower than irradiation by SSL and UVA, but again the rate of RA loss was greater than that of 13RA (Fig. 1E, F). Our results show that in ethanol solution that RA is less photostable than 13RA, that photoisomerization between RA and 13RA is a major but not exclusive component of degradation, and that the UVA portion of the SSL accounts for the majority of the degradation caused by SSL.
Figure 1.
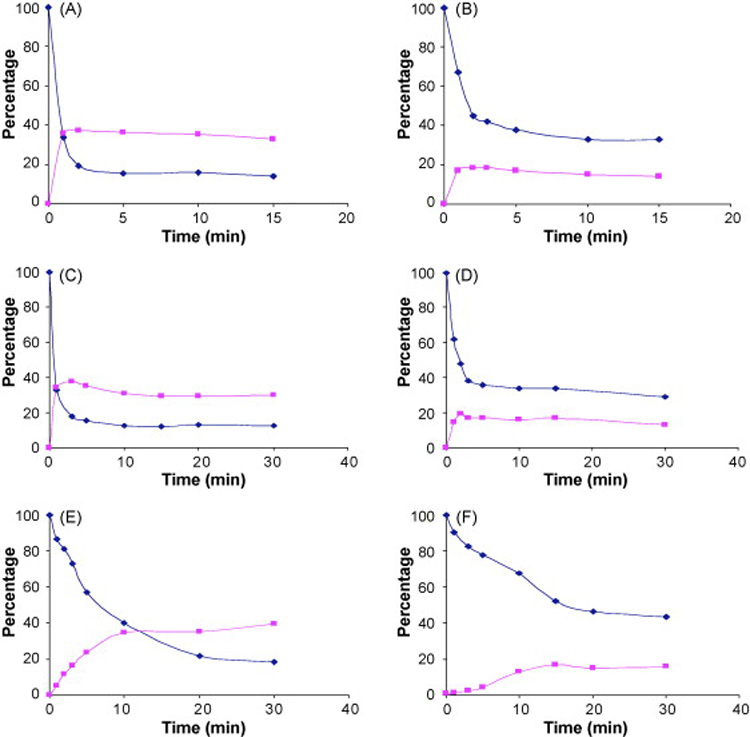
Concentration of RA (diamonds) and 13RA (triangles) as a function of time in irradiated ethanol solutions. Degradation of RA formulations is shown in panels A, C, and E and degradation of 13RA formulations is shown in panels B, D, and F. Panels A and B shown SSL, panels C and D show UVA and panels E and F show visible light.
3.2. Photostability of retinoic acids in cream preparations
The photodegradation of RA and 13RA cream preparations was also carried out under SSL, UVA and visible light irradiation. Irradiation of RA by SSL caused rapid isomerization to 13RA but 13RA also was degraded following its formation. The degradation of RA when irradiated by SSL showed that no detectable RA material remained after 45 min (Fig. 2A). Again, 13RA was degraded at a slower rate than RA as approximately 40% of the original material remained after a 45 min exposure (Fig. 2B). Qualitatively similar results were observed for UVA (Fig. 2C, D) and for visible light (Fig. 2, E, F) although the greater degree of photodegradation observed in the cream formulation for SSL and UVA was not observed for visible light.
Figure 2.
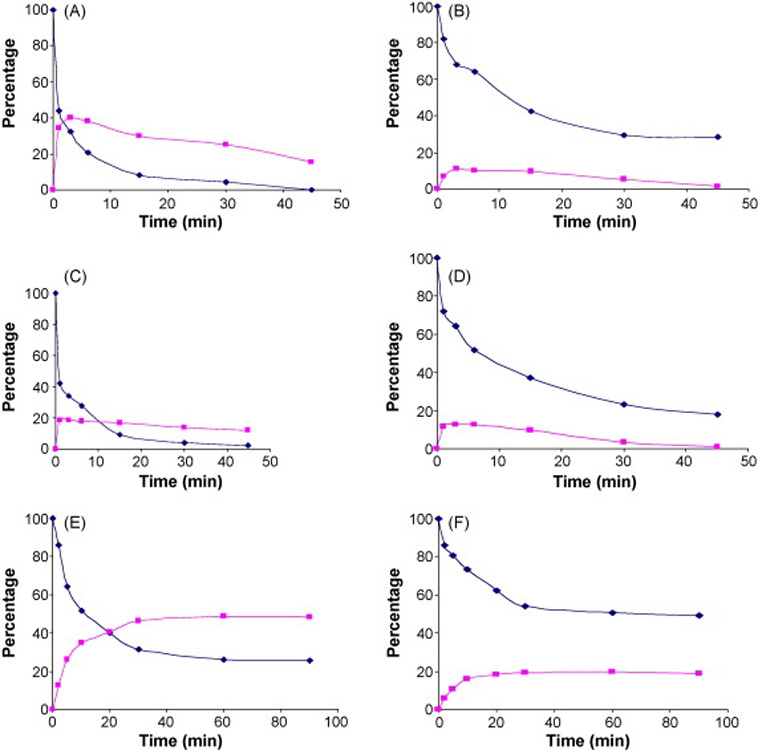
Concentration of RA (diamonds) and 13RA (triangles) as a function of time in cream formulations. Degradation of RA formulation is shown in panels A, C, and E and degradation of 13RA formulations is shown in panels B, D, and F. Panels A and B shown SSL, panels C and D show UVA and panels E and F show visible light.
3.3. Determination of kinetic parameters of photodegradation
The photochemical reaction in ethanol solution demonstrated that RA isomerizes to 13RA and vice versa, with both reactions reaching equilibrium by 15 minutes. Therefore the rate of photodegradation was calculated from the initial rate by measuring the concentration remaining of either RA or 13RA. As can be seen in Table 1, the rate constants were obtained from a slope of a curve fit of a plot of 1/concentration remaining versus time. The calculated half-life values were calculated according to the equation describing second-order kinetics and were found to be 7.21, 0.93, and 0.65 min when RA was irradiated by visible light, UVA, and SSL respectively. The corresponding half-life values for 13RA were 19.5, 2.40, and 2.26 min, demonstrating that degradation of RA is higher than 13RA under all conditions of irradiation. The rate constants and half-lives of RA and 13RA in cream formulations irradiated with different light sources (Table 2) show that, while the rates of degradation are more rapid in the cream formulations relative to ethanol, the same relative pattern of degradation for RA and 13RA was observed. The photodegradation of RA in ethanol and cream formulations was found to follow second-order kinetics as a plot of 1/ concentration remaining versus time gave a straight line with R2 = 0.998 (Fig. 3). This observation is in contrast to earlier studies (Brisaert and Plaizier-Vercammen 2000; Manconi et al. 2003; Ioele et al. 2005), most likely to due to the different conditions present in relatively dermatological preparations.
Table 1.
Rate constants of degradation of for RA and 13RA in ethanol solution*
| Retinoic acid | Source | k (liter/mmole. min) |
t0.5 (minute) |
r2 |
|---|---|---|---|---|
| SSL | 1.82 ± 0.17 | 0.65 ± 0.08 | 0.998 | |
| RA | UVA | 1.28 ± 0.13 | 0.93 ± 0.10 | 0.982 |
| Visible | 0.16 ± 0.02 | 7.22 ± 0.19 | 0.986 | |
| SSL | 0.53 ± 0.12 | 2.26 ± 0.12 | 0.985 | |
| 13RA | UVA | 0.49 ± 0.18 | 2.40 ± 0.27 | 0.997 |
| Visible | 0.06 ± 0.01 | 19.5 ± 0.93 | 0.985 | |
n=3
Table 2.
Rate constants of degradation of for RA and 13RA in cream preparations*
| Retinoic acid | Source | k (liter/mmole. min) |
t0.5 (minute) |
r2 |
|---|---|---|---|---|
| SSL | 1.11 ± 0.16 | 1.07 ± 0.20 | 0.997 | |
| RA | UVA | 0.92 ± 0.13 | 1.30 ± 0.15 | 0.986 |
| Visible | 0.09 ± 0.01 | 13.1 ± 0.32 | 0.988 | |
| SSL | 0.08 ± 0.01 | 14.0 ± 1.31 | 0.992 | |
| 13RA | UVA | 0.11 ± 0.01 | 11.3 ± 0.94 | 0.994 |
| Visible | 0.03 ± 0.005 | 41.0 ± 2.13 | 0.981 | |
n=3
Figure 3.
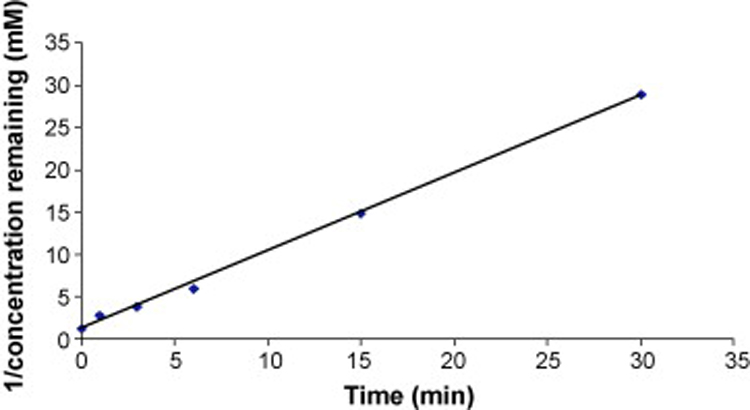
Kinetic analysis of photodegradation of RA in a cream formulation irradiated with SSL.
An advantage of the analytical method used in this study was that the relative rates of photoisomerization and photolysis could be determined. Since the degradation rate was much slower under visible light exposure, the rates of photolysis and photoisomerization were determined for this condition (Table 3). As shown in table 3, ktotal was calculated from the slope of a curve fit of a plot of 1/concentration remaining of either RA of 13RA versus time and kphotolysis was calculated from a slope of a curve fit of a plot of 1/concentration remaining of either (RA-13RA) in case of RA degradation or (13RA-RA) in case of 13RA degradation versus time. Finally kphotoisomerization was determined from the difference of (ktotal-kphotolysis). These results show that the rates of photoisomerization are higher than the rates of photolysis in ethanol solution but that the rates of photolysis are higher than the rates of isomerization in cream formulations.
Table 3.
Rate constants of total degradation, photolysis, and photoisomerization of RA and 13RA cream preparations irradiated by visible light*
| Retinoic acid | Preparation | Ktotal (liter/mmole. min) |
Kphotolysis (liter/mmole. min) |
Kphotoismerization (liter/mmole. min) |
|---|---|---|---|---|
| RA | Ethanol | 0.165 ± 0.021 | 0.056 ± 0.011 | 0.109 ± 0.020 |
| Cream | 0.091 ± 0.011 | 0.068 ± 0.006 | 0.023 ± 0.002 | |
| 13RA | Ethanol | 0.061 ± 0.012 | 0.007 ± 0.001 | 0.054 ± 0.003 |
| Cream | 0.029 ± 0.005 | 0.021 ± 0.007 | 0.008 ± 0.001 | |
n=3
Since Both RA and 13RA are biologically active, the rates of disappearance of total retinoid from ethanol solution and cream preparations were calculated as shown in Table 4 and Table 5, respectively. These results demonstrate that photodegradation occurs in RA and 13RA preparations upon exposure to light that can lead to loss of active material in such preparations.
Table 4.
Rate constants of degradation of retinoid from ethanol solution*
| Retinoic acid | Source | k (liter/mmole. min) |
t 0.5 (minute) |
r2 |
|---|---|---|---|---|
| SSL | 0.330 ± 0.021 | 3.60 ± 0.29 | 0.994 | |
| RA | UVA | 0.214 ± 0.017 | 5.57 ± 0.31 | 0.958 |
| Visible | 0.038 ± 0.009 | 31.7 ± 1.39 | 0.986 | |
| SSL | 0.247 ± 0.011 | 4.81 ± 0.73 | 0.958 | |
| 13RA | UVA | 0.246 ± 0.023 | 4.85 ± 0.36 | 0.987 |
| Visible | 0.030 ± 0.007 | 39.8 ± 1.16 | 0.976 | |
n=3
Table 5.
Rate constants of degradation of retinoid from cream preparation*
| Retinoic acid | Source | k (liter/mmole. min) |
t0.5 (minute) |
r2 |
|---|---|---|---|---|
| SSL | 0.116 ± 0.010 | 10.3 ± 0.76 | 0.996 | |
| RA | UVA | 0.156 ± 0.021 | 7.63 ± 0.89 | 0.978 |
| Visible | 0.012 ± 0.004 | 97.6 ± 1.32 | 0.980 | |
| SSL | 0.068 ± 0.007 | 17.6 ± 1.12 | 0.996 | |
| 13RA | UVA | 0.100 ± 0.023 | 11.9 ± 0.92 | 0.995 |
| Visible | 0.012 ± 0.002 | 96.0 ± 2.18 | 0.965 | |
n=3
4. Conclusions
Both RA and 13RA undergo both photoisomerization and photolysis following SSL, UVA and visible light exposure but RA is more sensitive to degradation than 13RA. Degradation of both retinoids by photolysis is considerably greater in cream formulations than in ethanol and the photodegradation follows second order kinetics. While studies in ethanol solution are valuable for determination of mechanisms of photodegradation of retinoids, the greater instability in cream formulations, where multiple components are present that can react with the retinoid, is of considerable practical significance as the identification and replacement of components of the formulations that accelerate RA photodegradation may allow the development of improved formulations. This possibility is supported by a recent study of Brisaert and co-workers (Brisaert and Plaizier-Vercammen 2006). The UVA component of SSL is the major contributor to photodegradation. Since UVA penetrates deeply into skin, our results suggest that photodegradation of RA may contribute to the photosensitivity associated with RA therapy. Our studies suggest that the use of effective UVA sunscreens also may reduce the side effects of RA therapy.
Acknowledgements
These studies were funded in part by Niadyne, Inc., and NIH grant R44 CA-90085. MKJ and ELJ are principals in Niadyne, Inc., whose sponsored research is managed in accordance with the University of Arizona conflict-of-interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brisaert M, Gabriels M, et al. Liposomes with tretinoin: a physical and chemical evaluation. J Pharm Biomed Anal. 2001;26(5–6):909–917. doi: 10.1016/s0731-7085(01)00502-7. [DOI] [PubMed] [Google Scholar]
- Brisaert M, Plaizier-Vercammen J. Investigation on the photostability of a tretinoin lotion and stabilization with additives. Int J Pharm. 2000;199(1):49–57. doi: 10.1016/s0378-5173(00)00366-5. [DOI] [PubMed] [Google Scholar]
- Brisaert M, Plaizier-Vercammen JA. Investigation on the photostability of tretinoin in creams. Int J Pharm. 2006 doi: 10.1016/j.ijpharm.2006.10.023. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Brisaert MG, Everaerts I, et al. Chemical stability of tretinoin in dermatological preparations. Pharm Acta Helv. 1995;70:161–166. [Google Scholar]
- Cahnmann HJ. A fast photoisomerization method for the preparation of tritium-labeled 9-cis-retinoic acid of high specific activity. Anal Biochem. 1995;227(1):49–53. doi: 10.1006/abio.1995.1251. [DOI] [PubMed] [Google Scholar]
- Chivot M. Retinoid therapy for acne. A comparative review. Am J Clin Dermatol. 2005;6(1):13–19. doi: 10.2165/00128071-200506010-00002. [DOI] [PubMed] [Google Scholar]
- Curley RW, Jr, Fowble JW. Photoisomerization of retinoic acid and its photoprotection in physiologic-like solutions. Photochem Photobiol. 1988;47(6):831–835. doi: 10.1111/j.1751-1097.1988.tb01667.x. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. Faseb J. 1996;10(9):1002–1013. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- Ioele G, Cione E, et al. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int J Pharm. 2005;293(1–2):251–260. doi: 10.1016/j.ijpharm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Kang S, Bergfeld W, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6(4):245–253. doi: 10.2165/00128071-200506040-00005. [DOI] [PubMed] [Google Scholar]
- Layton AM, Cunliffe WJ. Guidelines for optimal use of isotretinoin in acne. J Am Acad Dermatol. 1992;27(6 Pt 2):S2–S7. doi: 10.1016/s0190-9622(08)80252-6. [DOI] [PubMed] [Google Scholar]
- Lovat PE, Irving H, et al. 9-cis retinoic acid--a better retinoid for the modulation of differentiation, proliferation and gene expression in human neuroblastoma. J Neurooncol. 1997;31(1–2):85–91. doi: 10.1023/a:1005785431343. [DOI] [PubMed] [Google Scholar]
- Lucero MJ, Vigo J, et al. Stability of hydrophilic gels of tretinoin. Int J Pharm. 1994;110:241–248. [Google Scholar]
- Manconi M, Valenti D, et al. Niosomes as carriers for tretinoin. II. Influence of vesicular incorporation on tretinoin photostability. Int J Pharm. 2003;260(2):261–272. doi: 10.1016/s0378-5173(03)00268-0. [DOI] [PubMed] [Google Scholar]
- Orfanos CE, Zouboulis CC, et al. Current use and future potential role of retinoids in dermatology. Drugs. 1997;53(3):358–388. doi: 10.2165/00003495-199753030-00003. [DOI] [PubMed] [Google Scholar]
- Tan X, Meltzer N, et al. Solid-state stability studies of 13-cis-retinoic acid and all-trans-retinoic acid using microcalorimetry and HPLC analysis. Pharm Res. 1992;9(9):1203–1208. doi: 10.1023/a:1015816225127. [DOI] [PubMed] [Google Scholar]
- Tan X, Meltzer N, et al. Determination of the kinetics of degradation of 13-cis-retinoic acid and all-trans-retinoic acid in solution. J Pharm Biomed Anal. 1993;11(9):817–822. doi: 10.1016/0731-7085(93)80074-b. [DOI] [PubMed] [Google Scholar]
- Tashtoush BM, Jacobson EL, et al. A rapid HPLC method for simultaneous determination of tretinoin and isotretinoin in dermatological formulations. J Pharm Biomed Anal. 2006 doi: 10.1016/j.jpba.2006.08.027. [DOI] [PubMed] [Google Scholar]
- Yap KL, Liu X, et al. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci. 2005;25(1):49–56. doi: 10.1016/j.ejps.2005.01.021. [DOI] [PubMed] [Google Scholar]


