Abstract
Background
Early adolescent weight may impact the risk of postmenopausal breast cancer, and this association may be modified by a family history of breast or ovarian cancer in a first degree relative, and/or estrogen (ER) and progesterone (PR) receptor status of the disease.
Methods
Relative weight at age 12 (above, below, or average weight compared to peers) and family history were ascertained using a mailed questionnaire in 1986, in the Iowa Women’s Health Study, a prospective cohort study of postmenopausal women. Incident breast cancer cases (including ER and PR status) were identified using the Iowa SEER Cancer Registry. Relative risks (RR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression, and were adjusted for breast cancer risk factors including body mass index (BMI) at age 18 and BMI at study baseline.
Results
Through 2003, 2503 cases of postmenopausal breast cancer were identified among 35,941 women in the analytic cohort. Compared to women with average weight at age 12, there was no association of below average weight with risk of breast cancer (RR=1.02, 95% CI: 0.92, 1.13), while women with above average weight had a lower risk (RR=0.85, 95% CI: 0.74, 0.98). There was no evidence of an interaction between weight at age 12 and family history (p=0.44). The inverse association of above average weight with risk of breast cancer was strongest for PR– tumors (RR=0.62; 95% CI 0.43, 0.89), intermediate for ER+ (RR=0.80; 95% CI 0.67, 0.96) and ER– (RR=0.77; 95% CI 0.50, 1.19) tumors, and weakest for PR+ tumors (RR=0.90; 95% CI 0.74, 1.09). These associations were not modified by a family history (all p>0.18). In a joint ER/PR analyses, the strongest inverse association with above average weight at age 12 was seen for ER+/PR– (RR=0.49; 95% 0.29, 0.85)
Conclusion
Above average weight at age 12 was inversely associated with risk of postmenopausal breast cancer, and was not modified by a family history of the disease. The inverse association was strongest for ER+/PR– tumors.
Introduction
Breast cancer is the most common non-cutaneous cancer among women and the second leading cause of cancer death among women in US (1). Most studies suggest that higher adult body mass index (BMI) is associated with elevated risk of postmenopausal breast cancer, presumably due to increased estrogen levels produced by excess adipose tissue (2, 3). However, among pre-menopausal women, where the major source of endogenous estrogens is the ovary, higher body mass index (BMI) has consistently been inversely associated with the risk of premenopausal breast cancer. While the mechanism underlying this observation is not known, lower estrogen levels among obese premenopausal women has been suggested (2, 4–6).
In contrast, greater BMI in later adolescence (age 15–18 years) has been inversely associated with both premenopausal (4, 7–13) and postmenopausal (4, 8–10, 14–16) breast cancer risk, although there are exceptions for premenopausal (17) and postmenopausal (7, 11, 17) breast cancer risk. More limited data suggest that this inverse association extends into earlier adolescence (ages 9 to 14 years) as well for premenopausal (8, 10, 12, 18–20) and postmenopausal (8, 10, 18, 19, 21, 22) breast cancer risk, although there are a few exceptions (17, 23). Early life obesity may also be modified by a family history of breast cancer (21, 24, 25). Finally, risk factors may differ for biologic subtypes of breast cancer defined by estrogen and progesterone receptor (ER/PR) status (26, 27), but this has not been evaluated for early adolescent weight.
We evaluated the association of relative weight at age 12 with postmenopausal breast cancer risk in the Iowa Women’s Health Study. In addition, we investigated whether these associations differ by family history of breast cancer and/or biologic subtypes of breast cancer defined by estrogen and progesterone receptor (ER/PR) status.
Materials and Methods
The Iowa Women’s Health Study is a prospective cohort study of women aged 55–69 years at study baseline in 1986 (28). Briefly, a mailed survey was returned by 41,836 women in 1986 from a random sample of women with a valid Iowa driver’s license. The baseline survey included questions on a variety of potential breast cancer risk factors including medical and family history, anthropometrics, reproductive factors, and lifestyle characteristics. Participants were also asked: “Think back to when you were in 6th grade- or about the age of 12. Would you say at that time your weight was: below average for your age and height, about average for your age and height, or above average for your age and height”.
Breast cancers and their ER/PR status were ascertained through linkage to the State Health Registry of Iowa, part of the Surveillance, Epidemiology and End Results (SEER) program (29). On an annual basis, cohort members were linked to the registry based on name (first, last, maiden), zip code, birth date, and social security number. Deaths were identified through follow-up surveys, annual linkage to Iowa death certificates, and linkage to the National Death Index.
For this analysis, we excluded women if they reported any of the following on the baseline survey: being premenopausal (n=569), having a history of any cancer other than skin cancer (n=3830), or ever having had a total or partial mastectomy (n=1884). An additional 1164 women were excluded due to missing data on relative weight at age 12. This left a total of 35,941 women in the analysis (exclusions were not mutually exclusive).
Each woman accrued person-years of follow-up from the completion of the baseline questionnaire until a diagnosis of breast cancer, death, or emigration from Iowa; if none of these occurred, cohort members were censored on December 31, 2003. Relative risks (RR) and 95% confidence intervals (CI) were estimated using Cox regression, controlling for potential confounding factors as included in Table 1. Incidence was modeled as a function of age (30). Initially, we examined the overall association of weight at age 12 with breast cancer risk. Formal assessments of risk were assessed using tests for trend, calculated by ordering the three-level weight variable and including it in the Cox proportional hazards model as a linear term.
Table 1.
Distribution of breast cancer risk factors by relative weight at age 12, Iowa Women’s Health Study, 1986
| Relative Weight for Age and Height in 6th Grade or About the Age of 12
|
|||
|---|---|---|---|
| Below Average (N=8,082) | Average (N=23,127) | Above Average (N=4,732) | |
| Mean ± standard deviation | |||
| Age at study entry (years) | 61.7 ± 4.2 | 61.8 ± 4.2 | 61.2± 4.2 |
| Age of first menstruation (years) | 13.3 ± 1.6 | 12.8 ± 1.4 | 12.3 ± 1.4 |
| Age at menopause (years) | 47.6 ± 6.4 | 47.6 ± 6.4 | 47.8 ± 6.4 |
| Body mass index at age 18 (kg/m2) | 19.6 ± 2.3 | 21.6 ± 2.5 | 25.2 ± 4.1 |
| Body mass index in 1986 (kg/m2) | 25.8 ± 4.6 | 26.9 ± 4.8 | 29.6 ± 6.1 |
| Percent distribution | |||
| Body mass index at age 18 (kg/m2) | |||
| <18.0 | 30.0% | 6.6% | 2.2% |
| 18.0–24.9 | 67.9% | 85.6% | 53.6% |
| 25.0–29.9 | 1.9% | 7.0% | 32.0% |
| 30.0+ | 0.3% | 0.8% | 12.2% |
| Body mass index in 1986 (kg/m2) | |||
| <18.0 | 1.6% | 0.9% | 0.4% |
| 18.0–24.9 | 47.3% | 38.7% | 23.6% |
| 25.0–29.9 | 35.6% | 37.9% | 34.7% |
| 30.0+ | 15.5% | 22.5% | 41.4% |
| Education greater than high school | 41.0% | 37.1% | 44.5% |
| Family history of breast or ovarian cancer in a first degree relative | 14.0% | 13.6% | 12.8% |
| Any live births | 90.3% | 91.5% | 89.3% |
| Age at first live birth <20 years | 18.5% | 20.6% | 18.4% |
| Ever used oral contraceptives | 20.6% | 17.9% | 18.7% |
| Ever used hormone replacement therapy | 42.2% | 36.8% | 35.9% |
| Did not drink alcohol in 1986 | 56.7% | 55.8% | 58.8% |
| Never smoker | 68.4% | 66.0% | 60.6% |
| Physical activity index | |||
| Low | 47.3% | 47.1% | 48.5% |
| Medium | 27.4% | 27.9% | 26.2% |
| High | 25.3% | 25.0% | 25.3% |
We then investigated whether a family history of 1) breast or 2) breast or ovarian cancer in a first degree relative modified the association between weight at age 12 and risk of breast cancer. Results were similar, so we report results for a family history of breast or ovarian cancer in order to facilitate comparison with a previously published study (21). Formal tests of interaction were carried out by including the main effects of weight at age 12 (ordinal) and family history, and testing the statistical significance of the corresponding interaction term. Finally, we evaluated whether these associations varied by breast cancer subtypes defined by ER and PR status. In these receptor-specific analyses, events not of that specific cancer type were considered censored observations. All analyses were performed on SAS 8.02 (SAS Institute, Inc., Cary, NC) and Splus 7.0.6 (Mathsoft, Inc., Seattle, WA) software systems.
Results
Of the 35,941 women in the analytic cohort, 22.5% reported below average weight at age 12, 64.3% reported average weight, and 13.2% reported above average weight. Table 1 reports breast cancer risk factors by relative weight at age 12 years. Compared to women reporting below average weight, women reporting above average weight had an earlier age at first menstrual cycle (13.3 versus 12.3 years); higher BMI at age 18 (19.6 versus 25.2 kg/m2) and at study baseline in 1986 (25.8 versus 29.6 kg/m2); lower use of hormone replacement therapy (42.2% vs. 35.9%); and were never smokers (68.4% versus 60.6%). Other breast cancer risk factors were similar across categories of relative weight at age 12.
During 548,567 person-years of follow-up (through 2003), 2503 breast cancers were identified. The mean age at diagnosis was 71.4 years (SD=6.2 years). ER status was available on 1901 (75.9%) of the cases, and 84.4% of these cases were ER+. PR status was available on 1825 (72.9%) of the cases, and 73.0% of these cases were PR+.
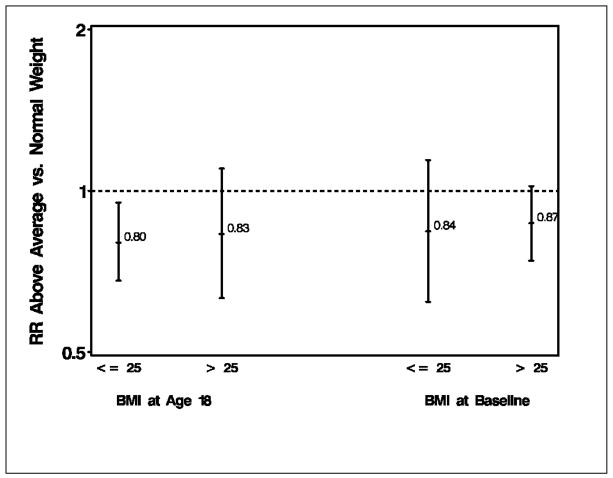
The association of weight at age 12 with breast cancer risk is reported in Table 2. Compared to women with average weight at age 12, there was no association of below average weight with risk of breast cancer, while women with above average weight had a lower risk of breast cancer (RR=0.85, 95% CI 0.74, 0.98) after adjustment for multiple risk factors including BMI at age 18 and BMI at study baseline. As shown in Table 1, while there was tracking of weight over the life course, this was not absolute. For example, of women reporting above average weight at age 12, 44% were overweight or obese at age 18 (but 56% had a BMI of <25) and 76% were overweight or obese at study baseline (but 24% had a BMI<25). As shown in Figure 1, results were unchanged when we stratified on BMI at age 18 (adjusting for baseline BMI) or BMI at baseline (adjusting for BMI at age 18).
Table 2.
Multivariate-adjusted relative risks of postmenopausal breast cancer by relative weight at age 12, overall and stratified by first degree family history of breast or ovarian cancer, and by breast cancer subtype based on ER and PR status
| All
|
No Family History
|
Family History
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Breast Cancer | Relative Weight at age 12 | Cases | RR | 95% CI | P trend | Cases | RR | 95% CI | P trend | Cases | RR | 95% CI | P trend |
| All | Below | 596 | 1.02 | 0.92,1.13 | 465 | 0.99 | 0.88,1.11 | 112 | 1.10 | 0.87,1.38 | |||
| Average | 1635 | 1.00 | reference | 1309 | 1.00 | reference | 282 | 1.00 | reference | ||||
| Above | 272 | 0.85 | 0.74,0.98 | 0.08 | 220 | 0.86 | 0.73,1.01 | 0.02 | 43 | 0.84 | 0.60,1.17 | 0.05 | |
| P Interaction (family history and weight 12) = 0.44 | |||||||||||||
| ER+ | Below | 383 | 1.01 | 0.89,1.16 | 296 | 0.95 | 0.82,1.11 | 72 | 1.25 | 0.94,1.67 | |||
| Average | 1055 | 1.00 | reference | 859 | 1.00 | reference | 165 | 1.00 | reference | ||||
| Above | 166 | 0.80 | 0.67,0.96 | 0.08 | 137 | 0.82 | 0.68,1.00 | 0.06 | 24 | 0.77 | 0.49,1.22 | 0.03 | |
| P Interaction (family history and weight 12) = 0.21 | |||||||||||||
| ER– | Below | 76 | 1.10 | 0.81,1.48 | 62 | 1.17 | 0.84,1.63 | 12 | 0.78 | 0.38,1.60 | |||
| Average | 190 | 1.00 | reference | 147 | 1.00 | reference | 35 | 1.00 | reference | ||||
| Above | 31 | 0.77 | 0.50,1.19 | 0.20 | 26 | 0.81 | 0.50,1.30 | 0.11 | 5 | 0.81 | 0.31,2.09 | 0.71 | |
| P Interaction (family history and weight 12) = 0.73 | |||||||||||||
| PR+ | Below | 315 | 1.00 | 0.86,1.16 | 240 | 0.94 | 0.80,1.11 | 63 | 1.27 | 0.93,1.74 | |||
| Average | 868 | 1.00 | reference | 701 | 1.00 | reference | 139 | 1.00 | reference | ||||
| Above | 149 | 0.90 | 0.74,1.09 | 0.48 | 122 | 0.91 | 0.73,1.12 | 0.31 | 23 | 0.94 | 0.59,1.48 | 0.06 | |
| P Interaction (family history and weight 12) = 0.19 | |||||||||||||
| PR– | Below | 123 | 1.08 | 0.85,1.37 | 100 | 1.08 | 0.83,1.40 | 18 | 0.91 | 0.52,1.59 | |||
| Average | 328 | 1.00 | reference | 264 | 1.00 | reference | 54 | 1.00 | reference | ||||
| Above | 42 | 0.62 | 0.43,0.89 | 0.24 | 36 | 0.69 | 0.47,1.01 | 0.02 | 6 | 0.53 | 0.21,1.34 | 0.45 | |
| P Interaction (family history and weight 12) = 0.81 | |||||||||||||
Cox proportional hazards regression analysis, accounting for age, education status, age at menopause. age at menarche, parity, age at first birth body mass index at age 18, body mass index in 1986, oral contraceptive use, hormone replacement therapy, smoking status, alcohol use, and physical activity level.
Figure 1.
Multivariate-adjusted relative risks and 95% CI intervals of post-menopausal breast cancer for above average weight at age 12 (compared to average weight), stratified by Body Mass Index (BMI) at age 18 (including adjustment for BMI at study baseline) and study baseline in 1986 (including adjustment for BMI at age 18)
The inverse association of weight at age 12 with breast cancer risk was not modified by a family history of breast or ovarian cancer in a first degree relative (p for interaction = 0.44) (Table 2). Similar results were seen if we restricted to a first degree relative with breast cancer only (data not shown).
The inverse association of above average weight at age 12 with risk of breast cancer was observed for all subtypes defined by ER and PR status, and was strongest for PR– tumors (RR=0.62), intermediate for ER+ (RR=0.80) and ER– (RR=0.77) tumors, and weakest for PR+ tumors (RR=0.90) (Table 2). These associations were not modified by a family history of breast or ovarian cancer (all p>0.18). In an analysis where ER and PR were jointly evaluated (not reported in the table), an inverse association with above average weight was seen for ER+PR+ (RR=0.91, 95% CI 0.75, 1.10), ER+PR– (RR=0.49, 95% CI 0.29, 0.85), ER–PR+ (RR=0.74, CI 0.24, 2.26) and ER–PR– (RR=0.77, 95% CI 0.47, 1.26). There was insufficient sample size to evaluate the interaction of these subtypes by a family history of breast or ovarian cancer.
Discussion
We confirmed an inverse association between relative weight at age 12 and risk of postmenopausal breast cancer after adjustment for a wide variety of breast cancer risk factors, including BMI at age 18 and BMI at study baseline. However, we were not able to confirm the interaction between relative weight at age 12 and family history of breast or breast or ovarian cancer we had reported previously in a different population (21). We also found inverse associations for relative weight at age 12 with all breast cancer subtypes defined by ER and PR status, and this was strongest for ER+PR– tumors.
Strengths of this study include the prospective cohort design, excellent follow-up of the cohort, case identification using a SEER cancer registry, ability to assess multiple potential confounders, and assessment of associations for breast cancer subtypes defined by ER and PR status. There are also limitations. Relative weight at age 12 was self-reported and involved recalling a relative weight more than 4 to 6 decades in the past. Indeed, this is why we did not collect actual weight. Recall of childhood and adolescent body build by elderly subjects has been shown to have reasonable validity (31, 32) and any bias introduced is expected to attenuate associations. Furthermore, we observed expected associations of relative weight at age 12 with age of first menstruation, which provided some internal consistency for our measure. Information about ER/PR status of breast cancer was obtained through multiple laboratories involved with SEER, rather than a single reference laboratory. However, the availability of receptor status and the ER/PR distribution in our study (ER+ 64.1%, ER– 11.9%, PR+ 53.2%, and PR– 19.7%) was similar to that reported by other studies (27, 33).
Most studies have reported that greater weight or obesity in early adolescence (age 9 to 14 years) is associated with a lower risk of postmenopausal breast cancer (8, 10, 18, 19, 21, 22), although there are exceptions (17, 23). This association appears to be independent of adult obesity (which is positively associated with postmenopausal breast cancer risk), particularly as demonstrated in our multivariate-adjusted analyses and analyses stratified on adult BMI. However, we were not able to confirm results of a prior study which found a strong interaction (p ≤ 0.001) between relative age at weight 12 with family history on the risk of postmenopausal breast cancer (15, 21). The latter study, conducted among a historical cohort of 426 families of breast cancer probands diagnosed between 1944 and 1952, found that women with above average weight at age 12 had a lower risk of breast cancer if they had no family history of breast cancer (OR=0.75, 95% CI 0.26, 2.16), while women with a family history had a greatly increased risk of breast cancer (OR=4.25, 95% CI: 1.71, 10.5). These latter findings, different from our study results, could be due to difference in the genetic risk, as the study was based on a historical cohort of families of breast cancer probands with a higher genetic risk of breast cancer, while our study was based on a population cohort of average genetic risk.
We found that the inverse association of relative weight at age 12 with postmenopausal breast cancer risk was apparent for all tumor subtypes defined by ER or PR status, although results were strongest for ER+/PR- tumors. To our knowledge, no studies have evaluated the association between early adolescent obesity and postmenopausal breast cancer by ER/PR tumor subtype.
The biological mechanisms for a putative inverse relationship between relative weight at age 12 and postmenopausal breast cancer risk is not known. In a 7-year longitudinal study of 286 girls initially aged 8 to 9 years, adiposity was associated with higher circulating concentrations of dehydroepiandrosterone sulfate (DHEAS) and lower concentrations of sex hormone-binding globulin (SHBG), but there were no consistent associations for circulating levels of estrogen or progesterone (34). The lack of an association for estrogen would suggests that early adolescent obesity is not likely to influence postmenopausal breast cancer risk through estrogen signaling, consistent with the similar association for ER+ (RR=0.80) and ER– (RR=0.77) tumors in our study. We did observe larger differences for PR+ (RR=0.90) versus PR– (RR=0.62) tumors, however, which is inconsistent with the finding of no differences in progesterone concentrations noted above. Obese adolescent and preadolescent girls also have elevated levels of insulin and insulin-like growth factor I (IGF-I), and this leads to impaired ovarian steroid metabolism and anovulation (35). Fewer ovulatory cycles are expected to protect against breast cancer (36, 37), although one recent study found that the inverse relationship between adult BMI and premenopausal breast cancer incidence was not likely to be explained by menstrual cycle characteristics of the women (38). However, the impact of fewer ovulatory cycles could be more pronounced during the time frame before first full-term pregnancy, and particularly in adolescence, due to the greater susceptibility to carcinogens of undifferentiated breast tissue (39, 40). There may also be other aspects of the hormonal milieu associated with obesity in the early teenage years that protects against breast cancer, and this requires further evaluation (34, 35).
We confirmed an inverse association between relative weight at age 12 and risk of postmenopausal breast cancer, and this was independent of adult BMI. The inverse association was strongest for ER+/PR– tumors. We did not find any interaction between relative weight at age 12 and family history of cancer as suggested in previous studies.
Acknowledgments
Funding Source: Supported in part by R01 CA39742
Footnotes
Financial Disclosure: None
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Key TJ, Appleby PN, Reeves GK, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 3.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–27. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–11. [PubMed] [Google Scholar]
- 5.Tworoger SS, Eliassen AH, Missmer SA, et al. Birthweight and body size throughout life in relation to sex hormones and prolactin concentrations in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15:2494–501. doi: 10.1158/1055-9965.EPI-06-0671. [DOI] [PubMed] [Google Scholar]
- 6.Ursin G, Longnecker MP, Haile RW, et al. A meta-analysis of body mass index and risk of premenopausal breast cancer. Epidemiology. 1995;6:137–41. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Choi NW, Howe GR, Miller AB, et al. An epidemiologic study of breast cancer. Am J Epidemiol. 1978;107:510–21. doi: 10.1093/oxfordjournals.aje.a112570. [DOI] [PubMed] [Google Scholar]
- 8.Hislop TG, Coldman AJ, Elwood JM, et al. Childhood and recent eating patterns and risk of breast cancer. Cancer Detect Prev. 1986;9:47–58. [PubMed] [Google Scholar]
- 9.Chu SY, Lee NC, Wingo PA, et al. The relationship between body mass and breast cancer among women enrolled in the Cancer and Steroid Hormone Study. J Clin Epidemiol. 1991;44:1197–206. doi: 10.1016/0895-4356(91)90152-y. [DOI] [PubMed] [Google Scholar]
- 10.Brinton LA, Swanson CA. Height and weight at various ages and risk of breast cancer. Ann Epidemiol. 1992;2:597–609. doi: 10.1016/1047-2797(92)90004-a. [DOI] [PubMed] [Google Scholar]
- 11.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body size and risk of breast cancer. Am J Epidemiol. 1997;145:1011–9. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- 12.Coates RJ, Uhler RJ, Hall HI, et al. Risk of breast cancer in young women in relation to body size and weight gain in adolescence and early adulthood. Br J Cancer. 1999;81:167–74. doi: 10.1038/sj.bjc.6690667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanderson M, Shu XO, Jin F, et al. Weight at birth and adolescence and premenopausal breast cancer risk in a low-risk population. Br J Cancer. 2002;86:84–88. doi: 10.1038/sj.bjc.6600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folsom AR, Kaye SA, Prineas RJ, et al. Increased incidence of carcinoma of the breast associated with abdominal adiposity in postmenopausal women. Am J Epidemiol. 1990;131:794–803. doi: 10.1093/oxfordjournals.aje.a115570. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson C, Baron J, Persson I, et al. Body size in different periods of life and breast cancer risk in post- menopausal women. Int J Cancer. 1998;76:29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Hilakivi-Clarke L, Forsen T, Eriksson JG, et al. Tallness and overweight during childhood have opposing effects on breast cancer risk. Br J Cancer. 2001;85:1680–4. doi: 10.1054/bjoc.2001.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pryor M, Slattery ML, Robison LM, et al. Adolescent diet and breast cancer in Utah. Cancer Res. 1989;49:2161–7. [PubMed] [Google Scholar]
- 18.Le Marchand L, Kolonel LN, Earle ME, et al. Body size at different periods of life and breast cancer risk. Am J Epidemiol. 1988;128:137–152. doi: 10.1093/oxfordjournals.aje.a114936. [DOI] [PubMed] [Google Scholar]
- 19.Berkey CS, Frazier AL, Gardner JD, et al. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–9. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Baer HJ, Colditz GA, Rosner B, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7:R314–25. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerhan JR, Grabrick DM, Vierkant RA, et al. Interaction of adolescent anthropometric characteristics and family history on breast cancer risk in a Historical Cohort Study of 426 families (USA) Cancer Causes Control. 2004;15:1–9. doi: 10.1023/B:CACO.0000016566.30377.4e. [DOI] [PubMed] [Google Scholar]
- 22.Ahlgren M, Melbye M, Wohlfahrt J, et al. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–26. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S, Favero A, La Vecchia C, et al. Body size indices and breast cancer risk before and after menopause. Int J Cancer. 1996;67:181–6. doi: 10.1002/(SICI)1097-0215(19960717)67:2<181::AID-IJC5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 24.Ursin G, Paganini-Hill A, Siemiatycki J, et al. Early adult body weight, body mass index, and premenopausal bilateral breast cancer: data from a case-control study. Breast Cancer Res Treat. 1994;33:75–82. doi: 10.1007/BF00666073. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson C, Colditz G, Rosner B, et al. Association of family history and other risk factors with breast cancer risk (Sweden) Cancer Causes Control. 1998;9:259–67. doi: 10.1023/a:1008817018942. [DOI] [PubMed] [Google Scholar]
- 26.Potter JD, Cerhan JR, Sellers TA, et al. Progesterone and estrogen receptors and mammary neoplasia in the Iowa Women's Health Study: how many kinds of breast cancer are there? Cancer Epidemiol Biomarkers Prev. 1995;4:319–26. [PubMed] [Google Scholar]
- 27.Colditz GA, Rosner BA, Chen WY, et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–28. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 28.Bisgard KM, Folsom AR, Hong CP, et al. Mortality and cancer rates in nonrespondents to a prospective cohort study of older women: 5-year follow-up. Am J Epidemiol. 1994;139:990–1000. doi: 10.1093/oxfordjournals.aje.a116948. [DOI] [PubMed] [Google Scholar]
- 29.Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review, 1975–2002. Bethesda, MD: National Cancer Institute; 2005. [Google Scholar]
- 30.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 31.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138:56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 32.Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155:672–9. doi: 10.1093/aje/155.7.672. [DOI] [PubMed] [Google Scholar]
- 33.Grann VR, Troxel AB, Zojwalla NJ, et al. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103:2241–51. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- 34.Baer HJ, Colditz GA, Willett WC, et al. Adiposity and sex hormones in girls. Cancer Epidemiol Biomarkers Prev. 2007;16:1880–8. doi: 10.1158/1055-9965.EPI-07-0313. [DOI] [PubMed] [Google Scholar]
- 35.Stoll BA. Teenage obesity in relation to breast cancer risk. Int J Obes Relat Metab Disord. 1998;22:1035–40. doi: 10.1038/sj.ijo.0800769. [DOI] [PubMed] [Google Scholar]
- 36.Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43. doi: 10.1016/0277-5379(88)90173-3. [DOI] [PubMed] [Google Scholar]
- 37.Henderson BE, Ross RK, Judd HL, et al. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56:1206–8. doi: 10.1002/1097-0142(19850901)56:5<1206::aid-cncr2820560541>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166:2395–402. doi: 10.1001/archinte.166.21.2395. [DOI] [PubMed] [Google Scholar]
- 39.Russo J, Hu YF, Silva ID, et al. Cancer risk related to mammary gland structure and development. Microsc Res Tech. 2001;52:204–23. doi: 10.1002/1097-0029(20010115)52:2<204::AID-JEMT1006>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Colditz GA, Frazier AL. Models of breast cancer show that risk is set by events of early life: prevention efforts must shift focus. Cancer Epidemiol Biomarkers Prev. 1995;4:567–71. [PubMed] [Google Scholar]