Abstract
Matrix metalloproteinases (MMPs) have been implicated in numerous tissue-remodeling processes. The finding that mice deficient in collagenase-2 (MMP-8) are more susceptible to develop skin cancer, prompted us to investigate the role of this protease in cutaneous wound healing. We have observed a significant delay in wound closure in MMP8−/− mice and an altered inflammatory response in their wounds, with a delay of neutrophil infiltration during the first days and a persistent inflammation at later time points. These changes were accompanied by alterations in the TGF-β1 signaling pathway and by an apoptosis defect in MMP8−/− mice. The delay in wound healing observed in MMP8−/− mice was rescued by bone marrow transplantation from wild-type mice. Analysis of other MMPs showed that MMP8−/− mice had a significant increase in the expression of MMP-9, suggesting that both proteases might act coordinately in this process. This possibility was further supported by the novel finding that MMP-8 and MMP-9 form specific complexes in vivo. Taken together, these data indicate that MMP-8 participates in wound repair by contributing to the resolution of inflammation and open the possibility to develop new strategies for treating wound healing defects.
Keywords: neutrophil, MMP-9, cancer, protease, degradome
Proteases perform a wide variety of functions in numerous physiological and pathological conditions, including development, cell migration, inflammation, and apoptosis (1, 2). Members of the matrix metalloproteinase (MMP) family of enzymes have been implicated in multiple processes that require extracellular matrix remodeling (3–5). Skin wound healing is a complex process in which the presence of a coordinated proteolytic activity is necessary to cleave collagens and other extracellular matrix proteins, to replace the provisional fibrin matrix and to allow keratinocyte migration (6, 7). The relevance of MMPs in skin wound healing was first demonstrated after the finding that MMP inhibitors severely impair this process in different animal models (7–9). Similarly, Col1a1tm1Jae mice, which are considered as a multiple MMP-deficient model as the main site of collagen I cleavage has been mutated, also exhibit a clear delay in cutaneous wound repair, underscoring the importance of collagenases in this process (10). However, studies with mutant mice deficient in specific MMPs possessing collagenolytic activity have failed to reveal significant defects in this process. Thus, wound closure is unaltered in mice deficient in MT1-MMP, a membrane-bound metalloproteinase that activates proMMP-2 and also exhibits collagenolytic activity (11–13). Likewise, mutant mice lacking collagenase-3 (MMP-13), a potent enzyme strongly over-expressed during the initial steps of wound healing, do not exhibit any apparent abnormality in the process of skin repair (14).
Collagenase-2 (MMP-8) is mainly produced by neutrophils, where it is concentrated in secretory granules that are degranulated on neutrophil activation. To date, the biological functions of this neutrophil collagenase are uncertain, although it is implicated in a variety of tissue remodeling processes associated with inflammatory conditions or in the course of cutaneous wound healing (15–19). The relevance of MMP-8 in wound repair is suggested by the finding that this neutrophil protease is the main collagenase produced in healing wounds as well as in nonhealing ulcers (19, 20). Interestingly, analysis of MMP expression patterns in wounds from MMP13−/− has shown an increased production of MMP-8 at the wound site. Therefore, this suggests that the lack of MMP-13 may be functionally compensated by an enhanced expression of MMP-8, finally resulting in normal healing (14). Taken together, these results indicate that MMP-8 participates in the process of skin wound healing, and its presence might be necessary for this process to be completed. However, beyond these correlative observations, to date no information is available about the precise functional role of this enzyme in cutaneous wound repair. In this study, we have taken advantage of mutant mice deficient in MMP-8 to study its role in skin wound healing.
MATERIALS AND METHODS
Animals
MMP8−/− mice of a mixed C57BL/6J/129 background (21) were used, and wild-type littermates served as controls. The generation of MMP9−/− mice in a C57BL/6J background has been previously described (22). All experiments were performed with 8–12 wk old male mice, and wild-type litter-mates were used as controls. All mice were kept under specific pathogen-free conditions, and the experiments were conducted in accordance with the guidelines of the Committee on Animal Experimentation of the Universidad de Oviedo, Oviedo-Spain.
Wound model
Skin wounds were performed as described previously (23). Briefly, mice were anesthetized by isoflurane inhalation, and after shaving the dorsal hair and cleaning the exposed skin with 70% ethanol, full-thickness excisional skin wounds were performed on either side of the dorsal middle line using an 8 mm biopsy-punch (Accuderm, Ft. Lauderdale, FL, USA). Usually, two wounds were made on the same animal, and healing was monitored by taking photographs at the indicated time points. Wound area was calculated for each time point, and wound closure was expressed as percentage of recovery with respect to the initial wound area. For biochemical and histological experiments, animals were sacrificed by cervical dislocation, and wounds and surrounding area were harvested and snap-frozen for biochemical analysis, or fixed for histological analysis.
Bone marrow transplantation
Bone marrow transplantation experiments were performed as described previously (24). Briefly, wild-type or MMP8−/− mice were treated with 25 mg/Kg of busulfan for 4 days, followed by injection of 200 mg/Kg of cyclophosphamide. Twenty-four hours later, bone marrow was collected from the femurs of donor mice, and 4 × 106 cells were injected in recipient animals via the lateral tail vein. Six weeks later the engraftment was evaluated by immunofluorescence in blood samples using a neutrophil-specific antibody against Ly-6G/Gr-1 (Pharmingen, La Jolla, CA, USA), and an antibody against MMP-8 (21). Transplant efficiency was more than 90% in all cases.
Histological analysis and in situ hybridization
Wound tissues were fixed overnight in 4% paraformaldehyde buffered with phosphate-buffered saline (PBS) (pH 7.4) and embedded in paraffin. Sections (4 μm width) were subjected to hematoxylin and eosin staining. For the in situ hybridization experiments, skin samples were fixed overnight in 4% paraformaldehyde at 4°C, then dehydrated in ethanol and embedded in paraffin. Sections (5 μm) were hybridized to 35S-labeled antisense MMP13 and MT1-MMP riboprobes as described previously (25). Slides were exposed to photographic emulsion at 4°C for 6 days, then developed, fixed, and cleared. Sections were counterstained with 0.02% toluidine blue. Sections hybridized with a labeled-sense riboprobe were used as negative controls. No positive hybridization signal was found in negative controls. Bright-field and dark-field images were captured with SPOT digital camera. Sense or antisense 35S-uridine triphosphate-labeled RNA probes were synthesized from the corresponding linearized DNA using the appropriate RNA polymerases. Detection of apoptotic cells in wound sections was performed using a fluorescent terminal transferase dUTP nick end-labeling (TUNEL) kit (Chemicon, Temecula, CA, USA) following manufacturer’s instructions. TUNEL-staining was visualized using a fluorescent microscope and photographed with a CCD camera.
Analysis of re-epithelialization
Re-epithelialization was quantified as described previously (26). Briefly, wound sections were stained with hematoxylin and eosin, and wound width and distance between the leading edges of keratinocyte migration was measured. Re-epithelialization was calculated as the ratio between the distance covered by the epithelium and the distance between wound ends.
Gelatin zymography and Western blot analysis
MMP-2 and MMP-9 activity was analyzed by gelatin zymography as described previously (27). Basically, wound extracts were homogenized in a modified RIPA lysis buffer (0.1 M Tris/HCl, 0.15 M NaCl, 1% Triton X-100, 0.1% SDS, pH 7.4) containing complete protease inhibitor cocktail without EDTA (Roche, Mannheim, Germany), and protein content was quantified using the BCA method (Pierce, Rockford, IL, USA). Protein (10 μg) were separated in a 8% SDS-PAGE gel containing 0.2% gelatin. Gels were washed twice for 30 min with 2.5% Triton X-100 and incubated at 37°C for 4 h in the presence of 10 mM Tris (pH 8.0) and 5 mM CaCl2 and stained with Coomassie brilliant blue to reveal gelatinolytic bands. Bone marrow cells were obtained from the femurs from 8-wk-old male mice, resuspended at 107 cells/ml in PBS, and 106 cells were degranulated by treatment with TPA (1 μM) or fMLP (1 μM). To analyze skin wound extracts by Western blot, 20 μg of protein were analyzed using rabbit IgGs against murine collagenase-2/MMP-8, pSmad2, pSmad3, Smad4, TGF-β RI, and actin, or sheep antibodies against murine MMP-9 (provided by G. Murphy, University of Cambridge, UK). For the detection of pSmad3, wound lysates were immunoprecipitated with a rabbit antibody against Smad2/3 (Cell Signaling, Beverly, MA, USA) and protein A/G-sepharose. We used secondary antibodies, donkey antibody to rabbit and goat antibody to sheep IgGs coupled to peroxidase, at 1:20,000 dilution. We developed the reaction with Supersignal Chemiluminescent Substrate (Pierce). For the detection of MMP-8/MMP-9 complexes, samples were run unreduced, otherwise samples were reduced with 2-mercaptoethanol.
Measurement of cytokines and chemokines by ELISA
Wound samples were homogenized in PBS containing 0.1% SDS, 1% Nonidet P-40, and 5 mM EDTA, pH 7.4, containing complete protease inhibitor mixture, and cell debris were cleared by centrifugation at 13,000 g for 15 min at 4°C. Levels of TGF-β1, KC, and MIP-2 proteins in wound extracts were quantified using commercial ELISA kits (R&D Systems and Promega, Madison, WI, USA) according to the manufacturers’ instructions. Total protein concentration was measured using the BCA assay as above, and the data were expressed as picograms of chemokine per milligram of sample.
Statistical analysis
Statistical differences were determined using the Student’s t test or analysis of variance. All data are presented as the mean ± SEM.
RESULTS
Loss of MMP-8 impairs wound healing in the skin
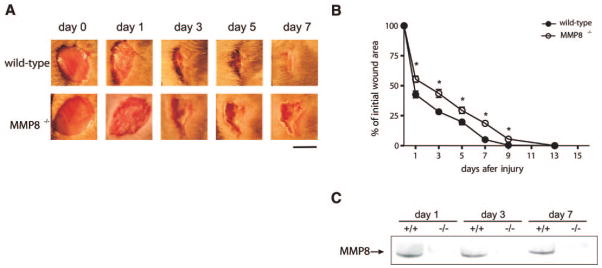
The ability of collagenases to degrade different components of the extracellular matrix, together with previous observations showing that MMP-8 is the predominant collagenase in healing wounds (19, 20), prompted us to investigate whether this neutrophil proteinase participates in the process of cutaneous wound repair. We used MMP8−/− mice and their corresponding wild-type littermate controls, and performed full-thickness skin excisional wounds as described under Materials and Methods. After monitoring the wound healing process at different time points, we observed that wound closure in MMP8−/− mice was significantly delayed when compared to wild-type littermates (Fig. 1A, B). This delay was observed from the first day and was sustained throughout the healing process, resulting in the complete closure of the wound 13–14 days after injury in MMP8−/− animals, in comparison to wild-type animals, in which healing was completed by days 8 and 9. These data indicate that MMP-8 participates in skin wound closure.
Figure 1.
Loss of MMP-8 results in delayed wound healing. Excisional wounds were performed in MMP8−/− mice and wild-type littermates, and the healing process was monitored at different time points. A) Representative images of wounds from wild-type and MMP8−/− mice at different time points. Wild-type mice completely healed the wound area between 8 and 9 days after injury. However, mice lacking MMP8 showed a delay in this process, and complete healing was not observed until 13–15 days postinjury. Scale bar: 5 mm. B) Wound area was quantified every two days and expressed as the percentage of wound closure for each genotype (n=8; *P<0.05). C) Western blot analysis of skin extracts from wild-type (+/+) and MMP8−/− (−/−) animals at different time points after injury. MMP-8 was detected in injured skin in wild-type animals from days 1 to 7 after injury.
To further evaluate the participation of MMP-8 during the wound healing process, we analyzed the presence of this protease in the wound area; we detected MMP-8 in wild-type animals as soon as 24 h after wounding, and it was present in the wound area up to 7 days after injury, while MMP-8 could not be detected in uninjured skin (Fig. 1C and data not shown). These expression data, together with the observation that the absence of MMP-8 results in wound closure delay, indicate that this neutrophil enzyme plays a nonredundant functional role during skin wound healing.
Delayed re-epithelialization at the wound sites in MMP8−/− mice
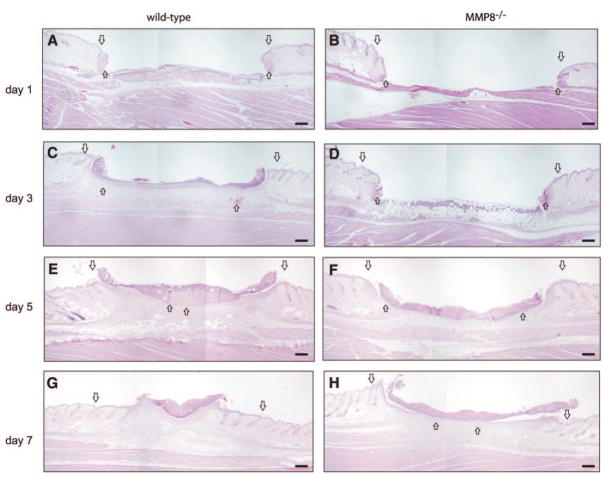
Re-epithelialization is a crucial event during the wound healing process, allowing the formation of a thick and hyperproliferative epithelium as well as granulation tissue that covers the wound (28, 29). The observed delay in skin wound healing in MMP8−/− mice prompted us to perform a detailed analysis of the re-epithelialization process as shown in Fig. 2. Three days after injury, re-epithelialization was significantly slower in MMP8-deficient animals compared with wild-type littermates. In addition, it was exclusively limited to the wound margins, while in wild-type animals re-epithelialization occurred at a normal rate, and by day 3 ~30% of the wound was already re-epithelialized (Fig. 2A–D). The delay in re-epithelialization continued throughout the wound-healing process, with the greatest differences being observed at day 5, in which re-epithelialization was starting in MMP8−/− mice, covering only about one-third of the wound area, whereas this process was almost completed in wild-type controls (Fig. 2E, F). Finally, by day 7, re-epithelialization was completed in control mice but it was still partial in MMP8−/− animals, indicating that these animals have a significant delay in this process (Fig. 2G, H).
Figure 2.
Re-epithelialization is delayed in MMP8−/− wounds. Wound tissue was obtained from wild-type and MMP8−/− mice at different time points, and histological analysis was performed to examine re-epithelialization. A–H) Representative hematoxylin and eosin-stained tissue sections illustrating the effect of MMP8 deletion in granulation tissue development. Wound ends are indicated by big arrows, and the leading edge of keratinocyte migration is indicated by small arrows. Scale bars: 200 μm.
Neutrophil recruitment is impaired in MMP8−/− wounds
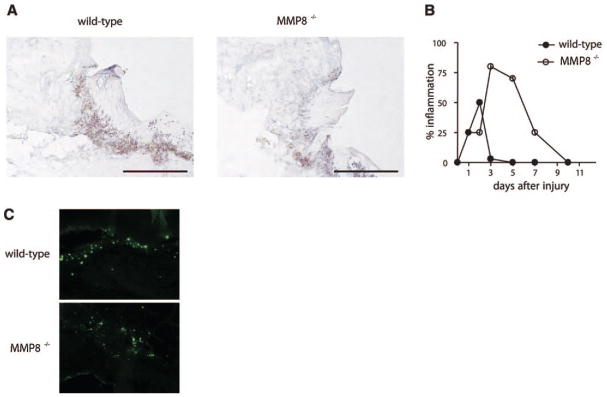
A characteristic feature following skin injury is the rapid release of proinflammatory cytokines and chemokines, including TNF-α, KC/CXCL1, MIP-2/CXCL2, and LIX/CXCL5, which promote the migration of a variety of inflammatory cells into the wound site (30). During this inflammatory phase, neutrophils and macrophages infiltrate the wound area, preventing the growth of pathogens and contributing to the healing process. As MMP-8 is produced mainly by neutrophils, we investigated whether neutrophil infiltration was altered in skin wounds from MMP8−/− mice. To determine the presence of neutrophils in the wound site, we performed histological analysis of wounded tissue at different time points. Similar to previous reports, neutrophil recruitment into the wound site started a few hours after injury in wild-type animals, reaching a maximum 2 days postinjury, and declining progressively afterward, returning to basal levels 3–5 days after injury (Fig. 3A, B). However, MMP8−/− mice showed a different pattern of inflammatory response, which could be divided into two different phases. During the first phase of inflammation, the influx of neutrophils into the wound site was delayed in MMP8−/− mice when compared to wild-type animals (Fig. 3B). In contrast with this reduced neutrophil recruitment observed during the first phase of healing, in a later phase, the number of neutrophils was significantly increased in MMP8−/− wounds (Fig. 3B), indicating that the resolution of inflammation was delayed in MMP8-deficient mice. On the one hand, these results suggest that the defect in neutrophil emigration to the site of injury could be due to a failure to produce the inflammatory signals necessary for neutrophil recruitment to the wound area. On the other hand, the delay in wound healing and re-epithelialization observed in MMP8−/− mice could be due to a defect in the inflammatory response that accompanies the healing process, either through the delay in neutrophil recruitment to the wound site or to the persistent inflammation in these mutant animals.
Figure 3.
Loss of MMP-8 results in altered recruitment of inflammatory cells into the wound site. A) Ly-6G staining of 1-day wounds reveals a massive neutrophil infiltrate in wild-type mice, while recruitment of inflammatory cells is delayed in MMP8−/− mice. Scale bars: 200 μm. B) Histological data quantification expressed as the percentage of inflammation in the wounds at different time points. C) Delayed apoptosis in MMP8-deficient wounds. TUNEL analysis of day 3 wounds reveals a reduced number of apoptotic cells (green) in MMP8 −/− wounds when compared to wild-type mice.
Sustained inflammation in MMP8−/− wounds is accompanied by a delay in neutrophil apoptosis
Inflammation plays an important role during the wound healing process. In fact, recruitment of inflammatory cells during the first hours after injury is necessary to release different molecules necessary for matrix remodeling and keratinocyte migration, as well as to prevent pathogen growth. However, after this initial response, which is mainly mediated by neutrophils, resolution of inflammation is required for the healing process to proceed. Failure to resolve this inflammatory response can impair the healing process (31). MMP8−/− skin wounds were characterized by an increased inflammation, and neutrophils were still present in skin wounds 5 and 7 days after injury (Fig. 3B). In contrast, it is well established that during normal wound healing neutrophils have already died by apoptosis and are cleared by macrophages at these later time points (31). Based on these findings, we investigated whether the increased inflammation observed in MMP8−/− wounds was due to a defect in neutrophil apoptosis. We analyzed the presence of apoptotic cells in the wound area in wild-type and MMP8−/− mice. We observed that the number of apoptotic cells determined by TUNEL assays was significantly reduced in MMP8−/− wounds when compared with wild-type wounds (Fig. 3C). These results reflect a defect in apoptosis in MMP8-deficient animals and are reminiscent of previous studies in an allergen-induced model of asthma (32), suggesting that the persistent inflammation observed in MMP8−/− skin wounds is likely due to a delay in neutrophil apoptosis.
MMP8 deficiency alters the TGF-β1 signaling pathway during cutaneous wound healing
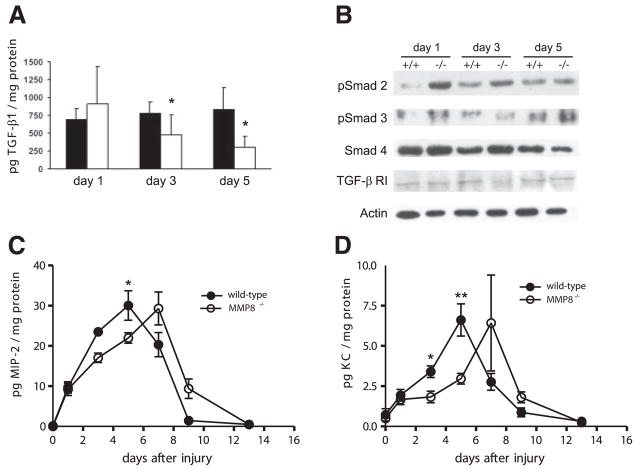
To identify molecular pathways that could be responsible for the altered inflammatory response and delay in wound healing observed in MMP8−/− animals, we analyzed the levels of different cytokines and growth factors previously implicated in wound healing and inflammation, including TNF-α, TGF-β1, IL-1β, KC, or MIP-2 (30). Changes in TGF-β1, as well as in its intracellular effectors, members of the Smad family of transcription factors have been previously associated with altered ability to heal skin wounds (33, 34). MMP8−/− mice showed reduced levels of active TGF-β1 at days 3 and 5 when compared to wild-type animals (Fig. 4A). In keeping with this, pSmad2 was significantly increased in MMP8−/− wounds when compared to wild-type mice, and a moderate increase in pSmad3 was also observed at 24 h, while TGF-β receptor I or total Smad4 levels did not change (Fig. 4B). These data suggest that the reduced levels of active TGF-β1, together with the increased activation of Smad2 and/or Smad3 might contribute to the observed differences in neutrophil infiltration in these animals, as well as to the delay in wound healing, as these processes have been shown to be dependent on TGF-β1 signaling (33, 34). We did not find significant changes in the levels of the proinflammatory cytokines TNF-α or IL-1β (data not shown). However, analysis of KC and MIP-2 in wound extracts revealed that while both chemokines reached maximum levels 5 days postinjury in wild-type mice, MMP8−/− animals showed a delay of two days in reaching their maximum levels (Fig. 4C, D). The delayed expression of KC and MIP-2 could be responsible for the persistence of inflammation in MMP8−/− mice, contributing to the overall delay in wound healing observed in these animals.
Figure 4.
TGF-β1 signaling is altered in MMP8−/− wounds. A) ELISA for active TGF-β1 in wound extracts from wild-type (closed bars) and MMP8−/− mice (open bars) at different time points (n=3; *P<0.05). B) Western blot analysis of protein extracts from wild-type and MMP8−/− wounds for pSmad2, pSmad3, Smad4, TGF-β RI, and actin. pSmad3 was detected after immunoprecipitation of Smad3 from wound homogenates. C, D) ELISA analysis of the proinflammatory chemokines MIP-2 and KC shows that there is a delay in the production of these chemokines during the wound healing process (n=4; *P<0.05, **P<0.01).
Bone marrow transplantation rescues the wound healing defect in MMP8−/− mice
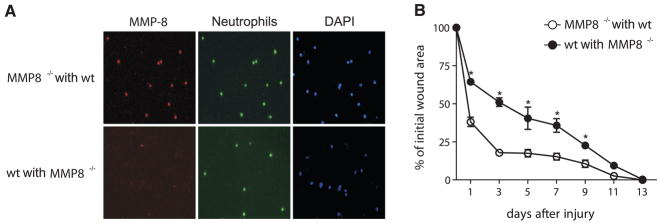
The increased inflammation observed in MMP8−/− wounds, and the fact that MMP-8 is confined to neutrophils, suggest that the wound healing defect in these animals is attributable to these inflammatory cells. To test this hypothesis, we asked whether bone marrow transplantation could rescue the healing defect observed in MMP8−/− mice. We transplanted MMP8−/− mice with bone marrow from wild-type donor mice, and vice versa. Six weeks later, engraftment efficiency was found to be more than 90% in all cases (Fig. 5). To investigate the wound healing ability of transplanted mice, excisional skin wounds were performed as above, and wound healing was followed until complete closure. Interestingly, bone marrow transplantation was sufficient to rescue the wound healing delay observed in MMP8−/− animals. In fact, wound healing in MMP8−/− mice transplanted with wild-type bone marrow was similar to that observed in wild-type animals, with more than 50% of the wound closed after day 1, and achieving complete wound closure in 9 days (Fig. 5B). Furthermore, wild-type animals transplanted with bone marrow from MMP8−/− donors showed a delay in the wound healing process similar to that observed in MMP8−/− animals (Fig. 5B), achieving complete closure in 13 days. These results demonstrate that the healing defect observed in MMP8−/− mice is solely due to the absence of MMP-8 in bone marrow-derived inflammatory cells and suggest that this metallo-protease has a positive effect during skin wound healing and contributes to resolve the inflammation associated with this process.
Figure 5.
Bone marrow transplantation rescues the wound healing defect observed in MMP8−/− mice. Wild-type mice and MMP8−/− animals were transplanted with bone marrow from MMP8−/− or wild-type mice, respectively. A) Immunofluorescence analysis of peripheral blood cells from these animals stained with specific antibody against MMP-8 (red) and the neutrophil-specific antigens (Ly6G, green). Cells were counterstained with DAPI (blue). B) Excisional wounds were performed in either MMP8−/− mice transplanted with bone marrow from wild-type mice (open bars), or in wild-type littermates transplanted with MMP8−/− -derived bone marrow (closed bars). Wound closure was monitored at different time points and expressed for each condition and genotype as the percentage of wound closure (n=7; *P<0.05).
Altered expression of MMP-9 and MMP-13 in wounds from MMP8−/− mice
It is well established that a dramatic remodeling of the extracellular matrix takes place during the wound healing process. These remodeling events are largely mediated by an array of proteolytic enzymes, which are secreted by inflammatory cells or keratinocytes, and facilitate migration of keratinocytes to cover the wound area (3). MMPs can cleave virtually all protein components of the extracellular matrix, and treatment with an MMP inhibitor delays the wound healing process (7, 8), suggesting that MMPs contribute to this process, either independently or coordinately. We next examined whether other MMPs potentially implicated in the wound healing process were altered in the absence of MMP8. By in situ hybridization (Fig. 6A), expression of MMP13 and MT1-MMP (MMP14) was detected in wild-type as well as in MMP8−/− wounds. No differences were observed in the distribution of MMP14 transcripts (Fig. 6A). The expression pattern of MMP13 was clearly altered in MMP8-deficient animals, where a more restricted distribution was observed compared to that in wild-type mice (Fig. 6A). Because MMP13 transcripts were associated with the leading edge of the re-epithelialization layer, this suggests that the differences observed in the expression pattern are likely the result of the delay in re-epithelialization in MMP8-deficient animals. It should be noted that MMP-8 and MMP-13 could compensate for each other, as a recent report hypothesized that MMP-8 compensates for the lack of MMP-13 during the wound healing process in MMP13−/− mice (14).
Figure 6.
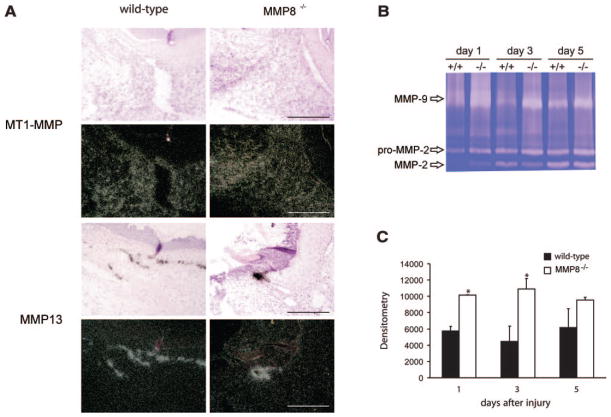
Altered expression of MMPs in MMP8−/− wounds. A) Representative in situ hybridizations of day 5 wounds with specific probes for MT1-MMP/MMP14 and MMP13 in wild-type and MMP8−/− mice. Top panels show dark-field images and bottom panels the corresponding bright-field images Differences in the expression pattern of MMP13 could be observed between wounds from wild-type and MMP8 deficient mice. Cells expressing MMP13 are confined to keratinocytes of the leading wound edge in both genotypes, but due to the delay in re-epithelialization in MMP8−/− wounds, the distribution pattern of MMP13 expression is broader in wild-type wounds. Scale bars: 200 μm. B) Gelatin zymography of wound extracts reveals that MMP-9 production at the wound site is increased in MMP8−/− animals, while production of MMP-2 does not show major differences. C) Densitometric quantification of MMP-9 gelatinolytic bands shown in B (n=3; *P<0.05).
We next examined the MMP-2 (gelatinase A) and MMP-9 (gelatinase B) levels in wounds from MMP8−/− mice by gelatin zymography. As can be seen in Fig. 6B, both metalloproteinases were detected at significant amounts in samples derived from wild-type and MMP8−/− mice. MMP-2 was present in skin wound extracts as soon as 24 h postinjury, however at these initial stages the 72 kDa proMMP-2 was mainly detected (Fig. 6B). By day 3, activated MMP-2 was detected as a 62 kDa band in wound extracts from both wild-type and MMP8−/− mice. The absence of significant changes suggests that MMP-2 does not contribute to the wound healing defect observed in MMP8-deficient animals. Interestingly, in the absence of MMP-8, there was increased MMP-9 in the wounded area 3 days after injury, compared with wild-type animals (Fig. 6B, C). This enhanced production of MMP-9 might be the result of the accumulation of neutrophils in these MMP8−/− animals, and/or a compensatory MMP9 up-regulation.
MMP-8 and MMP-9 form specific complexes in vivo
To determine if MMP-9 production was increased in MMP8−/− neutrophils, we compared the MMP-9 released from bone marrow cells obtained from MMP8−/− and wild-type mice. Degranulation of the same number of bone marrow cells (106) was induced by treatment with TPA, and secreted MMP-9 was analyzed by gelatin zymography (Fig. 7A). We observed no difference in the intensity of the of the 92 kDa gelatinolytic band released from induced bone marrow cells from MMP8−/− and wild-type mice. What we did find was the absence of a relatively low intensity gelatinolytic band (~150 kDa) present in extracts from cells from wild-type bone marrow but absent in extracts from MMP8−/− bone marrow (Fig. 7A). As MMP-9 has been shown to form homodimers as well as stable complexes with proteins like neutrophil gelatinase-associated lipocalin (Ngal) (35), we reasoned that this band could represent a complex containing MMP-9 and another protein absent in MMP8−/− mice. Western blot analysis of TPA-treated bone marrow supernatants with anti-MMP-9 specific antibodies revealed that the 150 kDa complex possessing gelatinolytic activity contained MMP-9 (Fig. 7B). Surprisingly, incubation of the same blot with an antibody against murine MMP-8 not only resulted in the detection of its 60 kDa band, but also in the detection of a specific signal associated with this 150 kDa complex (Fig. 7C), suggesting that this band is likely a complex containing MMP-8 and MMP-9.
Figure 7.
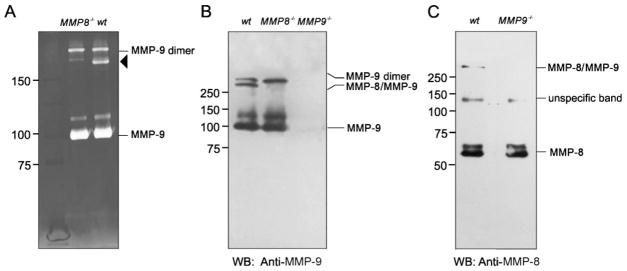
MMP-8 forms specific complexes with MMP-9. Bone marrow cells (106) from the indicated genotypes were treated with TPA to induce degranulation, and the presence of MMP-9 or MMP-8 was detected by gelatin zymography or Western blot. A) Gelatin zymography of supernatant from TPA-treated bone marrow cells reveals the presence of similar amounts of MMP-9 activity in wild-type and MMP8−/− mice. A high molecular mass gelatinolytic band (arrowhead) is present in wild-type cells but absent in MMP8-deficient cells. B) Western blot analysis of supernatant from TPA-treated bone marrow cells with antibodies against murine MMP-9 confirms that MMP-9 forms part of the high-molecular-mass gelatinolytic band absent in MMP8−/− cells. No hybridization bands are detected in the supernatant of MMP9−/− cells. C) Western blot analysis of the same supernatants as above using an antibody against murine MMP-8 shows that the high-molecular-mass gelatinolytic band contains MMP-8, and the complex is specifically formed between MMP-8 and MMP-9, as the complex is not present in MMP9−/− cells.
To further confirm that MMP-8 forms a stable complex with MMP-9, we obtained bone marrow cells from MMP9−/− mice, and these cells were analyzed by Western blot with specific antibodies against MMP-8. As expected, we detected a 60 kDa band corresponding to this MMP-8, but in the absence of MMP-9, the additional 150 kDa band containing MMP-8 was no longer detected, confirming that this high molecular weight complex is formed by the interaction between MMP-8 and MMP-9 (Fig. 7C). These results represent the first observation that MMPs can form heterodimeric complexes in vivo when secreted by neutrophils and open the possibility to perform additional studies to uncover the functional relevance of this newly identified complex.
DISCUSSION
MMPs are widely associated with processes in which extensive remodeling of the extracellular matrix is required (3, 4). Indeed, MMPs, including the three collagenases (MMP-1, MMP-8, and MMP-13), as well as MMP-2, -3, -9, -19, -26, and MMP14/MT1-MMP, have been shown to be expressed at wound sites during the healing process (14, 36–40). However, although blockade of their activity with MMP inhibitors delays wound healing (7, 8), all MMP-deficient animals previously described do not show mayor abnormalities in wound healing in the skin with the exception of MMP3 (stromelysin-1)-deficient animals, which show a defect in wound contraction (11, 14, 41, 42). This raised the question of whether the critical MMP had not been determined or whether no one MMP was essential for the wound healing. The recent availability of mutant mice deficient in MMP8 has allowed us to investigate its individual contribution to the wound healing process (21). In the present study, we demonstrate the functional relevance of this enzyme in cutaneous wound repair by showing that MMP8−/− mice exhibit a significant delay in wound closure and re-epithelialization. We also show that this delay is associated with marked alterations in the inflammatory response characteristic of the wound healing process. Moreover, this healing defect can be completely rescued by transplantation of bone marrow from wild-type animals to MMP8−/− mice, demonstrating that MMP-8 derived from inflammatory cells is necessary for the wound healing process to proceed normally.
The alterations observed in MMP8−/− mice differentially affect the two distinct phases of the cutaneous repair process. Thus, during the first 48 h after wounding, recruitment of inflammatory cells to the wound site is delayed in MMP8−/− mice, suggesting that inflammatory cells are unable to extravasate into the wound site. This could be due to an inability to degrade type I collagen, or to impaired production or mobilization of proinflammatory signaling molecules in MMP8−/− animals. However, a failure of MMP8−/− inflammatory cells to transmigrate to the wound site would be expected to result in an accumulation of inflammatory cells in surrounding blood vessels, an effect that we did not observe in MMP8−/− wounds.
The mechanism by which the delayed infiltration of neutrophils into the wound area retards the repair process in MMP8−/− mice appears to be dependent on TGF-β1 signaling, a central regulator of inflammation and wound healing. In fact, animals lacking Smad3 show accelerated wound healing and reduced local inflammation, while overexpression of Smad2 in the epidermis under the control of the keratin-14 promoter has been shown to delay wound healing (33, 34), indicating that these proteins are critical for normal healing in the skin. In this regard, our results show reduced levels of active TGF-β1 and an increased activation of Smad2 and moderate Smad3 phosphorylation in MMP8−/− mice when compared to wild-type animals from day 1 to day 3 postinjury, suggesting that this altered signaling pathway could be responsible for the abnormal inflammatory response and the delay in re-epithelialization and wound healing observed in these mutant mice. Additionally, we observed a delay of 2 days in the expression of the chemokines KC and MIP-2 in MMP8−/− wounds. However, this was evident later in the wound healing process around day 5, suggesting that these chemokines are not responsible for the initial defects observed in these animals. These results are in agreement with previous observations in TNF-α-induced lethal hepatitis in MMP8−/− mice, in which leukocyte infiltration is also impaired during the first hours (43), and confirm the importance of MMP-8 in neutrophil recruitment in vivo.
In marked contrast with the observed delay in the earliest steps of the wound healing process in MMP8−/− mice, a sustained inflammatory response was found in wounds from these mice at later steps. This phase of healing, extending from days 3 to 9, is characterized by a massive apoptotic death of neutrophils, which are then phagocytosed by invading macrophages (31). Accordingly, the sustained inflammation exhibited by MMP8−/− wounds is likely due to a defect in neutrophil apoptosis, since we observed decreased numbers of apoptotic cells in MMP8−/− wounds. The apoptosis defect observed during wound healing in MMP8−/− mice also confirms and extends previous findings showing a reduction in neutrophil apoptosis after induction of asthma in these mutant mice (32). This finding also suggests that MMP-8 might be implicated in other inflammatory diseases in which defective apoptosis and persistent inflammation is observed (43, 44).
Although previous reports had shown that MMP-8 expression was associated with healing wounds as well as with nonhealing ulcers, thereby suggesting that this enzyme might impair wound repair (20, 36), our data indicate that it plays an overall beneficial effect and is necessary for the healing process to proceed normally. The association between MMP-8 and nonhealing ulcers, such as those present in diabetic patients (45), might just reflect the sustained inflammatory response associated with these processes. The participation of MMPs in wound healing has been traditionally linked to their ability to cleave different components of the extracellular matrix. In this sense, although MMP-8 was first discovered as a collagenolytic enzyme, our results suggest it has additional functions independent of its collagen degrading activity. In fact, loss of MMP8 results in complete healing of skin wounds 13–15 days postinjury, 5 days later than in wild-type mice, indicating that its activity is necessary for this process to proceed normally, but is dispensable for wound closure, as this process is completed in MMP8−/− animals. Similarly, re-epithelialization is delayed in these mice, being complete about two days later than in their wild-type littermates.
As extensive remodeling of the extracellular matrix occurs during the re-epithelialization phase, we cannot rule out the possibility that the absence of collagenolytic activity provided by MMP-8 might affect remodeling of the extracellular matrix. However, due to the presence of numerous proteases released by keratinocytes, fibroblasts, and inflammatory cells in the wound area, it is possible that other extracellular matrix-cleaving proteases might compensate the loss of MMP-8, thus allowing the wound healing process to be completed despite its absence. When we examined the status of other members of the MMP family that have been previously associated with wound healing, we found that the expression of MMP13 (collagenase-3) and MMP9 showed some differences between wild-type and MMP8−/− wounds. MMP13 was highly expressed during the early phase of wound healing at the keratinocyte migration leading edge in wild-type animals (27), but its distribution pattern in MMP8−/− wounds was more confined to the leading edge, in agreement with the smaller size of the edge. These observations suggest that the absence of MMP-8 might be partially compensated by an altered expression of MMP13. The expression of MMP13 may be increased by the presence of unprocessed type I collagen in skin wounds (27), as shown for animals containing the collagenase-resistant collagen Col1a1tm1Jae. This suggests that the lack of MMP-8, which preferentially cleaves type I collagen (46), may lead to the accumulation of unprocessed type I collagen and therefore to the increased expression of MMP13. Although the altered expression of MMP13 in MMP8−/− wounds could compensate the lack of MMP-8, MMP13−/− animals do not show defects in wound healing (14). However, MMP13−/− mice show enhanced expression of MMP8 in the wound area (14), confirming the importance of the latter neutrophil enzyme in the wound healing process. These results, together with the observations presented herein, suggest that functional compensatory mechanisms between MMP-8 and MMP-13 might allow the completion of the wound healing process in the absence of either protease. Generation of double knockout mice deficient for both collagenases could contribute to clarify this possibility. Unfortunately, the genes encoding these enzymes are closely linked in a small region of mouse chromosome 9A1 (47, 48), thereby preventing the generation of double deficient mice by simply breeding of animals deficient in each protease.
In this study, we also observed that MMP8−/− wounds show an up-regulation of MMP-9. This effect was specific to the healing process and not the result of a general increase of this protease in MMP8−/− mice, because bone marrow cells from wild-type and MMP8−/− mice secreted similar amounts of MMP-9. Previous reports have shown that animals deficient in MMP9 show an enhancement in wound healing, indicating that this protease inhibits the rate of wound closure in cornea and skin (41). Together, these data suggest that the increased secretion of MMP-9 might contribute to the healing defects observed in MMP8−/− animals. Interestingly, the relationship between both proteases is further illustrated by the novel finding that MMP-8 and MMP-9 form specific complexes in vivo. The functional relevance of this complex in the biological activity of both proteases is currently under investigation. In this regard, it is interesting to notice that recent reports have shown that MMP-8 binding to the neutrophil cell membrane prevents inactivation of this protease by tissue inhibitors of metalloproteases-1 and -2 (TIMP-1 and -2) (49). Similarly, interaction of MMP-9 with neutrophil gelatinase-associated lipocalin (Ngal) protects MMP-9 from degradation, which results in an overall increase in MMP-9 enzymatic activity (50). The finding that MMP-8 and MMP-9 form protein complexes in vivo raises the possibility that this complex might represent an additional regulatory mechanism by which the activity of these MMPs is controlled, or by which both proteases could act coordinately to process specific substrates.
This work provides the first causal evidence that MMP-8 is necessary for the healing of skin wounds, as its absence results in a significant delay in wound closure. The defects in wound healing and re-epithelialization observed in MMP8−/− mice are due to an altered inflammatory response that can be fully restored by performing a bone marrow transplantation using wild-type donors. These results confirm and extend the importance of this metalloproteinase in the regulation of inflammation. Furthermore, we have shown that MMP-8 may function in a coordinate fashion with MMP-9 during the wound healing process, as both proteases form stable and specific complexes in vivo. Finally, these data might shed insights into the biological function of MMP-8, and the mechanisms implicated in the protective effect of this neutrophil enzyme against skin tumor progression.
Acknowledgments
We thank A. R. Folgueras for support and helpful comments and L. Santos, I. Fernández, and C. Garabaya for excellent technical assistance. This work was supported by grants from CICYT-Spain, Fundación M. Botín, Fundación Lilly, the European Union (Cancer Degradome-FP6), the National Cancer Institute, USA (CA072006 and CA105379 to Z.W.), and the National Institute of Arthritis and Musculo-skeletal and Skin Diseases (AR044815 to S.M.K.). The Instituto Universitario de Oncología is supported by Obra Social Cajastur-Asturias and Red de Centros de Cancer-Instituto Carlos III, Spain. X.S.P. belongs to the Ramón y Cajal Program from the MCyT (Spain).
References
- 1.Puente XS, Sánchez LM, Overall CM, López-Otín C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 4.Overall CM, López-Otín C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2:657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 5.Folgueras AR, Pendás AM, Sánchez LM, López-Otín C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 6.Lund LR, Green KA, Stoop AA, Ploug M, Almholt K, Lilla J, Nielsen BS, Christensen IJ, Craik CS, Werb Z, Dano K, Romer J. Plasminogen activation independent of uPA and tPA maintains wound healing in gene-deficient mice. EMBO J. 2006;25:2686–2697. doi: 10.1038/sj.emboj.7601173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund LR, Romer J, Bugge TH, Nielsen BS, Frandsen TL, Degen JL, Stephens RW, Dano K. Functional overlap between two classes of matrix-degrading proteases in wound healing. EMBO J. 1999;18:4645–4656. doi: 10.1093/emboj/18.17.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibro-blast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Salonurmi T, Parikka M, Kontusaari S, Pirila E, Munaut C, Salo T, Tryggvason K. Overexpression of TIMP-1 under the MMP-9 promoter interferes with wound healing in transgenic mice. Cell Tissue Res. 2004;315:27–37. doi: 10.1007/s00441-003-0814-1. [DOI] [PubMed] [Google Scholar]
- 10.Beare AH, O’Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–163. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- 11.Mirastschijski U, Zhou Z, Rollman O, Tryggvason K, Agren MS. Wound healing in membrane-type-1 matrix metalloproteinase-deficient mice. J Invest Dermatol. 2004;123:600–602. doi: 10.1111/j.0022-202X.2004.23230.x. [DOI] [PubMed] [Google Scholar]
- 12.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 13.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartenstein B, Dittrich BT, Stickens D, Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z, Angel P. Epidermal development and wound healing in matrix metallo-proteinase 13-deficient mice. J Invest Dermatol. 2006;126:486–496. doi: 10.1038/sj.jid.5700084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balbín M, Fueyo A, Knäuper V, Pendás AM, López JM, Jiménez MG, Murphy G, López-Otín C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998;273:23959–23968. doi: 10.1074/jbc.273.37.23959. [DOI] [PubMed] [Google Scholar]
- 16.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 17.Kiili M, Cox SW, Chen HY, Wahlgren J, Maisi P, Eley BM, Salo T, Sorsa T. Collagenase-2 (MMP-8) and collagenase-3 (MMP-13) in adult periodontitis: molecular forms and levels in gingival crevicular fluid and immunolocalisation in gingival tissue. J Clin Periodontol. 2002;29:224–232. doi: 10.1034/j.1600-051x.2002.290308.x. [DOI] [PubMed] [Google Scholar]
- 18.Pirila E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P. Gelatinase A (MMP-2), collagenase-2 (MMP-8), and laminin-5 gamma2-chain expression in murine inflammatory bowel disease (ulcerative colitis) Dig Dis Sci. 2003;48:93–98. doi: 10.1023/a:1021790532723. [DOI] [PubMed] [Google Scholar]
- 19.Pirila E, Ramamurthy N, Maisi P, McClain S, Kucine A, Wahlgren J, Golub L, Salo T, Sorsa T. Wound healing in ovariectomized rats: effects of chemically modified tetracycline (CMT-8) and estrogen on matrix metalloproteinases -8, -13 and type I collagen expression. Curr Med Chem. 2001;8:281–294. doi: 10.2174/0929867013373552. [DOI] [PubMed] [Google Scholar]
- 20.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–195. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 21.Balbín M, Fueyo A, Tester AM, Pendás AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, López-Otín C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 22.Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angio-genesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters T, Sindrilaru A, Hinz B, Hinrichs R, Menke A, Al-Azzeh EA, Holzwarth K, Oreshkova T, Wang H, Kess D, Walzog B, Sulyok S, Sunderkotter C, Friedrich W, Wlaschek M, Krieg T, Scharffetter-Kochanek K. Wound-healing defect of CD18(-/-) mice due to a decrease in TGF-beta1 and myofibroblast differentiation. EMBO J. 2005;24:3400–3410. doi: 10.1038/sj.emboj.7600809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westerhof GR, Ploemacher RE, Boudewijn A, Blokland I, Dillingh JH, McGown AT, Hadfield JA, Dawson MJ, Down JD. Comparison of different busulfan analogues for depletion of hematopoietic stem cells and promotion of donor-type chimerism in murine bone marrow transplant recipients. Cancer Res. 2000;60:5470–5478. [PubMed] [Google Scholar]
- 25.Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (MMP13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1alpha(–/–) and MCP-1(–/–) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beare AH, Krane SM, Ferguson MW. Variable impairment of wound healing in the heterozygous collagenase-resistant mouse. Wound Repair Regen. 2005;13:27–40. doi: 10.1111/j.1067-1927.2005.130105.x. [DOI] [PubMed] [Google Scholar]
- 28.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 29.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 30.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 31.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 32.Gueders MM, Balbín M, Rocks N, Foidart JM, Gosset P, Louis R, Shapiro S, López-Otín C, Noël A, Cataldo DD. Matrix metalloproteinase-8 deficiency promotes granulocytic allergen-induced airway inflammation. J Immunol. 2005;175:2589–2597. doi: 10.4049/jimmunol.175.4.2589. [DOI] [PubMed] [Google Scholar]
- 33.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 34.Hosokawa R, Urata MM, Ito Y, Bringas P, Jr, Chai Y. Functional significance of Smad2 in regulating basal keratinocyte migration during wound healing. J Invest Dermatol. 2005;125:1302–1309. doi: 10.1111/j.0022-202X.2005.23963.x. [DOI] [PubMed] [Google Scholar]
- 35.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 36.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–134. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahokas K, Skoog T, Suomela S, Jeskanen L, Impola U, Isaka K, Saarialho-Kere U. Matrilysin-2 (matrix metalloproteinase-26) is upregulated in keratinocytes during wound repair and early skin carcinogenesis. J Invest Dermatol. 2005;124:849–856. doi: 10.1111/j.0022-202X.2005.23640.x. [DOI] [PubMed] [Google Scholar]
- 38.Okada A, Tomasetto C, Lutz Y, Bellocq JP, Rio MC, Basset P. Expression of matrix metalloproteinases during rat skin wound healing: evidence that membrane type-1 matrix metalloproteinase is a stromal activator of progelatinase A. J Cell Biol. 1997;137:67–77. doi: 10.1083/jcb.137.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hieta N, Impola U, Lopez-Otin C, Saarialho-Kere U, Kahari VM. Matrix metalloproteinase-19 expression in dermal wounds and by fibroblasts in culture. J Invest Dermatol. 2003;121:997–1004. doi: 10.1046/j.1523-1747.2003.12533.x. [DOI] [PubMed] [Google Scholar]
- 40.Ravanti L, Toriseva M, Penttinen R, Crombleholme T, Foschi M, Han J, Kahari VM. Expression of human collagenase-3 (MMP-13) by fetal skin fibroblasts is induced by transforming growth factor beta via p38 mitogen-activated protein kinase. FASEB J. 2001;15:1098–1100. [PubMed] [Google Scholar]
- 41.Mohan R, Chintala SK, Jung JC, Villar WV, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J Biol Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- 42.Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, Ronan J, Werb Z, Banda MJ. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg. 1999;230:260–265. doi: 10.1097/00000658-199908000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Lint P, Wielockx B, Puimege L, Noël A, López-Otín C, Libert C. Resistance of collagenase-2 (matrix metalloproteinase-8)-deficient mice to TNF-induced lethal hepatitis. J Immunol. 2005;175:7642–7649. doi: 10.4049/jimmunol.175.11.7642. [DOI] [PubMed] [Google Scholar]
- 44.Van Lint P, Libert C. Matrix metalloproteinase-8: cleavage can be decisive. Cytokine Growth Factor Rev. 2006;17:217–223. doi: 10.1016/j.cytogfr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metallo-proteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- 46.Marini S, Fasciglione GF, de Sanctis G, D’Alessio S, Politi V, Coletta M. Cleavage of bovine collagen I by neutrophil collagenase MMP-8. Effect of pH on the catalytic properties as compared to synthetic substrates. J Biol Chem. 2000;275:18657–18663. doi: 10.1074/jbc.M000283200. [DOI] [PubMed] [Google Scholar]
- 47.Balbín M, Fueyo A, Knäuper V, López JM, Alvarez J, Sánchez LM, Quesada V, Bordallo J, Murphy G, López-Otín C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- 48.Freije JM, Díez-Itza I, Balbín M, Sánchez LM, Blasco R, Tolivia J, López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- 49.Owen CA, Hu Z, López-Otín C, Shapiro SD. Membrane-bound matrix metalloproteinase-8 on activated poly-morphonuclear cells is a potent, tissue inhibitor of metalloproteinase-resistant collagenase and serpinase. J Immunol. 2004;172:7791–7803. doi: 10.4049/jimmunol.172.12.7791. [DOI] [PubMed] [Google Scholar]
- 50.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]