Abstract
Retinal neurogenesis ceases by the early postnatal period, although retinal progenitor cells (RPCs) persist throughout life. In this study, we show that in the mammalian eye, the function of Toll-like receptor 4 (TLR4) extends beyond regulation of the innate immune response; it restricts RPC proliferation. In TLR4-deficient mice, enhanced proliferation of cells reminiscent of RPCs is evident during the early postnatal period. In vitro experiments demonstrate that TLR4 acts as an intrinsic regulator of RPC fate decision. Increased TLR4 expression in the eye correlates with the postnatal cessation of cell proliferation. However, deficient TLR4 expression is not sufficient to extend the proliferative period but rather contributes to resumption of proliferation in combination with growth factors. Proliferation in vivo is inhibited by both MyD88-dependent and -independent pathways, similar to the mechanisms activated by TLR4 in immune cells. Thus, our study attributes a novel role to TLR4 as a negative regulator of RPC proliferation.
Introduction
During retinal development, multipotent progenitor cells give rise to the neurons and Müller glia of the mature retina. In mammals, retinal neurogenesis ends by the early postnatal period (Reh and Fischer, 2006). Although a small number of quiescent retinal stem/progenitor cells persist at the margin of the mature retina near the junction of the ciliary epithelium (CE; Ahmad et al., 2000; Tropepe et al., 2000), progenitor cell proliferation and neuronal differentiation are no longer evident. As retinal stem cell therapy has promising therapeutic potential for eye pathologies (Young, 2005; MacLaren et al., 2006), it is necessary to identify the factors that regulate retinal progenitor cell (RPC) proliferative capacity.
The expression of Toll-like receptor 4 (TLR4) has been recently documented in the ciliary body of the mammalian eye (Brito et al., 2004). TLR4 is primarily identified as an innate immune receptor (Takeda and Akira, 2005); therefore, its function in the eye has been commonly attributed to the immune response (Kumar et al., 2004; Chang et al., 2006). However, because TLRs recognize patterns rather than specific molecules, along with their ability to recognize physiological compounds (Ohashi et al., 2000; Okamura et al., 2001; Johnson et al., 2003), they are endowed with the innate ability to mediate a rapid response to a wide range of signals in the microenvironment and not merely to pathogens.
Nonimmune functions of the TLR family have been reported in Drosophila melanogaster in establishing the dorsal–ventral axis polarity, in synaptogenesis, and in axon pathfinding during embryogenesis (Anderson et al., 1985; Halfon et al., 1995; Rose et al., 1997). Such nonimmune functions of this receptor family have only recently emerged in mammals. We have recently shown that in the adult mammalian central nervous system (CNS), TLRs, including TLR4, regulate adult hippocampal neurogenesis (Rolls et al., 2007). In mammalian brain development, other members of the TLR family, TLR3 and TLR8, were identified as negative regulators of axonal/neurite outgrowth (Ma et al., 2006; Cameron et al., 2007). Conversely, TLR4 was found to be absent in neurons during the developmental stages of CNS formation (Lehnardt et al., 2003); however, with age, its expression levels increase (Wadachi and Hargreaves, 2006).
Collectively, the functions that have been recently attributed to TLRs in the mammalian CNS, the changes in TLR expression pattern with development, and the evidence of TLR4 expression in the retinal ciliary body, a location known to harbor RPCs, raised the possibility that TLR4 may play a role in the mammalian retina in RPC fate determination.
In this study, we identified TLR4 as a negative regulator of RPC proliferation. During the early postnatal period, TLR4-deficient (TLR4D) mice exhibited enhanced proliferation of cells expressing molecular markers commonly attributed to RPCs. In vitro experiments demonstrated that TLR4 directly modulates RPC fate decision. The increase in TLR4 levels, which coincided with the cessation of proliferation in the ciliary body, was found to be one of the factors that contributed to the decrease in proliferation. Thus, we suggest that although TLR4 is not the primary factor that regulates RPC proliferative capacity throughout life, it does determine the sensitivity of these cells to the microenvironment.
Results and discussion
TLR4 deficiency results in increased proliferation and neuronal differentiation in the postnatal mammalian retina
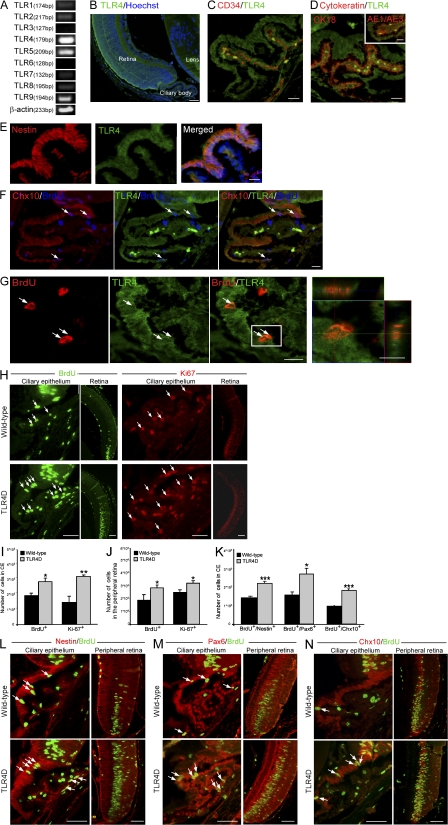
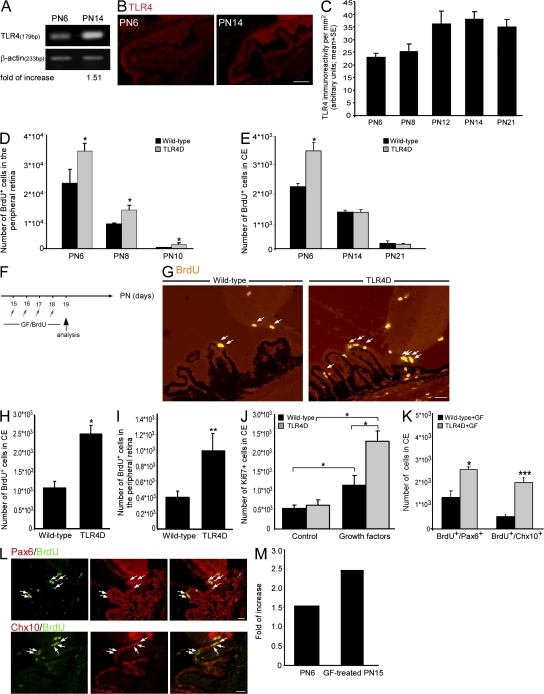
To assess the effect of TLR4 on RPC proliferation, we analyzed retinas from postnatal day 6 (PN6) mice, which is the latest time point at which intense proliferation in the mammalian retina has been described (Blanks and Bok, 1977; Young, 1985). We found by PCR that TLR4 is the dominant TLR family member expressed (Fig. 1 A). In agreement with Brito et al., (2004), immunohistochemical analysis revealed that TLR4 is expressed in the retina and the CE (Fig. 1 B), a location that has been shown to harbor a retinal progenitor population (Ahmad et al., 2000; Tropepe et al., 2000). We next identified the specific population of cells that expresses TLR4 in the ciliary body. TLR4-positive cells hardly expressed markers characteristic of endothelial (CD34; Fig. 1 C) or epithelial (cytokeratin 18 and AE1/AE3; Fig. 1 D) cells but expressed molecular markers characteristic of RPCs such as nestin, a neural progenitor marker (Fig. 1 E), and Chx10, a retinal progenitor marker (Fig. 1 F). To further strengthen our hypothesis that the TLR4-positive cells are proliferating RPCs, we injected the 6-d-old mice with the cell proliferation marker BrdU. BrdU-positive cells that coexpressed TLR4 were detected (Fig. 1 G).
Figure 1.
Deficiency in TLR4 results in increased proliferation of cells reminiscent of RPCs in the early postnatal retina. (A) Semiquantitative PCR of PN6 whole eyes for TLR1–9. (B) Staining for TLR4 of a PN6 eye. (C and D) Pictures of the CE labeled with TLR4 and the endothelial marker CD34 (C) or the epithelial markers cytokeratin 18 (CK18) and AE1/AE3 (D, inset). (E and F) Staining for nestin and TLR4 coexpression (E) or BrdU, Chx10, and TLR4 (F) in the CE. (G) Labeling of BrdU and TLR4 in the CE; right panel is a z axis projection of the boxed area. (H) Pictures of BrdU or Ki67 in the CE and the peripheral retina of WT and TLR4D PN6 mice. (I and J) Quantification of proliferating cells in the CE (I) and in the peripheral retina (J). (K) Quantification of BrdU+/nestin+, BrdU+/Pax6+, and BrdU+/Chx10+ cells. (L–N) Representative pictures of nestin/BrdU (L), Pax6/BrdU (M), and Chx10/BrdU (N) staining. Arrows indicate double-labeled cells. Asterisks denote significant differences between the indicated groups: *, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t test, n = 4. Error bars represent SEM. Bars: (B) 100 μm; (C–G) 20 μm; (individual cell in G) 10 μm; (H and L–N) 50 μm.
We next examined whether cell proliferation in the mammalian eye is affected by the absence of TLR4. To that end, we compared proliferation between wild-type (WT) and TLR4D PN6 mice assessed by BrdU incorporation and staining for the proliferation marker Ki67, a nuclear protein expressed in all phases of the cell cycle except the resting phase. The number of proliferating cells was higher in the TLR4D mice relative to the matched WT controls both in the CE and the peripheral retina (Fig. 1, H–J). Next, double immunohistochemical analysis confirmed that the observed increase in proliferation occurred in cells expressing the neural progenitor marker nestin and the retinal progenitor markers Pax6 and Chx10 (Fig. 1, K–N).
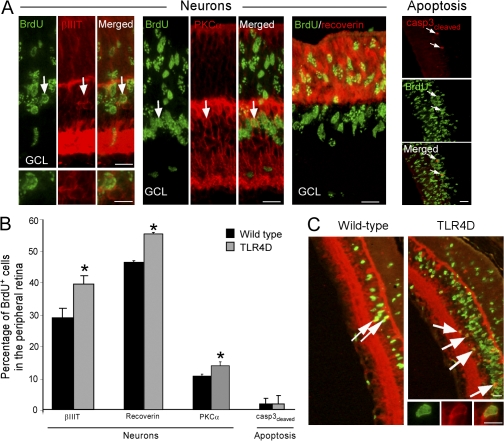
Quantitative analysis 7 d after injection revealed an increased number of BrdU+ cells expressing the neuronal marker βIII-tubulin (βIIIT) in the TLR4D mice (16,590 ± 1,872 vs. 8,480 ± 2,687, TLR4D vs. WT, respectively; Student's t test, *, P = 0.05). This increase was not solely a result of enhanced proliferation but also an outcome of increased differentiation into neuronal cells (Fig. 2, A–C). Because rod photoreceptors and bipolar cells are the major retinal neuronal cells formed during late histogenesis, we also quantified the percentage (out of total BrdU+ cells) of the bipolar-specific marker PKC-α and the photoreceptor-specific marker recoverin. Increased differentiation into the tested retinal neuronal cell types was evident in TLR4D mice (Fig. 2, A and B). Notably, these differences did not result from differential survival, as no effect on apoptosis (measured by cleaved caspase 3) was observed (Fig. 2 B). Collectively, these results suggest that in the PN6 retina, deficiency in TLR4 leads to both enhanced proliferation and neuronal differentiation of cells expressing markers characteristic of retinal progenitors.
Figure 2.
Deficiency in TLR4 in the early postnatal retina results in increased neuronal differentiation. (A) Staining for BrdU and the neuronal cell-specific markers βIIIT, PKC-α, and recoverin or the apoptotic marker cleaved caspase 3. (B) Percentage of BrdU+ cells coexpressing each of the tested markers out of the total BrdU+ cells (Student's t test, n = 4 per group; *, P < 0.05). Error bars represent SEM. (C) Staining for BrdU and the neuronal marker DCX in retinas of WT and TLR4D mice. Arrows indicate double-labeled cells. Bars: (A and C) 20 μm; (individual cells) 10 μm.
TLR4 activation has a direct effect on RPC fate decision
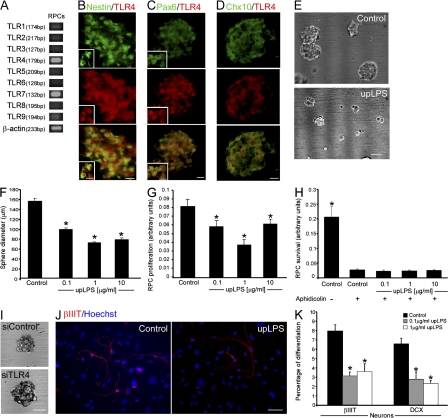
We next sought to determine whether the observed effects described in vivo could be attributed to a direct influence of TLR4 on RPCs. To address this issue, we first tested whether TLRs are expressed by RPCs in vitro. Undifferentiated RPCs express mRNA that encodes for members of the TLR family (TLR1–9), with pronounced expression of TLR4 (Fig. 3 A). Using TLR4-specific antibodies, we verified that RPCs indeed express this receptor (Fig. 3, B–D). To examine whether the TLR4 expressed on the RPCs affects their proliferation, we used an activator of TLR4, ultrapurified lipopolysaccharide (upLPS; Akira and Takeda, 2004). Reduction of sphere diameter and proliferation were observed in the presence of upLPS (Fig. 3, E–G). Treatment with upLPS did not affect RPC viability (Fig. 3 H). Furthermore, suppression of TLR4 expression using TLR4-targeted siRNA increased the sphere diameter by 19.5 ± 3.9% (n = 46–56; Student's t test, *, P = 0.0004; Fig. 3 I). Next, we examined the ability of TLR4 expressed on RPCs to direct their differentiation into neurons. Activation of TLR4 on RPCs using upLPS inhibited neuronal differentiation assessed by βIIIT and doublecortin (DCX; Fig. 3, J and K) and had no effect on differentiation to the other lineages. Collectively, these results suggest that TLR4 is an intrinsic regulator, restricting both the proliferation and neuronal differentiation of RPCs.
Figure 3.
Activation of TLR4 on RPCs directly restricts their proliferation and neuronal differentiation in vitro. (A) Semiquantitative PCR analysis of RPCs for TLR expression. (B–D) RPC spheres stained for TLR4 and the neural progenitor marker nestin (B) or the retinal progenitor markers Pax6 (C) and Chx10 (D). Insets depict single cells at higher magnification. (E) Pictures of RPC spheres with or without upLPS treatment. (F) Quantification of sphere diameter in the presence of upLPS (factorial ANOVA: F = 177.5; *, P = 0.0001). (G and H) Effect of upLPS on RPC proliferation (G; factorial ANOVA: F = 7.434; *, P = 0.0008) and survival (H; factorial ANOVA: F = 46.27; *, P = 0.0001) determined by XTT assay. (I) Pictures of RPCs treated with siRNA for TLR4. (J) Differentiated RPCs with or without upLPS treatment stained for βIIIT. (K) Percentages of RPC differentiation to βIIIT+ or DCX+ cells (factorial ANOVA: βIIIT, F = 16.92; *, P = 0.0001; and DCX, F = 14.73; *, P = 0.0003). Factorial ANOVA followed by Fisher's exact test; *, P = 0.05. Error bars represent SEM. Bars: (B–D) 20 μm; (individual cells) 10 μm; (E) 100 μm; (I and J) 50 μm.
Deficiency in the common adaptors of TLR signaling, MyD88 or TICAM1, results in increased proliferation in the postnatal mammalian retina
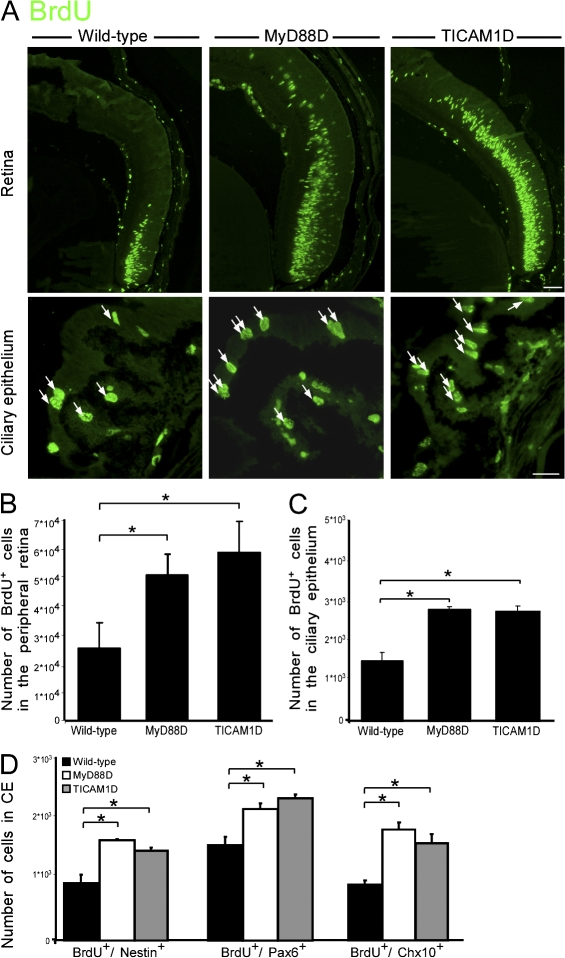
In the classical immune response, TLR signaling leads to the activation of the transcription factor NFκB, which acts as a master switch, regulating the transcription of many genes. TLR signaling, which relies on cytoplasmic adaptor molecules that can associate with the intracellular region of the TLR molecule, exerts its effect via two main signaling pathways, MyD88 (myeloid differentiation primary response protein 88)-dependent and -independent cascades. The former involves the activation of the intracellular adaptor MyD88, and the latter entails the participation of another adaptor, TICAM1 (TIR domain–containing adaptor protein inducing INF-β; Akira and Takeda, 2004; Krishnan et al., 2007).
Previous studies indicated that in developing neurons, TLR activation does not activate NFκB (Ma et al., 2006; Cameron et al., 2007), demonstrating that a different signaling pathway is induced in these cells. In contrast, TLR4 activation in adult neural progenitor cells results in the activation of both MyD88-dependent and -independent pathways (Rolls et al., 2007), similar to the pathways activated in the immune system. As the proliferation of RPCs can be considered a recapitulation of both neurogenesis and developmental processes, we wished to identify the signaling pathways used in this process. Therefore, we tested whether MyD88 and TICAM1 participate in the regulation of cell proliferation in the eye. We found increased numbers of BrdU+ cells in the peripheral retina and the CE of both MyD88- and TICAM1-deficient mice relative to WT PN6 mice (Fig. 4, A–C). We further confirmed that the proliferating cells expressed markers characteristic of RPCs, including nestin, Pax6, and Chx10 (Fig. 4 D). Our results therefore suggest that cell proliferation in the early postnatal eye is inhibited by both MyD88-dependent and -independent pathways, similar to the mechanism activated by TLRs in immune cells.
Figure 4.
Deficiency in the intracellular adaptors MyD88 or TICAM1 increases proliferation in the retinas of PN6 mice. (A) Representative pictures of MyD88-deficient (MyD88D), TICAM1-deficient (TICAM1D), and WT PN6 retinas stained for BrdU. Arrows indicate BrdU+ cells. Bars: (top) 50 μm; (bottom) 20 μm. (B and C) Quantification of BrdU+ cells in the peripheral retina (B; factorial ANOVA: F = 7.529; *, P = 0.0064) or in the CE (C; factorial ANOVA: F = 24.2; *, P = 0.0002). (D) Quantification of BrdU+/nestin+ (factorial ANOVA: F = 11.2; *, P = 0.003), BrdU+/Pax6+ (factorial ANOVA: F = 6.32; *, P = 0.019), and BrdU+/Chx10+ (factorial ANOVA: F = 43.8; *, P = 0.0001) cells. Factorial ANOVA followed by Fisher's exact test; *, P = 0.05; n = 4–8. Error bars represent SEM.
Deficiency in TLR4 promotes proliferation obtained by growth factor (GF) administration
Although adult retinal stem/progenitor cells exhibit self-renewal capacity in culture (Tropepe et al., 2000), their proliferation in the mature eye of rodents is not evident after the second postnatal week (Blanks and Bok, 1977; Young, 1985). The inhibitory role of TLR4 on cell proliferation at PN6 identified in this study along with the known progressive time-dependent decrease of proliferating progenitors prompted us to test whether TLR4 determines the time period at which proliferation occurs. Therefore, we explored whether TLR4 levels become elevated as animals enter adulthood and whether such an increase in TLR4 expression might explain the limited proliferation in the adult eye. We found elevated TLR4 levels in the CE from day 12 onwards relative to day 6 (Fig. 5, A–C).
Figure 5.
TLR4 regulates the responsiveness of cells reminiscent of RPCs after administration of GFs. (A) Semiquantitative PCR of PN6 and PN14 whole eyes for TLR4. (B and C) Pictures (B) and quantification (C; factorial ANOVA: F = 4.069; *, P = 0.0434) of TLR4 immunoreactivity at various time points. (D and E) Quantification of BrdU+ cells in the peripheral retina (D) and in CE (E; factorial ANOVA: F = 323; *, P = 0.0001 in D and F = 128; *, P = 0.0001 in E). (F) Experimental design. TLR4D and WT PN15 mice received intravitreal injections of FGF-2 and insulin. (G) Staining for BrdU after GF treatment. Arrows indicate BrdU+ cells. (H and I) Quantification of BrdU+ cells in the CE (H) and in the peripheral retina (I; Student's t test; *, P < 0.05; **, P < 0.01) after GF treatment. (J) Quantification of Ki67+ cells in the CE of untreated and GF-treated mice (factorial ANOVA: F = 7.529; *, P = 0.0064). (K) Quantification of BrdU+/Pax6+ and BrdU+/Chx10+ cells after GF treatment (Student's t test; *, P < 0.05; ***, P < 0.001). (L) Pictures of TLR4D retinas after GF treatment labeled for BrdU, Pax6, and Chx10. Arrows indicate double-positive cells. (M) Fold increase in BrdU+ cells in TLR4D retinas relative to control. Factorial ANOVA followed by Fisher's exact test; *, P = 0.05; n = 4–6. Asterisks denote significant differences. Error bars represent SEM. Bars, 25 μm.
The observed correlation between the elevated levels of TLR4 expression and the cessation of cell proliferation prompted us to test whether deficiency in the TLR4 receptor might enable extension of the time period in which proliferation is observed. As long as proliferation was detectable in the peripheral retina, deficiency in TLR4 resulted in increased proliferation relative to WT (Fig. 5, D and E). Because the absence of TLR4 had no effect on proliferation at later time points (Fig. 5 E), we suggest that TLR4 signaling represents a secondary mechanism restricting proliferation in the adult eye.
The absence of proliferating RPCs in the adult eye can be either attributed to the lack of proliferation-promoting factors or to the presence of inhibitory molecules. The former was recently demonstrated, as GF administration results in in vivo proliferation of cells expressing markers characteristic of RPCs (Fischer and Reh, 2003; Zhao et al., 2005). Therefore, we tested whether TLR4 participates in restricting proliferation when GFs are provided (Fischer and Reh, 2003; Zhao et al., 2005). We intravitreally injected FGF-2 and insulin as previously described (Zhao et al., 2005) to WT and TLR4D mice at PN15 (Fig. 5 F). Incorporation of BrdU, which was injected together with the GF, revealed, as expected, an extension of the proliferation period. As in the early postnatal stage, providing the eye with GF resulted in significantly higher proliferation in the TLR4D mice compared with the WT animals (Fig. 5, G–I), indicating that TLR4 had a negative impact on proliferation when conditions permissive for RPC proliferation were restored. To confirm these results and to demonstrate the effect of GFs on cell proliferation, we analyzed retinas for the expression of Ki67. A higher number of proliferating cells was seen in the GF-treated retinas relative to controls in both strains (Fig. 5 J); however, the effect was far more pronounced in the TLR4D mice (fourfold increase) than in their matched controls (2.5-fold increase; Fig. 5 J). We further confirmed that the observed increased proliferation involved cells expressing markers distinguishing RPCs, such as Pax6 and Chx10 (Fig. 5, K and L). These results suggest that under permissive conditions, TLR4 down-regulates proliferation. Importantly, deficiency in TLR4 had a more pronounced effect on proliferation in the PN15 eyes supplied with GF relative to PN6 eyes (Fig. 5 M). This pronounced effect is supported by the enhanced expression of TLR4 at PN15 relative to PN6 (Fig. 5, A–C). Thus, our findings suggest that even though it cannot be considered the primary limiting factor of RPC proliferation in adulthood, enhanced TLR4 expression in the eye contributes to the restriction of RPC proliferation.
In this study, we identified TLR4 as a novel player in the regulation of RPC proliferation in the mammalian eye. We found that TLR4 inhibits proliferation of retinal cells expressing progenitor markers in the early postnatal period and contributes to their lack of proliferation in the subsequent time period. In vitro experiments confirmed that TLR4 is expressed on the RPCs and directly affects their cell fate decision. Notably, TLR4 does not belong to any of the previously known classes of cell–cell signaling pathways used to determine the fate of RPCs (Yang, 2004). Interestingly, the major classes of known cell–cell signaling pathways share a common property with the TLR orthologue, as they all participate in dorsal–ventral patterning in Drosophila (Biemar et al., 2006). Moreover, because similar effects of TLR4 on neural progenitor cell proliferation are also evident in the adult hippocampus (Rolls et al., 2007), our results further emphasize the importance of this receptor family in neurogenic processes in general.
TLR4 is a member of a larger receptor family. Thus, our results suggest that additional members of this family may participate in retinogenesis and RPC fate regulation. Revealing the specific function of these family members necessitates further investigation. The unique features of the TLR family, including pattern recognition rather than identification of a single ligand and their ability to recognize stress-related compounds (Johnson et al., 2003) or pathogens, provide RPCs with the capacity to respond to various deviations from homeostasis, such as injury. Our finding that TLR4 restricts RPC proliferation might provide, at least in part, an explanation for the limited neurogenesis in response to injury (Nickerson et al., 2007), a condition that releases a variety of ligands that can ultimately be recognized by the TLR family (Vabulas et al., 2002). Revealing the mechanisms that constrain RPC proliferation has promising therapeutic potential for inducing neurogenesis in the adult eye under pathological conditions.
Materials and methods
Animals
TLR4D mice (C57BL/10ScNJ; The Jackson Laboratory) and their WT C57BL/10 counterparts (a gift of I. Cohen, Weizmann Institute of Science, Rehovot, Israel), MyD88-deficient mice (a gift of S. Akira and S. Uematsu, Research Institute for Microbial Diseases, Osaka University, Osaka, Japan), TICAM1-deficient mice (C57BL/6J-AW046014Lps2/J; The Jackson Laboratory), and WT C57BL/6 mice (supplied by the Animal Breeding Center of the Weizmann Institute of Science) were maintained at the Weizmann Institute Animal Facility. All animals were handled according to the regulations formulated by the Institutional Animal Care and Use Committee.
Administration of BrdU
Animals were given intraperitoneal injections of 0.125 mg/gram of body weight of BrdU (Sigma-Aldrich). For proliferation assays, mice were killed 6 h after injection, and their eyes were removed and prepared for histology as described in the Immunohistochemistry section. For differentiation assays, injected mice were maintained for 7 d and then killed.
GF administration
TLR4D and C57BL/10 PN15 mice were anesthetized, and GFs (20 ng/μl FGF-2 and 1 μg/μl insulin) together with 1 μg/μl BrdU were delivered into left eyes by intravitreal injection using a glass micropipette attached to a 10-μl Hamilton syringe (total volume of 1 μl/eye). Mice were injected on four consecutive days and killed 24 h after the last injection. Eyes were removed and prepared for histology as described in the next section.
Immunohistochemistry
Mice eyes were placed in 2.5% paraformaldehyde for 48 h and moved to 70% ethyl alcohol. The tissues were prepared for histology and stained as previously described (Shechter et al., 2007). The antibodies used are listed in Table I.
Table I. First antibodies used in this paper.
| Source | Dilution | Species | Antigen |
|---|---|---|---|
| Abcam | 1:50 | Rabbit | TLR4 |
| Cedarlane | 1:100 | Rat | CD34 |
| Abcam | 1:100 | Mouse | CK18 |
| Invitrogen | 1:75 | Mouse | CK AE1/AE3 |
| Cell Signaling Technology |
1:50 | Rabbit | Cleaved caspase 3 |
| Abcam | 1:200 | Sheep | Chx10 |
| AbD Serotec | 1:100 | Rat | BrdU |
| Millipore | 1:100 | Mouse | Nestin |
| Santa Cruz Biotechnology, Inc. |
1:100 | Goat | DCX |
| Millipore | 1:500 | Rabbit | Pax6 |
| Covance | 1:500 | Rabbit | βIIIT |
| Dako | 1:50 | Rat | Ki67 |
| Santa Cruz Biotechnology, Inc. |
1:200 | Rabbit | PKC-α |
| Millipore | 1:1,000 | Rabbit | Recoverin |
RNA purification, cDNA synthesis, and reverse-transcription PCR analysis
The primers used are listed in Table II. RNA procedures were performed as described previously (Butovsky et al., 2006); RNA was prepared by homogenation of the eyes using a homogenator in TRI reagent (PRO250; Sigma-Aldrich) on ice or by treating RPCs with TRI reagent on ice.
Table II. Primers used in this paper.
| Primer pairs | Sense and antisense sequences |
|---|---|
| β-Actin | 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′ |
| 5′-TGGAATCCTGTGGCATCCATGAAAC-3′ | |
| TLR1 | 5′-ATGATTCTGCCTGGGTGAAG-3′ |
| 5′-TCTGGATGAAGTGGGGAGAC-3′ | |
| TLR2 | 5′-CTCCCACTTCAGGCTCTTTG-3′ |
| 5′-AGGAACTGGGTGGAGAACCT-3′ | |
| TLR3 | 5′-AGCTTTGCTGGGAACTTTCA-3′ |
| 5′-GAAAGATCGAGCTGGGTGAG-3′ | |
| TLR4 | 5′-CAGCAAAGTCCCTGATGACA-3′ |
| 5′-AGAGGTGGTGTAAGCCATGC-3′ | |
| TLR5 | 5′-ATTCTCATCGTGGTGGTGGT-3′ |
| 5′-GCTATGGTTCGCAACTGGAT-3′ | |
| TLR6 | 5′-ACACAATCGGTTGCAAAACA-3′ |
| 5′-GGAAAGTCAGCTTCGTCAGG-3′ | |
| TLR7 | 5′-CCACAGGCTCACCCATACTT-3′ |
| 5′-CAAGGCATGTCCTAGGTG-3′ | |
| TLR8 | 5′-GGCACAACTCCCTTGTGATT-3′ |
| 5′-CATTTGGGTGCTGTTGTTTG-3′ | |
| TLR9 | 5′-GCTTTGGCCTTTCACTCTTG-3′ |
| 5′-AACTGCGCTCTGTGCCTTAT-3′ |
Preparation of RPCs
RPCs were isolated and grown as previously described (Neeley et al., 2008). In brief, PN0 eyes were removed and placed in HBSS. Neural retinas were carefully dissected away from the optic disc and ciliary marginal zone. Tissue was minced and digested in collagenase (0.1% in PBS, pH 7.2–7.4; Sigma-Aldrich) at room temperature. Liberated cells were collected through a 100-μm mesh strainer, centrifuged, and then resuspended in a neurobasal medium (Invitrogen) supplemented with 20 ng/ml EGF, 2% B-27 supplement (Invitrogen), 1% N-2 neural supplement (Invitrogen), 100 mg/ml penicillin/streptomycin (Biological Industries), 2 mM l-glutamine (Biological Industries), and 20 U/ml nystatin (Invitrogen). Cells were transferred to a 6-well plate and cultured at 37°C in a 5% CO2 incubator. 0.5 ml of fresh medium was added to each well every 2 d. Primary neurospheres formed within the first week in culture. These proliferating cultures were passaged weekly for 12 wk, at which point they were used for the experiments described here. siRNA transfection of RPCs as well as proliferation, differentiation, and survival assays were performed as described previously (Rolls et al., 2007). upLPS (Invitrogen) was added to the desired final concentration. For survival assays, 10 μg/ml aphidicolin (Sigma-Aldrich) was added to inhibit proliferation. Quantification of cell proliferation/viability was determined by XTT (TOX2; Sigma-Aldrich), a spectrophotometric measurement of cell viability based on mitochondrial dehydrogenase activity. Sphere diameter was calculated using Image-Pro software (Media Cybernetics, Inc.).
Image acquisition and quantification
Cyanine dye (Cy2 [A492nm and E510nm], Cy3 [A550nm and E570nm], or Cy5 [A650nm and E670nm])–conjugated antibodies (Jackson ImmunoResearch Laboratories) were used to visualize antibody-bound molecules. For nuclear staining, we used Hoechst 33342 fluorochrome (Invitrogen). The stained sections were mounted with glycerol vinyl alcohol mounting solution (Invitrogen). For microscopic analysis, a fluorescence microscope (E800; Nikon) or laser-scanning confocal microscope (Carl Zeiss, Inc.) was used. The fluorescence microscope was equipped with a digital camera (DXM 1200F; Nikon) and with either a 20× NA 0.50 or 40× NA 0.75 objective lens (Plan Fluor; Nikon). The confocal microscope was equipped with LSM 510 laser scanning (three lasers: Ar 488, HeNe 543, and HeNe 633) and with a 40× oil-immersion NA 1.3 Plan Neofluor objective lens. Recordings were made on postfixed tissues at 24°C using acquisition software (ACT-1 [Nikon]; or LSM [Carl Zeiss, Inc.]). Images were cropped and optimized in Photoshop 9.0 (Adobe Systems, Inc.) by making minor adjustments to contrast and arranged using Canvas X (Deneba Software). The number of cells in the peripheral retina and immunoreactivity in the CE was determined automatically with Image-Pro Plus 4.5 software (Media Cybernetics, Inc.). All measurements were performed by an observer blinded as to the identity of the examined tissues.
Statistical analysis
The results were analyzed by Student's t test or factorial analysis of variance (ANOVA) followed by Fisher's exact test and are expressed as means ± SEM.
Acknowledgments
We thank Prof. S. Akira, Dr. S. Uematsu, and Prof. I. Cohen for the TLR4D and MyD88-deficient mice. We thank S. Schwarzbaum and S.R. Smith for editing.
This study was supported, in part, by a grant from the Siegal Foundation and the Glaucoma Foundation, New York. M. Schwartz holds the Maurice and Ilse Katz Professorial Chair in Neuroimmunology. R. Shechter, A. Ronen, and A. Rolls designed and performed the experiments, analyzed the data, and took part in writing the manuscript. A. London contributed to the experimental work. S. Bakalash isolated the RPCs.
R. Shechter, A. Ronen, and A. Rolls contributed equally to this paper.
Abbreviations used in this paper: ANOVA, analysis of variance; βIIIT, βIII-tubulin; CE, ciliary epithelium; CNS, central nervous system; DCX, doublecortin; GF, growth factor; PN, postnatal day; RPC, retinal progenitor cell; TLR, Toll-like receptor; TLR4D, TLR4 deficient; upLPS, ultrapurified lipopolysaccharide; WT, wild type.
References
- Ahmad, I., L. Tang, and H. Pham. 2000. Identification of neural progenitors in the adult mammalian eye. Biochem. Biophys. Res. Commun. 270:517–521. [DOI] [PubMed] [Google Scholar]
- Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499–511. [DOI] [PubMed] [Google Scholar]
- Anderson, K.V., L. Bokla, and C. Nusslein-Volhard. 1985. Establishment of dorsal-ventral polarity in the Drosophila embryo: the induction of polarity by the Toll gene product. Cell. 42:791–798. [DOI] [PubMed] [Google Scholar]
- Biemar, F., D.A. Nix, J. Piel, B. Peterson, M. Ronshaugen, V. Sementchenko, I. Bell, J.R. Manak, and M.S. Levine. 2006. Comprehensive identification of Drosophila dorsal-ventral patterning genes using a whole-genome tiling array. Proc. Natl. Acad. Sci. USA. 103:12763–12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanks, J.C., and D. Bok. 1977. An autoradiographic analysis of postnatal cell proliferation in the normal and degenerative mouse retina. J. Comp. Neurol. 174:317–327. [DOI] [PubMed] [Google Scholar]
- Brito, B.E., D.O. Zamora, R.A. Bonnah, Y. Pan, S.R. Planck, and J.T. Rosenbaum. 2004. Toll-like receptor 4 and CD14 expression in human ciliary body and TLR-4 in human iris endothelial cells. Exp. Eye Res. 79:203–208. [DOI] [PubMed] [Google Scholar]
- Butovsky, O., Y. Ziv, A. Schwartz, G. Landa, A.E. Talpalar, S. Pluchino, G. Martino, and M. Schwartz. 2006. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol. Cell. Neurosci. 31:149–160. [DOI] [PubMed] [Google Scholar]
- Cameron, J.S., L. Alexopoulou, J.A. Sloane, A.B. DiBernardo, Y. Ma, B. Kosaras, R. Flavell, S.M. Strittmatter, J. Volpe, R. Sidman, and T. Vartanian. 2007. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J. Neurosci. 27:13033–13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J.H., P.J. McCluskey, and D. Wakefield. 2006. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 90:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, A.J., and T.A. Reh. 2003. Growth factors induce neurogenesis in the ciliary body. Dev. Biol. 259:225–240. [DOI] [PubMed] [Google Scholar]
- Halfon, M.S., C. Hashimoto, and H. Keshishian. 1995. The Drosophila toll gene functions zygotically and is necessary for proper motoneuron and muscle development. Dev. Biol. 169:151–167. [DOI] [PubMed] [Google Scholar]
- Johnson, G.B., G.J. Brunn, and J.L. Platt. 2003. Activation of mammalian Toll-like receptors by endogenous agonists. Crit. Rev. Immunol. 23:15–44. [DOI] [PubMed] [Google Scholar]
- Krishnan, J., K. Selvarajoo, M. Tsuchiya, G. Lee, and S. Choi. 2007. Toll-like receptor signal transduction. Exp. Mol. Med. 39:421–438. [DOI] [PubMed] [Google Scholar]
- Kumar, M.V., C.N. Nagineni, M.S. Chin, J.J. Hooks, and B. Detrick. 2004. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 153:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt, S., L. Massillon, P. Follett, F.E. Jensen, R. Ratan, P.A. Rosenberg, J.J. Volpe, and T. Vartanian. 2003. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA. 100:8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., J. Li, I. Chiu, Y. Wang, J.A. Sloane, J. Lu, B. Kosaras, R.L. Sidman, J.J. Volpe, and T. Vartanian. 2006. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 175:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren, R.E., R.A. Pearson, A. MacNeil, R.H. Douglas, T.E. Salt, M. Akimoto, A. Swaroop, J.C. Sowden, and R.R. Ali. 2006. Retinal repair by transplantation of photoreceptor precursors. Nature. 444:203–207. [DOI] [PubMed] [Google Scholar]
- Neeley, W.L., S. Redenti, H. Klassen, S. Tao, T. Desai, M.J. Young, and R. Langer. 2008. A microfabricated scaffold for retinal progenitor cell grafting. Biomaterials. 29:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson, P.E., J.G. Emsley, T. Myers, and D.B. Clarke. 2007. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Invest. Ophthalmol. Vis. Sci. 48:5266–5275. [DOI] [PubMed] [Google Scholar]
- Ohashi, K., V. Burkart, S. Flohe, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J. Immunol. 164:558–561. [DOI] [PubMed] [Google Scholar]
- Okamura, Y., M. Watari, E.S. Jerud, D.W. Young, S.T. Ishizaka, J. Rose, J.C. Chow, and J.F. Strauss III. 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229–10233. [DOI] [PubMed] [Google Scholar]
- Reh, T.A., and A.J. Fischer. 2006. Retinal stem cells. Methods Enzymol. 419:52–73. [DOI] [PubMed] [Google Scholar]
- Rolls, A., R. Shechter, A. London, Y. Ziv, A. Ronen, R. Levy, and M. Schwartz. 2007. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 9:1081–1088. [DOI] [PubMed] [Google Scholar]
- Rose, D., X. Zhu, H. Kose, B. Hoang, J. Cho, and A. Chiba. 1997. Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron growth cone in Drosophila. Development. 124:1561–1571. [DOI] [PubMed] [Google Scholar]
- Shechter, R., Y. Ziv, and M. Schwartz. 2007. New GABAergic interneurons supported by myelin-specific T cells are formed in intact adult spinal cord. Stem Cells. 25:2277–2282. [DOI] [PubMed] [Google Scholar]
- Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1–14. [DOI] [PubMed] [Google Scholar]
- Tropepe, V., B.L. Coles, B.J. Chiasson, D.J. Horsford, A.J. Elia, R.R. McInnes, and D. van der Kooy. 2000. Retinal stem cells in the adult mammalian eye. Science. 287:2032–2036. [DOI] [PubMed] [Google Scholar]
- Vabulas, R.M., H. Wagner, and H. Schild. 2002. Heat shock proteins as ligands of toll-like receptors. Curr. Top. Microbiol. Immunol. 270:169–184. [DOI] [PubMed] [Google Scholar]
- Wadachi, R., and K.M. Hargreaves. 2006. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J. Dent. Res. 85:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X.J. 2004. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin. Cell Dev. Biol. 15:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, M.J. 2005. Stem cells in the mammalian eye: a tool for retinal repair. APMIS. 113:845–857. [DOI] [PubMed] [Google Scholar]
- Young, R.W. 1985. Cell differentiation in the retina of the mouse. Anat. Rec. 212:199–205. [DOI] [PubMed] [Google Scholar]
- Zhao, X., A.V. Das, F. Soto-Leon, and I. Ahmad. 2005. Growth factor-responsive progenitors in the postnatal mammalian retina. Dev. Dyn. 232:349–358. [DOI] [PubMed] [Google Scholar]