Abstract
IFNγ induces cell death in epithelial cells, but the mediator for this death pathway has not been identified. In this study, we find that expression of Bik/Blk/Nbk is increased in human airway epithelial cells (AECs [HAECs]) in response to IFNγ. Expression of Bik but not mutant BikL61G induces and loss of Bik suppresses IFNγ-induced cell death in HAECs. IFNγ treatment and Bik expression increase cathepsin B and D messenger RNA levels and reduce levels of phospho–extracellular regulated kinase 1/2 (ERK1/2) in the nuclei of bik+/+ compared with bik−/− murine AECs. Bik but not BikL61G interacts with and suppresses nuclear translocation of phospho-ERK1/2, and suppression of ERK1/2 activation inhibits IFNγ- and Bik-induced cell death. Furthermore, after prolonged exposure to allergen, hyperplastic epithelial cells persist longer, and nuclear phospho-ERK is more prevalent in airways of IFNγ−/− or bik−/− compared with wild-type mice. These results demonstrate that IFNγ requires Bik to suppress nuclear localization of phospho-ERK1/2 to channel cell death in AECs.
Introduction
Although it is well established that IFNγ causes cell death in a variety of cell types (Deiss et al., 1995; Ossina et al., 1997; Wen et al., 1997; Ruiz-Ruiz et al., 2000; Trautmann et al., 2000; Horiuchi et al., 2006), the signal transduction downstream of STAT1 remains largely unknown (Barber, 2000). Unraveling the role of IFNγ in apoptosis remains a challenge because IFNγ may prime cells to apoptosis and through induction of many genes can concomitantly elicit an antiproliferative and a proliferative state (Xiang et al., 2008). The decision between life and death may depend on possible costimuli or the cell type. Enhanced expression and translocation of Diablo into the cytosol play a critical role in the promotion of IFNγ-induced apoptosis of IFNγ-sensitive B cells (Yoshikawa et al., 2001). Th1 cells that secrete high levels of IFNγ are more susceptible to activation-induced cell death than Th2 cells because Th2 cells express Fas-associated phosphatase, FAP-1 (Zhang et al., 1997). In keratinocytes, IFNγ induces apoptosis via increasing expression of Fas receptor (Trautmann et al., 2000), whereas the Fas ligand–Fas receptor pathway is not involved in the IFNγ-induced death of primary human airway epithelial cells (AECs [HAECs]; Shi et al., 2002; Trautmann et al., 2002). IFNγ induces cell death in AECs (Tesfaigzi, 2006) to remove hyperplastic epithelial cells after inflammation-induced epithelial cell hyperplasia by activating STAT1 (Shi et al., 2002), translocating Bax to the ER, and releasing ER calcium (Tesfaigzi et al., 2002; Stout et al., 2007). Disruption of the IFNγ-induced elimination of hyperplastic epithelial cells can be the source for chronic mucous secretions in asthma (Shi et al., 2002; Pierce et al., 2006) or for neoplastic growth over prolonged periods (Youn et al., 2005).
The Bcl-2 family of proteins consists of members with three to four Bcl-2 homology (BH) regions such as the proapoptotic proteins Bax and Bak (Lindsten et al., 2000) and the antiapoptotic members such as Bcl-2, Bcl-xL, and MCL-1. The interactions of these proteins are an essential gateway required for cell death in response to diverse stimuli (Wei et al., 2001) and under a wide variety of circumstances, suggesting that they act at a central control (CT) point in the pathway to apoptotic cell death (Adams and Cory, 1998; Cryns and Yuan, 1998; Thornberry and Lazebnik, 1998). Another group of Bcl-2 family members contains only the BH3 motif and displays some selectivity for multiple domain Bcl-2 members (Oda et al., 2000; Letai et al., 2002) and provides a link between various cell death initiators and the execution machinery of apoptosis (Coultas et al., 2002; Opferman and Korsmeyer, 2003). BH3-only proteins inactivate the antiapoptotic proteins and allow activation of the multidomain proapoptotic members Bax and Bak (Cheng et al., 2001; Naik et al., 2007; Shimazu et al., 2007; Willis et al., 2007). The proapoptotic activity of BH3-only molecules is kept in check by either p53-dependent transcriptional CT (Villunger et al., 2003), posttranslational modification (Verma et al., 2001; Lei and Davis, 2003), or by binding to the dynein light chain in myosin V filamentous actin and thereby being sequestered from binding to Bcl-2 (Puthalakath et al., 2001; Day et al., 2004).
Our goal for this study was to further characterize the IFNγ-induced cell death in AECs by identifying the BH3-only proteins involved in this pathway. Bik/Blk/Nbk was consistently induced by IFNγ, and its expression induced cell death. Loss of Bik but not p53, Bim, or Bax conferred resistance to IFNγ but not to thapsigargin-induced cell death. Primary mouse AECs (MAECs) from p53- but not bik-deficient mice were protected from DNA damage–induced cell death. We demonstrate that the conserved Leu residue within the BH3 domain of Bik is crucial for its cell death–inducing activity by interacting with and suppressing the nuclear localization of phospho–extracellular regulated kinase 1/2 (ERK1/2) in MAECs and HAECs. Furthermore, loss of Bik was accompanied by increased nuclear phospho-ERK1/2 and sustained epithelial cell hyperplasia in mouse airways, and blocking activated ERK1/2 with U0126 suppressed cell death in response to IFNγ treatment and Bik expression. Therefore, these experiments show that Bik is central in mediating IFNγ-induced cell death by retaining activated ERK1/2 in the cytosol in cultured AECs and during resolution of hyperplastic epithelial cells in mouse airways.
Results
IFNγ induces Bik expression to elicit cell death in AECs
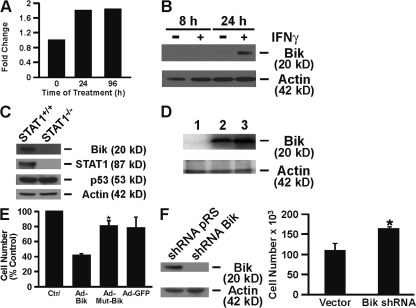
We have previously shown that proliferating but not resting HAECs undergo apoptosis in response to IFNγ (Shi et al., 2002) and that Bax plays a role in this cell death pathway (Tesfaigzi et al., 2002; Stout et al., 2007). To identify which of the BH3-only proteins initiate IFNγ-induced cell death upstream of Bax, primary HAECs and AALEB cells, a cell line derived from HAECs, were treated with IFNγ for 12, 24, 48, 72, and 96 h, and the changed expression of all the BH3-only members of the Bcl-2 family of proteins was screened using quantitative RT-PCR. Significant induction was consistently observed for Bik, but Puma, Hrk, Bid, and Bad remained unchanged. Bik mRNA levels were induced at 24–96 h (Fig. 1 A), and Bik protein levels were increased at 24 h after IFNγ treatment (Fig. 1 B) and remained elevated over 96 h (not depicted).
Figure 1.
IFNγ treatment induces Bik by activating STAT1 to cause cell death in AECs. (A and B) HAECs were treated with IFNγ for the indicated times, and Bik expression was analyzed by quantitative RT-PCR (A) and Western blotting (B). The relative standard curve method was used for analysis of unknown samples, and data are presented as fold change after averaging the ΔCT values for the untreated samples. (C) STAT1+/+ and STAT1−/− MAECs were treated with IFNγ for 24 h on Transwell culture inserts. Protein from harvested cells was immunoblotted with the indicated antibodies. (D) Detection of Bik in HBEC-2 cells infected with nothing (lane 1), Ad-Bik (lane 2), or Ad-BikL61G (lane 3) at 100 MOI by Western analysis. (E) HBEC-2 cells were infected with nothing, Ad-Bik, Ad-BikL61G, or Ad-GFP, and cells were counted 24 h after infection. (F) HBEC-2 cells were infected with a retroviral expression vector for Bik shRNA or an empty vector and 24 h later were treated with 50 ng/ml IFNγ. Cells were harvested 48 h later for Western blot analysis with anti-Bik and antiactin antibodies, and cell counts were determined for IFNγ-treated cells infected with a CT vector or Bik shRNA expression retroviruses. Data presented are means ± SEM for three independent experiments. *, P < 0.05; statistically significant difference from the untreated CT.
To determine whether Bik expression requires STAT1 activation, STAT1+/+ and STAT1−/− MAECs were treated with IFNγ for 24 h, and cell extracts were analyzed for Bik expression. Results showed that Bik expression was significantly reduced in STAT1−/− compared with STAT1+/+ MAECs (Fig. 1 C). Similarly, compared with Flag-expressing HBEC-2 cells, a significant reduction in Bik expression was observed in cells expressing a Flag-tagged dominant-negative construct for STAT1 that was previously shown to suppress IFNγ-induced cell death (unpublished data). However, p53, which has been shown to be important for inducing Bik (Han et al., 1996; Mathai et al., 2002), was not perceptibly affected in these cells (Fig. 1 C).
The BH3 domain of Bik is crucial for inducing death in proliferating epithelial cells
To investigate the role of Bik and the importance of the BH3 domain in inducing cell death, we expressed Bik adenoviral expression vector for Bik (Ad-Bik), mutant Bik (Ad-BikL61G), or adenoviral expression vector for GFP (Ad-GFP) in the immortalized AEC line, HBEC-2, and HAECs using an adenoviral expression system at an MOI of 100 (Fig. 1 D). Ad-Bik expression reduced the number of HBEC-2 cells significantly (Fig. 1 E) compared with Ad-BikL61G– or Ad-GFP–infected cells with an equal amount of MOI. As was previously observed for IFNγ (Shi et al., 2002), the ability of Bik to induce cell death was sensitive to cell growth conditions because confluent cultures of HBEC-2 cells or HAECs were unaffected by Ad-Bik infection, even though Bik expression was evident by Western blot analysis (unpublished data). To further investigate the role of Bik in IFNγ-induced cell death, IFNγ-induced Bik expression was suppressed in HAECs by infecting with a retroviral expression vector for Bik short hairpin RNA (shRNA), whereas CTs were infected with empty vector (Fig. 1 F). Suppression of IFNγ-induced Bik expression resulted in a significantly increased number of cells compared with cells infected with CT retrovirus (Fig. 1 F).
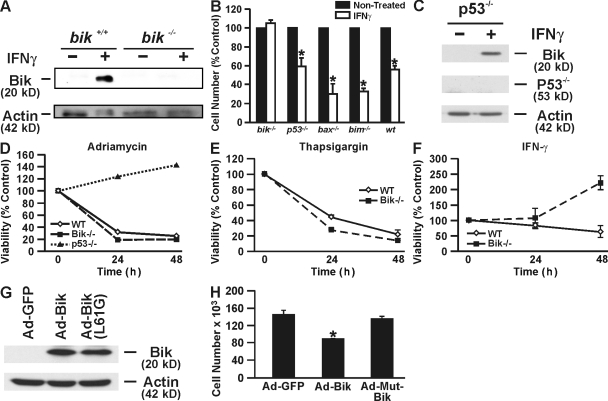
Overall, these studies showed that IFNγ induces cell death in AECs through Bik, although a previous study using bik−/− mice had shown that Bik has no role in hematopoietic cell death (Coultas et al., 2004). To determine whether Bik deficiency confers resistance to IFNγ-induced AEC death, we isolated MAECs from bik+/+ and bik−/− mice and placed them in culture on Transwell membranes. As expected, Bik expression was induced by recombinant murine IFNγ at 24 h in bik+/+ but not in bik−/− MAECs (Fig. 2 A). Consistent with the idea that IFNγ requires Bik to induce cell death, IFNγ significantly reduced the number of MAECs from wild-type but not from bik−/− mice (Fig. 2 B). MAECs from p53−/− mice died similarly to those from wild-type mice, whereas bim−/− or bax−/− MAECs appeared to be even more susceptible to IFNγ-induced cell death. IFNγ induced Bik expression in p53−/− MAECs (Fig. 2 C), suggesting that p53 is not a crucial player in the IFNγ-induced cell death process.
Figure 2.
Bik−/− MAECs are resistant to IFNγ-induced cell death. (A) Western blot analysis shows that MAECs from bik+/+ mice express Bik 24 h after IFNγ treatment, whereas bik−/− MAECs did not. (B) Primary MAECs isolated from bik−/−, p53−/−, bax−/−, bim−/−, and wild-type mice were placed in culture on Transwell membranes treated with 50 ng/ml recombinant murine IFNγ or were left untreated and counted 4 d later. MAECs from p53−/−, bax−/−, bim−/−, and wild-type mice showed significant reduction, whereas bik−/− AECs were unaffected. Data presented are means ± SEM for three independent experiments. (C) MAECs from p53−/− mice were either left untreated or were treated with IFNγ for 24 h, and protein extracts were analyzed for Bik expression by Western blotting. (D) p53−/−, bik−/−, and wild-type MAECs were treated with 0.5 μM adriamycin and counted at 0, 1, and 2 d. (E) Bik+/+ and bik−/− MAECs were treated with 1 μM thapsigargin, and cell viability was determined at 0, 1, and 2 d. (F) bik+/+ and bik−/− MAECs were treated with 50 ng/ml IFNγ, and cell viability was determined at 0, 1, and 2 d. (G and H) Bik−/− MAECs were either infected with Ad-GFP, Ad-Bik, or Ad-BikL61G, and 24 h later protein extracts were analyzed for Bik expression by Western blotting (G) and cells were counted (H). Error bars indicate ± SEM. *, P < 0.05; statistically significant difference from the untreated CT.
We next determined whether Bik deficiency affected the response of MAECs to proapoptotic stimuli other than IFNγ. Loss of Bik did not confer any protection on MAECs undergoing apoptosis after exposure to the DNA-intercalating agent adriamycin, whereas p53−/− MAECs were completely protected (Fig. 2 D). Bik+/+ and bik−/− MAECs were also equally susceptible to thapsigargin (Fig. 2 E). As was previously reported that HAECs are resistant to FasL (Hamann et al., 1998; Shi et al., 2002), treatment with FasL did not affect both bik−/− and bik+/+ MAECs (unpublished data). Therefore, loss of Bik did not sensitize MAECs to FasL-induced cell death. However, consistent with the described experiments, Bik deficiency completely protected MAECs from IFNγ observed over a period of 48 h, whereas ∼50% of bik+/+ MAECs died over this time period (Fig. 2 F). To further confirm that Bik is crucial for IFNγ-induced cell death, we reintroduced either wild-type Bik or mutant Bik into bik−/− MAECs using adenoviral infection and found significant cell death in bik−/− MAECs by expressing wild-type but not mutant Bik (Fig. 2, G and H).
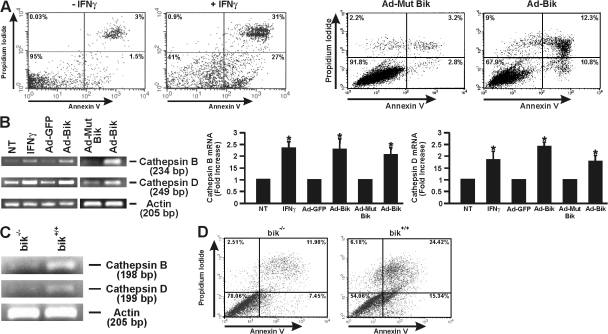
Translocation of phosphatidylserine from the inner leaflet of the cell's membrane to the outer leaflet is an early event in apoptotic cells that allows binding to annexin V (Vermes et al., 1995). Treating HBEC-2 cells with IFNγ for 48 h consistently showed a significant increase of cells that are positive for propidium iodide and annexin V FITC compared with nontreated CTs (Fig. 3 A). Similarly, the percentages of early and late apoptotic cells as determined by annexin V and propidium iodide staining were significantly increased from 5.5 ± 0.8% to 23.6 ± 2%, and the percentage of viable cells was reduced from 91.8 ± 0.8% to 67.9 ± 3.4% at 24 h of infection with Ad-BikL61G and Ad-Bik, respectively (Fig. 3 A). To ensure that cells were infected with equal titers of the adenoviral vectors, Bik expression was analyzed by Western blotting, and infection with Ad-GFP showed minimal cell death (unpublished data).
Figure 3.
INFγ and Bik induce annexin V positivity and expression of cathepsins B and D. (A) HBEC-2 cells were either left untreated, were treated with IFNγ for 48 h, or were infected with Ad-Bik or Ad-BikL61G and stained for annexin V positivity. Representative figures of six independent experiments are shown. (B) HBEC-2 cells were treated with nothing or IFNγ or were infected with Ad-GFP, Ad-BikL61G, or Ad-Bik, and mRNA levels for cathepsins B and D were assessed by RT-PCR. β-Actin mRNA expression was used as a loading CT, and expression levels were compared with CTs. A representative figure and the quantification of data expressed as mean ± SEM representing n = 3 for each group are shown. *, P < 0.05 compared with the respective CTs. (C) bik+/+ and bik−/− MAECs were treated with 50 ng/ml IFNγ, and mRNA levels for cathepsins B and D were assessed by RT-PCR. β-Actin mRNA expression was used as a loading CT. (D) Annexin V positivity of bik−/− and bik+/+ MAECs treated with 50 ng/ml IFNγ. NT, not treated.
If Bik is crucial for IFNγ-induced cell death, we reasoned that the known downstream effectors for IFNγ-induced cell death would be identically induced by Bik. Cathepsins B and D are increased in expression by IFNγ and mediate the resulting cell death (Deiss et al., 1996; Wang et al., 2000). Therefore, HBEC-2 cells were treated with IFNγ or nothing as a CT or were infected with Ad-Bik, Ad-BikL61G, or GFP (Ad-GFP), and expression of cathepsins B and D was analyzed by RT-PCR. Both cathepsins B and D were reproducibly induced by both IFNγ and Ad-Bik but not by Ad-GFP or Ad-BikL61G or in untreated CTs (Fig. 3 B). In addition, treatment with IFNγ increased expression of cathepsins B and D (Fig. 3 C) and annexin V positivity (Fig. 3 D) significantly more in bik+/+ compared with bik−/− MAECs, further confirming that Bik is a central mediator for this cell death pathway.
Bik blocks nuclear translocation of activated ERK1/2 to cause cell death
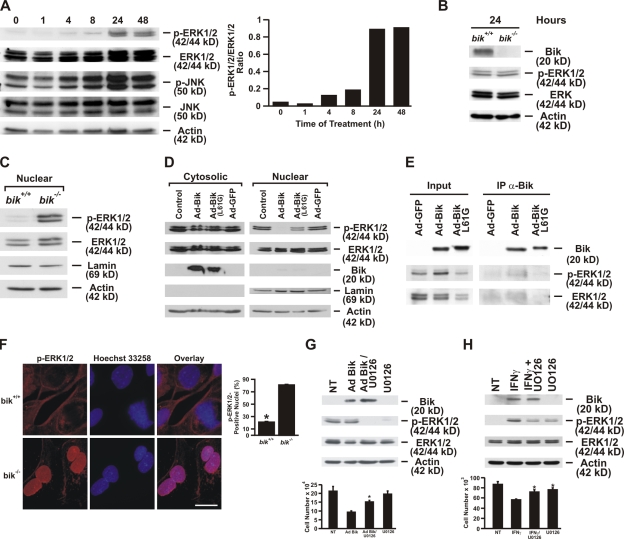
A previous study showed that IFNγ-induced cell death of oligodendroglial cells requires ERK1/2 activation (Horiuchi et al., 2006). To determine the mechanisms of how Bik mediates IFNγ-induced cell death, extracts from HBEC-2 cells treated with IFNγ for 0, 1, 4, 8, 24, and 48 h were subjected to Western blot analysis. ERK1/2 was significantly activated 24 h after IFNγ treatment (Fig. 4 A), but the extent of ERK1/2 activation was similar in bik+/+ and bik−/− MAECs when treated with IFNγ for 24 h and Bik expression was evident (Fig. 4 B), suggesting that Bik was not mediating ERK1/2 activation. Previous studies had demonstrated that nuclear ERK activation causes cells to proliferate, although cytosolic ERK is associated with cell death (Lai et al., 2002; Chen et al., 2005). Therefore, we examined the distribution of phospho-ERK1/2 in IFNγ-treated bik+/+ and bik−/− MAECs and found that activated ERK1/2 was reduced in the nuclear extract of bik+/+ compared with bik−/− MAECs (Fig. 4 C). To further confirm that Bik overexpression inhibits nuclear translocation of ERK1/2, primary HAECs were infected with nothing as a CT, Ad-Bik, Ad-BikL61G, or Ad-GFP, and the cytosolic and nuclear extracts were analyzed by Western blotting 48 h later. We had previously observed that leaving the medium unchanged for 48 h caused sustained activation of ERK1/2 in HAECs (unpublished data). Although phospho-ERK1/2 was detected in the cytosolic extracts from CT and Ad-Bik–infected cells, phospho-ERK1/2 was absent in the nuclear fraction of Ad-Bik–infected HAECs but present in CT cells transfected with Ad-GFP– and Ad-BikL61G–infected cells (Fig. 4 D). To investigate whether Bik binds to activated ERK1/2 to inhibit its nuclear translocation, we performed immunoprecipitation assays with proteins extracted from HBEC-2 that were infected with Ad-GFP, Ad-Bik, or Ad-BikL61G. Results showed that phospho-ERK1/2 is detected in pull-down products from Ad-Bik– but not from Ad-BikL61G– or Ad-GFP–infected HBEC-2 cells (Fig. 4 E), suggesting that Bik directly interacts with phospho-ERK1/2 to sequester ERK1/2 in the cytoplasm.
Figure 4.
Bik binds to activated ERK1/2 and inhibits its nuclear translocation. (A) HBEC-2 cells were treated with 50 ng/ml human recombinant IFNγ, and ERK1/2 activation was assessed at 1, 4, 8, 24, and 48 h of IFNγ treatment. The ratio of phospho-ERK1/2 to total ERK1/2 at different time points after IFNγ treatment was quantified. (B) ERK1/2 activation in MAECs from bik+/+ mice compared with those from bik−/− mice after IFNγ treatment. MAECs were treated with 50 ng/ml murine recombinant IFNγ for 24 h, and protein extracts were analyzed for Bik expression and phospho-ERK1/2. (C) Nuclear extract from IFNγ-treated bik+/+ and bik−/− MAECs was analyzed for activated ERK1/2. (D) Translocation of phospho-ERK1/2 is inhibited by Bik expression in primary HAECs. HAECs were either left untreated or were infected with Ad-Bik, Ad-BikL61G, or Ad-GFP at an MOI of 100, and ERK1/2 was activated by maintaining cells in unchanged media for 48 h. Nuclear and cytosolic extracts were analyzed for phospho-ERK1/2, total ERK1/2, Bik, lamin, and actin. The figure is representative of three independent experiments. (E) Bik interacts with phospho-ERK1/2. Cell lysates prepared from HBEC-2 cells infected with either Ad-GFP, Ad-Bik, or Ad-BikL61G were immunoprecipitated with anti-Bik antibody. The cell lysates (input) and immunoprecipitates were resolved by SDS-PAGE and analyzed by Western blotting using antibodies to Bik, phospho-ERK1/2, and total ERK1/2. (F) Representative photomicrographs and quantification showing that a higher percentage of MAECs from bik−/− compared with bik+/+ mice displays nuclear localization of activated ERK1/2. MAECs were treated with 50 ng/ml IFNγ for 24 h, fixed in paraformaldehyde, and immunostained for phospho-ERK1/2. The percentage of nuclei with phospho-ERK was quantified from three independent experiments. Bar, 10 μm. (G) HBEC-2 cells were infected with 100 MOI Ad-Bik and were cotreated with 1 μM U0126, the ERK-specific inhibitor. Cells infected with Ad-Bik showed a 60% decline in total cell number, and this decline was diminished when the HBEC-2 cells were cotreated with 0.1 or 1 μM U0126. The corresponding Western blot of proteins extracted from cells infected with Ad-Bik and treated with U0126 to suppress ERK1/2 activation. (H) Cell counts and Western blot analysis of protein extracted from HBEC-2 cells that were left untreated or treated with IFNγ either alone or with U0126. Treatment with 1 μM U0126 did not affect cell growth. NT, not treated. Error bars indicate group means ± SEM (n = 4 different treatments per group). *, P < 0.05; significantly different from Ad-Bik–infected cells.
Immunofluorescence staining showed that the percentage of cells with nuclear phospho-ERK1/2 was significantly higher in bik−/− MAECs compared with that observed in bik+/+ MAECs after 24 h of IFNγ treatment (Fig. 4 F). These results demonstrate that expression of Bik suppresses nuclear translocation of activated ERK1/2 and that the conserved Leu residue in the BH3 domain of Bik is crucial for the inhibition of translocation. Activated ERK1/2 in the presence of Bik was proapoptotic because Ad-Bik–induced cell death was suppressed by the ERK1/2 inhibitor U0126 (Fig. 4 G). Western blot analysis showed that in the presence of U0126, the levels of activation of cytosolic ERK1/2 were dramatically reduced in AECs infected with Ad-Bik (Fig. 4 G). Similarly, U0126 significantly attenuated IFNγ-induced reduction of cell numbers and suppressed IFNγ-induced cytosolic ERK1/2 activation (Fig. 4 H). Together, these experiments demonstrate that IFNγ through Bik inhibits nuclear translocation of activated ERK1/2 and that cytosolic ERK1/2 is proapoptotic.
Bik mediates removal of hyperplastic epithelial cells in mouse airways
Repeated exposure to allergen results in proliferation and epithelial cell hyperplasia associated with the mucous phenotype; however, when mice are continuously exposed for periods beyond 10 d, epithelial cell hyperplasia decreases (Tesfaigzi et al., 2002). Our previous experiments showed that IFNγ and STAT1 signaling are central for the resolution of allergen-induced epithelia cell hyperplasia (Stout et al., 2007). Because Bik was central for IFNγ-induced AEC death, we investigated whether this resolution process would be abrogated in IFNγ−/− and bik−/− mice. Interestingly, epithelial cell hyperplasia in IFNγ−/− airways after 5 d of allergen exposure was reduced compared with wild-type and bik−/− mice, suggesting that IFNγ plays a role in the allergen-induced proliferation of airway cells (Fig. 5, A and B). However, as expected, resolution of epithelial cell hyperplasia was abrogated in both IFNγ−/− (Fig. 5 A) and bik−/− (Fig. 5, B and C) mice during 15 d of allergen exposure, although it was significantly reduced in wild-type mice. Furthermore, the number of mucous cells per millimeter of basal lamina was significantly reduced in bik+/+ mice compared with bik−/− mice during 12 and 15 d of allergen exposure (Fig. 5, D and E), confirming that Bik is central for IFNγ-induced killing in mouse airways as was observed in cultured MAECs. The role of Bik in inhibiting nuclear translocation of ERK1/2 was investigated by assessing the distribution of phospho-ERK1/2 in lung tissues of bik+/+ and bik−/− mice at 5, 12, and 15 d of allergen exposure. Nuclear phospho-ERK1/2 was detected in airway cells of both bik+/+ and bik−/− mice exposed to allergen for 5 d but in significantly higher percentages in bik−/− airways at 12 and 15 d of exposure (Fig. 5 F). Interestingly, primarily the mucus-containing cells showed immunostaining for nuclear phospho-ERK1/2. Total ERK1/2 was found to be distributed similarly in both bik+/+ and bik−/− mouse lungs after exposure to allergen for 5, 12, or 15 d. These findings show that Bik expression suppresses nuclear translocation of phospho-ERK1/2 in airways when resolution of hyperplastic epithelial cells occurs.
Figure 5.
IFNγ and Bik are crucial for the resolution of epithelial cell hyperplasia and mucous cell metaplasia during prolonged exposure to allergen. Mice were immunized with ovalbumin/alum on days 1 and 7 and were exposed to ovalbumin aerosols for 5, 12, or 15 d. After sacrifice, the lungs of each mouse were fixed under constant pressure perfusion and cut in 4-mm slices from distal to caudal. Slices were embedded in paraffin, and tissue sections were stained with hematoxylin and eosin or AB/periodic acid Schiff to count the total cell number or mucous cell per millimeter of basal lamina, respectively. (A and B) The number of epithelial cells per millimeter of basal lamina was significantly reduced in wild-type mice but not in IFNγ−/− (A) or bik−/− (B) mice at 15 d of exposure. Error bars indicate ± SEM. (C) Representative micrographs from bik+/+- and bik−/−-sensitized mice exposed to allergen for 5, 12, and 15 d display that the density of epithelial cell nuclei is reduced in bik+/+ but not in bik−/− airways. (D) The number of mucous cells per millimeter of basal lamina was significantly reduced in bik+/+ but not in bik−/− mice. (E) Representative micrographs from bik+/+ and bik−/− mice. Error bars indicate group means ± SEM (n = 5 mice per group). (F) Representative photomicrographs and quantification showing that activated ERK1/2 is found in the nuclei in bik+/+ and bik−/− mice exposed to allergen for 5 d, but nuclear phospho-ERK is only observed in airways of bik−/− mice at 12 and 15 d. Arrows denote nuclear phospho-ERK1/2. Error bars indicate group means ± SEM (n = 3 mice/group). Representative photomicrographs showing that total ERK1/2 is uniformly distributed in airways from bik+/+ and bik−/− mice exposed to allergen for 5, 12, and 15 d. *, P < 0.05; significantly different from wild-type CTs.
Discussion
IFNγ-induced Bik expression and cell death requires STAT1 but is independent of p53
Our studies suggest that IFNγ-induced activation of STAT1 causes Bik expression because IFNγ failed to induce Bik expression in STAT1−/− MAECs, and the same truncation mutant of STAT1 that reduced IFNγ-induced Bik expression also significantly reduced IFNγ-induced cell death of AECs (Stout et al., 2007). The findings from the present study in bik−/− mice together with our previous findings that allergen-induced airway epithelial hyperplasia is sustained in STAT1−/− mice (Stout et al., 2007) places STAT1 activation upstream of Bik. Whether STAT1 activation leads to increased Bik promoter activity or causes stabilization of Bik mRNA is currently unclear. Support for direct interaction and activation of the Bik promoter by STAT1 could be based on the presence of seven STAT-binding consensus sequences, TTNCNNNAA (Bromberg and Chen, 2001), including two tandem sites within the core Bik promoter (Verma et al., 2000). However, our attempts to stimulate a Bik promoter luciferase construct with IFNγ were not successful, suggesting that other transcription factors or mRNA stability may play a critical role in the induction of Bik expression by IFNγ. Overall, the findings suggest that although STAT1 may affect many signaling pathways in vivo, its activation requires Bik expression to channel the apoptotic effect.
Several observations show that the pathway by which IFNγ induces cell death does not involve the p53 pathway. (1) Resistance of bik−/− MAECs to IFNγ did not include the resistance to adriamycin that is mediated by DNA damage and p53 activation. (2) During IFNγ-induced cell death, p53 levels were not affected in AECs. (3) IFNγ induced Bik expression in p53−/− MAECs, suggesting that IFNγ does not require p53 to induce Bik expression and cell death. Similarly, others have reported that Bik mediates cell death in the p53-deficient H1299 cells and does not require p53 (Mathai et al., 2002).
Loss of Bik mediates resistance to IFNγ but not to other cell death inducers
IFNγ induces Bik expression that is localized to the ER (Mathai et al., 2005), translocates Bax to the ER, and elicits ER stress as shown by release of ER calcium stores (Stout et al., 2007) by JNK activation and induction of CHOP levels (unpublished data). Bax plays a role in IFNγ-induced cell death (Tesfaigzi et al., 2002), but bax−/− MAECs succumb to IFNγ treatment, suggesting that Bak can substitute for the loss of Bax as was reported previously (Lindsten et al., 2000; Wei et al., 2001). In fact, data from the present studies suggest that loss of Bim or Bax may sensitize MAECs more to IFNγ-induced cell death. Future studies need to investigate the role of Bim and Bax in the IFNγ-induced expression of Bik and/or the ER-stress pathway.
Bik−/− MAECs are resistant to IFNγ-induced ER stress, although they are sensitive to thapsigargin, a selective inhibitor of the ER-associated Ca2+-ATPase that allows Ca2+ to flow from the ER lumen into the cytoplasm. This sensitivity may be the result of IFNγ causing activation of ER stress and calcium release by mechanisms different from those induced by thapsigargin or because the ER-associated Ca2+-ATPase may be downstream of Bik. IFNγ and Bik-induced ER stress may be caused by the inactivation of GRP78 (BiP), an ER-associated protein that has antiapoptotic properties (Fu et al., 2007). Bik binds to GRP78 and allows the release of the critical transmembrane ER signaling proteins PERK, Ire1, and ATF6 (Xu et al., 2005). Protein shutdown caused by the bacterial toxin MazF or cycloheximide was also shown to require Bik to mediate cell death in TRex-293 cells (Shimazu et al., 2007). It is possible that inhibition of protein synthesis causes Bik expression by blocking the proteasome degradation system and results in massive ER stress through the GRP78 system.
Bik blocks nuclear translocation of activated ERK1/2 to cause cell death
In HAECs, IFNγ activated ERK1/2 24 h after treatment, which coincides with the time of Bik expression. ERK1/2 activation was also associated with IFNγ-induced death in oligodendroglial cells (Horiuchi et al., 2006). Because ERK1/2 activation was similar in cytosolic extracts from bik+/+ and bik−/− MAECs, we started to analyze whether Bik may affect the localization of phospho-ERK1/2. Both Western blot and immunofluorescence analyses confirmed that the presence of nuclear ERK1/2 was suppressed when Bik was expressed. Not only IFNγ- but also starvation-induced phospho-ERK1/2 was inhibited from translocating to the nucleus when Bik was present. The conserved Leu residue in the BH3 domain that was crucial for Bik-induced cell death was also crucial for interacting and inhibiting phospho-ERK1/2 translocation. Furthermore, inhibition of ERK1/2 using U0126 suppressed IFNγ- and Bik-induced cell death, suggesting that cytosolic ERK1/2 is proapoptotic, whereas nuclear ERK1/2 promotes growth in AECs. The biological consequence of ERK activation in a given cell may be determined by the cell-specific cytosolic or nuclear substrates. ERK is generally considered to be antiapoptotic (Kolch, 2005) but can function as a stimulator of apoptosis in cells expressing death-associated protein (DAP) kinase (Chen et al., 2005), a kinase that promotes the cytoplasmic retention of ERK1/2. DAP-kinase was isolated from HeLa cells as a mediator of IFNγ-induced cell death (Deiss et al., 1995; Levy-Strumpf et al., 1997), but its role in affecting the BH3-only proteins is not known. DAP-kinase is constitutively expressed in human HAECs and MAECs (unpublished data). Therefore, IFNγ-induced ERK1/2 activation and Bik expression may initiate a feedback loop that initiates DAP-kinase–mediated cytoplasmic retention of ERK1/2 to promote the amplification of proapoptotic signals. Bik was shown to interact with Bcl-2 (Boyd et al., 1995), but this interaction is not sufficient for its apoptotic function (Elangovan and Chinnadurai, 1997). The present studies show that the proapoptotic function of Bik stems from its ability to inhibit nuclear translocation of phospho-ERK1/2.
The mechanisms of how Bik expression may induce expression of cathepsins B and D are unclear. Cathepsin B contains a TATA-less promoter but an E box that allows the formation of a transcription initiation complex involving the upstream stimulatory factors USF1 and ISF2 (Yan et al., 2003). Other transcription factors, including Ets1, Sp1, and EBS, regulate expression at two alternative promoters (Yan et al., 2000; Yan and Sloane, 2003). The TATA-containing promoter of cathepsin D is regulated by the hormone estrogen (Cavailles et al., 1993). Future studies will investigate whether Bik regulates expression of these mRNAs by reducing nuclear translocation of phospho-ERK1/2 that may have an inhibitory effect on these promoters or by increasing cytosolic phospho-ERK1/2 and prolonging mRNA half-life.
IFNγ can be proliferative but requires Bik to channel death
Exposure to allergen caused more AEC hyperplasia in bik−/− and wild-type mice compared with IFNγ−/− mice, suggesting that IFNγ plays a role in the proliferation of epithelial cells. However, once the hyperplastic stage was established, the resolution was abrogated in both IFNγ−/− and bik−/− mice. Such a double-sided effect for IFNγ has been previously reported (Hansen et al., 1999; Randolph et al., 1999; Xiang et al., 2008); however, this study demonstrates that IFNγ requires Bik to channel its cell death–inducing activity. So far, the murine Bik has only been suggested to represent a homologous equivalent of human Bik. The present studies show a role of human and murine Bik as the main mediator for IFNγ-induced cell death and, therefore, suggest that they are functionally homologous.
Although Bik is expressed in the liver, lung, heart, and kidneys and in granulocytes, macrophages, and developing as well as mature T and B lymphocytes (Coultas et al., 2004), the bik−/− mice develop and age normally, suggesting that IFNγ-induced cell killing is not required during normal development under injury- and pathogen-free conditions. Loss of Bik conferred no protection to mature T and B cells from spontaneous death in culture, from treatment with dexamethasone or etoposide, or from cytokine starvation of mitogen-activated B cells (Coultas et al., 2004). Bik inducing death in AECs rather than hematopoietic cells may result from airway cells expressing other genes such as DAP-kinase to facilitate Bik-mediated suppression of nuclear translocation of phospho-ERK1/2. The cell type–specific effect of BH3-only domain proteins is further supported by our previous studies showing that Bim, an essential initiator of apoptosis in negative selection of autoreactive thymocytes (Bouillet et al., 2002), and B cells (Enders et al., 2003), are not involved in the resolution of airway epithelial hyperplasia (Pierce et al., 2006).
The present studies show that loss of Bik or IFNγ leads to sustained epithelial cell hyperplasia after allergen-induced inflammation in mouse airways. In addition, proliferating rather than nonproliferating, resting AECs are prone to undergo cell death in response to IFNγ treatment or Bik overexpression. Therefore, Bik is ineffective in inducing cell death in confluent airway epithelial cultures (unpublished data). Primarily the mucus-containing cells showed nuclear phospho-ERK1/2 in lung tissues of mice exposed to allergen. Several studies have shown that after injury to the airway epithelium, a large proportion of the hyperplastic and cycling cells are secretory cells (Keenan et al., 1982a,b,c). Because mucous cells are the hyperplastic cells (Tesfaigzi et al., 2004), they may be by default susceptible to cell death and elimination in vivo during resolution. Susceptibility of proliferating cells to IFNγ or Bik-induced cell death may be a mechanism to selectively target hyperplastic epithelial cells that in airways usually represent mucus-producing cells (Lai et al., 2002). This selective elimination of only hyperplastic cells may ensure that resting AECs maintain the barrier function of the airway epithelium, may allow the CT of mucus production, and may prevent the development of cancerous lesions. Therefore, restoring Bik expression may be useful for eliminating hyperplastic airway cells. Bik was first identified as a protein that interacts with the adenoviral protein E1B 19K (Han et al., 1996; Mathai et al., 2002). The reason for E1B 19K or the viral homologue encoded by Epstein-Barr virus, BHRF1, singling out Bik for inhibition may be to sustain the viability of the host cells and promote replication and release of virus particles.
In summary, our analyses of HAECs and MAECs in culture and in airways of intact mice identify Bik as the central mediator of IFNγ-induced apoptosis. Furthermore, we provide evidence that Bik induces cell death by inhibiting nuclear translocation of activated ERK1/2 in a pathway that is independent of p53.
Materials and methods
Animals
Male-specific pathogen-free wild-type C57BL/6J and p53+/− mice were purchased from The Jackson Laboratory. Mice were housed in isolated cages under specific pathogen-free conditions. After a 14-d quarantine period, mice were acclimatized for 8 d and entered into the experimental protocol at 8–10 wk of age. The bax+/−, bim+/−, and bik+/− mice on C57BL/6 background were provided by S. Korsmeyer (Dana Farber Research Institute, Boston, MA) and A. Strasser (Walter and Eliza Hall Institute, Melbourne, Australia). Bax+/+ and bax−/−, p53+/+ and p53−/−, and bim−/− with bim+/+ and bik+/+ with bik−/− littermates were bred from the respective heterozygote mice at the Lovelace Respiratory Research Institute under specific pathogen-free conditions and genotyped as described previously (Coultas et al., 2004). STAT1−/− on C57BL/6 background were purchased from Taconic. All experiments were approved by the Institutional Animal Care and Use Committee and were performed at the Lovelace Respiratory Research Institute, a facility approved by the Association for the Assessment and Accreditation for Laboratory Animal Care International. Sensitization and exposure of mice to ovalbumin were performed as described previously (Tesfaigzi et al., 2002). Preparation of lung tissues for histopathological examination was performed as described previously (Shi et al., 2002). Tissue sections were stained with Alcian blue (AB) and periodic acid Schiff or hematoxylin and eosin as described previously (Shi et al., 2002). The number of AB-positive cells per millimeter of basal lamina was quantified using a light microscope (BH-2; Olympus) equipped with the Image analysis system (National Institutes of Health) as described previously (Harkema and Hotchkiss, 1991).
Cells
MAECs were harvested and cultured on Transwell membranes (Corning) after seeding with 4 × 104 or 9 × 104 cells as previously described (You et al., 2002). Cell viability was determined by trypan blue exclusion. Primary HAECs were purchased from Cambrex Bio Science Walkersville, Inc. The immortalized HAECs, AALEB, and HBEC-2 cells provided by S. Randell (University of North Carolina Chapel Hill, Chapel Hill, NC) and J. Shay (University of Texas Southwestern, Dallas, TX) were described previously (Lundberg et al., 2002; Ramirez et al., 2004). Cells were maintained in bronchial epithelial growth medium supplemented with growth factors as described previously (Shi et al., 2002).
Adenoviral expression vectors for Bik and BikL61G were provided by G. Shore (McGill University, Montreal, Quebec, Canada), and cells were infected as described previously (Mathai et al., 2002). The MAPK extracellular signal-regulated kinase inhibitor 4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio) butadiene (U0126) was purchased from EMD. The doxorubicin hydrochloride (adriamycin) was purchased from Thermo Fisher Scientific, and thapsigargin was purchased from Tocris Bioscience. Viability was assessed by trypan blue exclusion.
Retroviral silencing with Bik shRNA
Retroviral silencing vector encoding for Bik shRNA and the CT vector were purchased from Origene Technologies, Inc. The suppressing effect of the shRNA was established in HBEC-2 cells, and amplification and purification of plasmid DNA and packaging of the retroviral particles in Phoenix cells were performed as specified by the manufacturer's instructions. After infection with Bik or CT shRNAs, HBEC-2 cells were treated with 50 ng/ml human recombinant IFNγ, and 48 h later cells were harvested for Western blot analysis.
RT-PCR
For quantitative RT-PCR, the primer/probe sets (Applied Biosystems) were distributed into each well in duplicates, and target mRNAs were amplified by PCR in 20-μl reactions. Preamplification efficiency was assessed by performing amplification of nonamplifed cDNA with TaqMan Gene Expression Assays (Applied Biosystems). For all reactions, CT values >37 were eliminated for evaluation of preamplification efficiency. Uniform preamplification was demonstrated by a ΔΔCT value of −1.5 to 1.5 when comparing the CT values of each gene amplified from preamplified and nonamplified cDNA as described previously (Schwalm et al., 2008). Because all results were derived from the linear amplification curve, the use of the ΔΔCT method ensures that only mRNA amplification within the linear range was compared.
For semiquantitative RT-PCR, total RNA was extracted from HBEC-2 cells with TRIZOL (TRI Reagent; Invitrogen), first-strand cDNA was synthesized using an oligo(dT) primer and Omniscript reverse transcription (QIAGEN), and PCR for human and murine cathepsins B and D was conducted using the primers and conditions described previously (Dong et al., 2001; Tsukamoto et al., 2006). Amplified PCR products were quantified via densitometry using Fluor-S-Max Imager and Quantity One software (Bio-Rad Laboratories). Band intensities were normalized with the corresponding band intensities for β-actin.
Immunofluorescence and immunohistochemistry
Lung tissue sections were deparaffinized, rehydrated, washed, and, after antigen retrieval, incubated with the respective primary and secondary antibodies as described previously (Harris et al., 2005). Procedures for detection of ERK1/2 by immunofluorescence using rabbit antiphospho-ERK1/2 antibody and rabbit anti-ERK1/2 antibody (Cell Signaling Technology) at a 1:100 dilution and with a secondary goat anti–rabbit conjugated to Alexa Fluor 647 and the counterstain with Hoechst were described previously (Stout et al., 2007). Immunofluorescence was imaged using Axioplan 2 (Carl Zeiss, Inc.) with a Plan-Aprochromal 63×/1.4 oil objective and a charge-coupled device camera (SensiCAm; PCO), and the acquisition software used was digital microscopy software (Slidebook 4.2; Intelligent Imaging Innovation). Immunohistochemical stains were imaged using a microscope (Eclipse E600W; Nikon) with a Plan Fluor 60× NA 0.85 objective and a digital camera (DXM1200F; Nikon) with ACT-1 acquisition software (version 2.62; Nikon).
Western blot analysis
Protein lysates were prepared and analyzed by Western blotting as described previously (Tesfaigzi et al., 2002). Cytosolic and nuclear fractions were prepared by lysing cells in NP-40 to obtain the cytosolic fraction and extracting the nuclear proteins with a hypertonic extraction buffer (50 mM Hepes, pH 7.8, 50 mM KCl, and 300 mM NaCl) in the presence of protease and phosphatase inhibitors as described previously (Stout et al., 2007). The following antibodies were used: goat anti-Bik polyclonal antibody (Santa Cruz Biotechnology, Inc.), rabbit antiphospho-ERK1/2 antibody, and rabbit anti-ERK1/2 antibody (Cell Signaling Technology). Total ERK1/2 was detected with anti-ERK1/2 antibodies (1:1,000) in the same membrane used for antiphospho-ERK1/2 and total ERK1/2 antibodies, respectively, after deprobing. Equal protein loading was confirmed by subsequent probing with the mouse monoclonal antibody against actin (Santa Cruz Biotechnology, Inc.).
Pull-down assay
A size X protein A immunoprecipitation kit (Thermo Fisher Scientific) was used to cross-link 50 μg of purified anti-Bik antibody to protein A beads using disuccinmidyl suberate as described by the manufacturer. Bik-associated proteins were immunoprecipitated by incubating protein lysates prepared from Ad-GFP–, Ad-Bik–, or Ad-BikL61G–infected HBEC-2 cells with gentle mixing at 4°C overnight. After repeated washes, proteins bound to the Bik antibody on beads were eluted with 0.2 ml of ImmunoPure Elution buffer (Thermo Fisher Scientific) and analyzed by Western blotting and antiphospho-ERK1/2 and anti-Bik antibodies.
Statistical analysis
Grouped results from at least four different mice were expressed as means ± SEM. Data were analyzed using statistical analysis software (Statistical Analysis Software Institute). Results grouped by time point and genotype were analyzed using two-way analysis of variance. When significant main effects were detected (P < 0.05), Fisher's least significant difference test was used to determine the differences between groups. A p-value of 0.05 was considered to indicate statistical significance.
Acknowledgments
We thank J. Rir-Sim-Ah for technical assistance in selected experiments.
These studies were supported by grants from the National Institutes of Health (HL68111 and ES09237) and the University of New Mexico General Clinical Research Center (National Institutes of Health National Center for Research Resources General Clinical Research Center 5MO1 RR099).
Abbreviations used in this paper: AB, Alcian blue; AEC, airway epithelial cell; BH, Bcl-2 homology; CT, control; DAP, death-associated protein; ERK, extracellular regulated kinase; HAEC, human AEC; MAEC, mouse AEC; shRNA, short hairpin RNA.
References
- Adams, J.M., and S. Cory. 1998. The Bcl-2 protein family: arbiters of cell survival. Science. 281:1322–1326. [DOI] [PubMed] [Google Scholar]
- Barber, G.N. 2000. The interferons and cell death: guardians of the cell or accomplices of apoptosis? Semin. Cancer Biol. 10:103–111. [DOI] [PubMed] [Google Scholar]
- Bouillet, P., J.F. Purton, D.I. Godfrey, L.C. Zhang, L. Coultas, H. Puthalakath, M. Pellegrini, S. Cory, J.M. Adams, and A. Strasser. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926. [DOI] [PubMed] [Google Scholar]
- Boyd, J.M., G.J. Gallo, B. Elangovan, A.B. Houghton, S. Malstrom, B.J. Avery, R.G. Ebb, T. Subramanian, T. Chittenden, R.J. Lutz, et al. 1995. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 11:1921–1928. [PubMed] [Google Scholar]
- Bromberg, J., and X. Chen. 2001. STAT proteins: signal tranducers and activators of transcription. Methods Enzymol. 333:138–151. [DOI] [PubMed] [Google Scholar]
- Cavailles, V., P. Augereau, and H. Rochefort. 1993. Cathepsin D gene is controlled by a mixed promoter, and estrogens stimulate only TATA-dependent transcription in breast cancer cells. Proc. Natl. Acad. Sci. USA. 90:203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.H., W.J. Wang, J.C. Kuo, H.C. Tsai, J.R. Lin, Z.F. Chang, and R.H. Chen. 2005. Bidirectional signals transduced by DAPK-ERK interaction promote the apoptotic effect of DAPK. EMBO J. 24:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, E.H., M.C. Wei, S. Weiler, R.A. Flavell, T.W. Mak, T. Lindsten, and S.J. Korsmeyer. 2001. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 8:705–711. [DOI] [PubMed] [Google Scholar]
- Coultas, L., D.C. Huang, J.M. Adams, and A. Strasser. 2002. Pro-apoptotic BH3-only Bcl-2 family members in vertebrate model organisms suitable for genetic experimentation. Cell Death Differ. 9:1163–1166. [DOI] [PubMed] [Google Scholar]
- Coultas, L., P. Bouillet, E.G. Stanley, T.C. Brodnicki, J.M. Adams, and A. Strasser. 2004. Proapoptotic BH3-only Bcl-2 family member Bik/Blk/Nbk is expressed in hemopoietic and endothelial cells but is redundant for their programmed death. Mol. Cell. Biol. 24:1570–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryns, V., and J. Yuan. 1998. Proteases to die for. Genes Dev. 12:1551–1570. [DOI] [PubMed] [Google Scholar]
- Day, C.L., H. Puthalakath, G. Skea, A. Strasser, I. Barsukov, L.Y. Lian, D.C. Huang, and M.G. Hinds. 2004. Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem. J. 377:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiss, L.P., E. Feinstein, H. Berissi, O. Cohen, and A. Kimchi. 1995. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 9:15–30. [DOI] [PubMed] [Google Scholar]
- Deiss, L.P., H. Galinka, H. Berissi, O. Cohen, and A. Kimchi. 1996. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- Dong, Z., M. Katar, B.E. Linebaugh, B.F. Sloane, and R.S. Berk. 2001. Expression of cathepsins B, D and L in mouse corneas infected with Pseudomonas aeruginosa. Eur. J. Biochem. 268:6408–6416. [DOI] [PubMed] [Google Scholar]
- Elangovan, B., and G. Chinnadurai. 1997. Functional dissection of the pro-apoptotic protein Bik. Heterodimerization with anti-apoptosis proteins is insufficient for induction of cell death. J. Biol. Chem. 272:24494–24498. [DOI] [PubMed] [Google Scholar]
- Enders, A., P. Bouillet, H. Puthalakath, Y. Xu, D.M. Tarlinton, and A. Strasser. 2003. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J. Exp. Med. 198:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y., J. Li, and A.S. Lee. 2007. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 67:3734–3740. [DOI] [PubMed] [Google Scholar]
- Hamann, K.J., D.R. Dorscheid, F.D. Ko, A.E. Conforti, A.I. Sperling, K.F. Rabe, and S.R. White. 1998. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 19:537–542. [DOI] [PubMed] [Google Scholar]
- Han, J., P. Sabbatini, and E. White. 1996. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell. Biol. 16:5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, G., G. Berry, R.H. DeKruyff, and D.T. Umetsu. 1999. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema, J.R., and J.A. Hotchkiss. 1991. In vivo effects of endotoxin on nasal epithelial mucosubstances: quantitative histochemistry. Exp. Lung Res. 17:743–761. [DOI] [PubMed] [Google Scholar]
- Harris, J.F., M.J. Fischer, J.A. Hotchkiss, B.P. Monia, S.H. Randell, J.R. Harkema, and Y. Tesfaigzi. 2005. Bcl-2 sustains increased mucous and epithelial cell numbers in metaplastic airway epithelium. Am. J. Respir. Crit. Care Med. 171:764–772. [DOI] [PubMed] [Google Scholar]
- Horiuchi, M., A. Itoh, D. Pleasure, and T. Itoh. 2006. MEK-ERK signaling is involved in interferon-gamma-induced death of oligodendroglial progenitor cells. J. Biol. Chem. 281:20095–20106. [DOI] [PubMed] [Google Scholar]
- Keenan, K.P., J.W. Combs, and E.M. McDowell. 1982. a. Regeneration of hamster tracheal epithelium after mechanical injury. I. Focal lesions: quantitative morphologic study of cell proliferation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 41:193–214. [DOI] [PubMed] [Google Scholar]
- Keenan, K.P., J.W. Combs, and E.M. McDowell. 1982. b. Regeneration of hamster tracheal epithelium after mechanical injury. II. Multifocal lesions: stathmokinetic and autoradiographic studies of cell proliferation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 41:215–229. [DOI] [PubMed] [Google Scholar]
- Keenan, K.P., J.W. Combs, and E.M. McDowell. 1982. c. Regeneration of hamster tracheal epithelium after mechanical injury. III. Large and small lesions: comparative stathmokinetic and single pulse and continuous thymidine labeling autoradiographic studies. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 41:231–252. [PubMed] [Google Scholar]
- Kolch, W. 2005. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6:827–837. [DOI] [PubMed] [Google Scholar]
- Lai, J.M., S. Wu, D.Y. Huang, and Z.F. Chang. 2002. Cytosolic retention of phosphorylated extracellular signal-regulated kinase and a Rho-associated kinase-mediated signal impair expression of p21(Cip1/Waf1) in phorbol 12-myristate-13- acetate-induced apoptotic cells. Mol. Cell. Biol. 22:7581–7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei, K., and R.J. Davis. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 100:2432–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai, A., M.C. Bassik, L.D. Walensky, M.D. Sorcinelli, S. Weiler, and S.J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2:183–192. [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf, N., L.P. Deiss, H. Berissi, and A. Kimchi. 1997. DAP-5, a novel homolog of eukaryotic translation initiation factor 4G isolated as a putative modulator of gamma interferon-induced programmed cell death. Mol. Cell. Biol. 17:1615–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten, T., A.J. Ross, A. King, W.X. Zong, J.C. Rathmell, H.A. Shiels, E. Ulrich, K.G. Waymire, P. Mahar, K. Frauwirth, et al. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol. Cell. 6:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg, A.S., S.H. Randell, S.A. Stewart, B. Elenbaas, K.A. Hartwell, M.W. Brooks, M.D. Fleming, J.C. Olsen, S.W. Miller, R.A. Weinberg, and W.C. Hahn. 2002. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 21:4577–4586. [DOI] [PubMed] [Google Scholar]
- Mathai, J.P., M. Germain, R.C. Marcellus, and G.C. Shore. 2002. Induction and endoplasmic reticulum location of BIK/NBK in response to apoptotic signaling by E1A and p53. Oncogene. 21:2534–2544. [DOI] [PubMed] [Google Scholar]
- Mathai, J.P., M. Germain, and G.C. Shore. 2005. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J. Biol. Chem. 280:23829–23836. [DOI] [PubMed] [Google Scholar]
- Naik, E., E.M. Michalak, A. Villunger, J.M. Adams, and A. Strasser. 2007. Ultraviolet radiation triggers apoptosis of fibroblasts and skin keratinocytes mainly via the BH3-only protein Noxa. J. Cell Biol. 176:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda, E., R. Ohki, H. Murasawa, J. Nemoto, T. Shibue, T. Yamashita, T. Tokino, T. Taniguchi, and N. Tanaka. 2000. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 288:1053–1058. [DOI] [PubMed] [Google Scholar]
- Opferman, J.T., and S.J. Korsmeyer. 2003. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 4:410–415. [DOI] [PubMed] [Google Scholar]
- Ossina, N.K., A. Cannas, V.C. Powers, P.A. Fitzpatrick, J.D. Knight, J.R. Gilbert, E.M. Shekhtman, L.D. Tomei, S.R. Umansky, and M.C. Kiefer. 1997. Interferon-gamma modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J. Biol. Chem. 272:16351–16357. [DOI] [PubMed] [Google Scholar]
- Pierce, J., J. Rir-Sima-Ah, I. Estrada, J.A. Wilder, A. Strasser, and Y. Tesfaigzi. 2006. Loss of pro-apoptotic Bim promotes accumulation of pulmonary T lymphocytes and enhances allergen-induced goblet cell metaplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 291:L862–L870. [DOI] [PubMed] [Google Scholar]
- Puthalakath, H., A. Villunger, L.A. O'Reilly, J.G. Beaumont, L. Coultas, R.E. Cheney, D.C. Huang, and A. Strasser. 2001. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 293:1829–1832. [DOI] [PubMed] [Google Scholar]
- Ramirez, R.D., S. Sheridan, L. Girard, M. Sato, Y. Kim, J. Pollack, M. Peyton, Y. Zou, J.M. Kurie, J.M. Dimaio, et al. 2004. Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins. Cancer Res. 64:9027–9034. [DOI] [PubMed] [Google Scholar]
- Randolph, D.A., R. Stephens, C.J. Carruthers, and D.D. Chaplin. 1999. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Invest. 104:1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ruiz, C., C. Munoz-Pinedo, and A. Lopez-Rivas. 2000. Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria- operated apoptotic program. Cancer Res. 60:5673–5680. [PubMed] [Google Scholar]
- Schwalm, K., J.F. Stevens, Z. Jiang, M.R. Schuyler, R. Schrader, S.H. Randell, F.H. Green, and Y. Tesfaigzi. 2008. Expression of the pro-apoptotic protein bax is reduced in bronchial mucous cells of asthmatics. Am. J. Physiol. Lung Cell. Mol. Physiol. 294:L1102–L1109. [DOI] [PubMed] [Google Scholar]
- Shi, Z.O., M.J. Fischer, G.T. De Sanctis, M. Schuyler, and Y. Tesfaigzi. 2002. IFNg but not Fas mediates reduction of allergen-induced mucous cell metaplasia by inducing apoptosis. J. Immunol. 168:4764–4771. [DOI] [PubMed] [Google Scholar]
- Shimazu, T., K. Degenhardt, E.K.A. Nur, J. Zhang, T. Yoshida, Y. Zhang, R. Mathew, E. White, and M. Inouye. 2007. NBK/BIK antagonizes MCL-1 and BCL-XL and activates BAK-mediated apoptosis in response to protein synthesis inhibition. Genes Dev. 21:929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout, B.A., K. Melendez, J. Seagrave, M.J. Holtzman, B. Wilson, J. Xiang, and Y. Tesfaigzi. 2007. STAT1 activation causes translocation of Bax to the endoplasmic reticulum during the resolution of airway mucous cell hyperplasia by IFNγ. J. Immunol. 178:8107–8116. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi, Y. 2006. Roles of apoptosis in airway epithelia. Am. J. Respir. Cell Mol. Biol. 34:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaigzi, Y., M.J. Fischer, F.H.Y. Green, G.T. De Sanctis, and J.A. Wilder. 2002. Bax is crucial for IFNg-induced resolution of allergen-induced mucous cell metaplasia. J. Immunol. 169:5919–5925. [DOI] [PubMed] [Google Scholar]
- Tesfaigzi, Y., J.F. Harris, J.A. Hotchkiss, and J.R. Harkema. 2004. DNA synthesis and Bcl-2 expression during the development of mucous cell metaplasia in airway epithelium of rats exposed to LPS. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L268–L274. [DOI] [PubMed] [Google Scholar]
- Thornberry, N.A., and Y. Lazebnik. 1998. Caspases: enemies within. Science. 281:1312–1316. [DOI] [PubMed] [Google Scholar]
- Trautmann, A., M. Akdis, D. Kleemann, F. Altznauer, H.U. Simon, T. Graeve, M. Noll, E.B. Brocker, K. Blaser, and C.A. Akdis. 2000. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J. Clin. Invest. 106:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann, A., P. Schmid-Grendelmeier, K. Kruger, R. Crameri, M. Akdis, A. Akkaya, E.B. Brocker, K. Blaser, and C.A. Akdis. 2002. T cells and eosinophils cooperate in the induction of bronchial epithelial cell apoptosis in asthma. J. Allergy Clin. Immunol. 109:329–337. [DOI] [PubMed] [Google Scholar]
- Tsukamoto, T., J. Iida, Y. Dobashi, T. Furukawa, and F. Konishi. 2006. Overexpression in colorectal carcinoma of two lysosomal enzymes, CLN2 and CLN1, involved in neuronal ceroid lipofuscinosis. Cancer. 106:1489–1497. [DOI] [PubMed] [Google Scholar]
- Verma, S., M.L. Budarf, B.S. Emanuel, and G. Chinnadurai. 2000. Structural analysis of the human pro-apoptotic gene Bik: chromosomal localization, genomic organization and localization of promoter sequences. Gene. 254:157–162. [DOI] [PubMed] [Google Scholar]
- Verma, S., L.J. Zhao, and G. Chinnadurai. 2001. Phosphorylation of the pro-apoptotic protein BIK: mapping of phosphorylation sites and effect on apoptosis. J. Biol. Chem. 276:4671–4676. [DOI] [PubMed] [Google Scholar]
- Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J. Immunol. Methods. 184:39–51. [DOI] [PubMed] [Google Scholar]
- Villunger, A., E.M. Michalak, L. Coultas, F. Mullauer, G. Bock, M.J. Ausserlechner, J.M. Adams, and A. Strasser. 2003. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 302:1036–1038. [DOI] [PubMed] [Google Scholar]
- Wang, Z., T. Zheng, Z. Zhu, R.J. Homer, R.J. Riese, H.A. Chapman Jr., S.D. Shapiro, and J.A. Elias. 2000. Interferon γ induction of pulmonary emphysema in the adult murine lung. J. Exp. Med. 192:1587–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, M.C., W.X. Zong, E.H. Cheng, T. Lindsten, V. Panoutsakopoulou, A.J. Ross, K.A. Roth, G.R. MacGregor, C.B. Thompson, and S.J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, L.P., K. Madani, J.A. Fahrni, S.R. Duncan, and G.D. Rosen. 1997. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN- gamma and Fas. Am. J. Physiol. 273:L921–L929. [DOI] [PubMed] [Google Scholar]
- Willis, S.N., J.I. Fletcher, T. Kaufmann, M.F. van Delft, L. Chen, P.E. Czabotar, H. Ierino, E.F. Lee, W.D. Fairlie, P. Bouillet, et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 315:856–859. [DOI] [PubMed] [Google Scholar]
- Xiang, J., J. Rir-Sim-Ah, and Y. Tesfaigzi. 2008. IL-9 and IL-13 induce mucous cell metaplasia that is reduced by IFN-gamma in a Bax-mediated pathway. Am. J. Respir. Cell Mol. Biol. 38:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, C., B. Bailly-Maitre, and J.C. Reed. 2005. Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115:2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, S., and B.F. Sloane. 2003. Molecular regulation of human cathepsin B: implication in pathologies. Biol. Chem. 384:845–854. [DOI] [PubMed] [Google Scholar]
- Yan, S., I.M. Berquin, B.R. Troen, and B.F. Sloane. 2000. Transcription of human cathepsin B is mediated by Sp1 and Ets family factors in glioma. DNA Cell Biol. 19:79–91. [DOI] [PubMed] [Google Scholar]
- Yan, S., D.T. Jane, M.J. Dufresne, and B.F. Sloane. 2003. Transcription of cathepsin B in glioma cells: regulation by an E-box adjacent to the transcription initiation site. Biol. Chem. 384:1421–1427. [DOI] [PubMed] [Google Scholar]
- Yoshikawa, H., Y. Nakajima, and K. Tasaka. 2001. IFN-gamma induces the apoptosis of WEHI 279 and normal pre-B cell lines by expressing direct inhibitor of apoptosis protein binding protein with low pI. J. Immunol. 167:2487–2495. [DOI] [PubMed] [Google Scholar]
- You, Y., E.J. Richer, T. Huang, and S.L. Brody. 2002. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- Youn, C.K., H.J. Cho, S.H. Kim, H.B. Kim, M.H. Kim, I.Y. Chang, J.S. Lee, M.H. Chung, K.S. Hahm, and H.J. You. 2005. Bcl-2 expression suppresses mismatch repair activity through inhibition of E2F transcriptional activity. Nat. Cell Biol. 7:137–147. [DOI] [PubMed] [Google Scholar]
- Zhang, X., T. Brunner, L. Carter, R.W. Dutton, P. Rogers, L. Bradley, T. Sato, J.C. Reed, D. Green, and S.L. Swain. 1997. Unequal death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J. Exp. Med. 185:1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]