Abstract
Seven hundred and seventy nine infants were screened at 4 months of age for motor and emotional reactivity. At age 9 months, infants who showed extreme patterns of motor and negative (n = 75) or motor and positive (n = 73) reactivity and an unselected control group (n = 86) were administered the Laboratory Temperament Assessment Battery (Lab-TAB), and baseline electroencephalogram (EEG) data were collected. Negatively reactive infants showed significantly more avoidance than positively reactive infants and displayed a pattern of right frontal EEG asymmetry. Positively reactive infants exhibited significantly more approach behavior than controls and exhibited a pattern of left frontal asymmetry. Results support the notion that approach-withdrawal bias underlies reactivity in infancy.
Keywords: temperament, frontal EEG asymmetry, approach-withdrawal bias
The term temperamental reactivity refers to individual differences in physiological and behavioral response to the environment that are thought to be constitutional in origin. Operational definitions of reactivity vary across the literature. Rothbart (2004) emphasizes motor arousal, orienting, and emotionality. She and her colleagues posit a hierarchical structure to temperament that is grounded in general indices of emotionality and includes, among its broad factors, negative affectivity (i.e., fear, frustration, sadness, and discomfort) and extraversion or surgency (i.e., sensation seeking, positive anticipation, impulsivity, and activity level; Rothbart, Ahadi, Hershey, & Fisher, 2001). Another approach to temperamental reactivity focuses not on general emotionality, but on underlying motivational systems that may guide infant behavior (Fox, 1991; Gray, 1982). Specifically, approach-withdrawal tendencies may underlie reactivity in infancy. These tendencies may be represented by distinct neural profiles, including patterns of frontal EEG asymmetry (Fox, 1991; 1994; Fox et al., 1995).
Rothbart (2004) has suggested that these divergent approaches to reactivity, an emotion-based versus a motivation-based approach, may be the result of discrepant terminology and not true disagreement regarding the construct itself. However, no study to-date has examined the extent to which the broader emotion-based approach of Rothbart compliments a motivational approach to understanding infant reactivity. This report is based on data derived from a new and ongoing longitudinal sample of children who were identified on the basis of extreme positive and negative reactivity to auditory and visual stimuli. We screened 779 infants at age 4 months in order to identify groups of infants who displayed patterns of positive and negative reactivity. At 9 months the selected infants and an unselected control group underwent episodes of the Laboratory Temperament Assessment Battery (Lab-TAB; Rothbart & Goldsmith, 1990) which were later coded in terms of general emotionality, including fear, anger and joy; and indices of approach and avoidance. EEG was collected during a baseline state to measure frontal EEG asymmetry (FA).
Behavioral Reactivity
Kagan and his colleagues were the first to select infants on the basis of degree of motor and emotional reactivity in order to examine the temperament of behavioral inhibition. They showed that negatively reactive (NR) infants manifested fearfulness to unfamiliar events at 9 and 14 months and behavioral inhibition (García Coll, Kagan, & Reznick, 1984) at 21 months (Kagan & Snidman, 1991; Snidman, Kagan, Riordan, and Shannon, 1995). Using a similar selection procedure, Fox and his colleagues identified a sample of NR infants and found that at age four, 27% of these children were classified as behaviorally inhibited (Fox, Henderson, Rubin, Calkins, & Schmidt, 2001).
Fox and colleagues (2001; Calkins, Fox & Marshall, 1996) also selected a group of infants at four months of age who were motorically reactive and displayed positive affect in response to stimulation. These children were consistently low in fear and high in sociability throughout the first four years of life. As a group, the positively reactive (PR) infants showed greater continuity of their temperament, as 47 % of PR infants remained continuously non-inhibited and socially exuberant. Exuberance itself is not a maladaptive outcome, as parents likely reinforce displays of sociability and positive affect in their young children. Rothbart and Gunnar characterize approach-driven children as surgent (Ahadi, Rothbart, & Ye, 1993; Gunnar, Sebanc, Tout, Donzella, & van Dulmen, 2003), and approach-related biases, when coupled with emotion dysregulation, may predispose children to negative outcomes of an externalizing nature (Calkins et al., 1996; Putnam & Stifter, 2005; Rubin, Coplan, Fox, & Calkins, 1995).
Frontal EEG Asymmetry and Temperament
Davidson (1995) first suggested that the pattern of frontal EEG asymmetry (FA) might reflect an underlying motivation bias to respond to the environment in a particular hedonic manner. Resting left FA is associated with the propensity toward approach-related tendencies (Pizzagalli, Sherwood, Henriques, & Davidson, 2005); while resting right FA is associated with withdrawal motivation (Sutton & Davidson, 1997). This pattern of frontal EEG asymmetry is apparent in infancy. Infants who respond negatively to stimulation show a pattern of right FA (Buss et al., 2003; Calkins, Fox, & Marshall, 1996). Importantly, continuity in temperament is strongest for children whose behavioral profile is accompanied by a corresponding profile of FA (Fox et al., 2001; Henderson, Fox, and Rubin, 2001; Henderson, Marshall, Fox and Rubin, 2004).
Summary and Hypotheses
This is the first study to-date that has included the Lab-TAB measure as a follow-up to earlier behavioral reactivity coding. The inclusion of this measure at nine months allows for: 1) the validation of the early reactivity paradigm as a method of identifying infants who continue to show approach or withdrawal bias and 2) an elucidation of the nature of the manifestation of approach-withdrawal behaviors, inasmuch as general emotional responding (fear, anger and joy) and approach-withdrawal conflict (approach, avoidance) are examined.
We hypothesized that relative to infants in the unselected control group, NR infants would manifest heightened fear responses and more avoidant behavior to fear-evoking stimuli in the laboratory and would show a pattern of right FA at nine months. We also hypothesized that, relative to infants in the control group, PR infants would manifest high degrees of joy and approach during a pleasure-evoking paradigm and increased negative affect during an anger-evoking paradigm and a corresponding profile of left FA at age nine months.
Method
Participants
Families identified via commercially available mailing lists were sent a letter about the project and were asked to complete a form and send it back to the laboratory. Interested mothers of developmentally healthy infants were scheduled for a laboratory visit between their infant’s 15th and 17th weeks.
Four-Month Selection
We screened 779 infants for degree of reactivity to visual and auditory stimuli at four months (see Calkins, Fox, & Marshall, 1996; Fox et al., 2001). Infant behavior during the reactivity paradigm was subsequently coded as follows: A motor reactivity score was obtained by summing the frequencies of arm waves, arm wave bursts (several waves in rapid succession), leg kicks, leg kick bursts, back arches and hyper extensions throughout the paradigm. A negative affect score was derived by summing the frequencies of fussing and crying and a positive affect score was obtained by summing the frequencies of smiling and positive vocalizations.
The first 100 infants screened were used as a criterion group, i.e., their negative, positive, and motor reactivity scores were used to set the selection criteria for all subsequent infants as follows: Infants who scored above the criterion group mean on both negative affect and motor arousal and below the mean on positive affect served as the NR group (n = 75). Infants who scored above the criterion group mean on both positive affect and motor arousal and below the mean on negative affect served as the PR group (n = 73). Eighty-six infants who did not meet the criteria for either temperament group served as the control sample.
Four reliable raters coded the four-month reactivity paradigm, with pairs of coders achieving intraclass correlation coefficients ranging from .80 to .92. A MANOVA comparing the three temperament groups on the three reactivity dimensions was significant (p< .001). The NR group manifested significantly more negative affect than both the PR and control groups (F (2, 231) = 75.08, p < .001; Tukey’s HSD both p’s < .001). The PR group displayed significantly more positive affect than the NR and the control groups (F (2, 231) = 41.94, p < .001; Tukey’s HSD both p’s < .001). The control group showed significantly less motor activity than both the NR and PR groups (F (2, 231) = 51.17, p < .001; Tukey’s HSD both p’s < .001).
9 Month Laboratory Visit
Based on four-month temperament group status, 278 infants were invited to continue participation, and, of these, 234 participated at nine months. There was no differential attrition based on temperament group. Infants who dropped out were not significantly different from those who remained in the study in terms of negative affect, positive affect, or motor activity.
Of the 234 infants who participated at nine months, 152 (65.8 %) were Caucasian; 31 were African American (13.4 %); 14 Hispanic (6.1 %); 5 Asian (2.2 %), and the remaining 32 children were of other or mixed ethnicity. Eighty-four percent of the children (n = 187) came from intact homes and roughly half of the children (n = 111) had siblings. Mothers averaged 32 years of age (SD = 5.3) and fathers averaged 34 years (SD = 6.1) at the time of the child’s birth.
Observational Ratings of Infant Temperament
Laboratory Assessment Temperament Battery
Several episodes of the Lab-TAB (Goldsmith & Rothbart, 1999) were administered at age 9 months, including Attractive Toy behind Barrier (n = 182), Masks (n = 180), Puppets (n = 182), and Unpredictable Toy (n = 166). All were carried out in accordance with Lab-TAB guidelines (see Hane, Polak-Toste, Ghera, Gunner, & Fox, 2006). Termination due to infant protest was responsible for all of the missing Lab-TAB data. There were no significant group mean differences on four-month reactivity scores between the infants who were missing lab-TAB data and those who were not. The infants missing data were evenly distributed across the four month temperament groups. All raw Lab-TAB ratings were converted to z prior to composite derivation.
The puppet episode was used to assess the emotion of joy and the motivation of approach. A joy composite was created from the puppet episode by averaging the ratings on intensity of smiling (0–2) and the presence of positive vocalization (0, 1), with higher scores reflecting more joy, M = .00, SD = .76. An approach composite was derived by summing the scores for intensity of approach (0–3), intensity of positive motor activity (0–2), and duration of attention to puppets (in sec) and subtracting from this total the intensity of escape behavior (struggling to avoid the stimulus by attempting to crawl out of the highchair, [0–3]), with higher scores representing more behavioral approach relative to avoidance during the puppet paradigm, M = −.12, SD = 1.95.
Two Lab-TAB paradigms were used to assess the emotion of fear and the motivation of avoidance, including masks and unpredictable toy. A fear composite was derived by averaging the ratings for intensity of vocal distress (0–2), intensity of frowning (0–2), and intensity of facial fear (0–2) from the masks and unpredictable toy episodes, with higher scores reflecting more fear, M = .00, SD = .76. An avoidance score was obtained separately for masks and unpredictable toy by rating the intensity of escape (0–3) and subtracting from it intensity of positive motor behavior (0–2) and approach (0–3) from the masks and unpredictable toy paradigms. The avoidance scores from masks and unpredictable toy were then averaged, such that a higher score indicates more behavioral avoidance relative to approach during fear-evoking paradigms, M = −.02, SD = 2.02.
The toy behind the barrier paradigm was chosen to assess the emotion of anger and the motivation of approach. An anger composite was derived by averaging the ratings of intensity of facial anger (0–2), intensity of struggle (0–2), and intensity of vocal distress (0–2), with a higher score indicating more expressed anger, M = −.01, SD = .63.
Prior to coding, interrater reliability was achieved by two independent observers who were blind to all other data in the study. Reliabilities were achieved separately for each of the scales entering into the all lab-TAB composites. Kappas ranged from .86 to .99 (M = .94) for approach; .66 to .83 and (M = .74) for joy; .83 to .98 (M = .89) for avoidance; .72 to .97 (M = .91) for fear; and. 80 to .94 (M = .86) for anger.
Frontal EEG Asymmetry
During the nine-month laboratory visit, infants underwent EEG data collection during a baseline state. The procedure of EEG collection at 9 months has been described in detail elsewhere (Hane & Fox, 2006; Marshall, Bar-Haim, and Fox, 2002). In order to calculate the frontal and parietal asymmetry indices, natural log (ln) 6–9 Hz power data from the mid-frontal and parietal regions (electrodes F3/F4 and P3/P4) were used. Asymmetry was computed as power in the right lead minus power in the left lead for homologous leads. Inasmuch as activation and power in the alpha band are reciprocally related (Davidson, 1988), negative asymmetry index scores represent right EEG asymmetry (increased activation in the right frontal region) while positive index scores represent left EEG asymmetry (increased activation in the left frontal region).
Of the 219 infants who consented to participate in the collection of physiological data, data from 121 (55 %) infants are included in this report. Sixty-two infants had insufficient EEG data to be included in analysis (fewer than 29 DFT windows) due to excessive movement during data acquisition and 27 infants had data that were unusable due to technical problems with certain electrodes. Data from the remaining 130 were inspected for outliers and infants who had frontal asymmetry scores that exceeded (+/−) 3 SD’s were not included in further analyses (n = 9). Infants who were missing asymmetry data were compared to those who were not on all behavioral indices of temperament, including early reactivity and the Lab-TAB variables. There were no significant differences.
Results
Preliminary Analyses
Table 1 provides the relations among the Lab-TAB variables and frontal EEG asymmetry. Table 2 provides the descriptive statistics for the Lab-TAB variables, organized by temperament group. There was no differential placement across the three temperament groups for males or females, χ2 (2) = 3.97, p > .101.
Table 1.
Intercorrelations among the 9-month indices of observed temperament
| Avoidance | Fear | Anger | Joy | Frontal EEG Asymmetry | |
|---|---|---|---|---|---|
| Approach | −.269** | −.219** | .002 | .327** | .030 |
| Avoidance | .207 | −.062 | −.144 | −.116 | |
| Fear | .267** | −.122 | −.050 | ||
| Anger | −.131 | .012 | |||
| Joy | .013 |
Note. p < .05
p < .01
Table 2.
Means and standard deviations on laboratory measures of temperament at 9 months for the three temperament groups.
| Measures | Controls | Negatively Reactive | Positively Reactive | Significance | |||
|---|---|---|---|---|---|---|---|
| N | M (SD) | n | M (SD) | n | M (SD) | ||
| Fear | 53 | .05 (.64) | 54 | −.01 (.33) | 52 | −.09 (.33) | |
| Avoidance | 53 | .11 (1.19) | 54 | .16 (.82) | 49 | −.27 (.92) | NR> Control*; Control>PR* |
| Joy | 74 | −.09 (.92) | 63 | −.11 (.87) | 55 | .26 (1.19) | PR>Control* |
| Approach | 60 | −.11 (1.02) | 55 | −.08 (.77) | 52 | .28 (.97) | PR>NR* |
| Anger | 62 | .11 (.71) | 51 | −.14 (.51) | 57 | −.05 (.58) | |
Note: NR = negatively reactive; PR = positively reactive
The groups significantly differ, p < .05, (post-hoc least square difference test, two-tailed significance).
Four-Month Temperament Groups and Behaviors in the LAB-TAB
A multivariate analysis of variance (MANOVA) was computed, examining group differences on observed joy, approach, fear, avoidance, and anger. A significant Wilks’ Lambda was yielded, Lambda = .86, F (10, 258) = 2.11, p < .05. Univariate effects revealed that the groups differed in degree of avoidance, F (2, 156) = 3.00, p = .05, Eta 2 = .04 and approach, F (2, 164) = 2.70, p = .07, Eta2 = .032. Post-hoc comparisons using Least Squared Differences (LSD) showed that the NR group was significantly more avoidant than the PR group and that the PR group was significantly less avoidant and higher in approach than the control group. A nonsignificant trend also implicated differences in the temperament groups on joy, F (2, 189) = 2.65, p = .07, Eta 2 = .04. Post-hoc LSD comparisons revealed that the PR group manifested more joy than the control and NR infants (p’s < .05 for each comparison; See Table 2).
4 Month Temperament Groups and 9 Month Frontal EEG Asymmetry
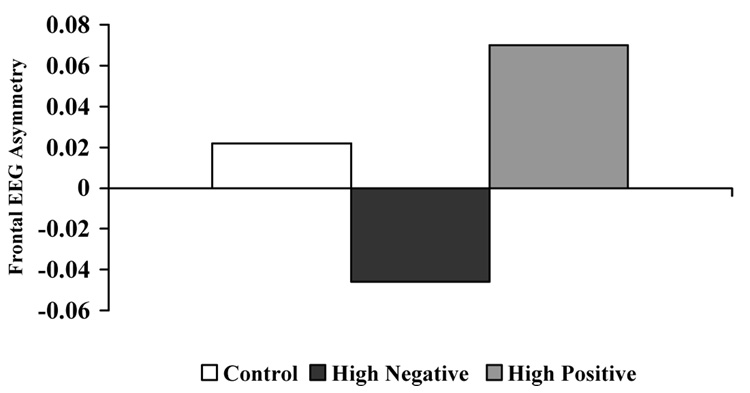
In order to examine the degree to which the temperament groups differed on degree of frontal EEG asymmetry, a univariate ANOVA was computed. The temperament groups showed differing patterns of FA at 9 months, F (2, 118) = 3.58, p < .05, Eta 2 = .06. Post-hoc LSD comparisons revealed that the NR group was significantly different from the PR group, with negative infants showing a pattern of right FA and positive infants showing a pattern of left FA (See Figure 1). A similar analysis comparing the temperament groups on parietal EEG asymmetry was examined and was not significant, F (2, 116) < 1.00, ns, suggesting that the findings regarding EEG asymmetry between the temperament groups are specific to the frontal region.
Figure 1.
Mean frontal EEG asymmetry scores for the temperament groups.
Discussion
We sought to elucidate the nature of temperamental reactivity in infancy by following groups of infants who were selected at 4 months on the basis of positive and negative affective and motor reactivity. At age 9 months, baseline EEG data were collected and these infants were assessed using the Lab-TAB (Rothbart & Goldsmith, 1990) to examine differences in emotional expressivity and approach-avoidance behavior.
We found that NR infants selected at 4 months were significantly more likely to show a pattern of avoidance at 9 months in response to fear-evoking stimuli that was characterized by intensely struggling to escape the situation while manifesting low levels of interest in, or approach toward, the aversive targets. NR infants did not manifest significantly higher levels of overt fear, as evidenced by negative facial affect and vocal distress. This null finding may be due to the ambiguity in the source of infant distress signals during the fear-evoking paradigms (Oster, Hegley, & Nagel, 1992) which may make the coding of fear more challenging than escape behavior. However, it is important to note that negative affect is not a central feature of either behavioral inhibition or social reticence. Instead, these two established sequelae of negative reactivity in infancy are characterized by hesitance to approach, or avoidance of, ominous stimuli and social situations. Hence, general measures of fear may not sufficiently capture approach-avoidant conflicts in infancy.
We hypothesized that infants who manifested a pattern of positive emotion reactivity in infancy would continue to display joy and approach during a playful encounter with an experimenter. This hypothesis was supported and these findings offer the first evidence indicating that infants selected on the basis of positive emotion reactivity display a unique pattern of behavior that is typified by both joyfulness and approach tendencies when presented with social stimuli later in infancy. Hence, it appears that broader indices of positive emotionality and more specific approach behavior are relevant dimensions of positive reactivity.
In contrast to our hypothesis, PR infants did not manifest significantly more anger than the other temperament groups. Previous research has found approach-driven children to be at-risk for problems of an externalizing nature (Calkins, Fox, & Marshall, 1996; Donzella et al., 2000; Putnam & Stifter, 2005). The factors that place approach-driven infants at-risk for the development of externalizing problems may not be apparent at 9 months. This finding is consistent with the report of Calkins et al. (1996), which showed no relation between positive reactivity at four months and maternal report of distress to limits at nine months. Derryberry and Rothbart (2001) suggest that approach tendencies in infancy contribute to the development of negative emotionality later in childhood, as the demand for voluntary self control increases and our findings support this notion.
Our hypothesis regarding FA was supported. The asymmetry findings, and the weak association between observed approach and avoidance (see Table 1), support of our view that approach and withdrawal behavior represent separate dimensions of reactivity and that bias on either dimension is represented by a distinct neurological profile (Calkins, Fox & Marshall, 1996; Fox, 1991; 1994; Fox et al., 2001), with withdrawal-prone infants showing a pattern of right FA and approach-driven infants showing left FA. Davidson (2000, 2004) has suggested that these asymmetries may partly reflect activation of specific areas of prefrontal cortex as they modulate or inhibit the activity of sub-cortical sites such as the amygdala, the limbic structure responsible for detecting and responding to novelty with fight or flight reactions. Observed approach and avoidance did not correlate significantly with FA, which may be a function of low statistical power or that FA was no obtained during the fear-inducing tasks themselves. Buss et al. (2003) found that right FA was associated with withdrawn behaviors only when EEG was recorded in-vivo during a stranger approach. Also, FA has been shown to indirectly predict social outcomes for these two temperament styles beyond infancy (e.g., Fox et al., 2001). Our future research will examine the role of FA as a mediator in the relations between early reactivity and later outcomes, including inhibition, exuberance, and social competence.
Limitations
Behavioral measures such as the Lab-TAB are not without limitation (Hane et al., 2006) and although the Lab-TAB paradigms are designed to elicit targeted emotions, it can’t be determined with any certainty that infant responses are a direct function of the Lab-TAB stimuli. For example, infants may have manifested negative affect that was not anger in response to limits, but distress due instead to carry-over effects from other Lab-TAB paradigms or to the broader testing situation, including restricted access to mother. This is supported by the positive association between the fear and anger variables. Additionally, although statistically significant, the effect sizes in the relations between early reactivity and the Lab-TAB and FA measures are quite modest, indicating that there are likely other contributors to the development of approach-withdrawal bias in infancy that have not been addressed in this report.
Summary and Conclusions
The findings reported here indicate that continuity of approach bias includes continued manifestation of approach behaviors and the expression of positive affect, but not anger. Withdrawal bias at nine months was expressed in terms of avoidance, and not the expression of fear. It has been suggested that the field of temperament is in need of a unified language, so that apparently discrepant, but conceptually similar constructs, do not give rise to debate that does not in fact exist (Rothbart, 2004). Findings of this report suggest that approach-withdrawal behavior is an important dimension of reactivity and that reliance on behavioral measures of general emotionality (i.e., fear and anger) may not successfully index the continued manifestation of approach or withdrawal tendencies from early to mid infancy.
Acknowledgements
This research was supported by National Institute of Health Grant HD 17899 to Nathan A. Fox. We thank Kristin Ross and Stacey Barton for their assistance with data collection and Laura Driscoll for her assistance with data collection and coding. We are deeply indebted to the families who have participated and continue to participate in this research.
Footnotes
Comparison of the break-down by gender within only the NR and PR groups shows a slight overrepresentation of females in the NR group (46 vs. 29) and males in the PR group (40 vs. 33), χ2 (1) = 3.87, p = .05.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at http://www.apa.org/journals/dev/
Contributor Information
Amie Ashley Hane, Williams College.
Nathan A. Fox, University of Maryland College Park
Heather A. Henderson, University of Miami
Peter J. Marshall, Temple University
References
- Ahadi SA, Rothbart MK, Ye R. Children’s temperament in the US and China: Similarities and differences. European Journal of Personality. 1993;7:359–377. [Google Scholar]
- Buss KA, Schumacher JR, Malmstadt, Dolski I, Kalin N, Goldsmith HH, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behavioral Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibition in infancy. Child Development. 1996;67:523–540. [PubMed] [Google Scholar]
- Davidson RJ. EEG measures and cerebral asymmetry: Conceptual and methodological issues. International Journal of Neuroscience. 1988;39:71–89. doi: 10.3109/00207458808985694. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Asymmetric brain function, affective style, and psychopathology: The role of early experience and plasticity. Development and Psychopathology. 1995;6:741–758. [Google Scholar]
- Davidson RJ. Affective style, psychopathology, and resilience: Brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biological Psychiatry. 2004;67:219–223. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Donzella B, Gunnar MR, Krueger WK, Alwin J. Cortisol and vagal tone responses to competitive challenge in preschoolers: Associations with temperament. Developmental Psychobiology. 2000:209–220. doi: 10.1002/1098-2302(2000)37:4<209::aid-dev1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Fox NA. If it’s not left, it’s right: Electroencephalograph asymmetry and the development of emotion. American Psychologist. 1991;46:863–872. doi: 10.1037//0003-066x.46.8.863. [DOI] [PubMed] [Google Scholar]
- Fox NA. Dynamic cerebral processes underling emotion regulation. In: Fox NA, editor. Emotion regulation: Behavioral and biological considerations. Monographs of the Society for Research in Child Development. Vol. 59. 1994. pp. 152–166. (Serial No. 2–3) [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins S, Marshall TR, Coplan RJ, Porges SW, et al. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Rubin KH, Calkins SD, Marshall TR, Coplan RJ, Porges SW, Long J, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. [PubMed] [Google Scholar]
- García Coll C, Kagan J, Reznick SJ. Behavioral inhibition in young children. Child Development. 1984;55:1005–1019. [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery (version 1.3; locomotor version) Eugene: University of Oregon; 1990. Unpublished manuscript. [Google Scholar]
- Gray JA. The Neuropsychology of Anxiety. London: Oxford University Press; 1982. [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, van Dulmen MMH. Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology. 2003;43:346–358. doi: 10.1002/dev.10144. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA. Ordinary variations in maternal caregiving of human infants influence stress reactivity. Psychological Science. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- Hane AA, Fox NA, Polak-Toste C, Ghera MM, Guner BM. The contextual basis of maternal perceptions of infant temperament. Developmental Psychology. 2006;42:1077–1088. doi: 10.1037/0012-1649.42.6.1077. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75:236–250. doi: 10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychological Science. 1991;2:40–44. [Google Scholar]
- Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Development. 1998;69:1483–1493. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition. Child Development. 1987;58:1459–1474. [PubMed] [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Oster H, Hegley D, Nagel L. Adult judgments and fine-grained analysis of infant facial expressions: Testing the validity of a priori coding formulas. Developmental Psychology. 1992;28:1115–1131. [Google Scholar]
- Pizzagalli DA, Sherwood R, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness. Psychological Science. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Stifter CA. Behavioral approach-inhibition in toddlers: Prediction from infancy, positive and negative affective components, and relations with behavior problems. Child Development. 2005;76:212–226. doi: 10.1111/j.1467-8624.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Commentary: Temperament and the pursuit of an integrated developmental psychology. 50th anniversary special issue - Merrill-Palmer Quarterly. 2004;50(4):492–505. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of Temperament at three to seven years: The Children's Behavior Questionnaire. Child Development. 2001;72(5):1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Coplan RJ, Fox NA, Calkins SD. Emotionality, emotion regulation, and preschoolers' social adaptation. Development and Psychopathology. 1995;7:49–62. [Google Scholar]
- Snidman N, Kagan J, Riordan L, Shannoon DC. Cardiac function and behavioral reactivity during infancy. Psychophysiology. 1995;32:199–207. doi: 10.1111/j.1469-8986.1995.tb02949.x. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. [Google Scholar]