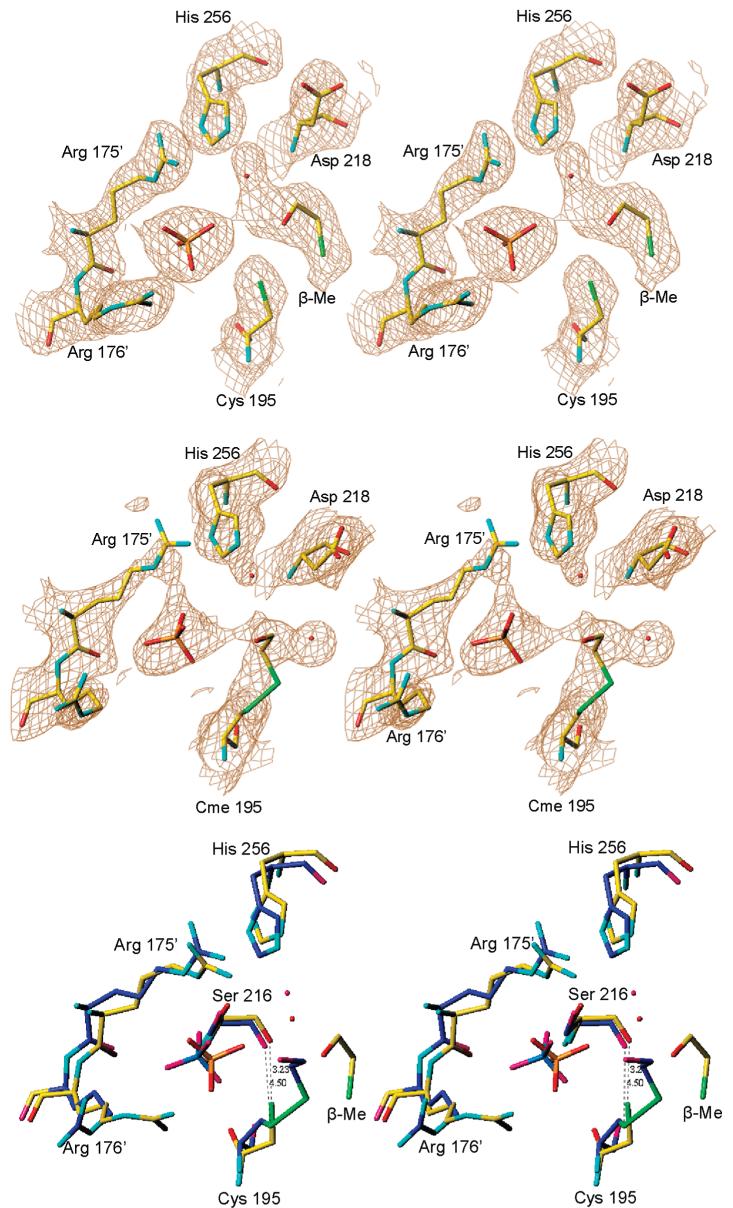
Figure 5.
Stereoview of the active sites of the dimer in crystal form 2 with 2Fo - Fc electron density, contoured at the 1.0σ level, for subunits A (top) and B (middle). Superposition of subunits A (in yellow) and B (in blue) is shown in the bottom panel. The β-mercaptoethanol molecule is connected to the side chain of Cys195 by a disulfide bond in subunit B while it forms a noncovalent adduct in subunit A. The distances from Cys195 to Ser216 are also different. Different oxidation states of Cys195 reflect its propensity to oxidation.