Abstract
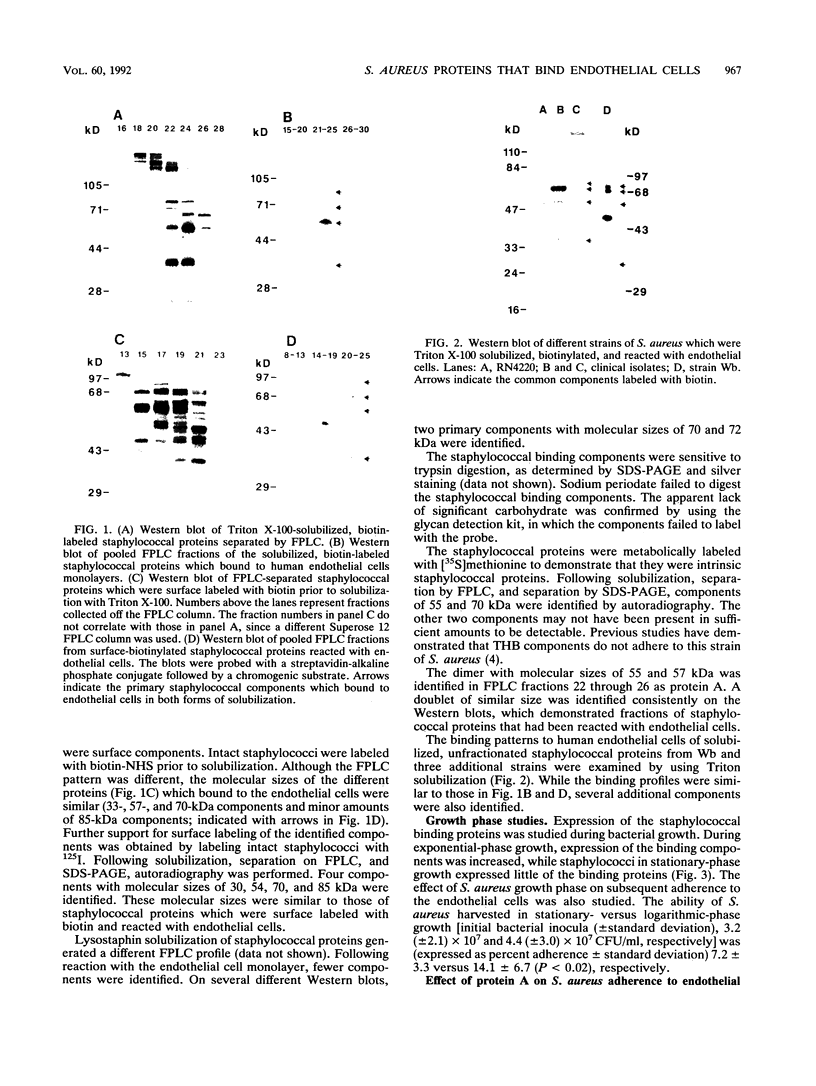
The adherence of Staphylococcus aureus to human endothelial cells is saturable in both dose- and time-dependent assays. Staphylococcal surface components which bound to endothelial cells in vitro were identified by using biotin-labeled, solubilized staphylococcal proteins. Four trypsin-sensitive components with molecular sizes of 30, 55 to 57, 70, and 85 kDa were recognized. These proteins did not label with the glycan detection system. When staphylococci were harvested during the exponential phase of growth, staphylococcal adherence to endothelial cells was significantly increased and increased expression of the S. aureus binding proteins was observed. Preincubation of endothelial cells with protein A did not reduce S. aureus adherence in an in vitro infection assay. Four S. aureus surface components whose expression is growth phase dependent adhere to human endothelial cells in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole G. W., Silverberg N. L. The adherence of Staphylococcus aureus to human corneocytes. Arch Dermatol. 1986 Feb;122(2):166–169. [PubMed] [Google Scholar]
- Coleman G., Jakeman C. M., Martin N. Patterns of total extracellular protein secretion by a number of clinically isolated strains of Staphylococcus aureus. J Gen Microbiol. 1978 Jul;107(1):189–192. doi: 10.1099/00221287-107-1-189. [DOI] [PubMed] [Google Scholar]
- Elliott D. A., Hatcher V. B., Lowy F. D. A 220-kilodalton glycoprotein in yeast extract inhibits Staphylococcus aureus adherence to human endothelial cells. Infect Immun. 1991 Jun;59(6):2222–2223. doi: 10.1128/iai.59.6.2222-2223.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Speziale P., Hök M. Isolation and characterization of a fibronectin receptor from Staphylococcus aureus. J Biol Chem. 1987 May 15;262(14):6564–6571. [PubMed] [Google Scholar]
- Gordon P. B., Sussman I. I., Hatcher V. B. Long-term culture of human endothelial cells. In Vitro. 1983 Sep;19(9):661–671. doi: 10.1007/BF02628957. [DOI] [PubMed] [Google Scholar]
- Gould K., Ramirez-Ronda C. H., Holmes R. K., Sanford J. P. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975 Dec;56(6):1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers J. M., Proctor R. A. Reduced adherence to traumatized rat heart valves by a low-fibronectin-binding mutant of Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2306–2312. doi: 10.1128/iai.57.8.2306-2312.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lopes J. D., dos Reis M., Brentani R. R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985 Jul 19;229(4710):275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A. H., Nowlan P., Weavers E. D., Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987 Dec;55(12):3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén C., Yacoub A. I., Maxe I., Heinegård D., Oldberg A., Franzén A., Ljungh A., Rubin K. Specific binding of bone sialoprotein to Staphylococcus aureus isolated from patients with osteomyelitis. Eur J Biochem. 1989 Sep 15;184(2):331–336. doi: 10.1111/j.1432-1033.1989.tb15023.x. [DOI] [PubMed] [Google Scholar]
- Sanford B. A., Davison V. E., Ramsay M. A. Staphylococcus aureus adherence to influenza A virus-infected and control cell cultures: evidence for multiple adhesins. Proc Soc Exp Biol Med. 1986 Jan;181(1):104–111. doi: 10.3181/00379727-181-42230. [DOI] [PubMed] [Google Scholar]
- Sheagren J. N. Staphylococcus aureus. The persistent pathogen (second of two parts). N Engl J Med. 1984 May 31;310(22):1437–1442. doi: 10.1056/NEJM198405313102206. [DOI] [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M. Isolation and characterization of a putative collagen receptor from Staphylococcus aureus strain Cowan 1. J Biol Chem. 1989 Dec 15;264(35):21080–21086. [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tompkins D. C., Hatcher V. B., Patel D., Orr G. A., Higgins L. L., Lowy F. D. A human endothelial cell membrane protein that binds Staphylococcus aureus in vitro. J Clin Invest. 1990 Apr;85(4):1248–1254. doi: 10.1172/JCI114560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P. T., Lai L. W., Drake T. A., Sande M. A. Effect of fibronectin on adherence of Staphylococcus aureus to fibrin thrombi in vitro. Infect Immun. 1985 Apr;48(1):83–86. doi: 10.1128/iai.48.1.83-86.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Weinstein L., Schlesinger J. J. Pathoanatomic, pathophysiologic and clinical correlations in endocarditis (first of two parts). N Engl J Med. 1974 Oct 17;291(16):832–837. doi: 10.1056/NEJM197410172911609. [DOI] [PubMed] [Google Scholar]
- von Boxberg Y., Wütz R., Schwarz U. Use of the biotin-avidin system for labelling, isolation and characterization of neural cell-surface proteins. Eur J Biochem. 1990 Jun 20;190(2):249–256. doi: 10.1111/j.1432-1033.1990.tb15569.x. [DOI] [PubMed] [Google Scholar]






