Introduction
The remineralization potential of a fluoride containing novel resin-based calcium phosphate (Ca-PO4) cement developed for pulp capping or lining purposes has recently been reported [1]. The cement consists of tetracalcium and dicalcium phosphates (TTCP and DCPA, respectively), an acidic (carboxylated) monomer, and a reinforcing hydrophobic co-monomer. The calcium phosphates employed as fillers in the cement are similar to those used in a water-based self-setting bone cement [2,3]. In the set cement, a polymer network surrounds the mineral phase consisting of hydroxyapatite and other calcium phosphates. The reasonably high strength of the cement suggests that it may be suitable as a base when placed under permanent restorative materials [1]. The conversion of the calcium phosphate components to hydroxyapatite, which is typically observed during the setting of water-based cements, is delayed in the resin-based cement. Therefore calcium and phosphate ions released from the cement are available and have been shown to remineralize demineralized tooth hard tissue [1].
The here reported cement has been designed to be placed directly on the cavity floor as a lining or direct or indirect pulp capping cement. After insertion, it will be covered with a restorative material to complete the restoration. While it has been shown that the cement moderately adheres to dentin without use of a bonding agent [4], it may be desirable to improve the adhesion between dentin and cement by placing a bonding agent prior to inserting the cement and the final restoration. However, bonding agents, which usually contain acidic monomers, could interact with calcium ions diffusing out of the cement and reduce the remineralization. The presence of fluoride in the cement could also affect the remineralization as calcium and fluoride ions could form calcium fluoride (CaF2), thereby reducing the calcium ions available for the remineralization of carious dentin lesions.
Therefore, it was the purpose of this study to examine in a 2 × 2 factorial design the hypothesis that fluoride in the cement (factor 1) or a barrier of a bonding agent (factor 2) could compromise the remineralization potential of the cement.
Materials and Methods
Materials
The solid phase of the cements consisted of TTCP and DCPA. To maintain a higher pH in the final cement mixture, a calcium-enriched TTCP, termed TTCP2.05, was prepared; its preparation was described in detail previously [1]. The subscript ‘2.05’ denotes that the TTCP preparation had a calcium (Ca) to phosphate (PO4) ratio of 2.05, as it contained a small amount of basic calcium oxide. The median particle diameter of ground TTCP2.05 was 16 µm and of ground DCPA 1.1 µm. The particle sizes were measured with a centrifugal particle size analyzer (Shimadzu SA-CP3, Columbia, MD, USA). Two monomers, the acidic pyromellitic glycerol dimethacrylate (PMGDM; Paffenbarger Research Center, Gaithersburg, MD, USA) and the co-monomer ethoxylated bisphenol A dimethacrylate (EBPADMA; Sartomer Co., West Chester, PA, USA) were used. The cement components (Table 1) were pre-mixed into two pastes. To prepare Paste 1, the respective ingredients were hand mixed. To prepare Paste 2, the ingredients were added to methylene chloride, tumbled for 30 min and the solvent was removed by vacuum suction. To enable a chemical cure after mixing benzoyl peroxide was added to Paste 1 and the aromatic tertiary amine, N,N-dimethylaminophenethanol was added to Paste 2. To make the cement light-curable camphorquinone was also used. Immediately before sample preparation, the pastes were mixed at a ratio of one part of Paste 1 to two parts of Paste 2 for about 30 s. Table 1 displays the composition of Cements A and B showing the components in each paste and proportions after mixing. Cement A is a fluoride-free cement, while Cement B contains fluoride.
Table 1.
Composition of Cements A and B showing the mass fraction % of components after mixing. (Components 1 through 6 are combined in Paste 1, components 7 through 9 in Paste 2.)
| Cement A | Cement B | |
|---|---|---|
| Paste 1: | ||
| 1. DCPA# | 19.6 | 19.3 |
| 2. PMGDM## | 8.2 | 8.0 |
| 3. water | 3.0 | 2.9 |
| 4. benzoyl peroxide | 0.4 | 0.4 |
| 5. camphorquinone | 0.1 | 0.1 |
| 6. sodium hexafluorosilicate | --- | 1.6 |
| Paste 2: | ||
| 7. TTCP2.05* | 60.4 | 59.5 |
| 8. EBPADMA** | 8.2 | 8.1 |
| 9. DMAPE*** | 0.1 | 0.1 |
dicalcium phosphate anhydrous
pyromellitic glycerol dimethacrylate
calcium enriched tetracalcium phosphate
ethoxylated bisphenol A dimethacrylate
N,N-dimethylaminophenethanol.
Methods
Four experimental treatments were designed: A = Cement A; A2 = Cement A + bonding agent; B = Cement B; B2 = Cement B + bonding agent. For the fluoride, calcium and phosphate analyses six disks, measuring 15 mm in diameter and 1 mm high, were prepared from Cement A and six disks from Cement B. The disks were made by curing the respective mixed cements for 1 min on each side in Teflon molds under the light guides of 3 dental curing lights (≈ 400 mW/cm2 to 600 mW/cm2; MAX, Dentsply Int., Milford, DE, USA). A piece of fishing line was inserted into the uncured cement to allow free suspension of the cement specimen in solution. For simulating the use of bonding agents (treatment A2 and B2), three disks of Cement A and three disks of Cement B were coated with the bonding agent Prime One (Mirage, Chameleon Dental Products, Kansas City, KS, USA). This bonding agent is comprised of the acidic, carboxylated monomer PMGDM, a hydrophilic diluent co-monomer, polymerization activators, and a solvent. Approximately 60 µL bonding agent was used to coat the cement surfaces. The bonding agent was brushed onto the specimen and dried gently with an air stream at a distance of 7 cm from the specimen surface. A Mylar film was laid over the bonding agent, which was then cured for 1 min. The edges were coated with bonding agent to which a chemical activator had been added. The edges were cured without light for 5 min. Each side was thereby covered with an approximately (30 to 45) µm thick bonding agent layer. Each disk was placed in 100 mL saliva-like solution (SLS) formulated without fluoride having a composition of 50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer, 1.14 mmol/L calcium chloride, 0.59 mmol/L potassium dihydrogenphosphate, and 30 mmol/L potassium chloride, adjusted to pH 7.0 with potassium hydroxide. The specimens were stored at 37 °C for up to 45 d. At given time intervals, 1.5 mL of the solution was removed for analysis and replaced by fresh solution. After mixing equal volumes of test solution and TISAB (total ionic strength adjustment buffer solution, Fisher Scientific, Fair Lawn, NJ, USA), the amount of fluoride released was measured with a combination of a fluoride ion selective electrode (all electrodes: Orion, Cambridge, MA, USA) and the reference part of a combination pH electrode. Fluoride standard solutions ranging from 1 × 10−6 mol/L to 1 × 10−4 mol/L were measured to form a calibration curve, which was used to calculate the fluoride concentration (mmol/L). The total calcium and the total phosphate ion concentrations were measured spectrophotometrically by using known standards and calibration techniques [5]. In the reagent for the calcium analysis, concentrated formic acid was used instead of glacial acetic acid and potassium hydroxide instead of tetrabutyl ammonium hydroxide. Computed standard deviations are reported as a measure of the standard uncertainty.
Remineralization of artificial dentin lesions
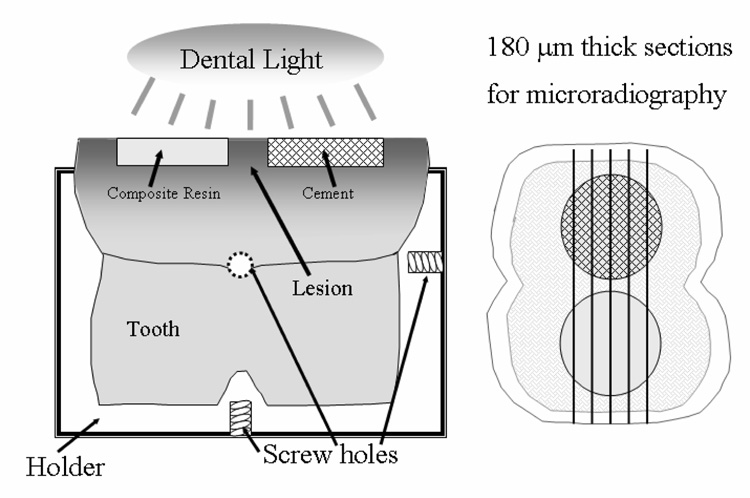
Sixteen extracted human molars, free of any blemishes, were sectioned from their roots about 5 mm below the cemento-enamel junction and embedded vertically with composite resin in square stainless steel mounting devices with machined screw holes (Figure 1) [1]. These devices allowed for repositioning of the teeth after the treatment with respect to their longitudinal axes. The upper third of the crowns were cut off parallel to the bottom of the holder, and two cavities, 3 mm in diameter and 1 mm deep, were machined into each dentin surface under water irrigation. The enamel was coated with wax and the dentin surfaces were exposed to 20 mL demineralizing solution for 48 h. The demineralizing solution (pH 4.3) consisted of an aqueous solution of 0.075 mol/L acetic acid, 0.002 mol/L calcium (from CaCl2), and 0.002 mol/L phosphate (from KH2PO4). After rinsing, the surfaces were kept slightly moist under moistened tissue paper. For the bonding agent-free treatments (groups A and B), the bottom of the cavity receiving the calcium- and phosphate-free control material was covered with an experimental adhesive composite resin, which was cured for 10 s.
Figure 1.
Experimental setup for the remineralization study.
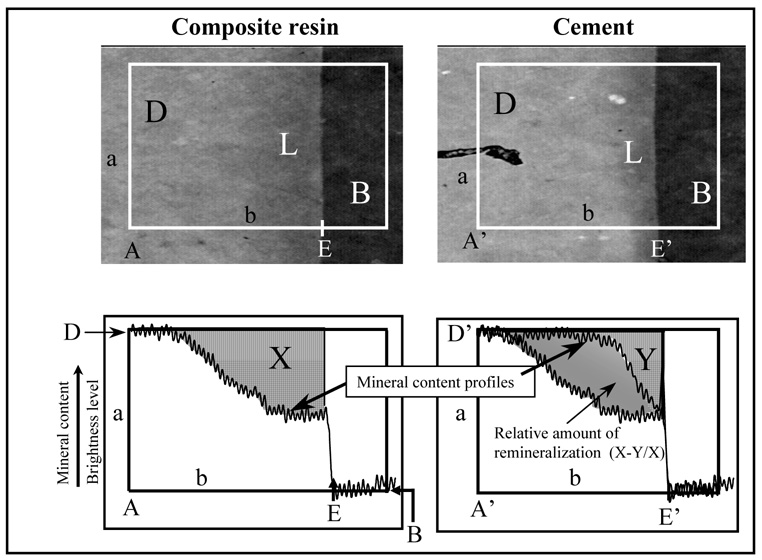
The restoration was completed with a hybrid composite resin (TPH, Dentsply Int., Milford, DE, USA). The other cavity received either Cement A or Cement B. For groups A2 and B2 (with bonding agent), each cavity was coated with two layers of Prime One (Chameleon Dental Products), which was dried for 15 s and light-cured for 10 s prior to inserting composite resin or the respective cement. Following the completion of the cement and control material restorations, the restorations were light cured simultaneously for 1 min. Each tooth was placed in 100 mL fluoride-free saliva-like solution (1.2 mmol/L calcium, 0.72 mmol/L phosphate, 30 mmol/L KCl, 50 mmol/L HEPES [4-(2-hydroxethyl)-1-piperazineethanesulfonic acid] adjusted to pH 7.0 with concentrated hydrochloric acid) and incubated at 37 °C for 6 weeks. From each tooth two to five transverse sections, about 180 µm thick, were cut as illustrated in Figure 1. Two sections from each tooth were randomly selected; on each section four images were analyzed. Contact microradiographs were obtained by placing the sections on Kodak SO343 film (Eastman Kodak Co., Rochester, NY, USA), and exposing them for 13 min to CuKα radiation generated at 40 kV and 3 mA (Faxitron Model #43855A, Hewlett Packard, McMinnville, OR, USA) at a distance of 14 cm. The film was developed according to manufacturer’s recommendations. The microradiographs were examined with a digital imaging analysis software (NIH, Bethesda, MD, USA) to quantitatively assess the mineral content of the lesions and establish if any remineralization had occurred [6,7,1]. This procedure entails a digital imaging technique that captures the gray levels (brightness) of rectangular sections (with sides a and b, Figure 2) of the radiographic image that extends from the cement or the highly filled composite resin into the unaltered dentin. The rectangle measures approximately 330 µm × 220 µm in size, using 256 (gray) levels of intensity resolution, and a spatial resolution of 1.33 µm in horizontal and 1.37 µm in vertical direction. The microradiographs were normalized using an aluminum step wedge as an internal reference standard as previously described [7]. In accordance with the International System of Units, the unit used for the measured mineral loss (Delta Z) is micrometers (µm) instead of the more frequently reported µm·vol % (1 µm = 100 µm·vol %) [6]. Mineral content profiles from the area underneath Cement B and from underneath the composite resin are shown in Figure 2, which was taken from a previous publication [1] solely to illustrate the concept. The relative mineral content of the lesion is equal to the area defined by the mineral profile and lines a and b. Accordingly, ΔZ under the composite resin is equal to area X and under the cement to area Y. From that, the percent remineralization can be calculated as: ((X–Y)/X)*100). The lesion depth was taken as the point at which the mineral content was 95 % of the mineral content of unaltered dentin (D, Figure 2) [6,7]. To minimize the influence from ions diffusing through gaps between restoration and tooth, only the bottoms of the cavities were analyzed. For each of the treatment groups A, A2, B, and B2 four teeth had been prepared. From each tooth two slices and on each slice four images (rectangles) across the cavity floor extending from sound dentin into the cement were acquired and analyzed. To establish the variations between neighboring cavities, two slices of each of five additional teeth having only the demineralized side-by-side cavities were also analyzed.
Figure 2.
Example microradiographs and the corresponding computed mineral density profiles of artificial lesions under the cements and under the composite resin (control material). The remineralization is calculated from ((X–Y)/X), where X is the mineral loss under the composite resin and Y is the mineral loss under the cement, D is the unaltered dentin, L is the lesion, and B is the background. (Profiles from Dickens et al., 2003)
Statistical analyses
Computed standard deviations are reported as a measure of the standard uncertainty. One way analysis of variance (ANOVA) was used to establish if significant differences existed between groups (one-way ANOVA, Sidak-Holmes post-hoc treatment). Pearson’s correlation coefficients and paired t-test were used to demonstrate that the 48 h demineralization treatment produced identical lesion depths and mineral loss in the two side-by side lesions. Two-way ANOVA was applied to determine whether the presence of fluoride (factor 1) or an acidic bonding agent (factor 2) affected the outcome of the dentin remineralization. The threshold for statistical significance was set at α = 0.05.
Results
Calcium, phosphate and fluoride ion release
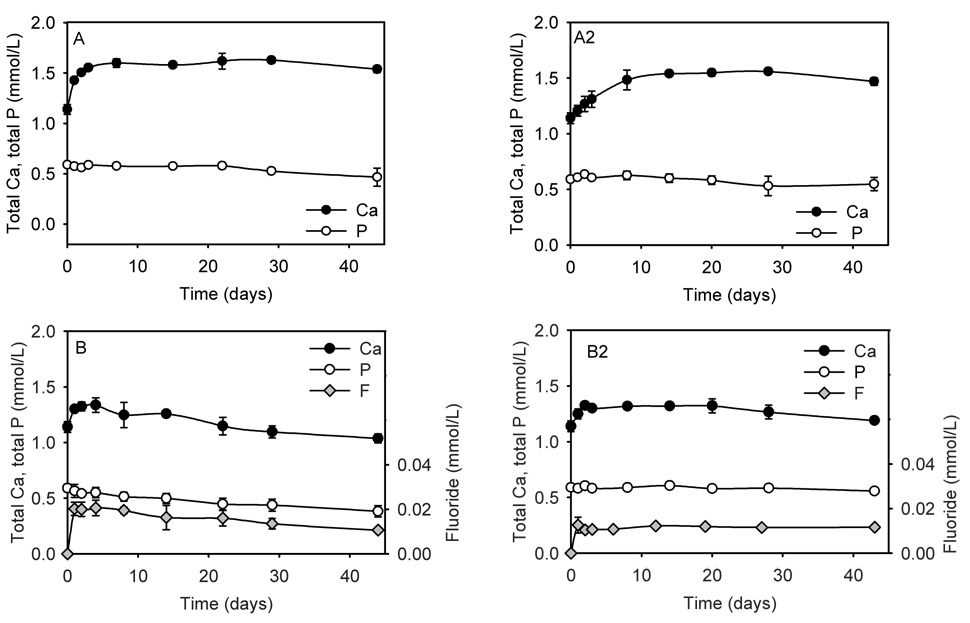
The cumulative calcium, phosphate and fluoride concentration profiles up to 6 weeks in SLS are shown in Figure 3. For cements A and A2 there was an initial increase from 1.14 mmol/L (SLS) to about 1.5 mmol/L in the calcium concentrations (please note that total calcium and total phosphate were measured), after which the level remained essentially stable. Due to the use of TTCP2.05 the calcium concentrations were much higher than the phosphate concentrations, which remained mostly unchanged from the 0.59 mmol/L in SLS over the experimental time period. Cement B and B2 showed an initial increase in calcium concentrations up to day 3. After that the concentrations steadily decreased for Cement B below, and for Cement B2 just slightly above, the calcium concentration in SLS (1.14 mmol/L). For Cement B the phosphate concentration decreased with time; for B2 it remained unchanged. The fluoride release from both B cements showed an initial burst and then slowly decreased (Cement B) or remained unchanged (Cement B2). The thickness of specimens before and after immersion was recorded and the percent increase was: Group A (1.8 ± 0.8); Group A2 (1.4 ± 0.2); Group B (3.6 ± 0.9); and Group B2 (5.8 ± 2.6). A two-way ANOVA showed that the presence of fluoride had a strong effect (p = 0.006) on the expansion, while the bonding agent had no effect (p > 0.05). The mass changes were also recorded. However, they are not reported, since under the experimental conditions the mass changes are effected by, water sorption, solubility and the surface deposition of hydroxyapatite (HAp) and fluorapatite (FAp). Of these events, water sorption and apatite deposition cannot easily be separated from each other.
Figure 3.
Total calcium and total phosphate ion concentrations after incubation of Cements A, A2 (A + bonding agent), B, and B2 (B + bonding agent) in saliva like solution. The fluoride concentrations of Cements B and B2 are also shown. The vertical bars show the standard deviation. If not visible they lie within the symbols.
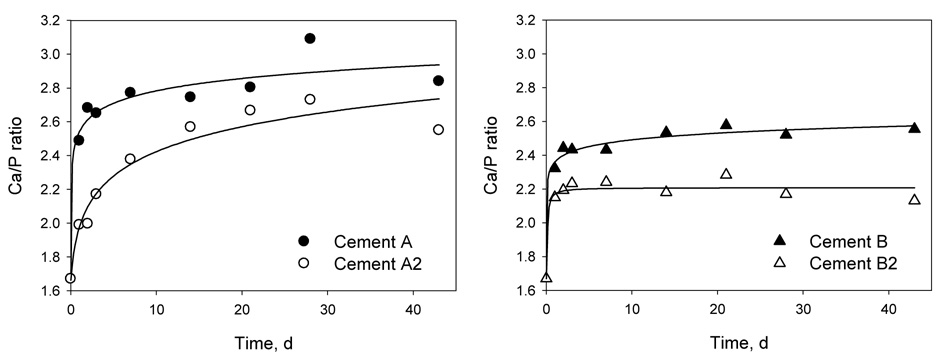
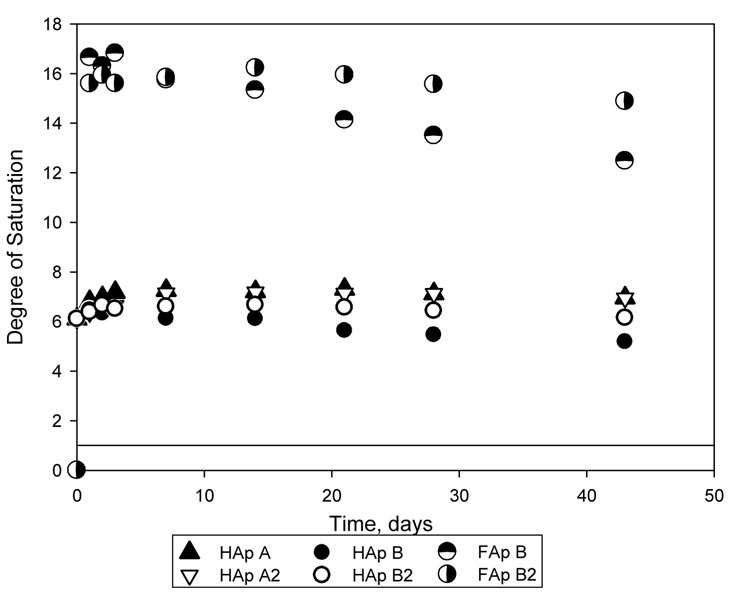
The ratios of Ca to PO4 in the ion solutions are shown in Figure 4. All reported Ca-to-P ratios were greater than 1.9, which was the Ca/P ratio of the SLS. The saturation levels for HAp or FAp calculated from ion solution concentrations are shown in Figure 5. A saturation level above 1 indicates that a solution is supersaturated with respect to a particular mineral. The dashed line represents the saturation level calculated for the SLS used in the experiments. The saturation levels for dicalcium phosphate dihydrate (DCPD), and for CaF2, minerals that could easily form and affect the precipitation of HAp and FAp, were below 0.5 for DCPD and 0.15 for CaF2. These results indicated that the solutions were undersaturated with respect to DCPA and CaF2. As shown in Figure 5, up to 42 days the solutions are supersaturated with respect to HAp and FAp, regardless of the presence of a bonding agent coating.
Figure 4.
Ratios of total calcium to total phosphate (Ca/P) ion concentrations as a function of time. The standard errors of the estimate for Cements A, A2, B and B2 are 0.113, 0.159, 0.041, and 0.053, respectively. (Solid symbols: Cements A and B; hollow symbols: Cements A2 and B2).
Figure 5.
Degree of saturation for hydroxyapatite (HAp) for Cements A, A2, B, and B2, and for fluorapatite (FAp) for Cements B and B2 calculated from the calcium, phosphate and fluoride ion solution concentrations. Solutions with saturation levels above 1 (solid line) are supersaturated with respect to the particular mineral.
Remineralization experiments
To determine whether the two side-by-side cavities had similar lesion depth and mineral loss values at the beginning of the experiment, the lesions that were created in the 48 h demineralization procedure were compared side-by-side in 10 slices obtained from 5 teeth. A paired t-test of the cavity pairs demonstrated that after demineralization two side-by-side cavities had statistically similar mineral loss (p = 0.464) and lesion depth values (p = 0.158). The Pearson correlation of the two neighboring cavities was performed and gave values of p < 0.001 and correlation coefficients of 0.981 and 0.843 for mineral loss and lesion depths, respectively. Table 2 presents the data for the mineral loss and lesion depths of the cements A, A2, B, B2, and the demineralized untreated lesions. To determine whether any remineralization had occurred in the lesions under the composite resin over the 6 week incubation time, mineral loss and lesion depth of the cavities that contained the composite resin were compared to the respective property of the 48 h-demineralized teeth. For both properties no statistically significant difference were found among the groups (One-way ANOVA, p > 0.05). Thus, it was assumed that the lesion mineral content under the control material represented the initial stage, and that it was acceptable to use the corresponding control mineral loss as an internal standard. The % remineralization, which was determined from the ratio of ‘mineral loss under the cement’ to ’mineral loss under the control’, is shown in Table 2. For this property, one-way ANOVA showed that Cement B2 had significantly lower remineralization than Cements A and B. There were no differences between Cements A, A2 and B. The factorial analysis using two-way ANOVA revealed a highly significant effect from the bonding agent (p < 0.001), while the presence of fluoride was not significant (p > 0.05).
Table 2.
Effects of fluoride (F) and bonding agent (BA) on mean and standard deviation in parenthesis of lesion depth, mineral loss (ΔZ), and % remineralization. The increase in specimen thickness after soaking is also shown.
| Lesion Depth (µm) | ΔZ (µm) | % Remineralization | Thickness (%) | ||||
|---|---|---|---|---|---|---|---|
| n | Control$ | Cement | Control$ | Cement | |||
| Cement A | 8 | 203 (35)# | 134 (33) | 69 (21) | 44 (22) | 38 (14)A* | 1.9 (0.8)a* |
| Cement A2 (+BA) | 8 | 164 (26) | 123 (19) | 56 (19) | 42 (10) | 23 (11)A,B | 1.4 (0.2)a |
| Cement B (F) | 8 | 202 (36) | 138 (30) | 63 (20) | 40 (15) | 35 (19)A | 3.6 (0.9)a,b |
| Cement B2 (F + BA) | 8 | 179 (16) | 158 (10) | 73 (12) | 64 (15) | 13 ( 7)B | 5.8 (2.6)b |
| 48 h lesions | 10 | 183 (29) | 73 (22) | ||||
n = number of slices, two from each tooth.
No significant differences were found comparing lesion depth or ΔZ under the control material among the four groups and the untreated 48 h lesions; one-way ANOVA, α = 0.05.
Numbers in parenthesis give one standard deviation.
Different superscript letters indicate significant differences, one-way ANOVA, Holm-Sidak, p < 0.05.
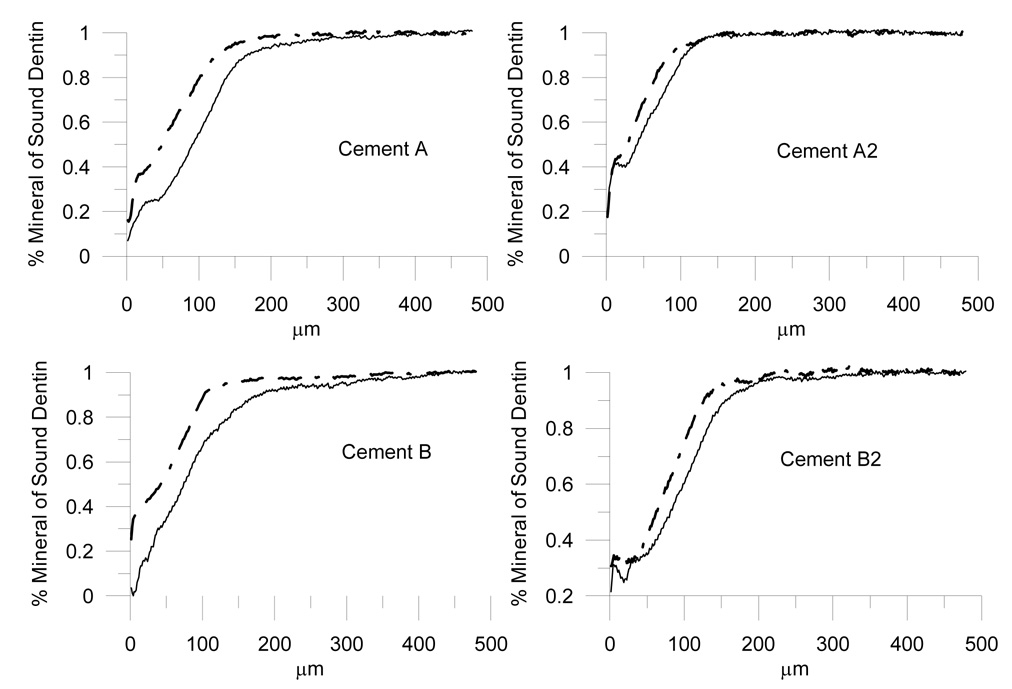
Figure 6 displays representative mineral profiles of cement and control from one section out of each group, for which the remineralization was approximately similar to the average calculated for that group.
Figure 6.
Representative mineral profiles under cements (dash-dot line) and control material (solid line) from one section out of each group, for which the remineralization was approximately similar to the average calculated for that group.
Discussion
Calcium, phosphate and fluoride have been shown to promote the induction of apatite [8] and increase the hardness of intact enamel [9]. Dentifrices containing calcium carbonate and fluoride were more effective to prevent enamel demineralization than silicate combined with fluoride [10]. Sullivan and others reported that calcium from dicalcium phosphate dihydrate was incorporated into enamel and plaque 18 h post-treatment [11]. A significant relationship between bovine dentin remineralization and calcium levels in calcium-containing mucin-based saliva substitutes has been reported; the phosphate level did not influence the outcome of the study [12]. In our dissolution experiments we showed that calcium, as well as phosphates and fluoride (where available), diffused out of the cements creating supersaturation of the solutions with respect to HAp and FAp, although the calcium concentrations were not correlated to the measured remineralization for reasons suggested below. Solution supersaturation with respect to HAp or FAp is a necessary condition to precipitate these particular minerals. Thus, the supersaturation levels in SLS calculated from the calcium, phosphate and fluoride ions after soaking indicate that the formation of HAp or FAp is possible. It should, however, be kept in mind that supersaturation does not necessarily predict whether or to which extent the minerals will precipitate. While the saturation levels indicate the potential for remineralization there is also a possibility that once the solutions became highly supersaturated towards HAp and FAp, these minerals may have precipitated in solution or on the specimen surface. The latter could explain the low level of calcium ions in some of the solutions at the end of the experiments, and is further corroborated by the fact that the amount of calcium present at the end of the soaking experiment corresponded inversely to an increase in thickness of the specimens which could have been caused in part by mineral deposits on the specimen surface (Pearson Product Moment Correlation: r = −0.60; p < 0.05). Swelling due to water sorption could also explain expansion but would not necessarily be correlated to the amount of calcium in solution, but rather depend on water absorbing components, i.e., the carboxylated monomer, which was kept constant among the cements, and the fluoride salt in the cement.
To actually afford remineralization in situ, the presence of a liquid phase is required to enable diffusion of ions out of the cement into the mineral deprived dentin. As remineralization occurred in our experiments at the bottom of the cavity, it is suggested that the necessary liquid phase was provided by SLS percolating through gaps between tooth and cement, or absorbed into the bulk of the cement. The saliva like solution used in this study is also supersaturated with respect to HAp. However, its role as a medium to promote remineralization at the bottom of the cavities may be neglected since the negative controls did not show significant differences in mineral content compared to the 48 h freshly demineralized tooth specimens.
In the results section we reported that a two-way ANOVA indicated the presence of the bonding agent resulting in significantly lower remineralization (p < 0.001). A study on polymeric substitutes in artificial saliva reported that poly(acrylic) acid caused further demineralization of enamel in remineralization experiments with substituted artificial saliva due to the high calciumbinding capacity of poly(acrylic) acid [13]. Analogous to the effects of the poly(acrylic) acid, it is suggested that in our study the bonding agent, containing an acidic monomer with an average of 2 acid groups per molecule, reduced the amount of calcium available to form HAp or FAp by binding the calcium as carboxylate salts [14].
In summary, the dissolution experiments and calculated saturations levels provided evidence that HAp or FAp could form from the ions leached out of the cements. There is, however, no correlation between the calcium levels in solution or the saturation levels and the actually measured remineralization. If such remineralizing cements are used in combination with bonding agents containing carboxylic acid moieties, a lower level of remineralization may be encountered, depending on whether or not fluoride is present in the cement. Predicting the remineralization potential from simple ion solution measurements of complex remineralizing materials where fluoride and other calcium binding compounds are present may underestimate their actual potential. As the use of bonding agents for improved adhesion between the remineralizing cements and dentin may compromise the remineralization, further investigations are underway to see whether mineral-enriched bonding agents, developed as a consequence of this study, could overcome this deficiency.
Acknowledgements
The support by the American Dental Association Foundation, the National Institute of Standards and Technology, and the National Institute of Dental and Craniofacial Research, Grant No. DE13298 is gratefully acknowledged. We also thank Esstech Co. for the supply of the EBPADMA monomer used in this study and Dentsply for supplying the composite resin.
Disclaimer
Commercial materials and equipment identified in this paper are for adequate definition of the experimental procedure. In no instance does such identification imply recommendation or endorsement by the National Institute of Standards and Technology or the ADA Foundation or that the material or equipment identified is necessarily the best available for the purpose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 2.Brown WE, Chow LC. In: A new calcium phosphate, water-setting cement, Cements Research Progress 1986. Brown PW, editor. 1987. pp. 352–379. [Google Scholar]
- 3.Chow LC, Hirayama S, Takagi S, Parry E. Diametral tensile strength and compressive strength of a calcium phosphate cement: effect of applied pressure. J Biomed Mater Res. 2000;53:511–517. doi: 10.1002/1097-4636(200009)53:5<511::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Dickens SH, Kelly SR, Flaim GM, Giuseppetti AA. Dentin adhesion and microleakage of a resin-based calcium phosphate pulp capping and basing cement. Eur J Oral Sci. 2004;112:452–457. doi: 10.1111/j.1600-0722.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 5.Vogel GL, Chow LC, Brown WE. A microanalytical procedure for the determination of calcium, phosphate and fluoride in enamel biopsy samples. Caries Res. 1983;17:23–31. doi: 10.1159/000260645. [DOI] [PubMed] [Google Scholar]
- 6.Chow LC, Takagi S, Shih S. Effect of a two-solution fluoride mouthrinse on remineralization of enamel lesions in vitro. J Dent Res. 1992;71:443–447. doi: 10.1177/00220345920710030401. [DOI] [PubMed] [Google Scholar]
- 7.Chow LC, Takagi S, Tung W, Jordan TH. Digital image analysis assisted microradiography-measurement of mineral content of caries lesions in teeth. J Res Natl Inst Stand Technol. 1991;96:203–214. doi: 10.6028/jres.096.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T, Toyooka H, Ito S, Crenshaw MA. In vitro study of remineralization of dentin: effects of ions on mineral induction by decalcified dentin matrix. Caries Res. 2003;37:445–449. doi: 10.1159/000073398. [DOI] [PubMed] [Google Scholar]
- 9.Munoz CA, Feller R, Haglund A, Triol CW, Winston AE. Strengthening of tooth enamel by a remineralizing toothpaste after exposure to an acidic soft drink. J Clin Dent. 1999;10:17–21. [PubMed] [Google Scholar]
- 10.Cury JA, Francisco SB, Simoes GS, Del Bel Cury AA, Tabchoury CP. Effect of a calcium carbonate-based dentifrice on enamel demineralization in situ. Caries Res. 2003;37:194–199. doi: 10.1159/000070444. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan RJ, Masters J, Cantore R, Roberson A, Petrou I, Stranick M, Goldman H, Guggenheim B, Gaffar A. Development of an enhanced anticaries efficacy dual component dentifrice containing sodium fluoride and dicalcium phosphate dihydrate. Am J Dent. 2001;14 Spec No:3A-11A. [PubMed] [Google Scholar]
- 12.Meyer-Lueckel H, Hopfenmuller W, von Klinggraff D, Kielbassa AM. Microradiographic study on the effects of mucin-based solutions used as saliva substitutes on demineralised bovine enamel in vitro. Arch Oral Biol. 2006;51:541–547. doi: 10.1016/j.archoralbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.van der Reijden WA, Buijs MJ, Damen JJ, Veerman EC, ten Cate JM, Nieuw Amerongen AV. Influence of polymers for use in saliva substitutes on de- and remineralization of enamel in vitro. Caries Res. 1997;31(3):216–223. doi: 10.1159/000262403. [DOI] [PubMed] [Google Scholar]
- 14.Dickens-Venz SH, Takagi S, Chow LC, Bowen RL, Johnston AD, Dickens B. Physical and Chemical Properties of Resin-Reinforced Calcium Phosphate Cements. Dent Mater. 1994;10:100–106. doi: 10.1016/0109-5641(94)90048-5. [DOI] [PubMed] [Google Scholar]