Abstract
Psychological stress increases the level of glucocorticoids in the circulating system. We found that dexamethasone administration in adult mice elevates the expression of COX-2 in the myocardium. With isolated neonatal cardiomyocytes, corticosterone (CT) at physiologically relevant doses (0.01–1 μM) induces the expression of COX-2 gene. The induction first appeared at 4 h and remained for at least 24 h with 1 μM CT treatment. This response is likely cardiomyocyte cell type specific since CT did not induce COX-2 expression in cardiac fibroblasts and glucocorticoids are known to suppress the expression of COX-2 in lymphocytes and several organs. Corticosteroids, but not estrogen or progesterone, induce COX-2 expression. The glucocorticoid receptor (GR) antagonist mifepristone (MF) prevented CT from inducing COX-2 gene, suggesting a GR-dependent induction in cardiomyocytes. COX-2 gene promoter deletion and mutation studies indicate a role of CCAAT/enhancer binding protein-β (C/EBP-β) in CT-induced COX-2 gene expression. Chromatin immunoprecipitation assays revealed that CT caused the binding of both GR and C/EBP-β to COX-2 promoter, while MF pretreatment blocked such binding. Coimmunoprecipitation experiments demonstrated that CT treatment induced the interaction of GR with C/EBP-β. Small interfering RNA against C/EBP-β prevented CT from activating COX-2 promoter or elevating COX-2 protein. Our data suggest that the interaction between GR and C/EBP-β contributes to elevated COX-2 gene transcription by CT in cardiomyocytes.
Keywords: gene expression, cyclooxygenase, transcription, protein-protein interaction
cyclooxygenase (COX) is a rate-limiting enzyme in the biosynthesis of prostaglandins (PGs). The importance of PGs in various biological systems has led to voluminous studies of two isoforms of COX enzyme: COX-1 and COX-2 (44, 52). While the COX-1 gene expresses constitutively in most cell types and is responsible for normal physiological functions of PGs, the expression of COX-2 gene is rapidly induced by proinflammatory cytokines, carcinogens, and mitogens in many cell types (50). Dysregulation of COX-2 gene expression has been reported in association with the pathogenesis of inflammatory diseases, carcinogenesis, neurodegenerative diseases, and a variety of other diseases.
In cardiomyocytes, the biological function of COX-2 remains controversial. Elevated expression of COX-2 gene has been detected in failing human hearts (55). While several studies indicate a detrimental role of COX-2 overexpression in the heart, the mainstream literatures suggest that COX-2 serves a protective function against cardiac injury (43). PGs, for example, PGI2 and PGE, have been shown to elicit a cardiac protective effect against ischemia-reperfusion injury in experimental animals (3, 7, 8, 42). PGE protects cardiomyocytes from cell injury induced by oxidants and doxorubicin (1, 29, 42, 56). In addition, PGs appear to mediate the protective effect of high-density lipoproteins on isolated rat hearts from ischemia-reperfusion injury (7). In the human population, recent clinical and epidemiologic studies have demonstrated an increased incidence of myocardial infarction in individuals taking the prescription drugs Vioxx and Celebrex, specific inhibitors of COX-2 (2, 14, 15, 33). These lines of evidence support the protective function of COX-2 gene in the heart.
Psychological stress is an inevitable event of our daily life. Stress increases the synthesis of glucocorticoids from the adrenal glands, causing an elevated level of glucocorticoids in the circulating system. Glucocorticoids regulate important physiological processes from body metabolism and biochemical homeostasis to immune responses. Synthetic glucocorticoids are among the most frequently used drugs because of their anti-inflammatory and immunosuppressive capability. The anti-inflammatory action of glucocorticoids largely results from downregulation of proinflammatory genes in immune cells, including COX-2. Despite the vast amount of information regarding the function and widespread pharmacological applications of glucocorticoids, the biological action of these steroids on the heart or cardiomyocytes has not been well studied, as evidenced by the limited number of publications in this area.
Previous studies from our lab (9) showed that glucocorticoids protect cardiomyocytes from apoptosis induced by doxorubicin, an antineoplastic drug known for its side effect of inducing cardiomyopathy. Microarray analyses found that corticosterone (CT) upregulates 140 genes and downregulates 108 genes in cardiomyocytes (9). Among the list of CT-induced genes, COX-2 mRNA has an average of 3.6-fold elevation (9). In this study, we characterized CT-induced COX-2 gene expression and investigated the mechanism underlying CT-induced COX-2 gene expression.
MATERIALS AND METHODS
Cell culture and drug treatment.
Cardiomyocytes and cardiac fibroblasts were prepared from 1- to 2-day-old neonatal Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) as previously described (36). Cardiomyocytes were seeded at a density of 2.5 × 106 cells per 100-mm dish or 0.3 × 106 cells per well of six-well plates. Cardiac fibroblasts were collected by differential plating, seeded in high-glucose DMEM with 10% FBS, and subcultured once to eliminate contamination by other cell types (36). Cells were placed in fresh DMEM containing 0% FBS for 24 h before experiments.
Animal experiments.
Male C57BL/6 mice (Jackson Lab, ME) weighing 20–26 g at the age of 4 wk were injected (ip) with vehicle or 20 mg/kg of dexamethasone. Animals were euthanized for collection of ventricular tissues 20 h later. Frozen tissues were ground with a pestle and mortar (VWR) in a liquid nitrogen bath, and resulting powders were dissolved in Laemmli lysis buffer for Western blot analyses. The protocol for animal usage was approved by the University of Arizona Institutional Animal Care and Use Committee.
Western blot analysis and coimmunoprecipitation.
Cells or tissues dissolved in Laemmli buffer were measured for protein concentration by the Warburg-Christian method (9, 23). After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane for incubation with antibodies against COX-2 (no. 160106, Cayman Chemical), glucocorticoid receptor (GR; sc-1004, Santa Cruz Biotechnology), CCAAT/enhancer-binding protein-β (C/EBP-β) antibody (sc-150x, Santa Cruz Biotechnology), or vinculin (V9131, Sigma-Aldrich). Horseradish peroxidase-conjugated secondary antibodies (Zymed) bound to the primary antibodies were detected with an enhanced chemiluminescence reaction.
Coimmunoprecipitation was performed with a nuclear extraction kit (Active Motif). The nuclear extracts containing 250 μg of proteins were incubated overnight at 4°C with 2 μg of anti-C/EBP-β antibody (sc-150x, Santa Cruz Biotechnology) in 500 μl of low-salt immunoprecipitation buffer (Active Motif). Protein G beads (60 μl, Sigma) were added to the mixture and incubated for an additional hour with rocking. The immunocomplexes were then washed six times with low-salt immunoprecipitation buffer. The immunoprecipitated proteins were dissolved in 20 μl of 2× Laemmli buffer and boiled for 5 min, before analysis by Western blot.
RNA isolation, semiquantitative RT-PCR, and real-time RT-PCR.
Total RNA (2 μg) was isolated with TRIzol (Invitrogen) for reverse transcription (RT) using hexanucleotide random primers. RT products (2.5 μl) were amplified in a reaction mixture (22.5 μl) containing 4× 200 μmol dNTPs, 20 pmol each of two oligonucleotide primers (forward 5′-TACAAGCAGTGGCAAAGGCC, reverse 5′-CAGTATTGAGGAGAACAGATGGG), and 0.2 U of Taq DNA polymerase with 30–35 cycles of 94°C for 30 s, 62°C for 30 s, and 72°C for 20 s. GAPDH was amplified in parallel PCR as an internal loading control with the primer pair of forward 5′-AGACAGCCGCATCTTCTTGT and reverse 5′-CCACAGTCTTCTGAGTGGCA.
For real-time PCR, hexanucleotide random primers were used for RT with 50 ng of RNA in a 50-μl reaction mixture. COX-2 cDNA was amplified with a TaqMan Universal PCR master mix and a COX-2 specific primer/probe mix set (ABI Rn00568225_m1). β-Glucuronidase was used as a reference gene (ABI Rn00566655_m1). The reporter fluorescence for newly synthesized DNA was detected with an ABI Biosystem 7300 sequencer during 40 cycles of 95°C for 15 s and 60°C for 1 min after 10-min denaturation at 95°C. The relative difference in the level of COX-2 cDNA/mRNA between samples was calculated based on 2−ΔCt, where Ct is threshold cycle.
Nuclear run-on assay.
A nonradioactive nuclear run-on assay was carried out with nuclei prepared from cardiomyocytes (2 × 107 cells) (35). Isolated nuclei were incubated 1 h at 30°C in a reaction mixture containing (in mM) 20 Tris·HCl (pH 8.0), 5 MgCl2, 200 KCl, 5 dithiothreitol, 4 each of ATP, CTP, and GTP, 4 biotin-16-UTP (Roche), and 200 sucrose with 20% glycerol. The resulting biotin-labeled RNA was extracted with TRIzol (Invitrogen) and isolated by magnetic Dynabeads M-280 covalently linked to streptavidin (Dynal Biotech, Brown Deer, WI). The beads were then resuspended in a reaction mixture for RT and real-time PCR.
COX-2 promoter luciferase construct, transient transfection, and luciferase assay.
To generate rat COX-2 promoter-luciferase construct, a fragment of rat COX-2 promoter DNA sequence (−449 to +24) was amplified by PCR using rat liver genomic DNA as a template. The forward primer (5′-GGG GTA CCA GAG CAG CAA GCA CGT CAG ACT) contains a KpnI restriction site, while the reverse primer (5′-CCT AGC TAG CAG CTC TCC GCT CAG TTT GAC AA) has an NheI restriction site, allowing restriction digestion and subcloning of the PCR product into a pGL3 Basic vector at 5′ upstream of the firefly luciferase gene (Promega, Madison, WI). The deletion mutant and point mutation of COX-2 promoter constructs were generated as described previously (19). The mutants of COX-2 promoter sequence were cloned into pGL2 Basic vector (Promega) (49). The promoter-luciferase reporter construct (0.2 μg DNA) was transfected into rat cardiomyocytes at 3 days after plating with Fugene 6 liposomes (Roche). After 5-h incubation with transfection mixtures, cells were placed in fresh DMEM containing 10% FBS for overnight recovery before serum starvation and experimental treatments. Luciferase activity was measured with an assay kit (Promega) and was normalized to the protein content unless indicated otherwise.
For transfecting small interfering RNA (siRNA), at 18–24 h after seeding in six-well plates, cardiomyocytes were cotransfected with 0.2 μg of rat COX-2 luciferase reporter construct and 0.04 μg of pRL-TK Renilla in the presence or absence of 100 pmol of C/EBP-β siRNA or scrambled sequence (siGenome SMARTpool M-099218-00, Dharmacon) with 3 μl of oligofectamine transfection reagent (no. 12252-011, Invitrogen). At 24 h after transfection, cells were placed in serum-free DMEM for 24 h before treatment with 1 μM CT for 12 h. Luciferase activities were measured with a dual luciferase kit (Promega), and the activity of firefly luciferase under the control of COX-2 promoter was corrected with Renilla luciferase.
Chromatin immunoprecipitation assay.
Chromatin immunoprecipitation (ChIP) assay was carried out with an enzymatic shearing kit according to the manufacturer's instruction (Active Motif) with antibodies against C/EBP-β (sc-150x, Santa Cruz Biotechnology), GR (sc-1002, Santa Cruz Biotechnology), or normal rabbit IgG. The DNA in immunoprecipitates was analyzed by PCR using the primer pair of forward 5′-CTCTCTTGGCACCACTTTGG-3′ and reverse 5′-AGCTCTCCGCTCAGTTTGACAA-3′ that recognize the −227 to +24 base pair (bp) region of COX-2 promoter. The PCR products were separated by agarose gel electrophoresis for detection by ethidium bromide staining.
Statistics.
Statistical analyses were performed with ANOVA (P < 0.05) and the Fisher's least significant difference procedure from STATLES software. Means that are not significantly different from each other are labeled in Figs. 3, 6, 8, 9, and 12 by a common letter symbol. Therefore, means in the “a” group are significantly different from means in the “b,” “bc,” or “c” group and so on. Means labeled with “ab” are not significantly different from those in the “a” or “b” group.
RESULTS
Corticosteroids induce COX-2 gene expression.
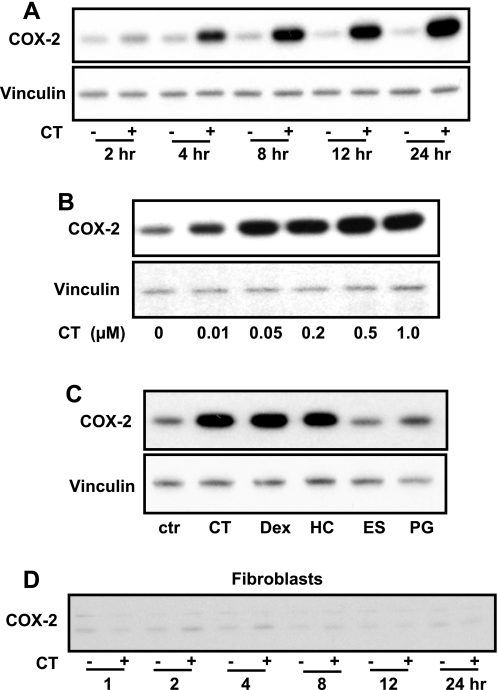
To characterize COX-2 induction by CT in cardiomyocytes, we examined the time course and dose response following CT treatment. Measurements of COX-2 protein indicate a clear increase of COX-2 at 4 h after CT treatment, and COX-2 remained elevated for at least 24 h (Fig. 1A). The dose-response studies found that CT can induce COX-2 protein at a concentration as low as 10 nM (Fig. 1B). To test the specificity of COX-2 induction by corticosteroids, we examined the effect of several steroids. Three glucocorticoids tested, i.e., hydrocortisone, CT, and dexamethasone, induced COX-2 expression (Fig. 1C). In contrast, estrogen and progesterone, two hormonal steroids that do not bind to GR, did not increase COX-2 expression (Fig. 1C). The myocardium contains fibroblasts in addition to cardiomyocytes. Cardiac fibroblasts were tested for induction of COX-2 by CT. In contrast to cardiomyocytes, fibroblasts did not show clear induction of COX-2 protein by CT treatment (Fig. 1D).
Fig. 1.
Corticosterone (CT) induces cyclooxygenase (COX)-2 protein in cardiomyocytes. A and B: cardiomyocytes were treated with CT (1 μM) for indicated times (A) or at various concentrations for 4 h (B). C: alternatively, cardiomyocytes were treated with 1 μM each of CT, dexamethasone (Dex), hydrocortisone (HC), estrogen (ES), or progesterone (PG) for 4 h. ctr, Control. D: cardiac fibroblasts prepared in parallel with cardiomyocytes were exposed to 1 μM CT for indicated times. The level of COX-2 protein was measured by Western blot (15 μg protein/lane) with vinculin as a loading control. Data show 1 experiment representative of 3.
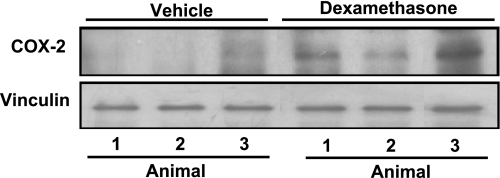
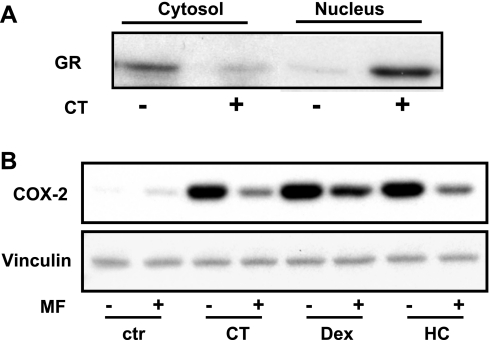
The in vitro finding suggests the possibility that elevating serum levels of glucocorticoids may cause an increase in COX-2 protein in the myocardium. Dexamethasone, a synthetic glucocorticoid having a longer half-life than CT and commonly used for in vivo glucocorticoid supplement, was administered in C57BL/6 mice. Ventricular tissues were harvested 20 h later from three vehicle control- or dexamethasone-treated animals for measurements of COX-2 protein. The results indicate an increased level of COX-2 protein in the myocardium of animals treated with dexamethasone (Fig. 2).
Fig. 2.
Dexamethasone induces COX-2 protein in the myocardium. Mice at 4 wk of age were injected with vehicle or dexamethasone (20 mg/kg ip). The ventricular tissues were collected 20 h later for Western blot analyses (100 μg protein/lane). Each lane represents COX-2 protein level from 1 animal. Data show 1 experiment representative of 3.
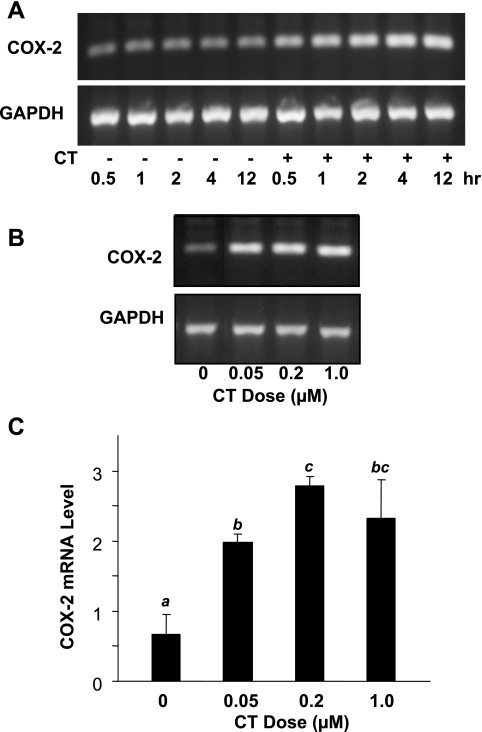
To determine whether CT induces transcriptional activation of COX-2 gene, the level of COX-2 mRNA in cardiomyocytes was analyzed by semiquantitative RT-PCR and real-time PCR. Figure 3A shows a time-dependent induction of COX-2 mRNA, with an increase of COX-2 mRNA first detected within 30 min of CT treatment and remaining for at least 12 h (Fig. 3A). In agreement with COX-2 protein data, CT at 50 nM–1 μM induced COX-2 mRNA (Fig. 3B). Induction of COX-2 at the mRNA level was confirmed by quantitative real-time RT-PCR, which revealed fourfold or more COX-2 mRNA in 50 nM–1 μM CT-treated cardiomyocytes compared with untreated cells (Fig. 3C).
Fig. 3.
CT induces COX-2 mRNA elevation. Cardiomyocytes were treated with 1 μM CT for various time points (A) or for 4 h with various doses of CT (B, C). The level of COX-2 mRNA was analyzed by RT-PCR (A, B) or real-time PCR (C). Data are presented from 1 of 3 independent experiments (A, B) or as means ± SD of triplicate samples from 1 experiment representative of 2 (C). C: superscript letter indicates significant difference (P < 0.05) from means labeled with a different letter as determined by ANOVA analysis from triplicate samples.
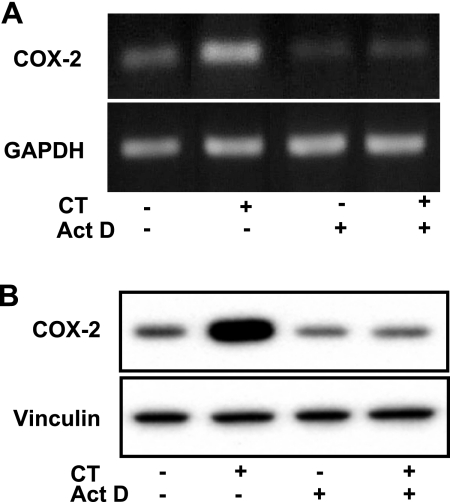
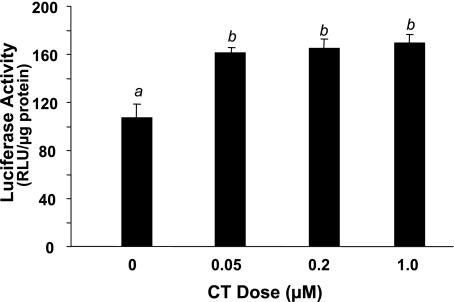
Three methods have been used to demonstrate that CT induces transcriptional activation of COX-2 gene: pharmacological inhibitors, nuclear run-on assay, and promoter reporter gene assay. Pretreatment with actinomycin D, an inhibitor of RNA synthesis, prevented CT from inducing COX-2 increases at the mRNA or protein level (Fig. 4). In nuclear run-on assays, the nuclei from CT-treated cardiomyocytes exhibited an increased rate of synthesis of COX-2 transcript (Fig. 5). The relative COX-2 transcription rate in vitro is 4.5 times faster with the nuclei from CT-treated cells compared with control (Fig. 5). A chimeric construct of luciferase gene under the control of rat COX-2 promoter was introduced into cardiomyocytes by transient transfection. Treatment with CT caused an increase in the activity of COX-2 promoter (Fig. 6). These data indicate that CT indeed causes transcriptional activation of COX-2 gene.
Fig. 4.
Actinomycin D (Act D) prevents CT from inducing COX-2. Cardiomyocytes were pretreated with Act D (0.5 μM) for 30 min before treatment with 1 μM CT for 2 (A) or 4 (B) h. COX-2 mRNA (A) or protein (B) was determined by RT-PCR or Western blot, respectively, with GAPDH (A) or vinculin (B) as a loading control. Data show 1 experiment representative of 3.
Fig. 5.
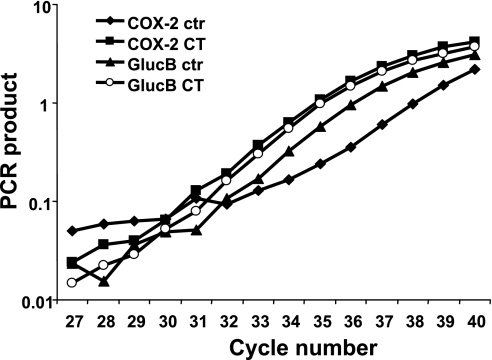
Nuclear run-on assays indicate that CT induces transcriptional activation of COX-2 gene. Nuclei were harvested from cardiomyocytes 6 h after 1 μM CT treatment. After the reaction of in vitro transcription as described in materials and methods, newly synthesized transcripts were detected by real-time PCR. Data show 1 experiment representative of 2. GlucB, β-glucuronidase.
Fig. 6.
CT activates COX-2 promoter. Cardiomyocytes were transfected with a rat COX-2 promoter (−449 to 24 bp) luciferase construct. The cells were treated with CT at the doses indicated for 12 h before harvesting for measurements of promoter activation. Data show means ± SD of triplicate samples from 1 experiment representative of 3. A superscript letter indicates significant difference (P < 0.05) from means labeled with a different letter as determined by ANOVA analysis. RLU, relative light unit.
GR and C/EBP-β in CT-induced COX-2 expression.
Glucocorticoids are known to enter cells through diffusion and bind to GR in the cytosol, causing nuclear translocation of the receptor. CT treatment caused nuclear translocation of GR in cardiomyocytes as expected (Fig. 7A). While activated GR turns on transcription of the genes containing the glucocorticoid response element (GRE), GR participates in chromatin remodeling and interacts with coregulators, resulting in changes in the expression of genes that do not have GRE in the promoters (12, 27, 28, 32, 51). A widely used GR antagonist, mifepristone (MF), occupies the ligand binding site of GR, preventing it from binding to glucocorticoids (6). MF provides a useful tool to test whether GR is essential for CT-induced COX-2 expression. Pretreatment with MF abolished COX-2 protein increases by three glucocorticoids (Fig. 7B). MF also prevented CT from inducing COX-2 mRNA or activating COX-2 promoter (Fig. 8). These data suggest that GR mediates CT-induced transcriptional activation of COX-2 gene.
Fig. 7.
Mifepristone (MF) inhibits glucocorticoids from inducing COX-2 protein. Cardiomyocytes were treated for 1 h with 1 μM CT for measurements of 94-kDa glucocorticoid receptor (GR) in the cytosol and nuclear fractions by Western blot (A). MF (1 μM) was added to cardiomyocytes for 60 min before 24-h 1 μM CT, Dex, or HC treatment for Western blots to measure COX-2 protein levels (B). Data show 1 experiment representative of 3 independent experiments.
Fig. 8.
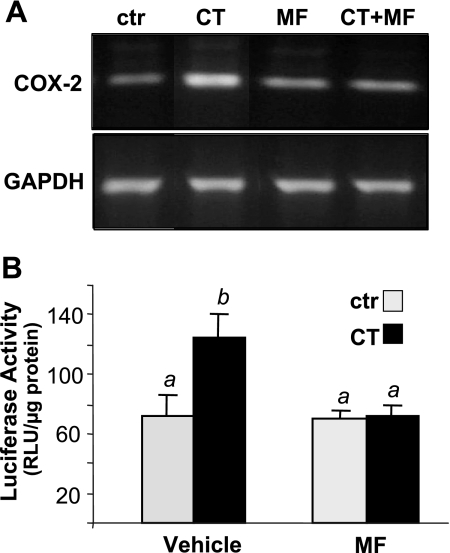
MF prevents CT from inducing COX-2 mRNA or activating COX-2 promoter. Cardiomyocytes were treated with 1 μM MF for 60 min before 4-h exposure to 1 μM CT. RNAs were collected to measure COX-2 mRNA levels by RT-PCR (A). Cardiomyocytes transfected with a rat COX-2 promoter (−449 to 24 bp) luciferase construct were pretreated 60 min with 1 μM MF before 12-h incubation of 1 μM CT for luciferase assay (B). Data show 1 experiment representative of 3 (A) or means ± SD from triplicate samples of 1 experiment representative of 3 independent experiments (B). B: a superscript letter indicates significant difference (P < 0.05) from means labeled with a different letter as determined by ANOVA analysis.
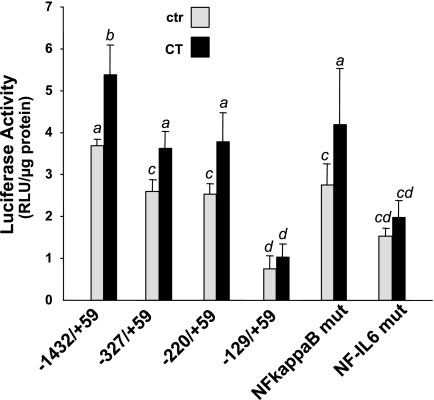
We have performed a promoter sequence search for a potential GRE within 3,000 bp upstream of the transcriptional start site and failed to found this consensus sequence in COX-2 gene promoter. Sequential deletion from the 5′ end or mutation at a specific cis-element site in COX-2 promoter allows us to identify the transcription factor responsible for CT-induced COX-2 gene. It appears that 5′ deletion upstream of −220 bp did not significantly affect CT from activating COX-2 promoter (Fig. 9). In contrast, the −129 bp COX-2 promoter construct did not show significant activation by CT (Fig. 9). Between −220 bp and −129 bp of COX-2 promoter, NF-IL6 stands out as a well defined cis-element. Mutating the NF-IL6 consensus sequence reduced the response of the promoter to CT stimulation (Fig. 9). In comparison, mutation of NF-κB, a cis-element located between −327 and −220 bp, did not affect CT-induced COX-2 promoter activation (Fig. 9).
Fig. 9.
NF-IL6 cis-element mediates CT-induced transcriptional activation of COX-2 gene. Cardiomyocytes were transfected with luciferase reporter constructs under the control of human COX-2 promoter fragments as indicated. The cells were incubated with 1 μM CT for 12 h before harvesting for measurements of luciferase activity. Data show means ± SD of 1 experiment representative of 3 independent experiments. A superscript letter indicates significant difference (P < 0.05) from means labeled with a different letter as determined by ANOVA analysis.
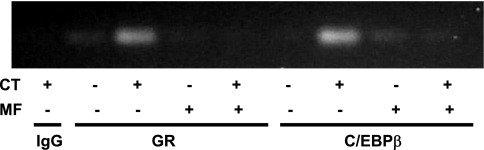
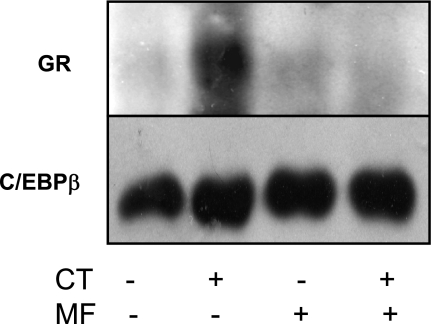
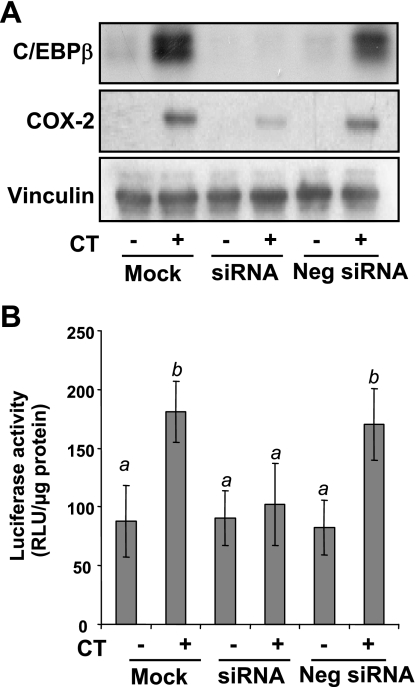
C/EBP-β is known to bind to NF-IL6 cis-element (25). GR has been shown to interact with C/EBPs to regulate gene expression (4, 41). ChIP assays were carried out to address whether CT treatment induced GR and C/EBP-β binding to COX-2 promoter. As shown in Fig. 10, CT treatment caused GR and C/EBP-β to bind to the −227/+24 region of COX-2 promoter. The binding was abolished by pretreating cells with MF (Fig. 10). To determine whether GR interacts with C/EBP-β, we performed a coimmunoprecipitation experiment. In the immunoprecipitates of C/EBP-β, GR was detectable in CT-treated cells (Fig. 11). MF was able to prevent GR from interacting with C/EBP-β (Fig. 11). To demonstrate that C/EBP-β indeed regulates CT-induced COX-2 transcription, we utilized siRNA of C/EBP-β. This siRNA was found to inhibit CT-induced COX-2 protein (Fig. 12A). Cotransfecting C/EBP-β siRNA with COX-2 promoter reporter construct indicated that C/EBP-β siRNA was capable of preventing CT from inducing COX-2 promoter activation (Fig. 12B). These data suggest that GR regulates COX-2 gene through C/EBP-β-mediated binding to COX-2 promoter.
Fig. 10.
GR and CCAAT/enhancer-binding protein-β (C/EBP-β) bind to COX-2 promoter. Cardiomyocytes were treated with vehicle or 1 μM CT in the absence or presence of 1 μM MF for 12 h for chromatin immunoprecipitation assay as described in materials and methods. The DNA in IgG, GR, or C/EBP-β immunoprecipitates was used as a template for PCR using the primers to generate −227/+24 fragment of COX-2 promoter. Band indicates the fragment of COX-2 promoter bound with GR or C/EBP-β in response to CT treatment. Data show 1 experiment representative of 3 independent experiments.
Fig. 11.
GR interacts with C/EBP-β protein. Cardiomyocytes were treated with vehicle or 1 μM CT in the absence or presence of 1 μM MF for 6 h. Nuclear proteins were extracted for immunoprecipitation using antibodies against C/EBP-β. Immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis for Western blot with antibodies against GR (top) or C/EBP-β (bottom). Data show 1 experiment representative of 3.
Fig. 12.
C/EBP-β small interfering RNA (siRNA) inhibits CT from inducing COX-2. Cardiomyocytes were transfected with C/EBP-β siRNA or negative control. To measure COX-2 luciferase activity, cells were cotransfected with a rat COX-2 promoter (−449 to 24 bp) luciferase construct and a TK-Renilla luciferase construct. At 48 h after transfection, cells were treated with 1 μM CT for 12 h before harvesting for Western blot (A) or luciferase activity assays (B). Data show 1 experiment representative of 3 (A) or means ± SD from triplicate samples of 1 experiment representative of 3 (B). B: a superscript letter indicates significant difference (P < 0.05) from means labeled with a different letter as determined by ANOVA analysis.
DISCUSSION
This study demonstrates that glucocorticoids at physiologically relevant doses induce elevation of COX-2 gene expression in cardiomyocytes in vitro. In vivo dexamethasone administration in mice caused elevation of COX-2 protein in the heart, suggesting the physiological significance of our finding. Mechanistic studies indicated that activation of GR was necessary for COX-2 induction. Both GR and C/EBP-β appeared to bind to COX-2 promoter within close proximity to the transcription start site. While MF prevented GR or C/EBP-β binding to COX-2 promoter, the physical interaction between GR and C/EBP-β occurred as a result of CT exposure. Inhibition of GR with MF or C/EBP-β with siRNA prevented CT from inducing COX-2 gene expression. These lines of evidence suggest that the interaction between GR and C/EBP-β on COX-2 promoter triggers transcriptional activation of COX-2 gene. Our data are consistent with the literature showing that activated GR interacts with C/EBP-β physically (4, 41) and that C/EBPs mediate the effect of glucocorticoids in gene expression (10, 13, 21, 31, 38, 57, 59, 61).
The finding of COX-2 induction by CT appears to be unique to cardiomyocytes. Cardiac fibroblasts from the same origin did not respond to CT in a manner similar to that in cardiomyocytes (Fig. 1D). In lymphocytes, pulmonary epithelial cells, airway smooth muscle cells, vascular endothelial cells, the kidney, and the lung, where glucocorticoids have been studied extensively, it is known that glucocorticoids suppress the expression of COX-2 gene (16–18, 22, 30, 34, 37, 60). Since C/EBP-β is a transcription factor expressed in many cell types and the expression level is high in human hematopoietic cells (Ref. 25; www.genome.ucsc.edu), this leads to the postulation that cardiomyocytes exhibit an additional factor collaborating with GR and C/EBP-β in turning on the transcription of COX-2 gene. Ligand-bound GR has been shown to interact with a large number of coregulators (12, 27, 28, 32). Some of these interactions cause chromatin remodeling and increase the accessibility of the transcriptional machinery to the promoter region of target genes. In addition to the possibility that cardiomyocytes exhibit cell type-specific coregulators of GR, we have found that CT activates p38 MAPK in cardiomyocytes but not cardiac fibroblasts in a parallel study (47a). Cardiomyocytes differ from fibroblasts in the signaling network (36). The unique nongenomic signaling response to CT may contribute to cell-type specific induction of COX-2 genes.
Psychological stress in general has been linked to an increased risk of hypertension and several other forms of cardiovascular diseases in humans (39, 40, 58). Elevation of circulating glucocorticoids due to either endogenous causes or pharmacological administration is known to contribute to hypertension in certain individuals (5, 54). Long-term pharmacological use of glucocorticoids is associated with an increased incidence of stroke, myocardial infarction, or heart failure (45, 53). In experimental animals, chronic administration of glucocorticoids causes cardiac enlargement, abnormal ST and T waves in electrocardiogram, and increases in the size of myocardial infarction (11, 20). Many of the detrimental effects are related to the activity of glucocorticoids in inducing vasoconstriction and altering biochemical metabolism, such as inducing hyperglycemia and dyslipidemia. Therefore, steroids that do not produce these undesirable effects may be useful for cardiac protection. Earlier experiments had demonstrated that short-term glucocorticoid treatment is cardiac protective (26). Pharmacological doses (50 mg/kg) of hydrocortisone prevented cardiomyocytes from progressing to necrosis due to ischemic injury in dogs (26). Methylprednisolone or dexamethasone, the synthetic glucocorticoids, protected the heart against ischemia-reperfusion injury or infarction in cats (24, 46). A few studies attributed the protective effect to induction of heat shock proteins (HSPs). For example, high doses (10–100 μM) of dexamethasone increase HSP72 gene expression in adult rat cardiomyocytes, and overexpression of HSPs protects against cardiac injury (48). Our finding here suggests a potential role of COX-2 or its product PGs in glucocorticoid-induced cytoprotection (9). However, a recent study from our laboratory (47) indicates that CT also induces COX-1 gene transcription in cardiomyocytes. Therefore the functional significance of COX-2 induction in the context of CT-induced cytoprotection requires further investigation considering COX-1 and other genes induced by CT.
GRANTS
This work was supported by National Institutes of Health Grants R01-ES-10826, R01-HL-076530, and T32-ES-007091 to Q. M. Chen. Real-time PCR was performed at the Genomics Core Facility supported by the Southwest Environmental Health Sciences Center (ES-06694).
Acknowledgments
Yan Lin and Michael Torris are acknowledged for technical assistance. We thank David Aguilar for statistics analyses.
Present address of H. Sun: Dept. of Anesthesiology, Div. of Molecular Medicine, University of California-Los Angeles, Los Angeles, CA 90095-7115.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem 274: 5038–5046, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Antman EM, DeMets D, Loscalzo J. Cyclooxygenase inhibition and cardiovascular risk. Circulation 112: 759–770, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bolli R, Shinmura K, Tang XL, Kodani E, Xuan YT, Guo Y, Dawn B. Discovery of a new function of cyclooxygenase (COX)-2: COX-2 is a cardioprotective protein that alleviates ischemia/reperfusion injury and mediates the late phase of preconditioning. Cardiovasc Res 55: 506–519, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boruk M, Savory JG, Hache RJ. AF-2-dependent potentiation of CCAAT enhancer binding protein beta-mediated transcriptional activation by glucocorticoid receptor. Mol Endocrinol 12: 1749–1763, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Brem AS Insights into glucocorticoid-associated hypertension. Am J Kidney Dis 37: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 48: 129–156, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res 92: 330–337, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Camitta MG, Gabel SA, Chulada P, Bradbury JA, Langenbach R, Zeldin DC, Murphy E. Cyclooxygenase-1 and -2 knockout mice demonstrate increased cardiac ischemia/reperfusion injury but are protected by acute preconditioning. Circulation 104: 2453–2458, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Chen QM, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes. Mol Pharmacol 67: 1861–1873, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Cram EJ, Ramos RA, Wang EC, Cha HH, Nishio Y, Firestone GL. Role of the CCAAT/enhancer binding protein-alpha transcription factor in the glucocorticoid stimulation of p21waf1/cip1 gene promoter activity in growth-arrested rat hepatoma cells. J Biol Chem 273: 2008–2014, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Czerwinski SM, Kurowski TT, McKee EE, Zak R, Hickson RC. Myosin heavy chain turnover during cardiac mass changes by glucocorticoids. J Appl Physiol 70: 300–305, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Deroo BJ, Archer TK. Glucocorticoid receptor-mediated chromatin remodeling in vivo. Oncogene 20: 3039–3046, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Fang HL, Abdolalipour M, Duanmu Z, Smigelski JR, Weckle A, Kocarek TA, Runge-Morris M. Regulation of glucocorticoid-inducible hydroxysteroid sulfotransferase (SULT2A-40/41) gene transcription in primary cultured rat hepatocytes: role of CCAAT/enhancer-binding protein liver-enriched transcription factors. Drug Metab Dispos 33: 147–156, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Fosslien E Cardiovascular complications of non-steroidal anti-inflammatory drugs. Ann Clin Lab Sci 35: 347–385, 2005. [PubMed] [Google Scholar]
- 15.Fries S, Grosser T. The cardiovascular pharmacology of COX-2 inhibition. Hematology Am Soc Hematol Educ Program 2005: 445–451, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Fu JY, Masferrer JL, Seibert K, Raz A, Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem 265: 16737–16740, 1990. [PubMed] [Google Scholar]
- 17.Hirst SJ, Lee TH. Airway smooth muscle as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med 158: S201–S206, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Inoue H, Umesono K, Nishimori T, Hirata Y, Tanabe T. Glucocorticoid-mediated suppression of the promoter activity of the cyclooxygenase-2 gene is modulated by expression of its receptor in vascular endothelial cells. Biochem Biophys Res Commun 254: 292–298, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem 270: 24965–24971, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Murata M, Kamiyama A. Experimental study of cardiomyopathy induced by glucocorticoids. Jpn Circ J 43: 1043–1047, 1979. [DOI] [PubMed] [Google Scholar]
- 21.Kimura T, Chowdhury S, Tanaka T, Shimizu A, Iwase K, Oyadomari S, Gotoh T, Matsuzaki H, Mori M, Akira S, Takiguchi M. CCAAT/enhancer-binding protein beta is required for activation of genes for ornithine cycle enzymes by glucocorticoids and glucagon in primary-cultured hepatocytes. FEBS Lett 494: 105–111, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Lasa M, Brook M, Saklatvala J, Clark AR. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol Cell Biol 21: 771–780, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layne E Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol 3: 447–454, 1957. [Google Scholar]
- 24.Lefer AM, Crossley K, Grigonis G, Lefer DJ. Mechanism of the beneficial effect of dexamethasone on myocardial cell integrity in acute myocardial ischemia. Basic Res Cardiol 75: 328–339, 1980. [DOI] [PubMed] [Google Scholar]
- 25.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem 273: 28545–28548, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Libby P, Maroko PR, Bloor CM, Sobel BE, Braunwald E. Reduction of experimental myocardial infarct size by corticosteroid administration. J Clin Invest 52: 599–607, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonard DM, Lanz RB, O'Malley BW. Nuclear receptor coregulators and human disease. Endocr Rev 28: 575–587, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Lonard DM, O'Malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27: 691–700, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Ma XQ, Fu RF, Feng GQ, Wang ZJ, Ma SG, Weng SA. Hypoxia-reoxygenation-induced apoptosis in cultured neonatal rat cardiomyocytes and the protective effect of prostaglandin E. Clin Exp Pharmacol Physiol 32: 1124–1130, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Madsen K, Stubbe J, Yang T, Skott O, Bachmann S, Jensen BL. Low endogenous glucocorticoid allows induction of kidney cortical cyclooxygenase-2 during postnatal rat development. Am J Physiol Renal Physiol 286: F26–F37, 2004. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy TL, Ji C, Chen Y, Kim K, Centrella M. Time- and dose-related interactions between glucocorticoid and cyclic adenosine 3′,5′-monophosphate on CCAAT/enhancer-binding protein-dependent insulin-like growth factor I expression by osteoblasts. Endocrinology 141: 127–137, 2000. [DOI] [PubMed] [Google Scholar]
- 32.McEwan IJ, Wright AP, Gustafsson JA. Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. Bioessays 19: 153–160, 1997. [DOI] [PubMed] [Google Scholar]
- 33.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 296: 1633–1644, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Newton R, Seybold J, Kuitert LME, Bergmann M, Barnes PJ. Repression of cyclooxygenase-2 and prostaglandin E2 release by dexamethasone occurs by transcriptional and post-transcriptional mechanisms involving loss of polyadenylated mRNA. J Biol Chem 273: 32312–32321, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Patrone G, Puppo F, Cusano R, Scaranari M, Ceccherini I, Puliti A, Ravazzolo R. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. Biotechniques 29: 1012–1014, 1016–1017, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Purdom S, Chen QM. Epidermal growth factor receptor-dependent and -independent pathways in hydrogen peroxide-induced mitogen-activated protein kinase activation in cardiomyocytes and heart fibroblasts. J Pharmacol Exp Ther 312: 1179–1186, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem J 318: 325–331, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth M, Johnson PR, Borger P, Bihl MP, Rudiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, Tamm M. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med 351: 560–574, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 45: 637–651, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99: 2192–2217, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Rudiger JJ, Roth M, Bihl MP, Cornelius BC, Johnson M, Ziesche R, Block LH. Interaction of C/EBPalpha and the glucocorticoid receptor in vivo and in nontransformed human cells. FASEB J 16: 177–184, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Shinmura K, Tamaki K, Sato T, Ishida H, Bolli R. Prostacyclin attenuates oxidative damage of myocytes by opening mitochondrial ATP-sensitive K+ channels via the EP3 receptor. Am J Physiol Heart Circ Physiol 288: H2093–H2101, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, Bhatnagar A, Bolli R. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA 97: 10197–10202, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69: 145–182, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts ACG, Leufkens HGM, Walker BR. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart 90: 859–865, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spath JA Jr, Lane DL, Lefer AM. Protective action of methylprednisolone on the myocardium during experimental myocardial ischemia in the cat. Circ Res 35: 44–51, 1974. [DOI] [PubMed] [Google Scholar]
- 47.Sun HP, Sheveleva E, Chen QM. Glucocorticoids induce COX-1 expression in cardiomyocytes: role of glucocorticoid receptor and Sp3 transcription factor. Mol Endocrinol (July 3, 2008); doi: 10.1210/me.2007-0302. [DOI] [PMC free article] [PubMed]
- 47a.Sun HP, Xu BB, Inoue H, Chen QM. p38 MAPK mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell Signal. doi: 10.1016/j.cellsig.2008.07.003. In press. [DOI] [PubMed]
- 48.Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and selective increase in heat-shock proteins by acute dexamethasone treatment. Am J Physiol Heart Circ Physiol 278: H1091–H1097, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Tang Q, Chen W, Gonzales MS, Finch J, Inoue H, Bowden GT. Role of cyclic AMP responsive element in the UVB induction of cyclooxygenase-2 transcription in human keratinocytes. Oncogene 20: 5164–5172, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Turini M, DuBois R. Cyclooxygenase-2: a therapeutic target. Annu Rev Med 53: 35–57, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Wallberg AE, Wright A, Gustafsson JA. Chromatin-remodeling complexes involved in gene activation by the glucocorticoid receptor. Vitam Horm 60: 75–122, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Warner T, Mitchell J. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J 18: 790–804, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med 141: 764–770, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Whitworth JA, Mangos GJ, Kelly JJ. Cushing, cortisol, and cardiovascular disease. Hypertension 36: 912–916, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation 98: 100–103, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 109: 2462–2468, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Yamada K, Duong DT, Scott DK, Wang JC, Granner DK. CCAAT/enhancer-binding protein beta is an accessory factor for the glucocorticoid response from the cAMP response element in the rat phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem 274: 5880–5887, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Yan LL, Liu K, Matthews KA, Daviglus ML, Ferguson TF, Kiefe CI. Psychosocial factors and risk of hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA 290: 2138–2148, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Yeagley D, Quinn PG. 3′,5′-Cyclic adenosine monophosphate response element-binding protein and CCAAT enhancer-binding protein are dispensable for insulin inhibition of phosphoenolpyruvate carboxykinase transcription and for its synergistic induction by protein kinase A and glucocorticoids. Mol Endocrinol 19: 913–924, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Zhang MZ, Harris RC, McKanna JA. Regulation of cyclooxygenase-2 (COX-2) in rat renal cortex by adrenal glucocorticoids and mineralocorticoids. Proc Natl Acad Sci USA 96: 15280–15285, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang N, Truong-Tran QA, Tancowny B, Harris KE, Schleimer RP. Glucocorticoids enhance or spare innate immunity: effects in airway epithelium are mediated by CCAAT/enhancer binding proteins. J Immunol 179: 578–589, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]