Abstract
Intercellular communication is important for cochlear homeostasis because connexin26 (Cx26) mutations are the leading cause of hereditary deafness. Gap junctions formed by different connexins have unique selectivity to large molecules, so compensating for the loss of one isoform can be challenging in the case of disease causing mutations. We compared the properties of Cx26 mutants T8M and N206S with wild-type channels in transfected cells using dual whole cell voltage clamp and dye flux experiments. Wild-type and mutant channels demonstrated comparable ionic coupling, and their average unitary conductance was ∼106 and ∼60 pS in 120 mM K+-aspartate− and TEA+-aspartate− solution, respectively, documenting their equivalent permeability to K+ and TEA+. Comparison of cAMP, Lucifer Yellow (LY), and ethidium bromide (EtBr) transfer revealed differences in selectivity for larger anionic and cationic tracers. cAMP and LY permeability to wild-type and mutant channels was similar, whereas the transfer of EtBr through mutant channels was greatly reduced compared with wild-type junctions. Altered permeability of Cx26 to large cationic molecules suggests an essential role for biochemical coupling in cochlear homeostasis.
Keywords: channel, selectivity, cochlear homeostasis, ethidium bromide, Lucifer yellow
gap-junctional communication facilitates the exchange of ions (K+ and Ca2+), second messengers [cAMP, cGMP, and inositol 1,4,5-trisphosphate (IP3)], and other small molecules (glucose, small interfering RNAs), providing a direct linkage between the cytoplasm of adjacent cells (7, 8, 24, 30, 31, 41, 42, 47, 56). Intercellular communication is essential for many physiological events, including cell synchronization, differentiation, cell growth, and metabolic coordination of avascular organs such as the epidermis and lens (36, 39, 44, 59, 63). In chordates, a family of genes called connexins (Cx) encodes gap junction channels. These integral membrane proteins share a similar structural topology, traversing the plasma membrane four times leaving the amino and carboxyl termini inside the cell. The four transmembrane domains constitute the wall/pore of the channel and are connected by two extracellular loops that play roles in docking processes and connexin recognition. A cytoplasmic loop connects the second and third transmembrane domains (18, 22, 71). Six connexins oligomerize within the cell into hemichannels, called connexons, which are then transported to the plasma membrane. These hemichannels either form nonjunctional channels in unopposed areas of the cellular membrane or interact with other connexons from the adjacent cell at regions of cell-to-cell contact to complete the formation of intercellular gap junctions (13, 28, 32, 49, 50).
There are 21 connexin isoforms in the human genome, and mutations in some of these genes have been implicated in several human hereditary diseases such as cataracts, X-linked Charcot-Marie Tooth disease, skin disorders, and sensorineural hearing loss (9, 25, 43, 46). Gap junctions were originally believed to be nonspecific porous structures, which would allow the free passage of any molecules smaller than 1.2 kDa (47). Since an individual cell can express more than one isoform, it has been thought that the loss of one gene in case of mutations/deletions might be compensated for by other connexins due to their high homology. For example, the transgenic expression of Cx26 from a modified bacterial artificial chromosome in Cx30−/− mice was shown to reverse the deafness phenotype observed in knockout animals, demonstrating that significant overexpression of Cx26 protein could be sufficient enough to maintain normal cochlear function (1). To date, gap junctions made of different connexins have been demonstrated to show little selectivity to monovalent ions, whereas permselectivity of each channel type to larger metabolites exhibited great variation (17, 20, 37, 53, 62, 64).
Mutations in at least three human connexin genes, Cx26, Cx30 and Cx31, which are widely expressed throughout cochlea, are the leading causes of nonsyndromic hereditary hearing loss (19, 25, 65). The function of gap-junctional communication in the inner ear is not fully understood. However, two mechanisms have been proposed regarding the role of cochlear intercellular communication. First, it has been suggested that the gap junction network between cochlear supporting cells plays a role in the recirculation of K+ back into the endolymph after the activation of auditory process (26, 27, 60). In this model, a cochlear supporting cell gap junction network is believed to remove K+ around the hair cells to maintain their sensitivity for the next stimuli and to recycle them back to endolymph to sustain the endolymphatic potential (61, 69). In the second model, biochemical coupling, in addition to ionic coupling between supporting cells, is thought to be important for normal cochlear function (12). It was proposed that the cochlear supporting cells take up the K+ released into the extracellular space between the hair cells and the supporting cells, which are then carried away from hair cells by means of a gap junction network. In addition to K+, the second messenger IP3 is also thought to be exchanged between supporting cells during the hearing process. IP3 transfer between the cells generates Ca2+ waves, which are in turn speculated to activate a K+/Cl− efflux system that pumps excess K+ back to the endolymph, thus sustaining the endolymphatic potential and high K+ concentration.
In this study, we analyzed two Cx26 recessive nonsyndromic deafness mutations, the NH2-terminal mutant Thr8Met (T8M) and the fourth transmembrane domain mutant Asn206Ser (N206S) by using dual whole cell voltage-clamp and flux experiments in mammalian expression systems. We verified that these mutant proteins were expressed and correctly targeted to the cell membrane. In addition, we analyzed the relative permeability of wild-type and mutant channels to five different molecules (Table 1). Wild-type and mutant Cx26 channels had similar single channel characteristics and voltage-gating properties in either K+-aspartate− or TEA+-aspartate−. The analysis of permeability of larger molecules demonstrated that mutant channels had differential selectivity to cations compared with wild-type Cx26 gap junctions. The transfer of anionic Lucifer Yellow (LY) and cAMP through the mutant channels was not affected, whereas their permeability to a cationic dye (ethidium bromide, EtBr) was considerably reduced relative to wild-type Cx26. These findings support the hypothesis that Cx26 permeability to larger molecules also plays a critical role in maintaining the auditory epithelium.
Table 1.
Physical properties of molecules assayed for permeability through wild-type and mutant Cx26 channels
| Molecule | Charge | Size, Da |
|---|---|---|
| Potassium | +1 | 39 |
| Tetraethylammonium | +1 | 130 |
| Ethidium bromide | +1 | 394 |
| cAMP | −1 | 329 |
| Lucifer yellow | −2 | 457 |
MATERIALS AND METHODS
Cell culture.
Experiments were performed using intercellular communication-deficient neuro-2A and HeLa cells that were individually transfected with human Cx26 wild-type, mutant T8M, or N206S cDNAs. Wild-type or mutant cDNAs were subcloned into the eukaryotic expression vector pIRES2-EGFP (Clontech Laboratories, Mountain View, CA). Cells were transiently transfected using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Briefly, 1 day before transfection cells were plated so that they would be 70–95% confluent the following day. Lipofectamine 2000 reagent, plasmids, and OPTI-MEM medium (GIBCO) were brought to room temperature. DNA (10 μg) and Lipofectamine 2000 (10 μl) were individually diluted in 0.75 ml OPTI-MEM. After 20 min incubation, the mix was added onto the cells drop by drop and incubated at 37°C incubator for 18–24 h. Transfection efficiency was verified by visualization of the GFP signal under a fluorescent microscope and protein expression was demonstrated by immunofluorescence staining. Transfected cells were then subcultured onto the micro coverglasses for electrophysiological measurements and flux studies within 24–48 h after being plated.
Immunofluorescent staining.
HeLa cells were grown on glass coverslips, transiently transfected with the corresponding DNAs, and cultured for 24 h. Cells were then fixed with 1% paraformaldehyde in PBS for 15 min at room temperature, permeabilized with PBS-0.1% Triton-X 100 for 10 min, and then blocked with 3% BSA in PBS with 0.1% Triton-X 100 for 30 min. A 1:500 dilution of a polyclonal antibody against Cx26 protein (Zymed Labs, South San Francisco, CA) was applied for 1 h followed by an application of 1:2,000 dilution of secondary Cy3-conjugated AffiniPure goat anti-rabbit antibody (Jackson ImmunoResearch Labs, West Grove, PA) for 30 min in the dark. Before being mounted, the coverslips were washed with PBS and dipped in distilled water, and mounted on slides using Vectashield with DAPI (Vector Laboratories, Burlingame, CA). The protein expression and localization was monitored with ×40 or ×60 objectives on an Olympus BX51 microscope (Olympus America, Center Valley, PA) and photographed with a MagnaFire digital camera (Optronics, Goleta, CA).
Electrophysiological recordings.
Experiments were carried out on transiently transfected N2A and HeLa cell pairs using the dual whole cell voltage-clamp method at room temperature. Cells on glass coverslips were transferred to the experimental chamber with a bath solution containing (in mM) 137.7 NaCl, 5.4 KCl, 2.3 NaOH, 1 MgCl2, 2 CsCl2, 2 CaCl2, 4 BaCl2 10 glucose, and 5 HEPES (pH 7.4). Patch pipettes were pulled from glass capillaries with a horizontal puller (Sutter Instruments, Novato, CA) and filled with a pipette solution of 120 mmol/l K+ aspartate−, 5 mM HEPES, 10 mM EGTA, and 3 mM NaATP (pH 7.2) or 120 mmol/l tetraethylammonium (TEA+)-aspartate−, 5 mM HEPES, 10 mM EGTA, and 3 mM NaATP (pH 7.2). At the beginning of each experiment, both cells were clamped at the same holding potential to provide a zero transjunctional voltage. Then one of the cells was stepped to different voltages (Vj of ±10–110 mV in 20-mV increments) (11, 54). The current from the cell held at constant potential was recorded and divided by the voltage to calculate conductance. The junctional current (Ij) values were determined at the beginning (Iinst) and at the end (Iss) of each pulse. The normalized steady-state conductance (gjss, normalized) was then calculated by taking the ratios between Iss and Iinst.
Fluorescent dye flux experiments.
Dye transfer through gap junction channels was investigated using cell pairs. LY and EtBr were individually dissolved in the pipette solution at a concentration of 1 and 0.5 mg/ml, respectively. One of the cells in a pair was patched with the pipette containing LY or EtBr for 12 min to allow the fluorescent dye passage from source cells to the adjacent cells. Fluorescent dye cell-to-cell spread was imaged at regular intervals using a 14-bit 16,000 pixel gray scale digital CCD-camera (HRm Axiocam, Carl Zeiss, Thornwood, NY). At the end of each experiment the second cell was patched and the junctional current between the cells was measured as indicated above. Our previous studies have shown similar results during LY flux studies when the junctional conductance was either measured throughout the experiments in perforated patch mode or measured at the end of the dye flux experiment by whole cell patch (53).
Fluorescent data analysis.
The fluorescent intensity in the cells was directly related to either the EtBr or LY concentration; the source cell intensity exponentially rose to steady state while the intensity in recipient cells increased linearly over 12 min. The outline of each cell and also an area of background were manually drawn in the brightfield image by using AxioVision Software (Zeiss). The fluorescent intensities of background, recipient cells, and source cells in the defined regions were then analyzed at specified time points. The averaged intensities for recipient and source cells were corrected by subtracting the background intensity from the respective images. The relative intensities were calculated at the 12-min time point by the ratio of the corrected fluorescent intensities of the recipient to the source cell. Then the relative intensities for both dyes were plotted as a function of junctional conductance and fit to a linear regression model using Spearman rank-order correlation.
cAMP transfer experiments.
Transiently transfected cell pairs were used for cAMP flux assays using dual voltage-clamp and a whole cell, perforated patch recording mode to control the membrane potential of both cells and to measure currents (23). SpIH, a cyclic nucleotide-modulated channel from sea urchin sperm, was subcloned into pDsRed2-C1 vector (Clontech Laboratories, Mountain View, CA), and HeLa cells were doubly transfected with pIRES2-EGFP Cx26T8M or Cx26N206S and pDsRed2-C1-SpIH as described above. Cell pairs consisting of one cell, which was yellow due to expression of both red SpIH and green enhanced green fluorescent protein (eGFP), and the other cell expressing only pIRES2-EGFP Cx26T8M or Cx26N206S were selected for cAMP transfer studies, which were performed as described by Kanaporis et al. (23). Briefly, cAMP was dissolved in the pipette solution at a concentration of 500 μM and was injected into the eGFP-expressing source cell containing only the Cx26 mutant channel via a patch pipette. SpIH-induced currents were recorded from the eGFP- and DsRed2-positive recipient cell that was transfected with both Cx26 mutant and SpIH channels. During these experiments, endogenous production and degradation of cAMP was prevented by addition of an adenylate cyclase inhibitor 2′,5′-dideoxyadenosine and a phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine, respectively.
Signal recording and analysis.
Patch-clamp amplifiers (Axopatch 200) were used to record voltage and current signals. The current signals were digitized with a 12-bit A/D-converter (Digidata 1322A, Axon Instrument) and stored with a personal computer. Data acquisition and analysis were performed with pClamp8 software (Axon Instrument). Statistical analyses and curve fitting were performed using Origin 6.1 (OriginLab, Northampton, MA). The results are presented as means ± SE.
RESULTS
Protein localization and functional characterization of T8M and N206S channels in mammalian cells.
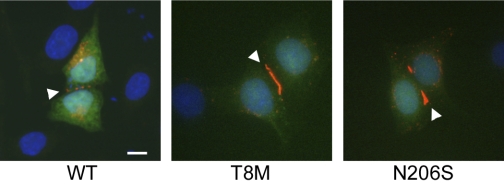
Previously, we demonstrated that two Cx26 deafness-associated recessive missense mutations, T8M and N206S, retained some electrical coupling activity in the paired Xenopus oocyte expression system by dual whole cell voltage-clamp experiments (35). We further characterized the permselectivity of these mutant channels to understand how they affect the function of gap-junctional communication in the inner ear. For this purpose, gap-junctional communication-deficient neuro-2A and HeLa cells were transiently transfected with Cx26 wild-type, T8M, and N206S pIRES2-EGFP constructs. Immunofluorescent staining of transiently transfected HeLa cells verified protein expression and localization for wild-type and mutant forms of Cx26 (Fig. 1). The mutant proteins were expressed in a comparable manner to wild-type Cx26 and were properly trafficked to the cell membrane, especially at the regions of cell-to-cell contact, shown by punctate staining (arrowheads). Therefore, the expression and membrane targeting of T8M and N206S mutant proteins were not altered due to corresponding mutations when expressed in mammalian cells.
Fig. 1.
Connexin 26 (Cx26) wild-type and NH2-terminal mutant Thr8Met (T8M) and transmembrane domain mutant Asn206Ser (N206S) protein expression and localization are shown. HeLa cells were transiently transfected with wild-type, T8M, and N206S constructs and were examined by immunostaining and fluorescence microscopy. Cells individually expressed wild-type and mutant T8M and N206S proteins and properly targeted them to the cell membrane especially at the region of cell-to-cell apposition. Arrowheads point to the punctate staining of Cx26 gap junction plaques at cell-cell contact areas. Green represents green fluorescent protein (GFP), blue shows DAPI staining of cell nuclei, and red is Cy3 staining of Cx26 protein. Scale bar = 10 μm.
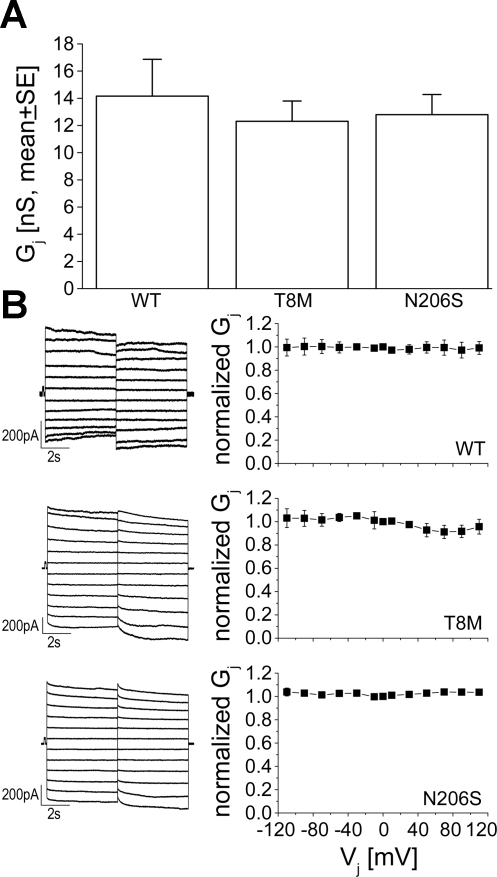
The ability of wild-type and mutant proteins to form functional channels was also analyzed by dual whole cell patch clamp in the transiently transfected HeLa cells. Cells were routinely treated with CO2 to differentiate between gap junctions and cytoplasmic bridges. When intercellular currents were unresponsive to CO2 exposure, implying the presence of cytoplasmic bridges, those cell pairs were excluded from further data analysis. HeLa cells transfected with wild-type Cx26 formed intercellular junctions with a mean conductance of 14.6 ± 1.3 nS (n = 15). The mean junctional conductance of T8M and N206S cell pairs were 12.3 ± 1.1 (n = 16) and 12.8 ± 1.2 (n = 20) nS, respectively, values that were not statistically different from either wild-type Cx26 or each other (Fig. 2A, ANOVA, followed by Student-Newman-Keuls post hoc test, P < 0.05). Comparable junctional conductance between cells expressing wild-type or mutant Cx26 proteins indicated that wild-type and mutant channels had similar ionic coupling activity in mammalian cells. Cx26 wild-type channels are known to show a very weak voltage-dependent response in junctional currents when expressed in mammalian cells (16, 55, 66). We also observed that Cx26 wild-type channels had very weak or absent voltage sensitivity, and junctional currents stayed nearly constant throughout the applied voltage steps (Fig. 2B). In contrast to our previous results in paired Xenopus oocytes, the response of junctional currents for T8M and N206S channels to a range of applied voltages was similar to wild-type Cx26 in mammalian cells, exhibiting little decay during the voltage steps (Fig. 2B).
Fig. 2.
Conductance and voltage-gating properties of wild-type, T8M, and N206S channels. A: comparison of macroscopic junctional conductance of Cx26 wild-type (n = 15), T8M (n = 16), and N206S channels (n = 20) in transiently transfected HeLa cells showed that they were not statistically different from each other (ANOVA, P < 0.05). Data are means ± SE. B: junctional currents from HeLa cell pairs transfected with wild-type Cx26, T8M, and N206S were recorded during application of a series of transjunctional voltages (Vjs) ranging from +110 to −110 mV in 20-mV increments (left). The normalized junctional conductance (means ± SE) versus Vjs was plotted (right). Cx26 wild-type and mutant T8M and N206S channels demonstrated very little voltage dependence.
Single channel properties of wild-type and mutant proteins.
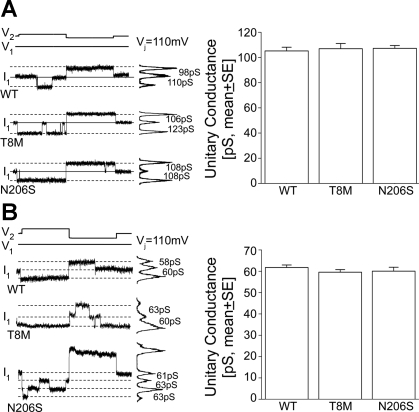
After verification of proper junction formation for the mutant channels, single channel events were examined to look for differences in the unitary conductance or gating between the mutant and wild-type connexins. In poorly coupled neuro-2A cell pairs, individual single channel events were recorded using dual whole cell voltage clamp. Representative current traces recorded at ± 110 in 120 mM K+-aspartate− and 120 mM TEA+-aspartate− pipette solution for Cx26 wild-type, T8M and N206S single channels are illustrated in Fig. 3A (left) and in Fig. 3B (left), respectively. The magnitude of unitary current change in these recordings represents K+ or TEA+ permeability through the corresponding single channels. Comparable changes in the traces of wild-type (110 and 98 pS for positive and negative voltages, respectively), T8M (123 and 106 pS), and N206S (108 pS) channels using K+ pipette solution implicated a similar potassium transfer between the cells (Fig. 3A, left). Furthermore, single channel recordings of Cx26 wild-type, T8M, and N206S channels using a larger cationic molecule TEA+ also yielded similar unitary conductances which were between 58–63 pS (Fig. 3B, left).
Fig. 3.
Single channel properties of wild-type, T8M, and N206S channels. Single channel currents recorded from cell pairs expressing wild-type, T8M, and N206S at a Vj of ±110 mV in transiently transfected Neuro-2A cells. A: current histograms of the single channels for wild-type and mutant connexins revealed unitary conductances between 98 and 123 pS in 120 mM K+-aspartate− (left). Solid line represents the zero junctional current and the dashed lines indicate open-state currents. The averaged unitary conductances of wild-type, T8M, and N206S channels were statistically indistinguishable from each other (ANOVA, P < 0.05) (right). B: with the use of 120 mM TEA+-aspartate− pipette solution (left), Cx26 wild-type, T8M, and N206S channels had unitary conductances between 58 and 63 pS and the mean unitary conductances for all three channels were comparable to each other (ANOVA, P < 0.05, right). Data represented as means ± SE.
The averaged unitary conductance of wild-type channels was 105 ± 2.9 pS (n = 16) in 120 mM K+-aspartate− pipette solution, which was consistent with previous reports (Fig. 3A, right) (6, 10, 21, 55), whereas Cx26 wild-type junctions had smaller mean unitary conductance in 120 mM TEA+-aspartate− solution compared with measurements using potassium (62 ± 1.2, n = 12). Ion substitution experiments have previously shown that Cx26 channels prefer cations to anions by a ratio of 2.6:1 and that permeability of large anions like aspartate or glutamate accounts for >10% of the measured unitary conductance for Cx26 (51). The ∼50% reduction in unitary conductance between K+ and TEA+ pipette solutions correlates well with the ∼50% difference in ionic mobility of TEA+ and K+ (4, 45). The unitary conductance of T8M and N206S were 106 ± 4.1 pS (n = 12) and 107 ± 2.2 pS (n = 15) in K+-aspartate− pipette solution; and 60 ± 1.2 pS (n = 13) and 60 ± 1.8 pS (n = 10) in TEA+-aspartate− solution, respectively (Fig. 3B). Comparable unitary conductance between wild-type and the mutant channels using both K+-aspartate− and TEA+-aspartate− pipette solutions (P < 0.05, ANOVA, followed by Student-Newman-Keuls post hoc test) demonstrated that T8M and N206S junctions were as permeable to K+ and TEA+ as wild-type Cx26, respectively. Thus the mutations did not affect the permeability of K+ and TEA+ transferred between the cells. In addition, since the average macroscopic conductance and the average unitary conductance were not statistically different, these data show that an equal number of operational channels (∼125) were present between the paired cells in each tested condition when K+-aspartate− pipette solution was used.
Permeability of wild-type and mutant channels to larger molecules.
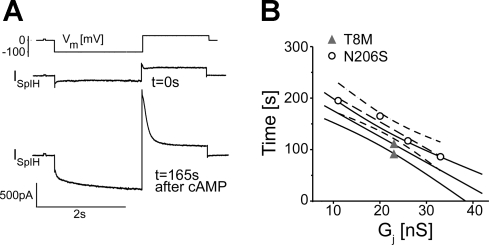
After the observation that Cx26 wild-type and mutant channels were each equally permeable to K+ and TEA+ respectively, the permeability of channels to second messengers and fluorescent dyes was examined to look for possible differences in their selectivity to larger molecules. We initially assessed the permeability of a biologically significant molecule through the channels. We recently developed a method to determine the permeability of gap junctions to cAMP by simultaneous measurements of junctional conductance and intracellular transfer of cAMP (23) and compared the permeability of cAMP through Cx26 wild-type and mutant channels using the same approach. Cells expressing only connexin channels (source cell) were patched in whole cell mode with the pipette containing cAMP, whereas the other cell transfected with both SpIH and Cx26 mutant channel (recipient) was patched in a perforated mode. SpIH currents were recorded in response to voltage steps from holding potentials of 0 to −100 mV, returning to a tail potential of +50 mV. Figure 4A illustrates an example of SpIH current from a cell pair expressing Cx26 mutant channel in response to a voltage step of −100 mV (Fig. 4A, top) when the whole cell patch was opened (t = 0s, middle) and at t = 165 s when SpIH current was increased and saturated due to cAMP transfer from source cell into recipient cell (bottom). To compare channel permeability to cAMP, saturation time points of SpIH currents were measured and plotted versus junctional conductance (Fig. 4B). The straight line corresponds to the first-order regression of SpIH saturation times for our previously published Cx26 wild-type channel data (23), and the SpIH saturation time was shown to be reciprocally proportional to junctional conductance. Closed triangles and open circles are the SpIH activation time points for Cx26 T8M (n = 2) and N206S (n = 4), respectively. The dashed line is the first-order regression of saturation times for N206S channels, which were not significantly different from Cx26 wild-type junctions. Two measurements for T8M channels also failed to yield significantly different saturation times from the Cx26 wild-type data, so we did not pursue further cAMP flux measurements. These data suggest that the T8M and N206S mutations in Cx26 did not alter the permeability of cAMP through the junctions.
Fig. 4.
Comparison of cAMP permeability through Cx26 wild-type, T8M, and N206S. A: SpIH current trace at the beginning of cAMP injection into source cell at t = 0 s and at t = 165 s when the current was saturated due to cAMP transfer into recipient cell from the injected cell, showing the SpIH-dependent current increase. B: SpIH saturation time for each channel was plotted against junctional conductance. Dark straight line represents our previously published SpIH activation times for wild-type channels (23), and light solid lines are the 95% confidence interval. Dashed line is the first-order regression for N206S channels, and the curved dashed lines are the 95% confidence interval. The SpIH activation times for N206S channels were not significantly different from wild-type and T8M channels whose saturation times overlap with wild-type Cx26.
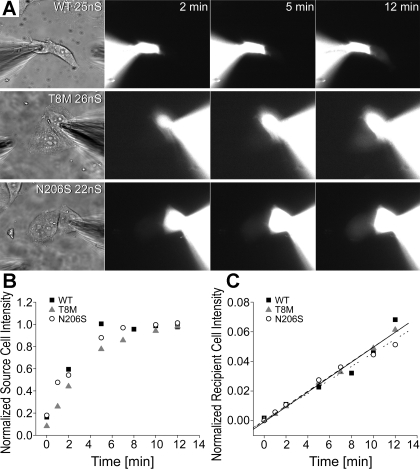
We then used fluorescent dyes with different molecular weights and charges to examine channel permeability since they do not require a reporter gene and can be directly quantified by epifluorescence. We first compared the permeation of LY, an anionic dye, through mutant and wild-type channels using dye flux experiments followed by measurements of junctional conductance (gj) in individual cell pairs (53). Figure 5A illustrates cell pairs expressing wild-type and mutant T8M and N206S channels as an example of LY passage at comparable macroscopic junctional conductance (22–26 nS). The fluorescent intensity in both recipient and source cells was monitored at regular intervals after the patch seal of the source cell was opened (Fig. 5A; 2, 5, and 12 min are shown). The transfer of LY through wild-type, T8M, and N206S channels could be observed by the increase in the fluorescent intensity in the recipient cells for all samples. Fluorescent values for source and recipient cells were normalized to the maximum intensities in the corresponding pairs and then plotted as a function of time. The source cells expressing wild-type, T8M, and N206S channels had similar loading patterns and stayed constant after reaching the saturation (Fig. 5B). However, there was a time-dependent increase in the fluorescent intensities of all recipient cells over 12 min (Fig. 5C). The normalized values in the recipient cells of wild-type, T8M, and N206S were not statistically different from each other (P < 0.05, ANOVA). The increase in the fluorescent intensity in the recipient cells could be fitted by linear regression with similar slopes for wild-type, T8M, and N206S channels (R2 values of 0.95, 0.99, and 0.98, respectively, Fig. 5C).
Fig. 5.
Permeability of wild-type and mutant channels to Lucifer yellow (LY). Measurements of LY flux from cell pairs expressing wild-type Cx26, T8M, and N206S followed by measurements of junctional conductance (gj). A: epifluorescent micrographs taken at 2, 5, and 12 min for cell pairs of similar junctional conductance (22–26 nS) demonstrated progressive fluorescent intensity increases in the recipient cells for each channel type. B: fluorescent intensities of the source cells of wild-type, T8M, and N206S were normalized to the maximum fluorescent value in the source cells and was plotted as a function of time. For all three examples shown in A, the source cells had similar loading patterns. C: normalized intensities of recipient cells expressing wild-type, T8M, and N206S showed a time-dependent increase in the intensities of recipient cells over the course of 12 min, implicating dye transfer from the source cells to the recipient cells.
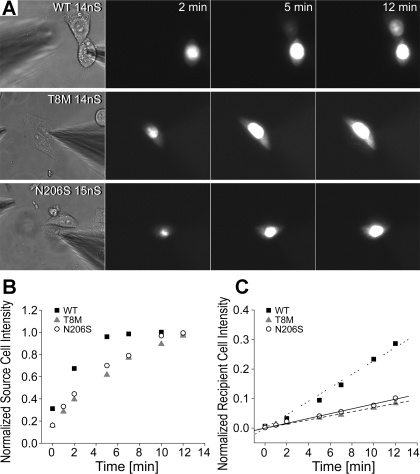
After verification of comparable LY dye transfer between the cell pairs expressing the wild-type and mutant channels, we studied the flux of a positively charged molecule, EtBr. Figure 6A illustrates examples of cells tested for EtBr transfer between pairs with similar junctional conductance (14–15 nS). The fluorescent intensity in the recipient cell transfected with wild-type Cx26 increased over time, and dye flux from the source cell to the recipient cell was evident starting from 5 min after opening the seal. On the other hand, there was little increase in the fluorescent intensity of recipient cells for T8M and N206S channels even at the 12-min time point (Fig. 6A). The normalized intensities versus time plots for the source cells of the examples shown in Fig. 5A revealed that there was some individual variation in the rate of cell loading, but all source cells reached a maximum intensity by 12 min (Fig. 6B). Furthermore, the mean time course of source cell loading was not statistically significant between wild-type and mutant experiments (Table 2). The normalized intensities for recipient cells of T8M and N206S showed only a very small increase over 12 min (R2 values for the linear regression were 0.99 for both, Fig. 6C). The EtBr fluorescent intensity increase in the recipient cells expressing wild-type Cx26 channels (the R2 value of linear regression was 0.98, with a six- to sevenfold increase in the slope) was much higher than that seen for mutant T8M and N206S channels. In dye transfer experiments, the diffusion of tracer from the patch pipettes into the source cells is the rate-limiting step for the equilibration of the patch pipette and the source cell (33, 38). To ensure that differences in the rate of dye delivery to source cells by the pipette were similar between mutant and wild-type expressing cells, we determined the time necessary for source cells of wild-type, T8M, and N206S to reach steady-state intercellular concentrations for both LY and EtBr. The averaged steady-state time for wild-type, T8M, and N206S was 183 ± 50, 185 ± 29, and 197 ± 17 s for LY experiments and 311 ± 53, 329 ± 32, and 332 ± 31 s for EtBr dye flux due to its high affinity to nucleic acids it took longer for cells to reach steady state (Table 2). The time constants for wild-type, T8M, and N206S in either LY or EtBr permeability experiments were not statistically different from each other (P < 0.05, ANOVA). Taken together, these data show that the observed reduction in the permeability of mutant channels to EtBr were not due to variations in the loading of the source cell with dye, or differences in the numbers of operational channels but resulted from intrinsic alterations of channel permeability to EtBr.
Fig. 6.
Permeability of wild-type and mutant channels to ethidium bromide (EtBr). EtBr transfer through cells expressing the wild-type and mutant channels was compared between pairs with similar junctional conductance. A: examples of epifluorescent micrographs (taken at 2, 5, and 12 min) of cell pairs expressing wild-type, T8M and N206S proteins during EtBr flux experiments. Transfer of EtBr through wild-type Cx26 channels was readily observed after the opening of the patch seal in the source cell, as seen by the increased intensity of the recipient cell. There was little visible fluorescent intensity in the recipient cells coupled by T8M and N206S channels. B: normalized fluorescent intensities of source cells expressing wild-type, T8M, and N206S Cx26 in A were plotted as a function of time (minutes) to show dye injection into the cells. C: fluorescent intensity of the recipient cells was increased over time. Both the T8M and N206S mutant channels had greatly reduced permeability relative to wild-type channels.
Table 2.
Comparison of properties of Cx26 wild-type, T8M, and N206S channels
| Channels | WT | T8M | N206S |
|---|---|---|---|
| Mean junctional conductance, nS | 14.6±1.3 (n=15) | 12.3±1.1 (n=16) | 12.8±1.2 (n=20) |
| Mean unitary conductance, K+, pS | 105±2.9 (n=16) | 106±4.1 (n=12) | 107±2.2 (n=15) |
| Mean unitary conductance, TEA+, pS | 62±1.2 (n=12) | 60±1.2 (n=13) | 60±1.8 (n=10) |
| Slope of LY transfer | 0.0029 | 0.0025 | 0.0021 |
| Slope of EtBr transfer | 0.021 | 0.0058 | 0.0059 |
| Source cell time constant to reach steady state, LY, s | 183±50 (n=7) | 185±29 (n=7) | 197±17 (n=9) |
| Source cell time constant to reach steady state, EtBr, s | 311±32 (n=16) | 329±53 (n=6) | 332±31 (n=7) |
Values are means ± SE; n is number of cell pairs. The apparent slopes of the fitted data for relative intensity of recipient cell versus junctional conductance in many cell pairs are presented for Lucifer yellow (LY) and ethidium bromide (EtBr) flux experiments. Time constants for source cells to reach steady state were determined for wild-type (WT), T8M, and N206S in both LY and EtBr permeability experiments and revealed that the source cell loadings were similar to each other (P < 0.05, ANOVA).
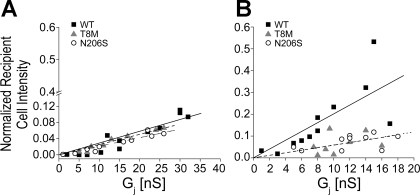
Measurements of LY or EtBr dye flux and junctional conductance (gj) in many cell pairs with different magnitudes of total conductance enabled further comparison of dye transfer among wild-type, T8M, and N206S channels (Fig. 7). If the dye concentration in the source cell is much greater than that of the recipient cell and the channels have equivalent unitary conductance, then the amount of dye flux in a fixed time would be expected to rise linearly as channel number increases. We observed a linear correlation between the gj and the relative intensity of the recipient cells expressing wild-type channels in LY transfer experiments (R2 for linear regression was 0.90); i.e., as the gj increased, more LY flux was observed at the end of the 12-min time period analyzed (Fig. 7A). A linear relation was also noticed for the mutant channels, where as the number of channels increased so did the amount of LY passing through channels (R2 values of T8M and N206S were 0.96 and 0.92, respectively). Comparison of LY transfer among all channel types revealed that the T8M and N206S mutations did not affect Cx26 channel permeability to LY since the LY flux through mutant channels was indistinguishable from wild-type junctions (P < 0.05, ANOVA, Fig. 7A). There was also a linear correlation between the gj and relative fluorescent intensity of recipient cells expressing wild-type Cx26 for EtBr flux (R2 value of 0.92, Fig. 7B). In contrast, mutant channels were poorly fit by linear regression (R2 values were 0.48 for T8M and 0.55 for N206S), likely due to their poor permeability to EtBr, which was greatly decreased relative to wild-type Cx26. There were 72.4% and 72.0% reductions in the apparent slopes of fitted lines of T8M and N206S, respectively (Fig. 7B). Thus, whereas T8M and N206S mutant channels passed negatively charged LY as well as wild-type junctions, their permeability to cationic EtBr was much lower.
Fig. 7.
Relative intensity of recipient cells for LY and EtBr as a function of junctional conductance (gj). The relative intensities of recipient cells of Cx26 wild-type, T8M, and N206S junctions in different cell pairs were analyzed at the 12-min time point and plotted against the gj. A: relationship between the relative intensity and the gj for wild-type, T8M, and N206S for LY was linear [R2 values were 0.90 (straight line), 0.96 (dashed line) and 0.92 (dotted line), respectively]. LY passage between pairs of cells expressing wild-type, T8M, and N206S was comparable to each other, and statistically were not different (ANOVA, P < 0.05). B: EtBr transfer between cell pairs expressing wild-type Cx26 showed a modest linear correlation between relative intensity of recipient cell and gj with a R2 value of 0.92 (straight line). T8M and N206S channels showed only a weak correlation between gj versus relative intensity of recipient cell and greatly reduced EtBr permeability. The slopes of fitted lines for T8M (dashed fitting line) and N206S (dotted fitting line) channels were 27.6% and 28% of that of wild-type Cx26.
DISCUSSION
Functional characterization of Cx26 missense mutations associated with nonsyndromic hearing loss may provide insights about the role of gap-junctional communication in the inner ear. To date, most of the analyzed Cx26 mutations were not able to form functional channels (6, 13, 34, 35, 40). However, a small subset of mutants has been shown to retain some level of ionic coupling (13, 35, 40, 67). These partially active mutants might help to characterize the type of molecules needed to be exchanged between cochlear supporting cells to maintain normal hearing. Here, we analyzed two partially functional Cx26 deafness-associated missense mutations T8M and N206S. We first verified the expression and proper membrane targeting of mutant channels. Then we demonstrated the ability of mutants to form functional channels in a mammalian expression system with a total junctional conductance comparable with wild-type Cx26. In addition, the wild-type and mutant channels were shown to have similar unitary conductance when measured with both a 120 mM K+ and TEA+ pipette solution, implicating that mutant junctions were as permeable to K+ and TEA+ as wild-type Cx26. Examination of their permeability to larger molecules revealed a differential selectivity of mutant channels for molecules with different molecular weight and charge. Although LY and cAMP transfer through the mutant junctions was comparable to wild-type channels, their permeability to EtBr was greatly reduced (summarized in Table 2). These observations provide support for the contribution of biochemical coupling within the supporting cell gap junction networks in the maintenance of cochlear homeostasis.
Cx26 wild-type channels are known to display weak voltage sensitivity in paired Xenopus oocytes where they have shown asymmetric junctional current decays during application of high voltages (higher than ± 80 mV) (2, 3, 48, 58). On the other hand, when expressed in mammalian cells, they often appear to lose this weak voltage dependence (16, 66). Here, we also report that Cx26 wild-type channels had no apparent voltage-dependent changes in their junctional currents, and the mutant channels had similar responses to the applied voltages. It is not clear why Cx26 channels show different macroscopic gating characteristics in these two model systems, although it may reflect differences in posttranslational processing between these different cell types.
At least four connexin isoforms (Cx26, Cx30, Cx31, and Cx43) have been shown to be expressed in the inner ear (14, 15, 26, 29). Cx26 and Cx30 are the main components of gap junction channels between the cochlear supporting cells where they were shown to be found in the same gap junction plaques. The ability of Cx26 and Cx30 to form heteromeric and/or heterotypic channels has been demonstrated by several labs (1, 66, 70). Cx26 and Cx30 share the highest homology among the members of connexin gene family. Therefore, it has been speculated that the loss of either gene in the inner ear due to mutations might be compensated for by the intact isoform (1). However, other studies have suggested that this may not always be the case. For example, Cx30 channels were shown to be more permeable to cationic dyes than to anionic ones (5, 52). On the other hand, Cx26 channels were demonstrated to be permeable to both cationic and anionic dyes and they were suggested to be primarily responsible for the permeability of anionic molecules in the inner ear (5, 37, 68). Here, we demonstrated that Cx26 mutant T8M and N206S channels had impaired permeability to a cationic dye, EtBr, while they retained their ability to transfer anionic LY and cAMP between cell pairs. Previously, Zhang et al. (67) showed that Cx26 mutations V84L, V95M, and A88S also lost their permeability to another cationic dye, propidium iodide. The altered permeability of Cx26 channels to positively charged molecules could help explain why these mutants generate deafness, despite remaining permeable to K+ and TEA+. Previous studies have suggested that there might be affinity binding sites within connexin channels that may facilitate their selective permeability to large molecules (20). Therefore, one possibility for the effect of T8M and N206S mutations on channel function might be due to alterations in the channel affinity for cationic molecules like EtBr, reducing their transfer through the channels.
Analysis of other functional Cx26 recessive nonsyndromic deafness mutations has shown reductions in permeability to second messangers. For example, V84L mutant channels were as permeable to potassium ions as wild-type junctions, but the passage of IP3 through V84L channels was impaired (6, 13), implicating the importance of biochemical coupling in the normal hearing process. Further support for this view came when Ca2+ wave generation was compared between Cx26 wild-type and deafness-causing mutants V84L, V95M, and A88S following IP3 injection (67). The IP3 transfer between cells expressing mutant proteins was abolished while the mutant junctions remained permeable to Ca2+ and Na+. We have not directly tested the IP3 permeability of T8M and N206S channels, although as they show a similar reduction in cationic dye permeability as V84L, V95M, and A88S, they may also share impaired IP3 flux. Although this would appear to contradict with the lack of permeability differences for the cAMP and LY anions we tested, it should be noted that the relationship between connexin channel conductance and ionic selectivity or dye permeability is highly complex (57), and that V84L channels, where the loss of IP3 permeability was first noted, retained normal permeability to the anionic dye LY (6, 13).
The role of intercellular communication in the inner ear is not fully understood. However, two hypotheses have been proposed. The first emphasizes the importance of ionic coupling between supporting cells where cochlear gap junctions play a role in the recirculation of K+ (27). The second model suggests a more local mechanism that included permeability of larger solutes such as IP3 (12). Partially functional mutants might provide an invaluable tool for differentiating the role of ionic coupling from biochemical coupling through the gap-junctional networks in the cochlea. The unitary conductance of Cx26 wild-type, T8M, and N206S channels were statistically indistinguishable from each other. This implicated that all channel types had the same K+ permeability. However, they showed differential selectivity to larger molecules. Hence, deafness associated with missense mutations in Cx26 cannot only be due to disruption of potassium permeability, rather abnormalities in the transfer of large molecules between the supporting cells must also play a role. Our data support the importance of biochemical coupling for normal cochlear functioning. Generation of mouse models using these Cx26 mutant variants might improve our understanding of their in vivo effect on intercellular communication and the molecules needed to be exchanged between the supporting cells during normal hearing.
GRANTS
This work was supported by National Institutes of Health Grants RO1 DC-006652 and RO1 EY-013163 (to T. W. White), GM-055263 and EY-014604 (to P. R. Brink), and American Heart Association Grant 0335236N (to V. Valiunas).
Acknowledgments
We thank Dr. Leon Moore and Engin Ozcivici for assistance with statistical analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmad S, Tang W, Chang Q, Qu Y, Hibshman J, Li Y, Sohl G, Willecke K, Chen P, Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci USA 104: 1337–1341, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrio LC, Suchyna T, Bargiello T, Xu LX, Roginski RS, Bennett MV, Nicholson BJ. Gap junctions formed by connexins 26 and 32 alone and in combination are differently affected by applied voltage. Proc Natl Acad Sci USA 88: 8410–8414, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beahm DL, Oshima A, Gaietta GM, Hand GM, Smock AE, Zucker SN, Toloue MM, Chandrasekhar A, Nicholson BJ, Sosinsky GE. Mutation of a conserved threonine in the third transmembrane helix of alpha- and beta-connexins creates a dominant-negative closed gap junction channel. J Biol Chem 281: 7994–8009, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Beblo DA, Veenstra RD. Monovalent cation permeation through the connexin40 gap junction channel. Cs, Rb, K, Na, Li, TEA, TMA, TBA, and effects of anions Br, Cl, F, acetate, aspartate, glutamate, and NO3. J Gen Physiol 109: 509–522, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltramello M, Bicego M, Piazza V, Ciubotaru CD, Mammano F, D'Andrea P. Permeability and gating properties of human connexins 26 and 30 expressed in HeLa cells. Biochem Biophys Res Commun 305: 1024–1033, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nat Cell Biol 7: 63–69, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bennett MV Physiology of electrotonic junctions. Ann NY Acad Sci 137: 509–539, 1966. [DOI] [PubMed] [Google Scholar]
- 8.Bennett MV, Trinkaus JP. Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol 44: 592–610, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergoffen J, Scherer SS, Wang S, Scott MO, Bone LJ, Paul DL, Chen K, Lensch MW, Chance PF, Fischbeck KH. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 262: 2039–2042, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Bicego M, Beltramello M, Melchionda S, Carella M, Piazza V, Zelante L, Bukauskas FF, Arslan E, Cama E, Pantano S, Bruzzone R, D'Andrea P, Mammano F. Pathogenetic role of the deafness-related M34T mutation of Cx26. Hum Mol Genet 15: 2569–2587, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brink PR, Cronin K, Banach K, Peterson E, Westphale EM, Seul KH, Ramanan SV, Beyer EC. Evidence for heteromeric gap junction channels formed from rat connexin43 and human connexin37. Am J Physiol Cell Physiol 273: C1386–C1396, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Bruzzone R, Cohen-Salmon M. Hearing the messenger: Ins(1,4,5)P3 and deafness. Nat Cell Biol 7: 14–16, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Bruzzone R, Veronesi V, Gomes D, Bicego M, Duval N, Marlin S, Petit C, D'Andrea P, White TW. Loss-of-function and residual channel activity of connexin26 mutations associated with non-syndromic deafness. FEBS Lett 533: 79–88, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Cohen-Salmon M, Maxeiner S, Kruger O, Theis M, Willecke K, Petit C. Expression of the connexin43- and connexin45-encoding genes in the developing and mature mouse inner ear. Cell Tissue Res 316: 15–22, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Forge A, Marziano NK, Casalotti SO, Becker DL, Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun Adhes 10: 341–346, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gemel J, Valiunas V, Brink PR, Beyer EC. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J Cell Sci 117: 2469–2480, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta 1662: 96–101, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Goodenough DA, Paul DL, Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol 107: 1817–1824, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grifa A, Wagner CA, D'Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet 23: 16–18, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Harris AL Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94: 120–143, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez VH, Bortolozzi M, Pertegato V, Beltramello M, Giarin M, Zaccolo M, Pantano S, Mammano F. Unitary permeability of gap junction channels to second messengers measured by FRET microscopy. Nat Methods 4: 353–358, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hertzberg EL, Disher RM, Tiller AA, Zhou Y, Cook RG. Topology of the Mr 27,000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J Biol Chem 263: 19105–19111, 1988. [PubMed] [Google Scholar]
- 23.Kanaporis G, Mese G, Valiuniene L, White TW, Brink PR, Valiunas V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J Gen Physiol 131: 293–305, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanno Y, Loewenstein WR. Low-resistance coupling between gland cells. Some observations on intercellular contact membranes and intercellular pace. Nature 201: 194–195, 1964. [DOI] [PubMed] [Google Scholar]
- 25.Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387: 80–83, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi T, Kimura RS, Paul DL, Adams JC. Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 191: 101–118, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi T, Kimura RS, Paul DL, Takasaka T, Adams JC. Gap junction systems in the mammalian cochlea. Brain Res Brain Res Rev 32: 163–166, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kovacs JA, Baker KA, Altenberg GA, Abagyan R, Yeager M. Molecular modeling and mutagenesis of gap junction channels. Prog Biophys Mol Biol 94: 15–28, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res 294: 415–420, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence TS, Beers WH, Gilula NB. Transmission of hormonal stimulation by cell-to-cell communication. Nature 272: 501–506, 1978. [DOI] [PubMed] [Google Scholar]
- 31.Loewenstein WR Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 61: 829–913, 1981. [DOI] [PubMed] [Google Scholar]
- 32.Marziano NK, Casalotti SO, Portelli AE, Becker DL, Forge A. Mutations in the gene for connexin 26 (GJB2) that cause hearing loss have a dominant negative effect on connexin 30. Hum Mol Genet 12: 805–812, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Mathias RT, Cohen IS, Oliva C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys J 58: 759–770, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melchionda S, Bicego M, Marciano E, Franze A, Morgutti M, Bortone G, Zelante L, Carella M, D'Andrea P. Functional characterization of a novel Cx26 (T55N) mutation associated to non-syndromic hearing loss. Biochem Biophys Res Commun 337: 799–805, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Mese G, Londin E, Mui R, Brink PR, White TW. Altered gating properties of functional Cx26 mutants associated with recessive non-syndromic hearing loss. Hum Genet 115: 191–199, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol 127: 2516–2524, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res 33: 369–378, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Oliva C, Cohen IS, Mathias RT. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J 54: 791–799, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima A, Doi T, Mitsuoka K, Maeda S, Fujiyoshi Y. Roles of Met-34, Cys-64, and Arg-75 in the assembly of human connexin 26. Implication for key amino acid residues for channel formation and function. J Biol Chem 278: 1807–1816, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Palmada M, Schmalisch K, Bohmer C, Schug N, Pfister M, Lang F, Blin N. Loss of function mutations of the GJB2 gene detected in patients with DFNB1-associated hearing impairment. Neurobiol Dis 22: 112–118, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Payton BW, Bennett MV, Pappas GD. Permeability and structure of junctional membranes at an electrotonic synapse. Science 166: 1641–1643, 1969. [DOI] [PubMed] [Google Scholar]
- 42.Revel JP, Yee AG, Hudspeth AJ. Gap junctions between electrotonically coupled cells in tissue culture and in brown fat. Proc Natl Acad Sci USA 68: 2924–2927, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH Jr, DiGiovanna JJ, Compton JG, Bale SJ. Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20: 366–369, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ. Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet 103: 393–399, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Robinson RA, Stokes R. Electrolyte Solutions (2nd ed.). London, UK: Butterworth, 1965.
- 46.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 62: 526–532, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson I, Rose B, Loewenstein WR. Size limit of molecules permeating the junctional membrane channels. Science 195: 294–296, 1977. [DOI] [PubMed] [Google Scholar]
- 48.Skerrett IM, Di WL, Kasperek EM, Kelsell DP, Nicholson BJ. Aberrant gating, but a normal expression pattern, underlies the recessive phenotype of the deafness mutant Connexin26M34T. FASEB J 18: 860–862, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Sosinsky GE, Nicholson BJ. Structural organization of gap junction channels. Biochim Biophys Acta 1711: 99–125, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia 54: 758–773, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Suchyna TM, Nitsche JM, Chilton M, Harris AL, Veenstra RD, Nicholson BJ. Different ionic selectivities for connexins 26 and 32 produce rectifying gap junction channels. Biophys J 77: 2968–2987, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol 288: C613–C623, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91: 104–111, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Valiunas V, Gemel J, Brink PR, Beyer EC. Gap junction channels formed by coexpressed connexin40 and connexin43. Am J Physiol Heart Circ Physiol 281: H1675–H1689, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Valiunas V, Niessen H, Willecke K, Weingart R. Electrophysiological properties of gap junction channels in hepatocytes isolated from connexin32-deficient and wild-type mice. Pflügers Arch 437: 846–856, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol 568: 459–468, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink PR. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ Res 77: 1156–1165, 1995. [DOI] [PubMed] [Google Scholar]
- 58.Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature 368: 348–351, 1994. [DOI] [PubMed] [Google Scholar]
- 59.Vinken M, Vanhaecke T, Papeleu P, Snykers S, Henkens T, Rogiers V. Connexins and their channels in cell growth and cell death. Cell Signal 18: 592–600, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Wangemann P K+ cycling and the endocochlear potential. Hear Res 165: 1–9, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Wangemann P Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576: 11–21, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber PA, Chang HC, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J 87: 958–973, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White TW, Paul DL. Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol 61: 283–310, 1999. [DOI] [PubMed] [Google Scholar]
- 64.Willecke K, Hennemann H, Dahl E, Jungbluth S, Heynkes R. The diversity of connexin genes encoding gap junctional proteins. Eur J Cell Biol 56: 1–7, 1991. [PubMed] [Google Scholar]
- 65.Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ. Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet 20: 370–373, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Scherer S. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am J Physiol Cell Physiol 293: C1032–C1048, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci USA 102: 15201–15206, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao HB Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signalling and metabolic communications. Eur J Neurosci 21: 1859–1868, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao HB, Kikuchi T, Ngezahayo A, White TW. Gap junctions and cochlear homeostasis. J Membr Biol 209: 177–186, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao HB, Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol 499: 506–518, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zimmer DB, Green CR, Evans WH, Gilula NB. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem 262: 7751–7763, 1987. [PubMed] [Google Scholar]