Abstract
Cultured confluent endothelial cells exhibit stable basal isometric tone associated with constitutive myosin II regulatory light chain (RLC) phosphorylation. Thrombin treatment causes a rapid increase in isometric tension concomitant with myosin II RLC phosphorylation, actin polymerization, and stress fiber reorganization while inhibitors of myosin light chain kinase (MLCK) and Rho-kinase prevent these responses. These findings suggest a central role for myosin II in the regulation of endothelial cell tension. The present studies examine the effects of blebbistatin, a specific inhibitor of myosin II activity, on basal tone and thrombin-induced tension development. Although blebbistatin treatment abolished basal tension, this was accompanied by an increase in myosin II RLC phosphorylation. The increase in RLC phosphorylation was Ca2+ dependent and mediated by MLCK. Similarly, blebbistatin inhibited thrombin-induced tension without interfering with the increase in RLC phosphorylation or in F-actin polymerization. Blebbistatin did prevent myosin II filament incorporation and association with polymerizing or reorganized actin filaments leading to the disappearance of stress fibers. Thus the inhibitory effects of blebbistatin on basal tone and induced tension are consistent with a requirement for myosin II activity to maintain stress fiber integrity.
Keywords: actin, blebbistatin, isometric tension, myosin light chain kinase, regulatory light chain phosphorylation, focal adhesions
myosin ii is the major cytoskeletal protein in muscle and nonmuscle cells with the ability to self-assemble into bipolar filaments and convert chemical energy of ATP into mechanical work. Myosin II-based contractile activity has been shown to play important roles in a host of cellular functions such as cell spreading, motility, and cell division. In addition, it has been implicated in the maintenance of cell shape and generation of basal and agonist-induced cytoskeletal tension (15, 25, 31, 91). While myosin II was initially considered a passive structural participant in cytoskeletal organization and maintenance of cell shape in nonmuscle cells, more recent immunolocalization studies have shown that it is arranged in stress fibers in a sarcomeric-like organization similar to muscle (31, 85, 86). This morphological organization has led to the hypothesis that stress fibers decorated with myosin II are contractile structures assembled for the generation of cellular tension (10, 33). Permeabilized cell preparations validated this hypothesis, demonstrating that cell contraction and tension generation are dependent on myosin II activation. Furthermore, the network contraction model now proposes that myosin II is responsible for both tension generation and the rearrangement of actin filaments into appropriately aligned and bundled contractile actin-myosin II networks (80, 87).
Understanding the mechanism of myosin II filament assembly, especially the spatial and temporal regulation in vivo, is essential to understanding the coordinated events that initiate and regulate cell contraction and motility. For endothelial cells this is particularly important for understanding the contribution of endothelial cell tone to blood vessel function and angiogenesis. The use of pharmacological probes has greatly facilitated our understanding of the biochemical, physiological, and biophysical properties of the enzymes and phosphatases that activate and initiate myosin II-based contraction and cell motility. Recently, blebbistatin, a cell-permeable, highly specific small-molecule inhibitor of myosin II (79), has become available, allowing the investigation of the role of myosin II in cell motility and muscle and nonmuscle cell contraction. Blebbistatin inhibits the Mg-ATPase activity of myosin IIA, IIB, and skeletal muscle myosin II, but it has little effect on smooth muscle myosin II and myosins I, V, and X (53, 54, 65). Two recent in vitro studies examined the mechanism of action of blebbistatin on myosin II and showed that this inhibitor did not affect ATP binding or hydrolysis, but instead, it bound to the myosin-ADP-Pi complex, interfering with phosphate release (1, 53, 65). Molecular simulation studies showed blebbistatin binding occurs within the cleft between the nucleotide pocket and actin-binding site, indicating that blebbistatin slowed ADP release thereby keeping myosin in an actin-detached state, preventing actomyosin interaction. Thus blebbistatin is capable of inhibiting myosin II function in a nonrigor state.
Myosin II activation is linked by signal transduction pathways that couple external stimuli to intracellular effector kinases and phosphatases. Both Ca2+-dependent and Ca2+-independent pathways activate myosin II motor activity (26, 50, 78). Ca2+-dependent cell contraction is mediated by the Ca2+/calmodulin (CaM)-dependent serine/threonine protein kinase, myosin light chain kinase (MLCK) (27, 50). MLCK catalyzes myosin II regulatory light chain (RLC) phosphorylation at two sites, Ser19 and Thr18 (31, 44, 45). Phosphorylation at these sites is required for myosin II filament formation (19, 31, 77), myosin II interaction with F-actin (2, 31, 56), and an increase in myosin II ATPase activity (44, 45). These phosphorylation-driven events are essential for initiating and maintaining myosin II-based contractions. Ca2+-independent regulation of myosin II motor activity and contraction has been documented for several kinases in vitro or in vivo, including Rho-kinase (25, 71, 78), p-21-associated protein kinase (8, 14, 81), and integrin-linked kinase (23, 43, 93). These kinases also catalyze myosin II RLC phosphorylation at one or two sites, Ser-19 and Thr-18, resulting in a net increase in RLC phosphorylation, myosin II activation, and contraction.
We have previously shown that endothelial cells and fibroblasts exhibit basal isometric tone associated with low levels of myosin II RLC phosphorylation (25, 31, 32). Upon addition of thrombin, a rapid increase in isometric tension and myosin II RLC phosphorylation occur and are accompanied by reorganization of actin and myosin II. Both the increase in isometric tension and RLC phosphorylation are inhibited by MLCK and Rho-kinase inhibitors, suggesting that the increase in isometric tension is mediated by myosin II activation. However, it is unclear whether the basal isometric tone is myosin II dependent or whether the stress fibers found in unactivated cells require myosin II bipolar filaments to maintain their integrity. It is also unclear whether or not other myosin types contribute to the development of tension that occurs upon thrombin activation. Therefore, to better understand the role of myosin II in endothelial cells, we undertook an in-depth characterization of the effect of blebbistatin on endothelial cell basal and agonist-induced tension development, actin and myosin II distribution, and myosin II RLC phosphorylation. As expected, blebbistatin inhibited the development of tension induced by thrombin but did not inhibit thrombin-induced RLC phosphorylation or actin polymerization. Unexpectedly, blebbistatin treatment abolished endothelial cell basal tension while causing an increase in myosin II RLC phosphorylation. The increase in RLC phosphorylation was Ca2+ dependent and mediated by MLCK. Blebbistatin did prevent myosin II filament formation and association with polymerizing actin filaments. Rotary-shadowed cytoskeletal preparations of treated cells showed loss of bipolar myosin II filament arrays accompanied by actin cytoskeletal reorganization.
MATERIALS AND METHODS
Reagents.
Bovine thrombin, BAPTA-AM, thapsigargin, jasplakinolide, and cytochalasin D were obtained from Sigma. KT5926 was a gift from Dr. John Payne, Kamiya Biomedical. (S)-(-)-blebbistatin was purchased from Toronto Research Chemicals (North York, ON, Canada). Purified isomers, (-)-blebbistatin, and (+)-blebbistatin were purchased from Calbiochem (San Diego, CA). Blebbistatin was dissolved at a final stock concentration of 100 mM in DMSO and stored at −70°C. (S)-(-)-blebbistatin from Toronto Research was used for all experiments except where indicated.
Cell culture.
The bovine pulmonary artery endothelial (BPAE) cell line isolated and characterized by Del Vecchio and Smith (22) was obtained from American Type Culture Collection (CLL-209). Cells were grown and maintained in minimal essential media (MEM) supplemented with 2 mM glutamine, 10% FCS, 50 U/ml penicillin, and 50 μg/ml streptomycin. BPAE monolayers used in these studies were 6 days postconfluent.
Cell labeling.
BPAE monolayers were washed in phosphate-free MEM containing 0.25% BSA and labeled with [32P]orthophosphoric acid as described previously (31). Monolayers were treated with 50 μM blebbistatin in low phosphate media containing 75 μCi/ml [32P]orthophosphoric acid for the indicated time periods.
Isometric tension measurements.
Isometric tension measurements were performed as described in detail previously (31, 52). BPAE cells were seeded onto precast collagen gels and maintained at 37°C in a humidified 5% CO2 atmosphere. Monolayers were confluent within 2–3 days after seeding and were used for experiments when basal isometric tension had stabilized (5–7 days after confluence).
Analysis of myosin light chain phosphorylation.
Myosin II RLC phosphorylation states were separated by urea/glycerol gel electrophoresis (14, 63). Control and treated monolayers were flooded with 1 ml of ice cold 10% TCA containing 10 mM DTT and processed as outlined previously (25, 30). Western blot analysis and detection of myosin II RLCs were carried out as described previously (14, 25).
Myosin II immunoprecipitation and one-dimensional tryptic peptide mapping.
[32P]orthophosphoric acid-labeled myosin II was immunoprecipitated from control and treated monolayers and was electrophoresed on 7.5% to 15% gradient SDS-polyacrylamide gels. For detection of 32PO4 incorporation in either myosin II heavy chains or light chains, phosphorylated bands were cut from the SDS polyacrylamide gels and processed for one-dimensional tryptic peptide mapping following methods outlined previously (30). Standards for one-dimensional tryptic peptide maps were generated as described previously (31).
Myosin light chain kinase activity.
Rabbit smooth muscle MLCK155 was expressed in Sf-9 cells and purified as previously outlined (14). MLCK210 was immunopurified from BPAE cells as described previously (30). Protein A beads containing MLCK210 were resuspended in PBS and 50 μl used for each phosphorylation reaction. For experiments determining whether blebbistatin directly activated MLCK, phosphorylation reactions were carried out in 50 μl of MLCK buffer (25 mM Tris pH 7.5, 150 mM KCl, 5 mM MgCl2, and 1 mM DTT) containing 1 μg recombinant RLC, 1 mM EGTA, and either 50 μl MLCK210 beads or 43 nM recombinant MLCK155. To assess whether blebbistatin inhibited MLCK activity, phosphorylation reactions were carried out in MLCK phosphorylation buffer containing 0.5 mM CaCl2 and 5 μg/ml calmodulin. Blebbistatin was added to the reaction mixtures (50 μM final concentration), and phosphorylation was initiated by addition of ATP to final concentrations of 50 μM ATP and 12.5 μM 32P-γ-ATP. Samples were incubated at 30°C for 15 min, and reactions were stopped by adding equal volumes of 20% TCA. Samples were washed with 100% acetone, air dried, resuspended in Laemmli sample buffer and RLCs separated by 12% SDS-PAGE.
Immunofluorescence microscopy.
For indirect immunofluorescence staining, control and treated monolayers were fixed and stained for actin and myosin II as described previously (31). All monolayers were double-labeled with affinity-purified rabbit anti-myosin heavy chain (MHC) antibody (94) followed by rhodamine phalloidin. For vinculin staining, endothelial cell cultures were fixed and permeabilized as previously described (31) and labeled with a 1:800 dilution of a mouse monoclonal anti-vinculin antibody (catalog no. V-9131; Sigma Chemical, St. Louis, MO). Monolayers were coverslipped in 90% glycerol/10% PBS containing 0.1 M n-propyl gallate (28). Cells were viewed with a Bio-Rad 1024 laser-scanning confocal microscope, and composite micrographs were constructed from 0.3-μm optical sections.
Vinculin plaque pixel intensity profiles were determined on TIFF images using Image Pro Plus computational software (Media Cybernetics, Bethesda, MD). The sum of the areas of vinculin staining was determined and expressed as a percentage of total cell area.
Preparation of endothelial cells for electron microscopy.
BPAE cells were grown on 0.5 × 0.5 mm collagen-coated glass coverslips and permeabilized in PHEM buffer (60 μM PIPES, 25 mM HEPES, 10 mM EGTA, and 2 mM MgCl2, pH 6.9) containing 25 μg/ml saponin and 5 μM phalloidin for 10 min at 37°C. After permeabilization, coverslips were washed in PHEM buffer, and in some samples, actin filaments were removed by treating cells with 0.05 μg/ml constitutively active recombinant gelsolin in PHEM buffer for 30 min at 37°C. Cells were further processed for rotary shadow electron microscopy (EM) analysis as described by Bridgman (7). Rotary-shadowed replicas were photographed at 100 KV in a JOEL 1200EX electron microscope.
F-actin quantitation.
BPAE monolayers were treated for the indicated time intervals, fixed in 3% formaldehyde, and stained for F-actin with rhodamine phalloidin. BPAE F-actin content was quantitated by methanol extraction of rhodamine phalloidin-stained cultures as described previously (31).
RESULTS
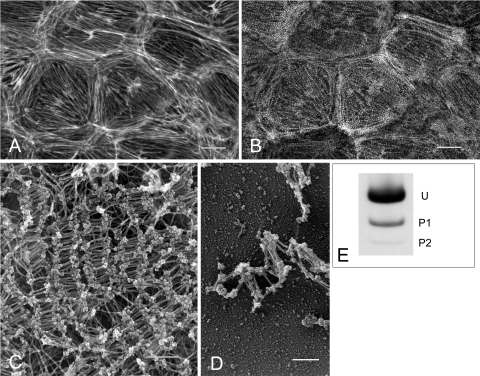
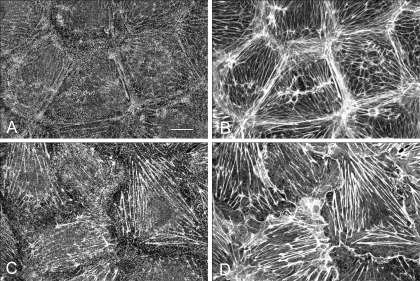
Similar to smooth muscle cells, endothelial cells and fibroblasts produce measurable baseline forces associated with low levels of constitutive myosin II RLC phosphorylation. To determine the contribution of myosin II to the maintenance of basal isometric tension, it is necessary to compare the extent and distribution of myosin II bipolar filament formation to the level of myosin II activity in postconfluent endothelial monolayers. BPAE monolayers were double-stained for myosin II with an affinity-purified rabbit anti-human platelet myosin II heavy chain antibody and for F-actin with rhodamine phalloidin and examined by confocal microscopy (31). Confluent endothelial monolayers (Fig. 1A) have a well-organized circumferential band of actin filaments at the cell margins and central interconnecting microfilaments bundles that join in a complex at the base of the cell. Myosin II (Fig. 1B) localizes as brightly staining spots and bands organized in a distinct sarcomeric-like pattern along actin filaments. In this report, these actin and myosin structures are referred to as stress fibers. To determine whether the bands and spots represent bipolar filaments, direct ultrastructural visualization of myosin II (Fig. 1, C and D) was achieved using saponin-treated monolayers that were incubated with constitutively active gelsolin to remove actin filaments. After fixation, membrane solubilization, and stabilization of filaments, the cells were critically point dried and rotary shadowed. Monolayers treated with gelsolin retained their normal overall morphology in the absence of intact actin filaments. Bipolar filaments characteristic of myosin II minifilaments of nonmuscle cells (80, 85, 86) were found to localize in clusters of varying sizes and configurations (Fig. 1C). Broad arrays of tightly packed bipolar filaments arranged parallel to one another were abundant in the center of the cell while narrower, long linear arrays were more prevalent along relatively straight cell edges. More loosely packed filaments were found at thin irregular shaped cell edges (Fig. 1D). The individual filaments exhibit a distinct bipolar morphology with a short smooth central rod region terminating in globular heads (Fig. 1, C and D). Such structures have previously been identified as bipolar myosin filaments using antibody labeling in a variety of cell types (7, 80). Ultrastructural analysis also revealed that the myosin II filaments formed a continuous interconnecting network that exhibited regional differences in complexity, packing density, and filament configuration.
Fig. 1.
Actin and myosin II distribution in bovine pulmonary artery endothelial (BPAE) monolayers. F-actin (A) and myosin II (B) colocalized in control monolayers. A rim of circumferential actin filaments delineates cell peripheries while bundles of microfilaments generally traverse the long axis of the cell interconnecting with filaments at the cell margins. Myosin II bipolar filaments (B) colocalize with underlying actin filaments. Bar, 10 μm. C: gelsolin treatment reveals myosin II bipolar filament arrays of varying sizes and configurations throughout the cytoplasm. Bar, 1 μm. D: small clusters of bipolar filaments were found at the cell periphery. Bar, 200 nm. E: constitutive regulatory light chain (RLC) phosphorylation was analyzed by glycerol/urea gel electrophoresis. Postconfluent monolayers exhibit low levels of RLC phosphorylation. U, unphosphorylated RLC; P1, monophosphorylated RLC; P2, diphosphorylated RLC.
Myosin II activation through RLC phosphorylation is believed to be a prerequisite for bipolar filament formation. Since both light and EM studies revealed extensive myosin II filament assembly and sarcomeric-like organization in postconfluent BPAE monolayers, the state of myosin II RLC phosphorylation was assessed as an index of myosin activation. RLC phosphorylation was analyzed by glycerol/urea gel electrophoresis. Untreated confluent BPAE monolayers have a constitutive level of RLC phosphorylation of 0.16 ± 0.02 mol PO4/mol RLC where 85% of the RLCs are unphosphorylated, 14% are monophosphorylated, and 1% are diphosphorylated (Fig. 1E). This indicates that low levels of RLC phosphorylation are sufficient to maintain myosin filament formation. Through their interaction with actin filaments, this low level of myosin II filament phosphorylation can potentially produce sufficient cellular force to generate and maintain basal cellular tension. To test this possibility, the effect of blebbistatin treatment on endothelial cell isometric tension was determined.
Blebbistatin and endothelial cell isometric tension.
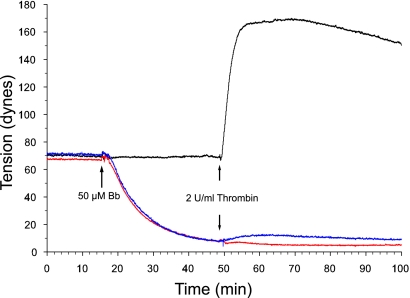
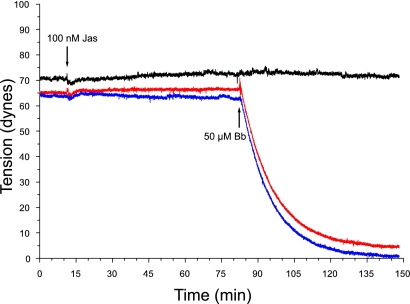
The effect of blebbistatin on basal and agonist-induced isometric tension was determined on BPAE monolayers grown on precast collagen gels as described previously (31, 52). A stable basal tension of 68–72 dyn is achieved within 4–5 days after cells are seeded onto collagen gels and monolayers are used within 24 h. The dose dependence of blebbistatin on basal and agonist-induced tension was determined by treating monolayers with 1, 10, 25, 50, and 100 μM blebbistatin and recording tension continuously for 120 min. Blebbistatin treatment resulted in a dose-dependent decrease in basal tension as well as inhibition of agonist (10 nM thrombin)-induced tension development (Fig. 2). Basal tension decreased by 25% within 30 min with 1 μM blebbistatin. The decline in basal tension increased with increasing doses of blebbistatin, reaching a maximum of 90–95% with 50 μM blebbistatin. Thrombin-stimulated tension development was also inhibited in a dose-dependent manner by blebbistatin, with 50 μM preventing 98% of thrombin-induced tension. On the basis of these results, a dose of 50 μM blebbistatin was chosen for all subsequent experiments.
Fig. 2.
Effect of blebbistatin (Bb) on BPAE basal and thrombin-induced isometric tension. Representative tracing of isometric tension produced by BPAE monolayers incubated with 50 μM blebbistatin is shown. A rapid drop in basal tension occurs within 1–2 min upon addition of blebbistatin. Tension continues to decline over the next 30 min, representing a 90% reduction from pretreatment levels (red). Monolayers pretreated with 50 μM blebbistatin, then stimulated with thrombin (in the presence of blebbistatin), do not contract. Direct inhibition of myosin II essentially eliminates thrombin-induced tension development; only a modest 3- to 5-dyn increase is seen (blue). Treatment with (+)-blebbistatin has no effect on basal thrombin-stimulated tension development. Thrombin induces a rapid rise in tension that is maintained for the duration of the experiment (black). Tension profile produced by BPAE cells incubated with DMSO followed by treatment with 2 U/ml thrombin shows that DMSO has no effect on basal or thrombin-induced tension (black).
When BPAE monolayers were treated with blebbistatin, isometric tension rapidly dropped within the first 2–3 min, decreased steadily over the next 20 min, and established a new baseline tension of 6 dyn (a 91% reduction) within 33 min. Treatment with (+)-blebbistatin or vehicle alone had no effect on basal tension.
Next, the effect of blebbistatin on agonist-induced tension development was determined. The addition of 10 nM thrombin in the continued presence of blebbistatin resulted in either an unsustained slight (3 dyn) increase in tension (Fig. 2) or no increase in tension within 10 min. In monolayers preincubated with either (+)-blebbistatin or vehicle alone, thrombin induced a rapid increase in tension of 100 dyn, typically peaking within 5–6 min (Fig. 2).
Effect of blebbistatin on myosin and actin organization.
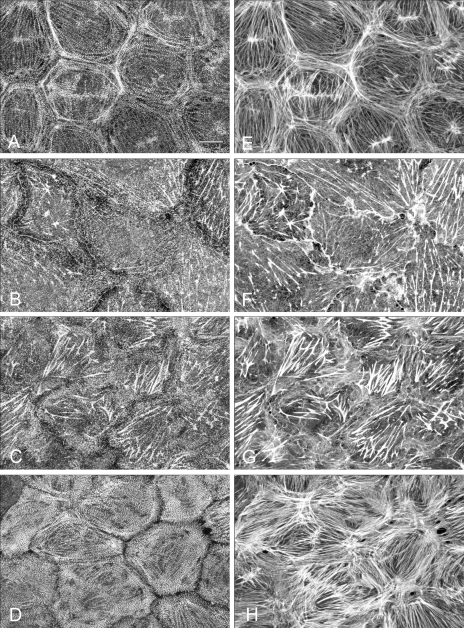
Control and blebbistatin-treated monolayers were stained for myosin II (Fig. 3, A–D) and F-actin (Fig. 3, E–H) and examined by confocal microscopy. Monolayers were composed of a cohesive sheet of polygonal cells that exhibit prominent myosin II staining at cell margins that delineate borders between individual cells (Fig. 3A). Myosin II associated with the peripheral band of actin and with central stress fibers exhibiting a distinct periodic bandlike pattern along actin filaments (Fig. 3, A and E). Blebbistatin induced a time-dependent reorganization of both myosin II and actin filaments (Fig. 3, B and F). Loss of myosin staining at the cell margins became apparent within 5 min, and by 15 min both the peripheral myosin II filaments and associated band of F-actin were lost. By 30 min (Fig. 3, B and F), myosin II was randomly localized in clumps or aggregates within the cytoplasm; residual punctuate myosin staining was present along the few remaining stress fibers, but the periodic filamentous pattern was completely lost. Stress fiber organization and structure was replaced with a fine cytoplasmic network of actin. Stress fibers that remained were distributed throughout the cytoplasm and exhibited variable lengths and thicknesses. Although no stress fibers were evident at the cell margins, F-actin staining was prominent along cell borders (Fig. 3F). Blebbistatin had no significant effect on either microtubule or intermediate filament distribution. In addition, blebbistatin treatment caused small gaps between cells randomly distributed throughout the monolayer.
Fig. 3.
Immunofluorescent localization of myosin II and F-actin in BPAE monolayers. Monolayers were fixed and double-labeled for myosin II (A–D) with affinity-purified antibodies to platelet myosin II and for F-actin by rhodamine phalloidin binding (E–H) as outlined in materials and methods. Cultures were treated as follows: incubation with vehicle only (DMSO) for 30 min (A and E); incubation with 50 μM blebbistatin for 30 min (B and F); incubation with 50 μM blebbistatin for 30 min followed by stimulation with 10 nM thrombin (in the presence of blebbistatin) for 5 min (C and G) and stimulation with 10 nM thrombin only (D and H). Blebbistatin treatment causes loss of myosin II bipolar filaments, resulting in disassembly of stress fibers, and prevents thrombin-induced myosin II filament formation. Bar, 10 μm.
BPAE monolayers incubated with blebbistatin for 30 min and then stimulated with 10 nM thrombin in the presence of the inhibitor showed small aggregates or clumps of myosin II within the cytoplasm and a fine network of actin filaments throughout the cell cytoplasm (Fig. 3G). A slight thickening of stress fibers was apparent in some cells. In comparison, monolayers stimulated with thrombin alone for 5 min showed polymerization of both myosin II and actin filaments (Fig. 3, D and H). The myosin II localized in a sarcomeric-like periodicity along actin stress fibers.
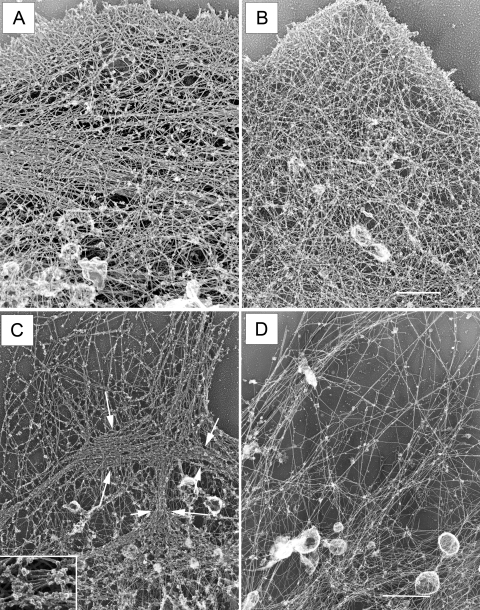
To further examine the effects of blebbistatin on myosin II bipolar filaments and the endothelial cell actin cytoskeleton, two sets of experiments were performed (Fig. 4). In the first set, control and blebbistatin-treated endothelial cell preparations were analyzed at the ultrastructural level. Rotary-shadowed replicas revealed a complex cytoskeletal organization in untreated endothelial cells (Fig. 4A). This included a fine meshwork of actin filaments at the cell periphery and a mixture of oriented stress fibers and bundled filaments throughout the cytoplasm. Blebbistatin caused complete loss of normal cytoskeletal organization within 30 min (Fig. 4B); actin stress fibers and filamentous bundles were no longer present and were replaced by a meshwork of more randomly dispersed filaments. In a second set of experiments, actin was removed by gelsolin treatment to directly visualize myosin II bipolar filaments. Gelsolin-treated control monolayers showed myosin II organized in clusters and elongated structures of varying sizes that form a continuous interconnected network of bipolar filament arrays (Figs. 1C and 4C). Following a 30-min blebbistatin incubation (Fig. 4D), the myosin II bipolar filament arrays were no longer detectable. Only an occasional loosely packed myosin filament could be found.
Fig. 4.
Blebbistatin treatment alters the distribution of F-actin. A: F-actin in untreated cells is a mixture of bundled filaments and meshwork. B: in contrast, cultures treated with 50 μM blebbistatin for 30 min lose their stress fibers and smaller filamentous bundles. F-actin reforms in a meshwork of fine filaments. Bar, 10 μm. C: following gelsolin treatment, bipolar filament arrays are apparent throughout the cell cytoplasm in control monolayers Inset: myosin II bipolar filaments. D: no bipolar filament arrays are detectable in blebbistatin-treated cells. Bar, 2.2 μm.
To determine whether actin disruption was involved in mediating the drop in basal tension, BPAE monolayers were treated with the actin stabilization cyclic peptide, jasplakinolide (38, 88, 89). Treatment of monolayers with 100 nM jasplakinolide for 90 min had little effect on myosin II and actin distributions (Fig. 5, A and B). Cultures incubated with jasplakinolide for 60 min and then treated with 50 μM blebbistatin (Fig. 5C) for 30 min (in the presence of jasplakinolide) showed loss of the organized periodic myosin staining pattern compared with cultures treated with jasplakinolide only (Fig. 5A). Discrete myosin II staining was lost at the cell margins but remained detectable as randomly dispersed aggregates within the cytoplasm. Jasplakinolide pretreatment significantly preserved actin filament integrity in the presence of blebbistatin (Fig. 5D) compared with monolayers treated with blebbistatin only (Fig. 3F). Whereas the majority of the central stress fibers remained intact, jasplakinolide did not protect the dense peripheral band of actin filaments from disassembly. Myosin II remained associated with the stabilized stress fibers but exhibited a clumped or aggregated staining pattern rather than the organized discrete periodic localization evident in control cultures (Fig. 5A). Since jasplakinolide pretreatment protected actin filaments, we sought to determine whether stabilization of actin inhibited or blunted the drop in basal tension upon blebbistatin treatment. As shown in Fig. 6, 100 nM jasplakinolide treatment alone had no effect on basal tension. The rapid drop in tension seen with blebbistatin treatment alone was not inhibited by pretreating monolayers with jasplakinolide for as long as 60 min.
Fig. 5.
Effect of jasplakinolide treatment on blebbistatin-induced cytoskeletal alterations. No significant alterations in myosin II (A) and F-actin (B) distribution were detected after 90-min incubation with 100 nM jasplakinolide. Monolayers pretreated with jasplakinolide for 60 min and then incubated with 50 μM blebbistatin for 30 min in the presence of inhibitor show loss of organized periodic myosin II staining (C) while the majority of actin filaments remain intact (D). Bar, 10 μm.
Fig. 6.
Effect of jasplakinolide (Jas) on endothelial cell basal isometric tension. Representative isometric tension profiles produced by BPAE monolayers incubated with 1) 100 nM jasplakinolide only (black), 2) pretreatment with 100 nM jasplakinolide followed by incubation with 50 μM blebbistatin (Bb) (red), and 3) incubation with 50 μM blebbistatin only (blue) are shown. Jasplakinolide treatment alone had minimal effect on endothelial cell basal tension. Blebbistatin resulted in a rapid drop in tension within the first 5 min that continues to decline over the ensuing 30 min. Jasplakinolide pretreatment did not prevent the decline in basal tension caused by blebbistatin.
Blebbistatin and focal adhesions.
Focal adhesions are the specialized sites of substrate adhesion that connect the extracellular matrix with the cytoskeleton, providing the structural link needed for generation of isometric tension (10, 11). Since disruption of cell-matrix interactions have been implicated in the decline in cellular tension, we sought to determine whether blebbistatin induced loss or disruption of focal adhesions by monitoring the status of the focal adhesion protein, vinculin.
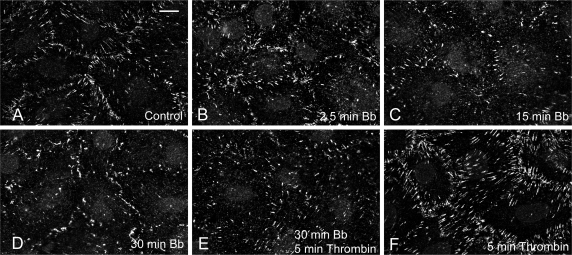
Control and blebbistatin-treated monolayers were fixed and immunostained for vinculin and actin. The majority of vinculin in control monolayers (Fig. 7A) is localized at the cell margins in discrete plaques outlining the borders between individual cells. Approximately 1% of the cell area is occupied by vinculin plaques in control cultures (Table 1). After 2.5 min of incubation with blebbistatin (Fig. 7B), a 10% increase in vinculin plaque area was detected, while minimal alteration in vinculin localization was apparent when compared with control monolayers (Fig. 7A). By 15 and 30 min (Fig. 7, C and D), 17% and 31% decreases, respectively, in total vinculin plaque area occurred. The number and size of the vinculin plaques decreased in conjunction with loss of their prominent localization at cell margins.
Fig. 7.
Effect of blebbistatin on vinculin localization in BPAE monolayers. In control monolayers (A), vinculin localizes as discrete plaques predominantly at cell margins. No significant change in vinculin distribution was detected after 2.5-min incubation with blebbistatin (B). By 15 and 30 min, blebbistatin caused loss of vinculin plaques and their localization became more disorganized (C and D). Incubation with 50 μM blebbistatin for 30 min followed by stimulation with 10 nM thrombin (in the presence of blebbistatin) for 5 min showed no increase in vinculin plaque formation (E). In contrast, thrombin stimulation alone (F) induced an increase in the number and size of vinculin plaques. Bar, 10 μm.
Table 1.
Effect of blebbistatin on vinculin plaques
| Control |
Blebbistatin |
Thrombin, 5 min | Blebbistatin, 30 min + Thrombin, 5 min | |||
|---|---|---|---|---|---|---|
| 2.5 min | 15 min | 30 min | ||||
| Vinculin staining, % of cell area | 0.93±0.03 | 1.02±0.05 | 0.77±0.09 | 0.64±0.04 | 1.17±0.05 | 0.69±0.05 |
| No. of images | 14 | 8 | 5 | 16 | 13 | 13 |
Results are expressed as the percentage (means ± SE) of total cell area positive for vinculin staining. Endothelial cell monolayers were treated as indicated, fixed, permeabilized, and immunostained for vinculin. Photomicrographs were taken with a Bio-Rad 1024 confocal microscope and were analyzed with Image Pro Plus software.
Treatment of monolayers with thrombin for 5 min resulted in pronounced formation of vinculin plaques (Fig. 7F). Thrombin stimulated a 26% increase in plaque area, with both the number and size of plaques at the cell periphery significantly increasing when compared with controls (Fig. 7A). In contrast, BPAE monolayers preincubated in blebbistatin for 30 min (Fig. 7E) and then stimulated with thrombin did not form additional vinculin plaques. A 26% loss was measured in these cultures, a decline comparable to cultures treated with blebbistatin alone (Table 1). No significant change in the localization of the focal adhesions was seen compared with cultures treated with blebbistatin alone (Fig. 7D).
Effect of blebbistatin on RLC and MHC phosphorylation.
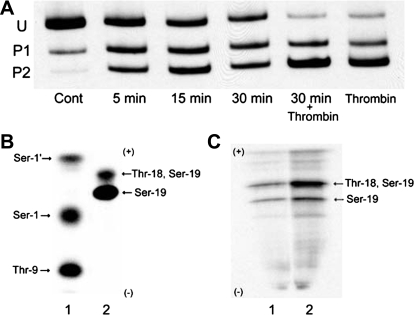
Blebbistatin inhibition of nonmuscle myosin II has been attributed to its ability to prevent myosin's interaction with actin filaments (1, 53, 65). Our data indicate that myosin II bipolar filaments disassemble or are released from the actin cytoskeleton and are unable to reform or reincorporate into the actin cytoskeleton upon agonist stimulation. It is possible that preventing the interaction of actin and myosin as well as inhibiting myosin activity accounts for these observations but may not explain the inability to form filaments upon thrombin stimulation. In vivo blebbistatin could either directly or indirectly affect the state of myosin II RLC or heavy chain (MHC) phosphorylation and thereby filament formation. To address this question, we assessed the effect of blebbistatin treatment on myosin RLC and MHC phosphorylation. Figure 8A shows a representative Western blot from a time course of BPAE RLC phosphorylation after incubation with 50 μM blebbistatin. Untreated cultures have a basal level of RLC phosphorylation of 0.14 mol PO4/mol RLC. Blebbistatin caused a time-dependent increase in RLC phosphorylation, with increases in phosphorylation detectable within 2.5 min (data not shown). By 15 min after the addition of blebbistatin, phosphorylation rose by 7.2-fold, achieving a maximal level of 1.04 mol PO4/mol RLC, and stayed elevated over the ensuing 15 min (0.97 mol PO4/mol RLC). Blebbistatin had no effect on MHC phosphorylation.
Fig. 8.
Blebbistatin-induced RLC phosphorylation. A: monolayers incubated with 50 μM blebbistatin showed a time-dependent increase in RLC phosphorylation. Incubation with 50 μM blebbistatin for 30 min followed by stimulation with 2 U/ml thrombin (in the presence of inhibitor) for 5 min resulted in a greater degree of RLC phosphorylation than occurs with thrombin treatment alone. RLC phosphorylation was analyzed by glycerol/urea gel electrophoresis as outlined in materials and methods. Cont, control. B and C: one-dimensional tryptic peptide map of RLC standards (B) and BPAE myosin RLC (C). B: RLC standards were phosphorylated by PKC or recombinant rabbit myosin light chain kinase (MLCK) as described previously (31). Ser1′, Ser1, and Thr9 are sites phosphorylated by PKC, whereas Ser19 and Thr18 are sites phosphorylated by MLCK. C: monolayers were labeled with [32P]orthophosphoric acid and myosin II immunoprecipitated from untreated monolayers (lane 1) and monolayers incubated with 50 μM blebbistatin for 30 min (lane 2), myosin digested with trypsin, and phosphopeptides analyzed by one-dimensional isoelectric focusing. Phosphopeptides obtained from control monolayers (C; lane 1) exhibit phosphorylation at Ser19 and Ser19/Thr18, sites that correspond to MLCK phosphorylation (21, 31). Myosin II immunoprecipitated from cultures treated with 50 μM blebbistatin (C; lane 2) shows an increase in 32PO4 incorporation at mono- (Ser19) and diphosphorylated (Ser19/Thr18) sites. No PKC phosphorylation (21, 31) was detected in control or treated monolayers (31).
Since monolayers preincubated with blebbistatin and then stimulated with thrombin produced minimal tension and showed marginal morphological evidence of filament formation, we sought to determine whether blebbistatin prevented an increase in agonist-stimulated RLC phosphorylation. BPAE monolayers incubated in blebbistatin alone for 30 min show a sevenfold increase in RLC phosphorylation, with 0.97 mol PO4/mol RLC compared with 0.14 mol PO4/mol RLC in unstimulated monolayers. Five minutes after the addition of thrombin to blebbistatin-treated cultures, there was a 1.6-fold further increase in RLC phosphorylation, from 0.97 mol PO4/mol RLC to 1.64 mol PO4/mol RLC, which is a greater increase in RLC phosphorylation than occurs in thrombin-treated cultures alone. To determine whether this increase in RLC phosphorylation occurred at the appropriate myosin II RLC activation sites, one-dimensional tryptic peptide maps of phosphorylated myosin II RLC from control and treated monolayers were generated. (Fig. 8C). The phosphopeptides resolved are identical to those produced by MLCK-catalyzed phosphorylation of recombinant RLC (14) and human umbilical vein endothelial cells (31) or platelet myosin II standards (Fig. 8B) (21, 31). Untreated monolayers exhibit a phosphorylation pattern that corresponds to myosin II RLC monophosphorylation at Ser19 and diphosphorylation at Thr18/Ser19 (Fig. 8C, lane 1). Incubation with blebbistatin for 30 min produces an increase in 32PO4 incorporation into both phosphopeptides (Fig. 8C, lane 2), with the predominant increase occurring in the diphosphorylated peptide, suggesting that MLCK mediates the increase in RLC phosphorylation. Phosphopeptides corresponding to residues phosphorylated by PKC were not detected in control or treated monolayers. These results indicate that blebbistatin treatment induces an increase in RLC phosphorylation at residues responsible for myosin II activation. In addition, blebbistatin caused no change in MHC phosphorylation, suggesting that neither the loss of bipolar filaments nor the inhibition of filament formation is dependent on phosphorylation at anomalous sites within the MHC.
Effect of inhibition of MLCK on blebbistatin-induced RLC phosphorylation.
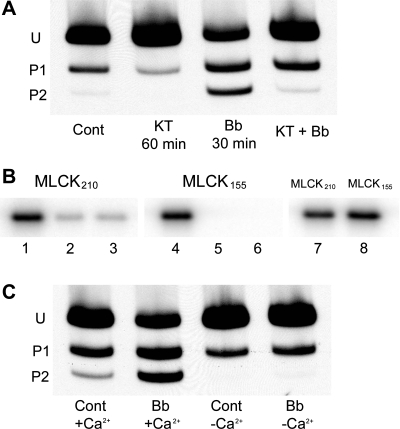
Since blebbistatin resulted in phosphorylation of myosin II RLC at Ser19 and Ser19/Thr18, sites known to be phosphorylated by MLCK, we sought to determine whether MLCK was responsible for blebbistatin-induced RLC phosphorylation. To inhibit MLCK activity, KT5926, a highly selective and potent inhibitor of MLCK (61), was used. Figure 9A illustrates the effect of KT5926 on blebbistatin-induced myosin RLC phosphorylation. KT5926 treatment alone for 60 min decreases RLC phosphorylation from 0.20 mol PO4/mol RLC to 0.08 mol PO4/mol RLC, whereas incubation with blebbistatin alone for 30 min increases RLC phosphorylation to 0.85 mol PO4/mol RLC (Fig. 9A). Monolayers pretreated with 100 nM KT5926 for 30 min, then incubated with 50 μM blebbistatin for 30 min (in the continuous presence of KT5926), showed a 65% inhibition in RLC phosphorylation. The small increase in RLC phosphorylation was due primarily to monophosphorylation, whereas the increase in diphosphorylation was essentially abolished.
Fig. 9.
Effect of MLCK inhibition on blebbistatin induced-RLC phosphorylation. A: cultures were incubated with 100 nM KT5926 (KT) for 60 min, and RLC phosphorylation was analyzed by glycerol/urea gel electrophoresis. Inhibition of MLCK caused a significant reduction in constitutive RLC phosphorylation. Monolayers preincubated with 100 nM KT5926 for 30 min and then treated with blebbistatin for an additional 30 min in the presence of inhibitor showed that MLCK was responsible for blebbistatin-induced RLC phosphorylation. B: effect of blebbistatin on MLCK catalytic activity. Both MLCK210 (lane 1) and MLCK155 (lane 4) catalyze RLC phosphorylation in the presence of Ca2+/CaM, whereas in the absence of Ca2+/CaM, no 32PO4 was incorporated into myosin II RLCs (lanes 2 and 5). Blebbistatin does not directly activate MLCK since incubation of either isoform of MLCK in phosphorylation buffer containing 50 μM blebbistatin in the absence of Ca2+/CaM resulted in no RLC phosphorylation (lanes 3 and 6). Blebbistatin does not inhibit MLCK activity in the presence of Ca2+/CaM (lanes 7 and 8). Lane 1, MLCK210 + Ca2+/CaM; lane 2, MLCK210 + EGTA; lane 3, MLCK210 + EGTA + 50 μM blebbistatin; lane 4, MLCK155 + Ca2+/CaM; lane 5, MLCK155 + EGTA; lane 6, MLCK155 + EGTA + 50 μM blebbistatin; lane 7, MLCK210 + Ca2+/CaM + 50 μM blebbistatin; lane 8, MLCK155 + Ca2+/CaM + 50 μM blebbistatin. C: effect of Ca2+ depletion on blebbistatin-induced RLC phosphorylation. For Ca2+ chelation, monolayers were preincubated for 1 h at room temperature in Ca2+-free HBSS containing 10 mM BAPTA-AM/1 μM thapsigargin (25). Cells were treated with blebbistatin for 30 min; samples were separated by glycerol/urea gel electrophoresis, transferred to nitrocellulose, and probed with anti-myosin RLC antibody. Ca2+ depletion prevents blebbistatin-induced RLC phosphorylation.
KT5926 studies identified MLCK, a Ca2+/CaM-dependent enzyme, to be involved in blebbistatin-induced RLC phosphorylation. To determine whether blebbistatin directly activates MLCK catalytic activity independent of Ca2+/CaM, the ability of blebbistatin to activate MLCK210 (nonmuscle isoform) and MLCK155 (smooth muscle isoform) catalytic activity was assessed. As shown in Fig. 9B, both MLCK210 (lane 1) and MLCK155 (lane 4) catalyze phosphorylation of RLCs in the presence of Ca2+/CaM. No RLC phosphorylation occurs in the absence of Ca2+ (Fig. 9B, lanes 2 and 5). Incubation of MLCK210 (Fig. 9B, lane 3) or MLCK155 (Fig. 9B, lane 6) in phosphorylation buffer containing 50 μM blebbistatin (final concentration) in the absence of Ca2+/CaM resulted in no RLC phosphorylation, indicating that blebbistatin does not directly activate MLCK. Furthermore, blebbistatin did not affect the activity of either MLCK isoform in the presence of Ca2+/CaM (Fig. 9B, lanes 7 and 8).
Because MLCK has a strict dependence on Ca2+ for activation, we next sought to determine whether Ca2+ was required for blebbistatin-induced RLC phosphorylation. Since recent studies (51, 73) have shown that blebbistatin is inactivated and cytotoxic at ultraviolet wavelengths that excite Ca2+-sensitive fluorescent dyes, intracellular Ca2+ was quenched by using the Ca2+ chelators BAPTA and thapsigargin. BPAE monolayers were preincubated in 10 μM BAPTA-AM/1 μM thapsigargin in media free of extracellular Ca2+ as outlined previously (25), and RLC phosphorylation was assessed 30 min after the addition of blebbistatin. Figure 9C demonstrates a typical phosphorylation experiment in the absence of Ca2+, showing the inhibition of RLC phosphorylation upon addition of blebbistatin. Unstimulated monolayers in the presence of Ca2+ have a basal level of phosphorylation of 0.20 mol PO4/mol RLC, whereas monolayers treated with blebbistatin in Ca2+ complete media show a 4.2-fold increase in RLC phosphorylation to 0.85 mol PO4/mol RLC. Chelation of cytosolic Ca2+ caused a 50% drop in baseline phosphorylation from 0.20 mol PO4/mol RLC (lane 1) to 0.10 mol PO4/mol RLC. Ca2+ chelation prevented the increase in blebbistatin-induced RLC phosphorylation, indicating that this increase is dependent on the influx of extracellular Ca2+.
F-actin content.
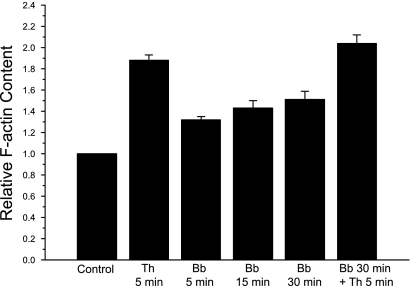
Light and EM micrographs showed that blebbistatin-treated cells contained a meshwork of F-actin, but actin bundles and stress fiber formation were not evident. To ascertain whether these filaments represented an increase or decrease in actin filament content, the relative F-actin content was measured after incubation with blebbistatin. As shown in Fig. 10, blebbistatin induces a time-dependent increase in F-actin. Within 5 min, blebbistatin induced a 30% increase in F-actin, reaching a maximal level of 50% above controls within 30 min. Thrombin treatment alone stimulated an 88% increase in F-actin content, whereas monolayers pretreated with blebbistatin for 30 min and then stimulated with thrombin showed an increase of 110% compared with controls. Blebbistatin did not affect depolymerization of pyrene-labeled actin filaments nor the rate of actin polymerization.
Fig. 10.
Effect of blebbistatin on F-actin content. Monolayers were treated as indicated, fixed, permeabilized, and stained with rhodamine phalloidin. Results are expressed as relative F-actin content: the ratio of fluorescence intensity of experimental monolayers to fluorescence intensity of control monolayers. The monolayers were treated as follows: incubation with 50 μM blebbistatin alone for 5, 15, and 30 min; incubation with 10 nM thrombin (Th) alone for 5 min; incubation with 50 μM blebbistatin for 30 min followed by stimulation with 10 nM thrombin for 5 min. Each point is the mean ± SE of 4 separate experiments.
DISCUSSION
In the present study, the cell-permeable inhibitor of myosin II blebbistatin was used to define the role of myosin II in endothelial cell stress fiber integrity, maintenance of basal tension, and agonist-induced force development. We report that blebbistatin treatment of endothelial cell monolayers results in 1) complete ablation of basal endothelial cell tension, 2) disassembly of actin stress fibers, 3) inhibition of myosin II association with actin filaments and bipolar filament formation, and 4) a Ca2+-dependent increase in RLC phosphorylation, but 5) no inhibition of thrombin-induced increases in RLC phosphorylation or actin polymerization. Our findings directly implicate myosin II as a central structural protein in endothelial cells responsible for maintaining stress fiber structure and regulating basal and agonist induced tension.
The few stress fibers in motile cells are dynamic contractile actomyosin bundles essential for adhesion to the substratum and for changes in cell morphology, specifically, retraction of the trailing edge during migration (3, 13, 18, 41, 58). Our studies have focused on the function of myosin II in nonmotile, postconfluent monolayers in which the nonmotile cells contain thick nondynamic stress fibers (20, 36, 70, 76).
Confluent monolayers have a consistent cytoskeletal organization and exhibit a highly organized myosin II filamentous structure (Fig. 1). Endothelial cells seeded onto preformed collagen gels generate tension within hours, which steadily increases over the ensuing 5 days until a stable basal tension is established. The cessation of active force development correlates with the formation of a confluent nonmotile sheet of endothelial cells (68). Myosin II normally exhibits a periodic sarcomeric-like distribution colocalizing with actin filaments; upon removal of actin, EM analysis revealed a lattice of myosin II bipolar filaments distributed throughout the cytoplasm. This structural organization implicates myosin II as a major cross-linking protein with the potential of generating tension or prestress within the cytoskeleton. Analysis of myosin RLC phosphorylation indicates that basal tension is associated with low levels of RLC phosphorylation. On addition of thrombin (or other inflammatory agonists), a rapid increase in tension occurs that is maintained for several hours before returning to prestimulation levels. This tension development is preceded by a rapid increase in RLC phosphorylation.
Blebbistatin abolishes both basal and agonist-induced tension, identifying myosin II as the molecular motor responsible for generating these forces. In addition, blebbistatin treatment induces a gradual loss of stress fibers without causing depolymerization of actin filaments. Ultrastructural analysis showed that the cytoplasm in blebbistatin-treated cells contained a tangled array of F-actin devoid of myosin II. Thrombin stimulation in blebbistatin-treated cells induced actin polymerization into filaments but without myosin II associated with these filaments. The newly formed F-actin in the absence of myosin II fails to bundle into stress fibers. Since blebbistatin blocks myosin II in an actin-detached state (53, 65), it appears that actin stress fibers cannot form in the absence of myosin binding.
Our studies corroborate those of Hotulainen and Lapppalainen (41) in live cells showing that blebbistatin caused a progressive loss of stress fibers and prevented stress fibers from forming. A number of earlier studies are also consistent with our observations on the role of myosin II in maintaining stress fiber integrity. Inhibition of myosin II activity by microinjecting affinity-purified antibodies to myosin II heavy chains (16, 17, 39) and light chains (40), ATP depletion (5, 6, 29, 74), N-ethylmaleimide-inactivated myosin II fragments, and small interfering RNA myosin knockdowns (3, 13, 42, 49) all show loss of stress fiber integrity.
Shu et al. (75) investigated the effects of blebbistatin on myosin II-dependent processes in Dictyostelium and showed that blebbistatin-inactivated myosin II localizes properly to the rear of polarized cells and to the cleavage furrow of dividing cells and did not interfere with localization of F-actin in the cortex of vegetative cells or at the leading edge of motile and dividing cells. It appears that these processes in Dictyostelium are not dependent on active myosin II, whereas in endothelial cells, both myosin ATPase activity and binding to F-actin are required for formation and bundling of stress fibers.
Inhibition of myosin II and disruption of stress fibers in nonconfluent cells has been associated with drastic changes in cell morphology, loss of focal adhesions, and detachment from the substratum (11, 13, 15–17, 81, 39, 92), whereas blebbistatin-treated endothelial cells were only slightly irregular in shape, exhibited their typical flattened morphology, and developed random small gaps between adjacent cells. These studies detected no extreme changes in morphology, detachment from the substratum, or significant loss of focal adhesions. It is possible that the junctional proteins in confluent endothelial cells served to maintain the cohesiveness of the monolayers.
In preconfluent well-spread cells, stress fibers terminate at focal adhesion sites that provide a structural link between the actin cytoskeleton and the extracellular matrix (4, 11, 15, 69). Intracellular tension develops as a result of myosin II interacting with actin anchored to focal adhesions, and the amount of tension generated correlates with the number and size of focal adhesions (4, 72, 83). Intracellular tension is transmitted to the extracellular matrix through these adhesion sites. Several studies have suggested that perturbation of stress fibers or inhibition of contractile activity leads to loss of focal adhesions (11, 37, 84, 90) and that inhibition of focal adhesion assembly blocks stress fiber formation (55, 62), suggesting that these structures are interdependent. In confluent endothelial cells, inhibition of myosin II and loss of stress fiber structure had minimal effects on vinculin localization, although a gradual decrease in vinculin staining was present. Also, the blebbistatin-induced drop in tension occurred before any detectable effect on focal adhesions. The focal adhesions in these circumstances were not sufficient to preserve stress fibers in the absence of myosin II-dependent cross-linking. In the presence of thrombin stimulation, no additional focal adhesions were detected, indicating that although myosin II is not required to maintain already formed focal adhesions, it is needed for their assembly.
To determine whether stabilizing actin would prevent the decline in tension and preserve stress fiber integrity, we stabilized actin with the cyclic peptide jasplakinolide (9, 38, 89). Jasplakinolide had little effect on myosin II and actin localization in control cultures. Jasplakinolide did not inhibit the blebbistatin-induced decline in basal tension nor stabilize myosin II bipolar filaments associated with stress fibers. However, jasplakinolide pretreatment preserved actin filament integrity and prevented any minor changes in focal adhesions seen in blebbistatin-treated monolayers. These results further indicate that in the absence of active myosin II, neither basal tension nor the structural organization of stress fibers can be maintained.
Previous studies from our lab have shown that inhibition of MLCK with KT5926 (32) has negligible effects on basal tension even though basal RLC phosphorylation was inhibited by 90%. In addition, inhibition of MLCK had little effect on endothelial cell stress fiber distribution and did not prevent thrombin-induced actin polymerization. Thus, inhibition of myosin II phosphorylation does not ablate basal tension or cause the breakdown of stress fibers. We propose that the initial rapid drop in tension evident with blebbistatin occurs in response to inhibition of myosin ATPase activity, while the slower continuous decline in force occurs because of stress fiber disassembly that results from the inability of myosin, as a result of blebbistatin inactivation, to rebind, cross-link, and stabilize actin filaments.
The decline in force in smooth muscle and nonmuscle cells has been shown to be associated with a decrease in myosin RLC phosphorylation. Unexpectedly, we found that blebbistatin treatment caused an increase in RLC phosphorylation, which was detectable within 2.5 min and increased 740% above vehicle controls within 15 min accompanied by a decline in basal tension. Blebbistatin did not prevent thrombin-stimulated increases in RLC phosphorylation. Even though blebbistatin induced an increase in RLC phosphorylation, significant aggregation of myosin II was not detected by immunofluorescent staining nor was bipolar filament formation detected by EM. While it is possible that small myosin II aggregates are lost during the fixation process, when supernatants from blebbistatin-treated monolayers were centrifuged at 100,000 g in an attempt to pellet such aggregates, no significant increase in myosin II was detected in pellets compared with vehicle controls. In addition, we detected no increase in MHC phosphorylation in blebbistatin-treated cultures, suggesting the lack of bipolar filaments formation is not a result of COOH-terminal heavy chain phosphorylation inhibiting myosin II polymerization (12, 46, 59, 60). These results show that blebbistatin interferes with bipolar filament formation without inhibiting RLC phosphorylation.
Blebbistatin-induced RLC phosphorylation was shown to occur at Ser19 and Thr18, sites known to be phosphorylated by MLCK and responsible for activating myosin II (44, 45, 47) in contrast with the Ser1, Ser2, and Thr9 sites associated with inhibition of myosin II activation (45). KT5926, a highly selective potent inhibitor of MLCK that interacts with the enzyme at its catalytic site (61), inhibited >65% of the blebbistatin-induced increase in RLC phosphorylation. Incubation of both purified nonmuscle MLCK210 and smooth muscle MLCK155 with blebbistatin in the presence or absence of Ca2+/CaM did not result in activation of MLCK, showing that blebbistatin did not directly activate MLCK, which is in agreement with an earlier report (79). Because MLCK has a strict dependence on Ca2+/CaM for activation, we determined whether Ca2+ was required for blebbistatin-induced RLC phosphorylation. Chelation of Ca2+ before and during blebbistatin treatment blunts the increase in RLC phosphorylation, indicating that the blebbistatin-induced increase in RLC phosphorylation is Ca2+ dependent. These results identify MLCK as the enzyme responsible for the blebbistatin-induced RLC phosphorylation. We speculate that loss of basal tension and/or disassembly of stress fibers triggers stretch-activated ion channels (57, 82) or modifies cell adhesion complexes (4, 48, 64) resulting in an influx of Ca2+ which activates MLCK.
In resting cells, the majority of myosin II is believed to be in soluble form, and upon phosphorylation of the RLC, it assembles into bipolar filaments, interacts with actin, and generates tension. Basal tension is associated with minimal RLC phosphorylation, suggesting that a mechanism for sustained basal tension in endothelial cells may be akin to that proposed for smooth muscle. Several laboratories (24, 35, 67) have shown that tissue-sustained force maintenance in smooth muscle is associated with low levels of RLC phosphorylation and myosin cross-bridge cycling rates. This cellular response is referred to as the “latch state” (24, 34, 35). Since phosphorylation of the myosin II RLC is closely correlated with myosin cross-bridge cycling rates, “latch bridges” are hypothesized to be generated by dephosphorylation of attached phosphorylated cross-bridges, resulting in attached dephosphorylated cross-bridges (latch bridge). These noncycling cross-bridges maintain force as a result of their slow detachment rate from actin filaments. In tissue culture cells, FRAP analysis (41) has shown that the association/dissociation rates for myosin heavy chains and myosin light chains are slow, indicating that myosin in stress fibers is relatively stable. The presence of bipolar filament arrays coupled with the low levels of myosin II RLC phosphorylation and slow dissociation rate lend support to the idea that force-maintaining myosin latch bridges may form and be responsible for maintenance of basal tone within a monolayer. Physiologically, endothelial cells line large and small blood vessels, forming a selective barrier regulating the movement of proteins, cells, and solutes between the vascular and extravascular compartments. Endothelial cells are required to withstand shear stress forces exerted by changes in blood pressure, and pulmonary capillaries must withstand the changes incurred during normal breathing. We suggest that maintenance of endothelial cell basal tension is critical for maintaining patency of vessels by regulating their diameter and distensibility.
In summary, our evidence indicates that blebbistatin interferes with both basal and agonist-induced tension by blocking formation of myosin II bipolar filaments and preventing the association of myosin II with actin filaments rather than through inhibition of myosin phosphorylation or stimulation of inhibitory phosphorylation.
GRANTS
This work was supported by National Institutes of Health Grants HL-45788, P20-RR16440, and NS26150.
Acknowledgments
We thank David Lagunoff for helpful discussions and critical reading of the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12: 378–379, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Applegate D, Pardee JD. Actin-facilitated assembly of smooth muscle myosin induces formation of actomyosin fibrils. J Cell Biol 117: 1223–1230, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao J, Jana SS, Adelstein RS. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J Biol Chem 280: 19594–19599, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Bershadsky AD, Gelfand VI. Role of ATP in the regulation of stability of cytoskeletal structures. Cell Biol Int Rep 7: 173–187, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Bershadsky AD, Gelfand VI, Svitkina TM, Tint IS. Destruction of microfilament bundles in mouse embryo fibroblasts treated with inhibitors of energy metabolism. Exp Cell Res 127: 421–429, 1980. [DOI] [PubMed] [Google Scholar]
- 7.Bridgman PC Growth cones contain myosin II bipolar filament arrays. Cell Motil Cytoskeleton 52: 91–96, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Rac-induced increase of phosphorylation of myosin regulatory light chain in HeLa cells. Cell Motil Cytoskeleton 58: 186–199, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Bubb MR, Spector I, Beyer BB, Fosen KM. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275: 5163–5170, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Burridge K Are stress fibres contractile? Nature 294: 691–692, 1981. [DOI] [PubMed] [Google Scholar]
- 11.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Dev Biol 12: 463–518, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Buxton DB, Adelstein RS. Calcium-dependent threonine phosphorylation of nonmuscle myosin in stimulated RBL-2H3 mast cells. J. Biol Chem 275: 34772–34779, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, Sheetz MP. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J 91: 3907–3920, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK). J Muscle Res Cell Motil 19: 839–854, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133: 1403–1415, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Citi S, Cross RA, Bagshaw CR, Kendrick-Jones J. Parallel modulation of brush border myosin conformation and enzyme activity induced by monoclonal antibodies. J Cell Biol 109: 549–556, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citi S, Kendrick-Jones J. Studies on the structure and conformation of brush border myosin using monoclonal antibodies. Eur J Biochem 165: 315–325, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci 121: 11–18, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Craig R, Smith R, Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. Nature 302: 436–439, 1983. [DOI] [PubMed] [Google Scholar]
- 20.Cramer LP Organization and polarity of actin filament networks in cells: implications for the mechanism of myosin-based cell motility. Biochem Soc Symp 65: 173–205, 1999. [PubMed] [Google Scholar]
- 21.Daniel JL, Sellers JR. Purification and characterization of platelet myosin. Methods Enzymol 215: 78–88, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Del Vecchio PJ, Smith JR. Expression of angiotensin-converting enzyme activity in cultured pulmonary artery endothelial cells. J Cell Physiol 108: 337–345, 1981. [DOI] [PubMed] [Google Scholar]
- 23.Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J Biol Chem 276: 16365–16373, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Dillon PF, Aksoy MO, Driska SP, Murphy RA. Myosin phosphorylation and the cross-bridge cycle in arterial smooth muscle. Science 211: 495–497, 1981. [DOI] [PubMed] [Google Scholar]
- 25.Emmert DA, Fee JA, Goeckeler ZM, Grojean JM, Wakatsuki T, Elson EL, Herring BP, Gallagher PJ, Wysolmerski RB. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am J Physiol Cell Physiol 286: C8–C21, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22: 32–39, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher PJ, Herring BP, Stull JT. Myosin light chain kinases. J Muscle Res Cell Motil 18: 1–16, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Giloh H, Sedat JW. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217: 1252–1255, 1982. [DOI] [PubMed] [Google Scholar]
- 29.Glascott PA, McSorley KM, Mittal B, Sanger JM, Sanger JW. Stress fiber reformation after ATP depletion. Cell Motil Cytoskeleton 8: 118–129, 1987. [DOI] [PubMed] [Google Scholar]
- 30.Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J Biol Chem 275: 18366–18374, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol 130: 613–627, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goeckeler ZM, Wysolmerski RB. Myosin phosphatase and cofilin mediate cAMP/cAMP-dependent protein kinase-induced decline in endothelial cell isometric tension and myosin II regulatory light chain phosphorylation. J Biol Chem 280: 33083–33095, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Gordon WE Immunofluorescent and ultrastructural studies of “sarcomeric” units in stress fibers of cultured non-muscle cells. Exp Cell Res 117: 253–260, 1978. [DOI] [PubMed] [Google Scholar]
- 34.Hai CM, Kim HR. An expanded latch-bridge model of protein kinase C-mediated smooth muscle contraction. J Appl Physiol 98: 1356–1365, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Hai CM, Murphy RA. Cross-bridge phosphorylation and regulation of latch state in smooth muscle. Am J Physiol Cell Physiol 254: C99–C106, 1988. [DOI] [PubMed] [Google Scholar]
- 36.Heath JP, Holifield BF. On the mechanisms of cortical actin flow and its role in cytoskeletal organisation of fibroblasts. Symp Soc Exp Biol 47: 35–56, 1993. [PubMed] [Google Scholar]
- 37.Helfman DM, Levy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, Lachish-Zalait A, Elbaum M, Bershadsky AD. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell 10: 3097–3112, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holzinger A Jasplakinolide. An actin-specific reagent that promotes actin polymerization. Methods Mol Biol 161: 109–120, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Honer B, Citi S, Kendrick-Jones J, Jockusch BM. Modulation of cellular morphology and locomotory activity by antibodies against myosin. J Cell Biol 107: 2181–2189, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honer B, Jockusch BM. Stress fiber dynamics as probed by antibodies against myosin. Eur J Cell Biol 47: 14–21, 1988. [PubMed] [Google Scholar]
- 41.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell 16: 649–664, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J, Mahavadi S, Sriwai W, Hu W, Murthy KS. Gi-coupled receptors mediate phosphorylation of CPI-17 and MLC20 via preferential activation of the PI3K/ILK pathway. Biochem J 396: 193–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem 260: 10027–10031, 1985. [PubMed] [Google Scholar]
- 45.Ikebe M, Hartshorne DJ, Elzinga M. Identification, phosphorylation, and dephosphorylation of a second site for myosin light chain kinase on the 20,000-Dalton light chain of smooth muscle myosin. J Biol Chem 261: 36–39, 1986. [PubMed] [Google Scholar]
- 46.Ikebe M, Hewett TE, Martin AF, Chen M, Hartshorne DJ. Cleavage of a smooth muscle myosin heavy chain near its C terminus by alpha-chymotrypsin. Effect on the properties of myosin. J Biol Chem 266: 7030–7036, 1991. [PubMed] [Google Scholar]
- 47.Ikebe M, Koretz J, Hartshorne DJ. Effects of phosphorylation of light chain residues threonine 18 and serine 19 on the properties and conformation of smooth muscle myosin. J Biol Chem 263: 6432–6437, 1988. [PubMed] [Google Scholar]
- 48.Ingber DE Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116: 1397–1408, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Jana SS, Kawamoto S, Adelstein RS. A specific isoform of nonmuscle myosin II-C is required for cytokinesis in a tumor cell line. J Biol Chem 281: 24662–24670, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Kamm KE, Stull JT. Dedicated myosin light chain kinases with diverse cellular functions. J Biol Chem 276: 4527–4530, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Kolega J Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun 320: 1020–1025, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol 117: 73–82, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Limouze J, Straight AF, Mitchison T, Sellers JR. Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil 25: 337–341, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol 138: 913–926, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahajan RK, Vaughan KT, Johns JA, Pardee JD. Actin filaments mediate Dictyostelium myosin assembly in vitro. Proc Natl Acad Sci USA 86: 6161–6165, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinac B Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 117: 2449–2460, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Mittal B, Sanger JM, Sanger JW. Visualization of myosin in living cells. J Cell Biol 105: 1753–1760, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murakami N, Kotula L, Hwang YW. Two distinct mechanisms for regulation of nonmuscle myosin assembly via the heavy chain: phosphorylation for MIIB and mts 1 binding for MIIA. Biochemistry 39: 11441–11451, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Murakami N, Singh SS, Chauhan VP, Elzinga M. Phospholipid binding, phosphorylation by protein kinase C, and filament assembly of the COOH terminal heavy chain fragments of nonmuscle myosin II isoforms MIIA and MIIB. Biochemistry 34: 16046–16055, 1995. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi S, Yamada K, Iwahashi K, Kuroda K, Kase H. KT5926, a potent and selective inhibitor of myosin light chain kinase. Mol Pharmacol 37: 482–488, 1990. [PubMed] [Google Scholar]
- 62.Neff NT, Lowrey C, Decker C, Tovar A, Damsky C, Buck C, Horwitz AF. A monoclonal antibody detaches embryonic skeletal muscle from extracellular matrices. J Cell Biol 95: 654–666, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perrie WT, Perry SV. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J 119: 31–38, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. Am J Physiol Cell Physiol 286: C518–C528, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Ramamurthy B, Yengo CM, Straight AF, Mitchison TJ, Sweeney HL. Kinetic mechanism of blebbistatin inhibition of nonmuscle myosin IIb. Biochemistry 43: 14832–14839, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Ramos E, Wysolmerski RB, Masaracchia RA. Myosin phosphorylation by human cdc42-dependent S6/H4 kinase/gammaPAK from placenta and lymphoid cells. Recept Signal Transduct 7: 99–110, 1997. [PubMed] [Google Scholar]
- 67.Rembold CM Relaxation, [Ca2+]i, and the latch-bridge hypothesis in swine arterial smooth muscle. Am J Physiol Cell Physiol 261: C41–C50, 1991. [DOI] [PubMed] [Google Scholar]
- 68.Rickard A, Portell C, Siegal J, Goeckeler Z, Lagunoff D. Measurement of the motility of endothelial cells in confluent monolayers. Microcirculation 10: 193–203, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Ridley AJ Stress fibres take shape. Nat Cell Biol 1: E64–E66, 1999. [DOI] [PubMed] [Google Scholar]
- 70.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4: 446–456, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakamoto T, Limouze J, Combs CA, Straight AF, Sellers JR. Blebbistatin, a myosin II inhibitor, is photoinactivated by blue light. Biochemistry 44: 584–588, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Sanger JW, Sanger JM, Jockusch BM. Differential response of three types of actin filament bundles to depletion of cellular ATP levels. Eur J Cell Biol 31: 197–204, 1983. [PubMed] [Google Scholar]
- 75.Shu S, Liu X, Korn ED. Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc Natl Acad Sci USA 102: 1472–1477, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Small JV, Rottner K, Kaverina I, Anderson KI. Assembling an actin cytoskeleton for cell attachment and movement. Biochim Biophys Acta 1404: 271–281, 1998. [DOI] [PubMed] [Google Scholar]
- 77.Smith RC, Cande WZ, Craig R, Tooth PJ, Scholey JM, Kendrick-Jones J. Regulation of myosin filament assembly by light-chain phosphorylation. Philos Trans R Soc Lond B Biol Sci 302: 73–82, 1983. [DOI] [PubMed] [Google Scholar]
- 78.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299: 1743–1747, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol 139: 397–415, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szczepanowska J, Korn ED, Brzeska H. Activation of myosin in HeLa cells causes redistribution of focal adhesions and F-actin from cell center to cell periphery. Cell Motil Cytoskeleton 2006. [DOI] [PubMed]
- 82.Tamada M, Sheetz MP, Sawada Y. Activation of a signaling cascade by cytoskeleton stretch. Dev Cell 7: 709–718, 2004. [DOI] [PubMed] [Google Scholar]
- 83.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci USA 100: 1484–1489, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian B, Millar C, Kaufman PL, Bershadsky A, Becker E, Geiger B. Effects of H-7 on the iris and ciliary muscle in monkeys. Arch Ophthalmol 116: 1070–1077, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Verkhovsky AB, Borisy GG. Non-sarcomeric mode of myosin II organization in the fibroblast lamellum. J Cell Biol 123: 637–652, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J Cell Biol 131: 989–1002, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verkhovsky AB, Svitkina TM, Borisy GG. Network contraction model for cell translocation and retrograde flow. Biochem Soc Symp 65: 207–222, 1999. [PubMed] [Google Scholar]
- 88.Visegrady B, Lorinczy D, Hild G, Somogyi B, Nyitrai M. The effect of phalloidin and jasplakinolide on the flexibility and thermal stability of actin filaments. FEBS Lett 565: 163–166, 2004. [DOI] [PubMed] [Google Scholar]
- 89.Visegrady B, Lorinczy D, Hild G, Somogyi B, Nyitrai M. A simple model for the cooperative stabilisation of actin filaments by phalloidin and jasplakinolide. FEBS Lett 579: 6–10, 2005. [DOI] [PubMed] [Google Scholar]
- 90.Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskeleton 29: 321–338, 1994. [DOI] [PubMed] [Google Scholar]
- 91.Wakatsuki T, Wysolmerski RB, Elson EL. Mechanics of cell spreading: role of myosin II. J Cell Sci 116: 1617–1625, 2003. [DOI] [PubMed] [Google Scholar]
- 92.Wei Q, Adelstein RS. Conditional expression of a truncated fragment of nonmuscle myosin II-A alters cell shape but not cytokinesis in HeLa cells. Mol Biol Cell 11: 3617–3627, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson DP, Sutherland C, Borman MA, Deng JT, Macdonald JA, Walsh MP. Integrin-linked kinase is responsible for Ca2+-independent myosin diphosphorylation and contraction of vascular smooth muscle. Biochem J 392: 641–648, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wysolmerski RB, Lagunoff D. Regulation of permeabilized endothelial cell retraction by myosin phosphorylation. Am J Physiol Cell Physiol 261: C32–C40, 1991. [DOI] [PubMed] [Google Scholar]