Abstract
High concentrations of cytosolic Na+ ions induce the time-dependent formation of an inactive state of the Na+/Ca2+ exchanger (NCX), a process known as Na+-dependent inactivation. NCX activity was measured as Ca2+ uptake in fura 2-loaded Chinese hamster ovary (CHO) cells expressing the wild-type (WT) NCX or mutants that are hypersensitive (F223E) or resistant (K229Q) to Na+-dependent inactivation. As expected, 1) Na+-dependent inactivation was promoted by high cytosolic Na+ concentration, 2) the F223E mutant was more susceptible than the WT exchanger to inactivation, whereas the K229Q mutant was resistant, and 3) inactivation was enhanced by cytosolic acidification. However, in contrast to expectations from excised patch studies, 1) the WT exchanger was resistant to Na+-dependent inactivation unless cytosolic pH was reduced, 2) reducing cellular phosphatidylinositol-4,5-bisphosphate levels did not induce Na+-dependent inactivation in the WT exchanger, 3) Na+-dependent inactivation did not increase the half-maximal cytosolic Ca2+ concentration for allosteric Ca2+ activation, 4) Na+-dependent inactivation was not reversed by high cytosolic Ca2+ concentrations, and 5) Na+-dependent inactivation was partially, but transiently, reversed by an increase in extracellular Ca2+ concentration. Thus Na+-dependent inactivation of NCX expressed in CHO cells differs in several respects from the inactivation process measured in excised patches. The refractoriness of the WT exchanger to Na+-dependent inactivation suggests that this type of inactivation is unlikely to be a strong regulator of exchange activity under physiological conditions but would probably act to inhibit NCX-mediated Ca2+ influx during ischemia.
Keywords: ischemia; cytosolic calcium concentration; cytosolic sodium concentration; cellular phosphatidylinositol-4,5-bisphosphate
sodium/calcium exchange (NCX) is subject to complex modes of regulation that are initiated by the transported ions themselves. Thus Ca2+ activates NCX activity allosterically by binding to sites in the large central “regulatory” loop of the exchanger, which contains two interacting Ca2+-binding domains (3, 8, 12). In contrast, Na+ ions induce an inactive state of the exchanger when they bind to the cytosolically disposed translocation sites, a process termed “Na+-dependent inactivation.” This inactivation process produces time-dependent changes in exchange currents measured in electrophysiological experiments with excised patches. Thus application of 100 mM Na+ to the cytosolic membrane surface leads to rapid development of an outward current when Ca2+ is present in the extracellular solution. With the wild-type (WT) NCX, outward currents typically reach a peak value within the mixing time of the cytosolic solution change and then decline over a 10- to 20-s interval to a steady-state value ranging from 10 to 50% of the peak amplitude. The time-dependent decline in outward current is a consequence of the inactivation process described above. Entry and exit of exchangers from the inactive state were detected in the noise analysis experiments conducted by Hilgemann's group (9). Na+-dependent inactivation has been demonstrated in cardiac ventricular myocytes (19), as well as in excised patches. The characteristics of Na+-dependent inactivation in excised patches have been described in detail by Hilgemann et al. (13) and Matsuoka et al. (20).
Na+-dependent inactivation is promoted by high cytosolic Na+ concentrations ([Na+]i), low cytosolic pH (pHi), and positively charged amphiphilic agents. Antagonists of Na+-dependent inactivation include high pH, negatively charged amphiphilic agents, high (∼10 μM) cytosolic Ca2+ concentration ([Ca2+]i), and ATP (11). The effects of [Ca2+]i are complex. In the Na+-inactivated state, there appears to be a 4- to 10-fold increase in the half-maximal [Ca2+]i (Kh) for allosteric Ca2+ activation (12, 20). High [Ca2+]i also stimulates recovery from the Na+-inactivated state, but whether Ca2+ is acting through the regulatory sites for allosteric Ca2+ activation is unclear.
ATP protects against Na+-dependent inactivation by stimulating phosphatidylinositol-4,5-bisphosphate (PIP2) synthesis (10). PIP2 interacts with the so-called exchange inhibitory peptide (XIP) region of the exchanger, an amphiphilic stretch of 20 amino acids that is highly enriched in basic and hydrophobic residues and found following the fifth transmembrane segment at the beginning of the large cytosolic regulatory domain. It is thought that the XIP region is an autoinhibitory domain that blocks activity when it interacts with a docking site elsewhere on the exchange protein. This docking site has not been identified, although there are indications that it might lie within residues 562–679 of the central hydrophilic domain (17). PIP2 appears to disrupt this autoinhibitory interaction by binding to the XIP region. Various mutations in the XIP region, involving basic or hydrophobic residues, result in enhanced (e.g., F223E) or reduced (e.g., K229Q) susceptibility to Na+-dependent inactivation (20). An XIP corresponding to the mutant with increased sensitivity to Na+-dependent inactivation (F223E) was shown to have a reduced affinity for binding to PIP2 compared with the WT peptide. Conversely, an XIP corresponding to the mutant that was resistant to Na+-dependent inactivation (K229Q) bound PIP2 with a higher affinity than the WT peptide (7). Thus Na+-dependent inactivation is antagonized by PIP2 binding to the XIP region. This is not necessarily a highly specific interaction, however, since phosphatidylserine and anionic amphiphiles, such as dodecylsulfate, also antagonize Na+-dependent inactivation (11).
In the present study, we examine the effects of elevated [Na+]i on NCX activity in transfected Chinese hamster ovary (CHO) cells expressing the WT canine exchanger or various mutants, including the XIP mutants described above. The results demonstrate that, as expected, inhibition of exchange activity was observed when [Na+]i was elevated, the F223E mutant was more sensitive than the WT exchanger, whereas the K229Q mutant was resistant, and inactivation was promoted by cytosolic acidification. Unexpectedly, however, we found that the WT NCX was quite resistant to Na+-dependent inactivation, even at 140 mM cytosolic Na+, unless pHi was reduced. The WT exchanger remained resistant to Na+-dependent inactivation at physiological pH values, even after depletion of cellular PIP2. We were surprised to find that Na+-dependent inactivation did not change the Kh for allosteric Ca2+ activation and that elevated [Ca2+]i appeared to offer little or no protection against inactivation, in contrast to the findings in excised patches. Finally, Na+-dependent inactivation could be partially, but transiently, reversed by an increase in the extracellular Ca2+ concentration during the exchange assay. Thus Na+-dependent inactivation of NCX expressed in CHO cells shows some similarities to, but also important differences from, the inactivation process as measured in excised patches.
METHODS
Cells.
CHO T cells [CHO K1 cells expressing the human insulin receptor (15), kindly provided by Dr. Michael Czech, University of Massachusetts Medical Center, Worcester, MA] were stably transfected with the mammalian expression vector pcDNA3 containing cDNA inserts coding for the canine WT exchanger (NCX1.1), the canine F223E mutant, the canine K229Q mutant, or the bovine Δ(241-680) mutant. CHO T cells were chosen because their NCX activities tend to be higher than those of other CHO lines. Canine WT, F223E, and K229Q cDNAs were kindly provided by Drs. Debora Nicoll and Kenneth Philipson (University of California Los Angeles). (Cells expressing the WT exchanger or various mutants will occasionally be designated “WT cells” or “K229Q cells.”) The transfected CHO cells were grown in F-12 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 20 μg/ml gentamicin.
Solutions.
Na+-containing physiological saline solution (Na-PSS) contains 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM glucose, and 20 mM MOPS, buffered to pH 7.4 with Tris. The compositions of other PSS solutions are identical, except for the NaCl and KCl concentrations: 10/130 Na/K-PSS contains 10 mM NaCl and 130 mM KCl, 100/40 Na/K-PSS contains 100 mM NaCl and 40 mM KCl, and K-PSS contains 140 mM KCl and no NaCl. For solutions at pH 6.9, MOPS-Tris was used as the buffering system; for solutions at pH 6.4, 20 mM MES-Tris was used. Biochemicals were purchased from Sigma, unless otherwise indicated, and cell culture medium, including fetal bovine serum, was obtained from Life Technologies.
Fura 2 imaging.
Cells were grown on 25-mm circular coverslips and loaded with fura 2 by incubation of the coverslips for 30–40 min at room temperature in Na-PSS containing 1 mM CaCl2, 0.25 mM sulfinpyrazone (to retard fura 2 transport from the cell), and 3 μM fura 2-AM (Molecular Probes). The coverslips were then washed in Na-PSS + 1 mM CaCl2, placed in a stainless steel holder (bath volume ∼0.8 ml; Molecular Probes), and viewed in a Zeiss Axiovert 100 microscope coupled to an Attofluor digital imaging system. Forty to 60 individual cells were selected and monitored simultaneously as 8 × 8 pixel regions of interest for each coverslip. At the end of each experiment, 10 mM MnCl2 was added, along with 1 μM ionomycin to quench fura 2 fluorescence and determine background fluorescence levels. Results are presented as the ratio of fluorescence intensities, after correction for background emission, at excitation wavelengths of 334 and 380 nm. Emission was monitored at >510 nm.
Measurement of NCX activity.
Most experiments used the monovalent cation channel-forming ionophore gramicidin to clamp [Na+]i at the desired level. Cells were placed in Na-PSS containing 0.3 mM EGTA ∼10 min before the recordings were begun. Ca2+ was released from the endoplasmic reticulum by application of 100 μM ATP + 2 μM thapsigargin, an irreversible and selective inhibitor of the sarco(endo)plasmic reticulum Ca2+-ATPase (16). At ∼8 min before the recordings were begun, the coverslip was washed with 5 ml of the desired Na/K-PSS solution containing 0.3 mM EGTA, and 1 ml of the same solution containing 1 μg/ml gramicidin was added. Exchange activity was initiated by superfusion of 5 ml of 0.1 mM CaCl2, or other concentrations as specified, in the same Na/K-PSS solution used for the preincubation. Excess solution was removed by constant aspiration during the solution changes. Occasional departures from this procedure are fully described.
M1 receptor expression.
For PIP2 depletion experiments, CHO T cells expressing canine NCX1.1 or mutant exchangers [Δ(241-680), F223E, or K229] were transiently transfected with pcDNA3 vector containing the coding sequence for human muscarinic acetylcholine (M1) receptor (kindly provided by Dr. Donald Hilgemann, University of Texas Southwestern Medical Center) using the Lipofectamine 2000 reagent (Invitrogen) and following the procedure recommended by the manufacturer. At 24 h after addition of the Lipofectamine-cDNA mixture, the cells were split, placed on 25-mm coverslips, and cultured for an additional 48 h before assay for NCX activity.
Analysis of Kh and Vmax.
After the initiation of reverse-mode NCX activity, cells expressing the WT, F223E, and K229Q exchangers exhibit lag periods of varying duration before the rate of Ca2+ uptake attains a maximal value. As described previously (23), the lags represent the time required for [Ca2+]i to increase to the range where allosteric Ca2+ activation occurs. Data for individual cells were analyzed by spreadsheet analysis, as described elsewhere (23). Briefly, the rate of change of the fura 2 signal for each cell was determined over a rolling interval of three data points (∼1.6 s for each data point) spread over the duration of the experiment. The maximal slope (in ratio units per second) was determined and is designated Vmax. The time point at which the slope was equal to 0.5 Vmax was then identified, and a look-up table based on calibration curves obtained previously was used to convert the fura 2 ratio at that time to a Ca2+ concentration, as described elsewhere (23); the Ca2+ concentration so determined was taken to be equal to Kh for allosteric Ca2+ activation. Because the assay conditions in the excised patch and cellular Kh measurements are quite different, it is not clear that the Kh values determined in the two assays are entirely equivalent, although the Kh values themselves are remarkably similar. The dissociation constant for Ca2+ binding by fura 2 was taken to be 274 nM at room temperature (24) and 224 nM at 37°C (6); at pH 6.4, the dissociation constant for fura 2 was assumed to be 284 nM (1, 18). Vmax and Kh values for individual cells were averaged to determine the mean value per coverslip; data are presented means ± SE for the number of coverslips indicated. Corrections for mitochondrial Ca2+ uptake or for Ca2+ buffering (23) were not applied to the data presented here. Statistical testing employed Student's two-tailed t-test.
RESULTS
Na+-dependent inactivation has been defined principally in terms of the time-dependent decline in outward NCX currents in excised patches after application of Na+ to the cytosolic membrane surface (see the introduction). Comparable experimental conditions are not feasible in fura 2-based experiments with intact cells, and it is not obvious, a priori, how Na+-dependent inactivation would be manifest in fura 2 experiments. In this report, two experimental approaches were combined to characterize Na+-dependent inactivation in fura 2-loaded CHO cells stably expressing the canine NCX. 1) Gramicidin, a channel-forming ionophore that permeabilizes membranes to monovalent cations, was used to equilibrate Na+ across the plasma membrane, allowing [Na+]i to be varied over a wide range. We assumed that Na+-dependent inactivation of NCX activity should be evident at high, but not low, Na+ concentrations. 2) NCX activity in cells expressing the WT NCX was compared with NCX activity in cells expressing NCX mutants that are more (F223E) or less (K229Q) susceptible than the WT NCX to Na+-dependent inactivation. With this approach, Na+-dependent inactivation should be manifest as a reduction in activity at high [Na+]i that is exacerbated in the susceptible F223E mutant and alleviated in the resistant K229Q mutant.
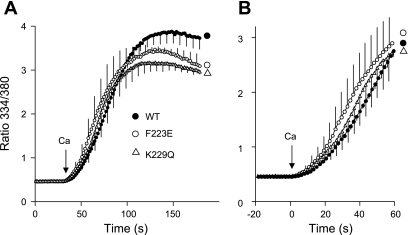
The data in Fig. 1 compare the activities of cells expressing WT, F223E, and K229Q exchangers when [Na+]i is 10 mM, a concentration at which Na+-dependent inactivation should be minimal. The cells were treated with ATP + thapsigargin to release Ca2+ from internal stores and block the activity of sarco(endo)plasmic reticulum Ca2+-ATPase. Then they were preincubated for 8 min in 10/130 Na/K-PSS containing gramicidin (see methods). The reverse (Ca2+-influx) mode of NCX activity was initiated by application of 0.1 mM CaCl2 in 10/130 Na/K-PSS. The rates and extents of Ca2+ uptake were similar for all three types of cells. Each trace shows a slight lag in Ca2+ uptake; the lag periods are attributable to the positive feedback exerted by allosteric Ca2+ activation (see methods) (23). Kh for allosteric Ca2+ activation (see methods) was 236 ± 13, 234 ± 15, and 219 ± 8 (SE) nM for WT, K229Q, and F223E cells, respectively (n = 3); none of these values were statistically different. The corresponding values for the maximal rate of Ca2+ uptake (Vmax; see methods) were 0.32 ± 0.04, 0.29 ± 0.05, and 0.33 ± 0.05 ratio units/s; again, the differences were not statistically significant. Thus, at 10 mM cytosolic Na+, when Na+-dependent inactivation would not be expected to occur, the cells expressing the three NCX variants behaved very similarly. These results did not verify a previous report that the Kh for allosteric Ca2+ activation was increased to 1.2 μM in the F223E mutant compared with the WT value of 0.2 μM (20).
Fig. 1.
Ca2+ uptake by cells expressing wild-type (WT) and F223E and K229Q mutant exchangers in 10 mM Na+. Fura 2-loaded Chinese hamster ovary (CHO) cells expressing WT and mutant exchangers were treated with ATP + thapsigargin to deplete internal Ca2+ stores and then incubated for 8 min in 10 mM NaCl-130 mM KCl (10/130 Na/K)-physiological saline solution (PSS) + 0.3 mM EGTA containing 1 μg/ml gramicidin. Na+/Ca2+ exchanger (NCX) activity was initiated by application of 4–5 ml of 0.1 mM CaCl2 in 10/130 Na/K-PSS. Values (means, with SE for every 4th data point) are expressed as ratio of fluorescence at 334 nm to fluorescence at 380 nm; n = 3 coverslips for each cell type. B: data from A on an expanded time scale. Ca2+ (0.1 mM) was applied at time 0. Experiments were carried out at room temperature.
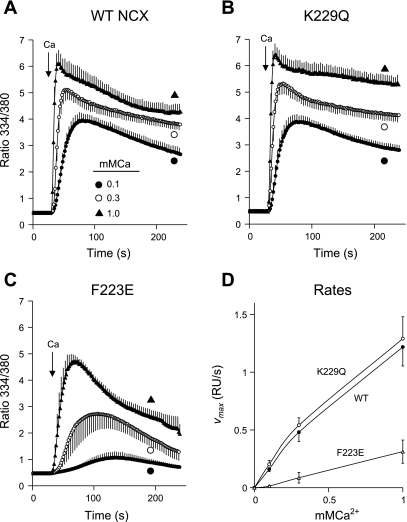
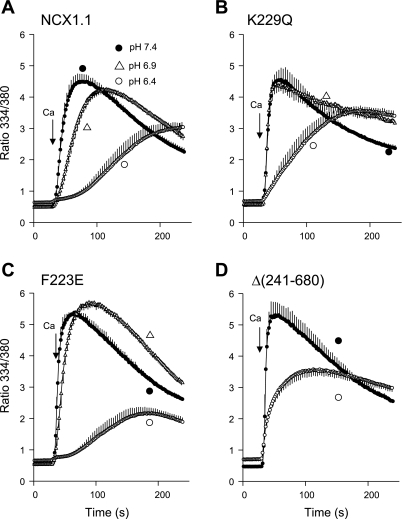
To determine whether high [Na+]i would lead to exchanger inactivation, we preincubated the gramicidin-treated cells in Na-PSS, which contains 140 mM Na+. NCX activity was then initiated by application of 0.1, 0.3, or 1.0 mM Ca2+ in Na-PSS. The data shown in Fig. 2 for the WT NCX and the K229Q mutant were very similar. [Ca2+]i increased, with little or no lag, to a peak value and then declined gradually. The absence of a lag period in these traces reflects the influence of the high [Na+]i, which induces a constitutive mode of activity that does not require allosteric Ca2+ activation (25). Ca2+ uptake by the F223E mutant (Fig. 2C) was strongly inhibited at each Ca2+ concentration compared with the WT NCX or the K229Q mutant, but inhibition was most pronounced at the lower Ca2+ concentrations. Thus Vmax values for Ca2+ uptake by the F223E mutant at 0.1, 0.3, and 1.0 mM Ca2+ were 7.6, 16.9, and 25.9% of the average values for the WT and K229Q cells (Fig. 2D). In similar experiments conducted at 140 mM Na+ with parental CHO cells, which do not exhibit NCX activity, no Ca2+ uptake was observed at 0.1 mM Ca2+; at 1.0 mM Ca2+, the ratio increased to a maximal value of only 0.7 (data not shown). Thus only NCX activity contributed to Ca2+ uptake in the experiments shown in Fig. 2.
Fig. 2.
Ca2+ uptake by cells expressing WT, F223E, and K229Q exchangers in 140 mM Na+. Cells expressing WT NCX (A), K229Q mutant (B), or F223E mutant (C) were pretreated with ATP + thapsigargin and incubated for 8 min before assay in Na-PSS containing 1 μg/ml gramicidin; NCX activity was initiated by application of 0.1, 0.3, or 1 mM CaCl2 in Na-PSS. D: maximal rates of Ca2+ uptake [Vmax, expressed as ratio units (RU)/s] determined from slopes of individual cells for each coverslip and then averaged. Values are means, with SE for every other data point; n = 4 coverslips for all except WT at 0.1 mM Ca2+, where n = 5. Experiments were conducted at room temperature.
The Ca2+ uptake traces for the F223E mutant also showed qualitative differences from those for the WT or K229Q cells. Thus the rise in [Ca2+]i displayed lags at 0.1 and 0.3 mM Ca2+, and the postpeak decline in [Ca2+]i was more pronounced in the F223E cells. The appearance of the lag periods suggests that, despite the high [Na+]i, allosteric Ca2+ activation was required for activity of the F223E mutant. Kh for allosteric Ca2+ activation in the experiment with 0.3 mM Ca2+ was 269 ± 31 nM (n = 4), which was not significantly different from Kh at 10 mM Na+ (219 ± 8 nM; see above). The duration of the lag periods was not significantly different from that in the experiments at 10 mM Na+ (data not shown). This behavior is markedly different from that in excised patches, where Na+-dependent inactivation is characterized by a 4- to 10-fold increase in the apparent Kh for allosteric Ca2+ activation (12, 20).
The behavior of the F223E mutant provides insight into the nature of Na+-dependent inactivation in the transfected cell system. As expected, inhibition of activity was noted only at high [Na+]i (cf. Figs. 1 and 2). Increasing the extracellular Ca2+ concentration appeared to lead to a partial recovery of NCX activity, as shown by the 22-fold increase in Vmax at 1 mM Ca2+ compared with 0.1 mM Ca2+ (Fig. 2D); the corresponding Vmax values for the WT and K229Q exchangers increased only 7.6- and 6.4-fold, respectively. However, activity remained strongly inhibited in the F223E mutant compared with the WT and K229Q exchangers, even at 1 mM Ca2+, indicating that the recovery from inactivation was incomplete. Moreover, the recovery appeared to be transient as well, since cells expressing the F223E mutant showed a marked falloff in [Ca2+]i after the peak value; this was also observed, but to a smaller extent, with the WT NCX and the K229Q mutant. The sharp postpeak decline in [Ca2+]i suggests that elevated [Ca2+]i did not protect the mutant NCX from Na+-dependent inactivation; in this experiment, the peak value of the fura 2 ratio (4.7) at 1 mM extracellular Ca2+ corresponds to [Ca2+]i of ∼2.6 μM.
PIP2 depletion: stimulation of NCX activity at low [Na+]i.
PIP2 protects NCX from Na+-dependent inactivation (10). In healthy, unstressed cells, high cellular PIP2 levels might explain why the cells expressing the WT NCX did not appear to undergo Na+-dependent inactivation in the experiment shown in Fig. 2A, even though they had been preincubated for several minutes with 140 mM cytosolic Na+. We sought to determine whether Na+-dependent inactivation could be induced in the WT NCX by reduction of PIP2 levels through stimulation of a nondesensitizing receptor, the M1 receptor, which activates phospholipase C activity.
Horowitz et al. (14) showed that, in CHO cells expressing the M1 receptor, carbachol administration leads to depletion of 90% of the cellular PIP2 within 1 min. We verified this result qualitatively using CHO cells stably expressing the M1 receptor (kindly provided by Dr. Donald Hilgemann). We stably transfected these cells to express the canine NCX1.1, but the NCX activity was low, so they were not used in activity measurements. We then transiently transfected the cells to express a fusion protein of green fluorescent protein (GFP) with the plekstrin homology (PH) domain of phospholipase Cδ1 (PLCδ1PH-GFP; kindly provided by Dr. T. Balla, National Institutes of Health). The PH domain of the fusion protein strongly associates with PIP2 in the plasma membrane, giving rise to a membrane-localized distribution of fluorescence (26, 27). Carbachol (100 μM) rapidly induced a profound redistribution of the fusion protein to the cytosol (see supplemental Fig. S1 in the online version of this article), consistent with a sharp decline in cellular PIP2. Importantly, when similar studies were conducted using the purinergic agonist ATP, instead of carbachol, no decline in cellular PIP2 was observed (see supplemental Fig. S1), perhaps because ATP is a weaker agonist than carbachol and/or the purinergic receptors rapidly desensitize. In the following experiments, we compare the effect of carbachol with that of ATP to discern the effects of PIP2 depletion.
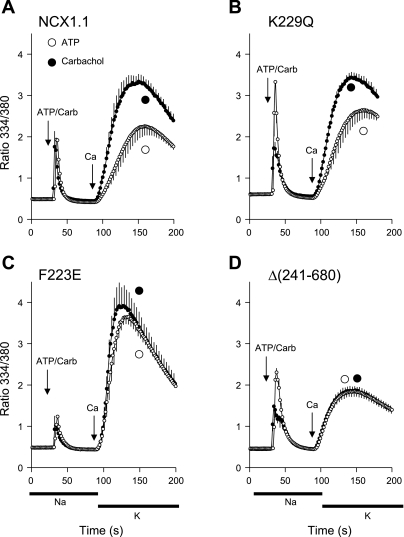
For the experiments shown in Fig. 3, cells expressing the WT NCX or the mutants were transiently transfected to express the M1 receptor. The cells were loaded with fura 2 and then preincubated in Na-PSS + 0.3 mM EGTA but, in this case, without gramicidin. ATP or carbachol was added, and NCX activity was initiated 60 s later by application of 5 ml of 0.1 mM CaCl2 in K-PSS. In CHO cells that had not been transfected with the M1 receptor, carbachol did not elicit Ca2+ release from the endoplasmic reticulum (see supplemental Fig. S2), consistent with the absence of an endogenous M1 receptor. In the transfected cells, carbachol and ATP induced [Ca2+]i transients of roughly the same amplitude (Fig. 3), indicating that both agonists were able to fully release Ca2+ from the internal stores. NCX activity was stimulated, rather than inhibited, by carbachol treatment in cells expressing the WT NCX and the K229Q mutant (Fig. 3, A and B). Vmax values increased 2.0- and 1.5-fold in carbachol- vs. ATP-treated cells for the NCX and the K229Q exchanger, respectively (P ∼ 0.01); there was no significant change in the Kh values for either cell type (see supplemental Fig. S3 for Vmax, Kh, and time-to-Vmax data).
Fig. 3.
Effect of phosphatidylinositol-4,5-bisphosphate (PIP2) depletion with carbachol (Carb) on NCX activities of WT and mutant cells at 10 mM Na+. Cells expressing WT (NCX1.1, A), K229Q (B), F223E (C), or Δ(241-680) (D) exchangers were transfected to transiently express the human M1 receptor and assayed for NCX activity after 72 h. Fura 2-loaded cells were incubated in Na-PSS + 0.3 mM EGTA for 8 min. ATP (100 μM) or carbachol (100 μM) in Na-PSS + 0.3 mM EGTA was applied to separate coverslips and, 60 s later, NCX activity was initiated by application of 0.1 mM CaCl2 in 140 mM KCl-PSS (K-PSS, horizontal bars). Only cells responding to carbachol (8–32% of total) were included in analysis for carbachol-treated coverslips; for ATP-treated coverslips, all cells responded to ATP and were analyzed. Values are means; n = 7 coverslips each for carbachol and ATP in A, n = 6 for carbachol and 5 for ATP in B, n = 5 for carbachol and 4 for ATP in C, and n = 5 for carbachol and n = 3 for ATP in D. Experiments were carried out at room temperature.
Coverslips exposed to ATP and carbachol were subjected to the identical transfection procedure. Only cells responding rapidly to carbachol (8–32% of the total) were included in the analysis for the carbachol coverslips in Fig. 3; the nonresponding cells behaved identically to cells that had not been subjected to the transfection procedure (see supplemental Fig. S2). For the ATP-treated coverslips, all cells responded to ATP and were analyzed.
Hilgemann's group reported that NCX activity is under dual control by PIP2 and that PIP2 depletion, while promoting Na+-dependent inactivation at high [Na+]i, stimulates activity at low [Na+]i. Our results confirm that finding. However, carbachol stimulated NCX activity of the F223E mutant to a much smaller degree, if at all. In this case, Vmax was substantially higher for the ATP-treated F223E cells (0.35 ± 0.04 ratio units/s) than for the WT (0.13 ± 0.02 ratio units/s) or K229Q (0.13 ± 0.02 ratio units/s) cells, perhaps explaining why the stimulatory effect of carbachol was less evident in the F223E cells.
We also examined the effects of PIP2 depletion in cells expressing a mutant with a large deletion, Δ(241-680), in the cytosolic regulatory domain. This mutant shows neither allosteric Ca2+ activation nor Na+-dependent inactivation (21). The results in Fig. 3D show that carbachol had no effect on the activity of the Δ(241-680) mutant.
The above-described experiments (Figs. 1–3) were carried out at room temperature. The remaining experiments were carried out at 37°C. At the higher temperature, the activity of the F223E mutant in 140 mM Na+ is substantially greater than at room temperature, although strong inhibition is still observed compared with the WT and K229Q exchangers.
Effect of PIP2 depletion at high [Na+]i.
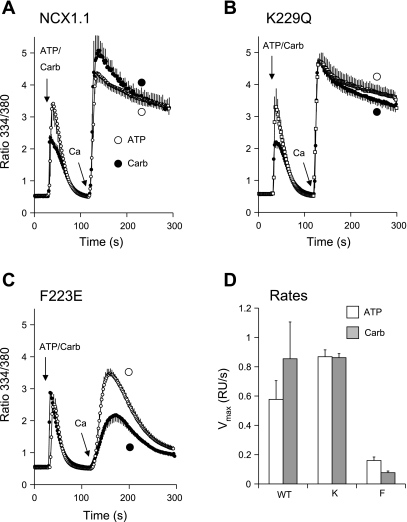
Does PIP2 depletion induce Na+-dependent inactivation in the WT exchanger under conditions of high [Na+]i? To address this question, we treated cells transiently expressing the M1 receptor with gramicidin and preincubated them for 8 min in Na-PSS (140 mM Na+). As shown in Fig. 2, this treatment does not by itself induce Na+-dependent inactivation in cells expressing the WT exchanger. As shown in Fig. 4, A and B, under these conditions, carbachol addition had very little effect on reverse-mode NCX activity in cells expressing the WT NCX or the K229Q mutant. The rates of Ca2+ uptake were quite high under these conditions and were not well resolved kinetically. However, a 50% reduction in the rate of Ca2+ uptake (e.g., induced by carbachol) should have been readily detectable; a possible 50% increase in the rate would have been more difficult to discern. In cells expressing the F223E mutant (Fig. 4C), preincubation with the high [Na+]i alone induced substantial inhibition of NCX activity. The mean Vmax for ATP-treated F223E cells was 22% of the average Vmax of the WT and K229Q cells (Fig. 4D). The addition of carbachol induced additional inhibition of NCX activity compared with ATP (Fig. 4C); Vmax for the F223E cells after carbachol addition was 48% of Vmax obtained with ATP (P ∼ 0.003). Thus PIP2 depletion exacerbated Na+-dependent inhibition in the F223E mutant but had no discernable effect on NCX activity in the cells expressing the WT exchanger.
Fig. 4.
Effect of PIP2 depletion with carbachol on NCX activities of WT and mutant cells at 140 mM Na+. Cells expressing WT (A), K229Q (B), or F223E (C) exchanger were transiently transfected to express the human M1 receptor and assayed after 72 h. Cells were incubated for 8 min in Na-PSS containing 1 μg/ml gramicidin and then treated with 100 μM carbachol or 100 μM ATP; NCX activity was initiated 90 s later by application of 4–5 ml of 0.1 mM CaCl2 in Na-PSS. D: difference in Vmax values between ATP-treated WT and K229Q (K) cells was not significant (P ∼ 0.09); corresponding difference between F223E (F) and WT cells was highly significant (P ∼ 0.003). Values are means, with SE bars for every other point; n = 5 coverslips each for carbachol and ATP in A, n = 7 for carbachol and 4 for ATP in B, and n = 9 for carbachol and 5 for ATP in C. Experiments were carried out at 37°C.
pHi and Na+-dependent inactivation.
Na+-dependent inactivation is promoted by cytosolic acidification and blocked by alkalinization (4). Therefore, we examined the role of pHi in Na+-dependent inactivation in cells expressing the WT and mutant exchangers. Although gramicidin channels conduct protons in addition to other monovalent cations, pHi measured using the pH probe 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein showed that the rate of change in pHi was slow in gramicidin-treated cells subjected to a change in extracellular pH (data not shown). Therefore, we supplemented gramicidin with the Na+/K+/H+ exchange ionophores nigericin (10 μM) and monensin (10 μM), which ensured that pHi would equilibrate within 60–90 s with the extracellular pH (data not shown). The general features of the pH dependence of NCX activity, first under conditions of low [Na+]i (10 mM) and then under conditions of high [Na+]i (140 mM), are discussed below. (For details of the analysis of the results in terms of Vmax and Kh for allosteric Ca2+ activation, see supplemental Figs. S4 and S5.)
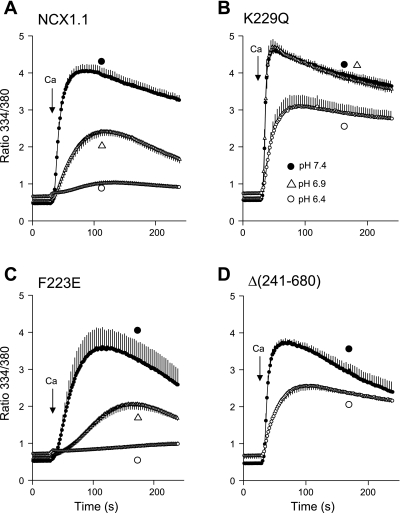
Gramicidin-treated cells expressing the WT NCX or various mutants were equilibrated in 10/130 Na/K-PSS with monensin and nigericin at pH 7.4, 6.9, and 6.4 and then assayed for reverse-mode NCX activity (Fig. 5). There was a strong inhibition of Ca2+ uptake for all cell types at pH 6.4 compared with pH 7.4; Ca2+ uptake was also somewhat reduced at pH 6.9 for the NCX and F223E cells, but not for the K229Q cells. At pH 6.4, Vmax was reduced four- to fivefold compared with pH 7.4 for the WT, K229Q, and Δ(241-680) exchangers and eightfold for the F223E mutant. For the WT, K229Q, and F223E exchangers, the average Kh for allosteric Ca2+ activation increased from 167 nM at pH 7.4 to 320 nM at pH 6.4 (P < 0.05). There was no significant difference in Kh among WT, K229Q, and F223E exchangers at either pH. The combination of the reduced Vmax and increased Kh accounts for the prolonged delay in the time courses of Ca2+ uptake for the NCX and F223E cells at pH 6.4. Although a corresponding delay in the average Ca2+ uptake curve for K229Q cells appeared to be absent in the pooled data, inspection of individual cells revealed typical delays for all cells. However, delays for the K229Q cells were reduced compared with those for the NCX and F223E cells (see supplemental Fig. S4). Vmax at pH 6.4 was 50% higher for the K229Q mutant than for the WT NCX or the F223E mutant (P ∼ 0.02). In general, the K229Q mutant was more resistant to the effects of reduced pH at 10 mM Na+ than the WT NCX or the F223E mutant.
Fig. 5.
Ca2+ uptake at 10 mM Na+ at pH 7.4, 6.9, or 6.4 in cells expressing WT (A), K229Q (B), F223E (C), or Δ(241-680) (D) exchanger. Cells were pretreated with ATP + thapsigargin and incubated for 4 min in 10/130 Na/K-PSS + 0.3 mM EGTA at pH 7.4 containing 1 μg/ml gramicidin. Medium was replaced with 10/130 Na/K-PSS + 0.3 mM EGTA at pH 7.4, 6.9, or 6.4; after an additional 2 min, monensin (10 μM) and nigericin (10 μM) were added, and cells were incubated for an additional 2 min before assay. NCX activity was initiated by application of 4–5 ml of 0.1 mM CaCl2 in 10/130 Na/K-PSS at pH 7.4, 6.9, or 6.4. Experiments with the pH indicator 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) showed that additional monensin or nigericin was not needed after initial application; pHi remained unchanged throughout the assay. Values are means, with SE bars at every other data point; n = 4–5 coverslips for all traces. Experiments were carried out at 37°C.
When similar experiments were conducted in cells equilibrated with high concentrations of Na+ (Fig. 6), acid conditions profoundly inhibited exchange activity in cells expressing the WT and F223E exchangers, but the K229Q and Δ(241-680) exchangers were much less affected. The WT, K229Q, and Δ(240–680) cells were equilibrated with 140 mM Na+; for the F223E cells, 100/40 Na/K-PSS was used, because activities in 140 mM Na+ were too low for analysis at the lower pH values. In 140 mM Na+, cells expressing the WT exchanger showed a fourfold reduction in Vmax at pH 6.9 compared with pH 7.4 and nearly complete abolition of exchange activity at pH 6.4 (Fig. 6A). The F223E cells, at 100 mM Na+, behaved similarly (Fig. 6C). Exchange activity in cells expressing the K229Q mutant, however, was unaffected by reduction of pH to 6.9, whereas at pH 6.4 the rate of Ca2+ uptake was reduced nearly fivefold (Fig. 6B; see supplemental Fig. S5). For the Δ(241-680) mutant, the maximal rate of Ca2+ uptake was reduced fourfold at pH 6.4 compared with pH 7.4 (Fig. 6D; see supplemental Fig. S5). The effects described above were greatly reduced in magnitude in cells that were not treated with monensin and nigericin (data not shown), indicating that extracellular acidification alone had little effect on exchange activity and that inhibition was therefore induced by acidification of the cytosol. The strong inhibition of Ca2+ uptake at the lower pH values in cells expressing the WT and F223E exchangers undoubtedly represents the induction of Na+-dependent inactivation. As shown in Fig. 6, A and B, at pH 7.4, Vmax values were nearly threefold higher for the K229Q than for the WT exchanger (see supplemental Fig. S4). Although the reason for this difference is not known with certainty, it might suggest that the WT exchanger shows a tendency toward inactivation at pH 7.4 under these experimental conditions.
Fig. 6.
Ca2+ uptake at 140 mM Na+ and pH 7.4, 6.9, or 6.4 in cells expressing WT (A), K229Q (B), F223E (C), or Δ(241-680) (D) exchanger. Cells expressing WT, K229Q, and Δ(241-680) exchangers were pretreated and equilibrated at pH 7.4, 6.9, or 6.4 and assayed as described Fig. 5 legend, except Na-PSS was substituted for 10/130 Na/K-PSS; for the F223E mutant, 100/40 Na/K-PSS was used. Values are means; n = 4–5 coverslips for A, B, and C; n = 3 for D. Experiments were carried out at 37°C.
Transient recovery from acid-induced Na+-dependent inactivation.
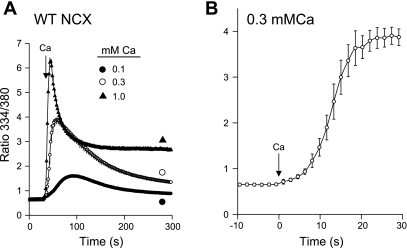
In cells expressing F223E, increasing the external Ca2+ concentration in the assay for NCX activity produced a transient recovery from Na+-dependent inactivation, as shown in Fig. 2C. Cells expressing the WT exchanger behaved similarly when Na+-dependent inactivation was induced by 140 mM Na+ at pH 6.4 (Fig. 7A). The stimulation of activity was only transient, however, as shown by the sharp decline in [Ca2+]i following the peak values of uptake, again recapitulating the results with the F223E cells in Fig. 2C. At 1 mM Ca2+, the peak value of the fura 2 ratio (6.2) corresponds to ∼11 μM cytosolic Ca2+ at pH 6.4. [Ca2+]i then declined to a plateau of 980 nM (ratio = 2.7). The plateau value might reflect some degree of protection against Na+-dependent inactivation or simply the residual activity of the exchanger in the inactivated state.
Fig. 7.
Ca2+ uptake at 140 mM Na+ and pH 6.4 in cells expressing WT NCX. A: cells expressing WT NCX were pretreated and equilibrated in Na-PSS at pH 6.4 as described in Fig. 6 legend; NCX activity was initiated by application of 0.1, 0.3, or 1.0 mM Ca2+ in Na-PSS. Values are means ± SE; n = 4 for all traces; error bars are smaller than symbols for 0.1 and 1.0 mM Ca2+. B: trace in A at 0.3 mM Ca2+ on an expanded time scale. Ca2+ was added at time 0. Experiments were carried out at 37°C.
Results for 0.3 mM Ca2+ are shown on an expanded time scale in Fig. 7B. There was a substantial lag period in the trace before maximal rates of Ca2+ uptake were attained, suggesting, as discussed above for the F223E cells (Fig. 2C), that allosteric Ca2+ activation was required to activate the WT exchanger under these conditions. Analysis of the results reveals an average Kh of 233 ± 27 nM (n = 4). As with the F223E cells, Na+-dependent inactivation did not increase the Kh for allosteric Ca2+ activation; in fact, Kh was actually less than that obtained at 10 mM Na+ (342 ± 15 nM; see supplemental Fig. S2). As discussed above, this behavior is markedly different from that observed in excised patches.
DISCUSSION
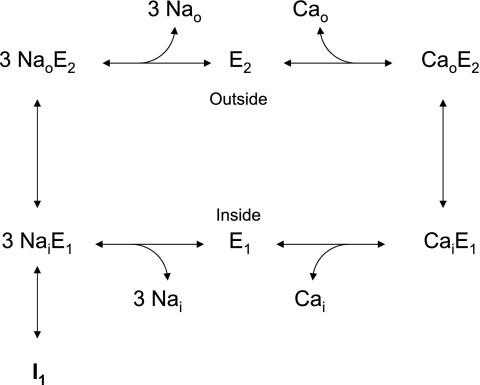
Figure 8 shows the conventional state diagram for the Na+/Ca2+ exchange process, as put forth by Hilgemann and colleagues (13). Entry into the Na+-inactivated (I1) state is thought to proceed from the cytosolically disposed, Na+-loaded configuration (3NaiE1). In our experiments, cells were incubated for several minutes with high concentrations of internal and external Na+. Data obtained with excised patches suggest that these conditions should lead to a substantial population of exchangers in the I1 state. Our results suggest that either this does not occur to a great extent with the WT NCX in the CHO cells or reversal of inactivation occurs within a very few seconds when exchange activity is initiated by application of extracellular Ca2+. Substantial Na+-dependent inactivation of the WT exchanger can be induced by cytosolic acidification (Figs. 6 and 7), in accordance with the well-known promotion of Na+-dependent inactivation by acidic conditions (4, 5). As expected from the results of Matsuoka et al. (20), the F223E mutant was more susceptible than the WT exchanger to Na+-dependent inactivation (Fig. 2C), whereas the K229Q mutant was resistant, even when pHi was reduced to 6.4 (Fig. 6B).
Fig. 8.
Scheme I: conventional state diagram for the NCX process, as proposed by Hilgemann et al. (13). I1, Na+-inactivated state.
With the F223E mutant (Fig. 2C) or with the WT exchanger at low pHi (Fig. 7), activity could be partially restored by an increase in the extracellular Ca2+ concentration. For the F223E mutant, NCX activity at 1 mM Ca2+ was still much less than that for the WT or K229Q exchanger (Fig. 2D), indicating that the mutant exchangers remained highly inactivated, despite the increased extracellular Ca2+ concentration. One interpretation of these results is that an increase in extracellular Ca2+ concentration draws exchangers out of the I1 state by mass action. Thus populating the CaoE2 and CaiE1 states shown in Fig. 8 could lead to a transient depopulation of the I1 state. The subsequent postpeak decline in activity, seen with the F223E mutant (Fig. 2C) and the WT exchanger at low pHi (Fig. 7), could be due to a gradual repopulation of the I1 state under the new steady-state conditions.
A second contributing factor in the transient recovery from inactivation could be the mechanical stimulation of exchange activity that occurs when cells are superfused with Ca2+-containing solutions to initiate exchange activity, as we recently reported (22). Thus exchange activity would be stimulated during the superfusion period, but activity would decline again after cessation of flow. Preliminary experiments (data not shown) suggest that the magnitude of this effect is small and that strong postpeak declines are still observed in the Na+-inactivated state after initiation of NCX activity by rapid solution exchange, rather than superfusion.
We considered the possibility that the relative insensitivity of the WT exchanger to Na+-dependent inactivation at pH 7.4 was due to the high levels of PIP2 in unstressed CHO cells. However, when profound PIP2 depletion was induced by carbachol in cells expressing the M1 receptor, we still did not observe significant Na+-dependent inactivation of the WT exchanger (Fig. 4A), although the expected effect of carbachol could be readily demonstrated with the F223E mutant (Fig. 4C). Perhaps PIP2 remains tightly bound to the WT NCX protein and is not available for hydrolysis by phospholipase C after receptor activation, whereas PIP2 might dissociate from the F223E mutant more readily, considering its lower affinity for PIP2 (7).
Yaradanakul and colleagues (28) recently investigated the effects of PIP2 depletion on exchange currents in CHO and baby hamster kidney cells expressing the M1 receptor. They found, as expected, that carbachol often produced a rapid inhibition of outward currents at 40 mM cytosolic Na+, but they noted that the effect was highly variable and that strong (≥60%) inhibition occurred in only one-third of the trials. They suggested that, in cases where no inhibition was observed, other anionic phospholipids might substitute for PIP2 in activating NCX activity. Similar considerations could possibly explain the lack of effect of PIP2 depletion in our experiments.
Yaradanakul and colleagues (28) also found that NCX activity was under dual control by PIP2. Thus, in contrast to the effects on reverse-mode NCX activity, carbachol-induced PIP2 depletion enhanced forward-mode (Ca2+ efflux) activity in the M1 receptor-expressing cells. The stimulatory effect of PIP2 depletion appeared to require high [Ca2+]i (≥5 μM). They suggested that PIP2 depletion altered membrane trafficking in a way that increased the surface density of exchanger proteins. The data shown in Fig. 3 also reveal a stimulatory effect of PIP2 depletion on NCX activity at low [Na+]i, but our results differ in several ways from those reported by Yaradanakul and colleagues. Thus PIP2 depletion stimulated reverse-mode activity in our experiments and clearly did not require sustained levels of elevated [Ca2+]i. Moreover, carbachol-induced PIP2 depletion stimulated NCX activity in cells expressing the WT and K229Q exchangers but had no effect on the “unregulated” Δ(241-680) mutant. The Δ(241-680) mutant undergoes more rapid endocytosis than the WT exchanger in CHO cells, probably because the WT NCX is tethered to the filamentous actin, whereas the mutant is not (2). Therefore, one might have expected that alterations in membrane trafficking induced by PIP2 depletion would stimulate the Δ(241-680) mutant even more effectively than the WT NCX. It is possible, of course, that specialized endocytic mechanisms dependent on an intact cytosolic domain might mediate PIP2-dependent internalization of the WT NCX, but not the Δ(241-680) mutant. In any event, it seems clear from our results with the Δ(241-680) mutant that the stimulatory effect of PIP2 on exchange activity involves interactions of some kind with the regulatory cytosolic domain of the exchanger.
In the same series of experiments, carbachol did not significantly stimulate the activity of the F223E mutant (Fig. 3C). However, this was most likely due to a higher Vmax for Ca2+ uptake in the “negative control” traces stimulated with ATP. The reasons for this behavior are unknown.
The data presented here, taken together with previous results, suggest that elevated [Na+]i has two opposing effects on exchange activity: the promotion of Na+-dependent inactivation and, as described previously (25), the induction of a “constitutive” mode of activity that does not require allosteric Ca2+ activation. The latter effect can be seen in the absence of lag periods in 140 mM cytosolic Na+ for the Ca2+ uptake data in Fig. 2, A and B, for the WT and K229Q exchangers. In the case of the F223E cells at 140 mM Na+, the lags reappeared in the traces for 0.1 and 0.3 mM Ca2+ (Fig. 2C). Lag periods were also evident for Ca2+ uptake traces for the WT exchanger in 140 mM Na+ when Na+-dependent inactivation was strongly promoted by low pHi (Fig. 7). These results suggest that, in the Na+-inactivated state, the constitutive behavior of the exchangers is lost, and they again require allosteric Ca2+ activation for activity.
The interaction between allosteric Ca2+ activation and Na+-dependent inactivation is not well understood. In excised patches, Na+-dependent inactivation induces up to a 10-fold increase in the apparent Kh for allosteric Ca2+ activation, and Na+-dependent inactivation can be blocked by high [Ca2+]i (12, 20). The second effect could be implicated in the first. Thus, in excised patches, Kh values are typically measured by the effects of Ca2+ on the peak current amplitudes measured shortly after application of cytosolic Na+. In rapidly inactivating mutants such as F223E, peak currents would tend to be reduced compared with the WT exchanger because of the rapidity of the inactivation process. Increasing [Ca2+]i could therefore increase the amplitude of the peak currents by reversing Na+-dependent inactivation, in addition to its effects on allosteric Ca2+ activation.
Thus the increase in the apparent Kh in excised patch studies might reflect the Ca2+-promoted recovery from Na+-dependent inactivation, rather than a true change in the Ca2+ dependence of allosteric Ca2+ activation. Our data from the CHO cells are consistent with this interpretation, since the Kh for allosteric Ca2+ activation was not increased as a result of Na+-dependent inactivation. Thus, for the F223E cells in 140 mM cytosolic Na+ (Fig. 2C), the average Kh (269 ± 31 nM) was not significantly different from Kh at 10 mM Na+ (219 ± 6 nM). For the WT cells at pH 6.4 (Fig. 7B), the average Kh (233 ± 27 nM) in the Na+-inactivated state was actually less than that in 10 mM Na+ (342 ± 15 nM; see supplemental Fig. S4). There is a need for caution, however, in comparing Kh values in excised patches and CHO cells, since the ionic conditions and experimental basis for the measurements are quite different in the two types of assays.
The mechanism by which high [Ca2+]i protects against Na+-dependent inactivation is not known, although it has been suggested that it might be related to the requirement for increased Ca2+ for allosteric Ca2+ activation (20). This is clearly not the case for the CHO cells, since [Ca2+]i far higher than the Kh for allosteric Ca2+ activation did not fully alleviate inactivation, as seen by the pronounced postpeak declines in Ca2+ uptake in Figs. 2C, 4C, and 7A. The reasons for the different responses of excised patches and CHO cells to elevated [Ca2+]i are not known, although, again, the experimental conditions are quite different in the two types of assays.
In summary, Na+-dependent inactivation of the NCX is manifest in transfected CHO cells as a reduced Vmax for Ca2+ uptake with no change in the Kh for allosteric Ca2+ activation. The WT canine exchanger used in these studies was quite resistant to Na+-dependent inactivation, even after extensive PIP2 depletion, but was strongly inactivated when pHi was reduced. The resistance of the WT exchanger to Na+-dependent inactivation suggests that this mode of NCX regulation is of little importance under normal physiological conditions. However, Na+-dependent inactivation could be an important protective response during ischemia, when high [Na+]i, low ATP/PIP2, and low pHi would strongly promote inactivation, thereby reducing NCX-mediated Ca2+ influx and toxic Ca2+ overload.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-49932 and American Heart Association Grant 0555759T.
Supplementary Material
Acknowledgments
We thank Drs. Kenneth Philipson and Debora Nicoll (UCLA) for the cDNAs coding for the WT, F223E, and K229Q canine exchangers. We thank Dr. Donald Hilgemann, UT Southwestern Medical Center, Dallas, Texas, for providing the cDNA for the human M1 receptor and the CHO cells stably expressing the M1 receptor. Thanks also to Dr. Tamas Balla, NICHD, NIH, Bethesda, Maryland, for the gift of the cDNA for the PLCδ1PH-GFP fusion protein.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Batlle DC, Peces R, LaPointe MS, Ye M, Daugirdas JT. Cytosolic free calcium regulation in response to acute changes in intracellular pH in vascular smooth muscle. Am J Physiol Cell Physiol 264: C932–C943, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Condrescu M, Reeves JP. Actin-dependent regulation of the cardiac Na+/Ca2+ exchanger. Am J Physiol Cell Physiol 290: C691–C701, 2006. [DOI] [PubMed] [Google Scholar]
- 3.DiPolo R Calcium influx in internally dialyzed squid giant axons. J Gen Physiol 73: 91–113, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doering AE, Lederer WJ. The mechanism by which cytoplasmic protons inhibit the sodium-calcium exchanger in guinea-pig heart cells. J Physiol 466: 481–499, 1993. [PMC free article] [PubMed] [Google Scholar]
- 5.Doering AE, Lederer WJ. The action of Na+ as a cofactor in the inhibition by cytoplasmic protons of the cardiac Na+-Ca2+ exchanger in the guinea-pig. J Physiol 480: 9–20, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 7.He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD. Interaction of PIP2 with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 278: C661–C666, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Hilge M, Aelen J, Vuister GW. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol Cell 22: 15–25, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Hilgemann DW Unitary cardiac Na+, Ca2+ exchange current magnitudes determined from channel-like noise and charge movements of ion transport. Biophys J 71: 759–768, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Hilgemann DW, Collins A. Mechanism of cardiac Na+-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol 454: 59–82, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilgemann DW, Collins A, Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol 100: 933–961, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilgemann DW, Matsuoka S, Nagel GA, Collins A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol 100: 905–932, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol 126: 243–262, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langille SE, Patki V, Klarlund JK, Buxton JM, Holik JJ, Chawla A, Corvera S, Czech MP. ADP-ribosylation factor 6 as a target of guanine nucleotide exchange factor GRP1. J Biol Chem 274: 27099–27104, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266: 17067–17071, 1991. [PubMed] [Google Scholar]
- 17.Maack C, Ganesan A, Sidor A, O'Rourke B. Cardiac sodium-calcium exchanger is regulated by allosteric calcium and exchanger inhibitory peptide at distinct sites. Circ Res 96: 91–99, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Zaguilan R, Martinez GM, Lattanzio F, Gillies RJ. Simultaneous measurement of intracellular pH and Ca2+ using the fluorescence of SNARF-1 and fura-2. Am J Physiol Cell Physiol 260: C297–C307, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Matsuoka S, Hilgemann DW. Inactivation of outward Na+-Ca2+ exchange current in guinea-pig ventricular myocytes. J Physiol 476: 443–458, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka S, Nicoll DA, He Z, Philipson KD. Regulation of cardiac Na+-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109: 273–286, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuoka S, Nicoll DA, Reilly RF, Hilgemann DW, Philipson KD. Initial localization of regulatory regions of the cardiac sarcolemmal Na+-Ca2+ exchanger. Proc Natl Acad Sci USA 90: 3870–3874, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves JP, Abdellatif M, Condrescu M. The sodium-calcium exchanger is a mechanosensitive transporter. J Physiol 586: 1549–1563, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves JP, Condrescu M. Allosteric activation of sodium-calcium exchange activity by calcium: persistence at low calcium concentrations. J Gen Physiol 122: 621–639, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuttleworth TJ, Thompson JL. Effect of temperature on receptor-activated changes in [Ca2+]i and their determination using fluorescent probes. J Biol Chem 266: 1410–1414, 1991. [PubMed] [Google Scholar]
- 25.Urbanczyk J, Chernysh O, Condrescu M, Reeves JP. Sodium-calcium exchange does not require allosteric calcium activation at high cytosolic sodium concentrations. J Physiol 575: 693–705, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol 143: 501–510, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varnai P, Lin X, Lee SB, Tuymetova G, Bondeva T, Spat A, Rhee SG, Hajnoczky G, Balla T. Inositol lipid binding and membrane localization of isolated pleckstrin homology (PH) domains. Studies on the PH domains of phospholipase Cδ1 and p130. J Biol Chem 277: 27412–27422, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+/Ca2+ exchange by PIP2: electrophysiological analysis of direct and indirect mechanisms. J Physiol 582: 991–1010, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.