Abstract
Platelets play an important role in hemostasis, with inappropriate platelet activation being a major contributor to debilitating and often fatal thrombosis by causing myocardial infarction and stroke. Although current antithrombotic treatment is generally well tolerated and effective, many patients still experience cardiovascular problems, which may reflect the existence of alternative underlying regulatory mechanisms in platelets to those targeted by existing drugs. In this study, we define a role for peripherally distributed members of the tachykinin family of peptides, namely substance P and the newly discovered endokinins A and B that are present in platelets, in the activation of platelet function and thrombus formation. We have reported previously that the preferred pharmacologically characterized receptor for these peptides, the NK1 receptor, is present on platelets. Inhibition or deficiency of the NK1 receptor, or SP agonist activity, resulted in substantially reduced thrombus formation in vitro under arterial flow conditions, increased bleeding time in mice, and a decrease in experimentally induced thromboembolism. Inhibition of the NK1 receptor may therefore provide benefit in patients vulnerable to thrombosis and may offer an alternative therapeutic target.
Introduction
Tissue damage results in the local exposure, generation, or release of factors such as collagen and thrombin that trigger the function of platelets, and thereby initiate hemostasis.1 Through the function of multiple cell adhesion and signaling receptors, platelets become entrapped at the injury site and become activated.2 This leads ultimately to the up-regulation in affinity of the integrin αIIbβ3 which, through binding ligands that include fibrinogen and von Willebrand factor, support the formation of a stable platelet thrombus.3 Effective thrombus formation in the arterial circulation is dependent on the secretion and release of factors such as ADP and thromboxane (TX) A2 from activated platelets, which through binding G protein–coupled receptors on the platelet surface, stimulate positive feedback activation.4
Roles in thrombus formation have been discovered for a number of newly identified regulatory molecules, including gas6,5 CD40L,6 semaphorin 3A,7 semaphorin 4D,8 and ephrins/eph kinases.9 We recently demonstrated that substance P (SP), a member of the tachykinin family, may also contribute to platelet regulation.10 We showed that SP can stimulate activation and aggregation of platelets and that platelets contain SP immunoreactivity that is released upon activation.
Tachykinins are a family of peptides characterized by the conserved C-terminal motif Phe-X-Gly-Leu-Met-NH2 (X is hydrophobic),11 which is central to their biological activity. Originally classified as neurotransmitters, this family of peptides comprising substance P, neurokinin A (NKA), and neurokinin B (NKB) mediates a variety of peripheral biological functions, including smooth muscle contraction, vasodilatation, plasma extravasation, neurogenic inflammation, and hematopoiesis, and there is a growing body of evidence to suggest that they may mediate their actions through endocrine/paracrine modes.12–14 The secretion of NKB by the placenta and the SP-like immunoreactivity released by platelets upon activation (and the observation that synthetic substance P can stimulate aggregation) are 2 such examples. This has been reinforced by the identification of a new tachykinin gene TAC4, which is almost exclusively expressed in peripheral tissues of nonneuronal origin.11,15 In mice, it gives rise to a single transcript containing an NK1 receptor agonist hemokinin; in humans, it encodes 4 transcripts containing endokinins A and B (EKA and EKB) with 2 transcripts (EKC and EKD) containing additional cryptic tachykinin sequences Phe-X-Gly-Leu-Leu-NH2 where X is the hydrophilic amino acid glutamine and thus lack activity at the known tachykinin receptors11,15,16 (Table 1). Unlike SP, NKA, and NKB, hemokinin and endokinins are not conserved throughout mammalian species. Tachykinins are believed to exert their actions via the 3 tachykinin receptors that have been cloned and characterized: NK1, NK2, and NK3, which bind preferentially to SP, NKA, and NKB, respectively, although each of these peptides is able to bind each of the receptors, albeit at lower affinity.11 We have reported previously the expression of NK1 and NK3 receptors on human platelets, and that the level of activation of platelets by SP is reduced in NK1 receptor–deficient mouse platelets.10
Table 1.
Alignment of the amino acid sequences of the mammalian tachykinins
| Tachykinin | Amino acid sequence |
|---|---|
| SP | RPKPQQFFGLM-NH2 |
| NKA | HKTDSFVGLM-NH2 |
| NKB | DMHDFFVGLM-NH2 |
| HK-1 | SRTRQFYGLM-NH2 |
| hHK-1 | TGKASQFFGLM-NH2 |
| hHK-1 (4–11) | ASQFFGLM-NH2 |
| EKA/B | GKASQFFGLM-NH2 |
| EKC | KKAYQLEHTFQGLL-NH2 |
| EKD | VGAYQLEHTFQGLL-NH2 |
HK-1 indicates hemokinin 1; hHK-1, human hemokinin; hHK-1 (4-11), a truncated version of hemokinin 1; and EKA/B, C-terminal sequence shared by endokinins A,B.
Due to the expression of all 4 TAC4 transcripts in a human megakaryocytic cell line,11 and the inability of immunoassays to distinguish between EKA/B and SP,10 suggesting human platelets as potential peripheral sources of each of these peptides, we have characterized the effects of these peripheral NK1 receptor agonists on human platelet function and demonstrate their prothrombotic potential. We illustrate the importance of the NK1 receptor in thrombogenesis and confirm that disruption of this axis impairs thrombus formation, hemostasis, and thrombosis.
Methods
Materials
Gq-deficient transgenic mice were provided by F. Lanza (Strasbourg, France) and S. Offermanns (Heidelberg, Germany). NK1- and TAC1-deficient mice were generated as described previously.17,18 All protocols involving the use of animals were approved by University of Reading or Imperial College London Local Ethical Review Panels and covered by Home Office licences. Male Balb/c mice (20/30 g) and 111Indium oxine were purchased from Harlan (Bicester, United Kingdom) and GE Healthcare (Chalfont St Giles, United Kingdom), respectively. Peptides were synthesized by Cambridge Research Biochemicals (Cleveland, United Kingdom), [3H] arachidonic acid was purchased from GE Healthcare, 3,3′-dihexyloxacarbocyanine iodide (DIOC6) was obtained from Sigma-Aldrich (Dorset, United Kingdom), and microcapillaries were supplied by Camlab (Cambridge, United Kingdom). YM254890 and Cangrelor were kindly provided by Astellas (Tokyo, Japan) and The Medicines Company (Parsippany, NJ), respectively. MRS2179 and L733060 were purchased from Sigma (Poole, United Kingdom), and SQ29548 was purchased from Alexis (Axxora, Nottingham, United Kingdom). Anti–P-selectin antibodies (AK-4) were purchased from BD Biosciences (Oxford, United Kingdom). Anti-FcγRIIa antibodies (mAbIV.3) were produced as described previously.19 Texas red– and phycoerythrin-conjugated secondary antibodies were purchased from Abcam (Cambridge, United Kingdom) and BD Biosciences, respectively. All other reagents used were from sources described previously.10,20,21
Peptides
EKA/B (GKASQFFGLM-NH2; Table 1) was synthesized using standard Fmoc methodology. EKA/B and SP were dissolved in acetic acid that was present in functional assays at 0.001% (vol/vol).
Human platelet aggregation
Washed platelets were prepared from fresh blood obtained from aspirin-free donors by differential centrifugation and aggregation measured by optical aggregometry as described previously.20 Informed consent from all human subjects donating blood was obtained, and procedures were approved by the University of Reading Local Ethics Committee.
Dense granule secretion and arachidonic acid release
Platelets were loaded with [3H] 5-HT or [3H] arachidonic acid, and release following secretion was measured as described previously.22
Alpha-granule secretion
As a measure of alpha-granule secretion, surface exposure of P-selectin was assessed by flow cytometry using an anti-CD62P (P-selectin) antibody using standard methodology.10
Immunofluorescence studies
Antibodies were raised in sheep to the peptide sequence AETWEGAGPSIQLQEVK, a unique sequence that is not present in SP, which lies upstream of the common C-terminal sequence of both EKA and EKB (precursor residues 30-47).23 Antisera titers were monitored using an enzyme-linked immunosorbent assay (ELISA) and high-titer bleeds selected for immunofluorescence studies. Human platelets were labeled with DIOC6 and applied to glass coverslips using a cytospin3 system (Shandon, Thermo Scientific, High Wycombe, United Kingdom). Platelets were fixed with 2% paraformaldehyde and blocked with modified Tyrode-HEPES buffer containing 0.1% (wt/vol) bovine serum albumin (BSA/Tyrode). Slides were incubated with a 1:100 dilution in BSA/Tyrode of antiserum, preimmune serum, or antiserum to which immunogen peptide (10 μg/mL) had been added 30 minutes prior to use, and incubated for 30 minutes. Slides were washed and incubated with Texas red–conjugated anti–sheep IgG antibody for 20 minutes and examined using a Leica DMIRE2 inverted confocal microscope (× 63 glycerol immersion objective lens; Leica, Milton Keynes, United Kingdom) to visualize platelets (488 nm) and endokinin expression (594 nm).
Mouse platelet preparation and aggregation assay
Platelets were isolated from mouse blood and washed, and aggregation assays were performed using levels of single platelets (using a Z2 Coulter Counter; Beckman Coulter, Hialeah, FL) following stimulation as a measure of aggregation, as described previously.10
Flow microscopy
Whole citrated blood obtained from aspirin-free donors was incubated with fluorescent dye (DIOC6) and perfused through collagen-coated (100 μg/mL) microcapillaries at a sheer rate of 1000 s−1 in the presence of NK1 antagonist L733060 or vehicle control. Alternatively, blood obtained from NK1-deficient transgenic mice or litter-matched controls was incubated with DIOC6 and perfused through collagen-coated capillaries. Tyrode-HEPES buffer was perfused through the microcapillaries to wash out red blood cells, leaving thrombi formed on the immobilized collagen. Thrombi were then visualized using a Leica DMIRE2 inverted confocal microscope (using N PLANL 20×/0.4 objective lens with 0- to 2-mm correction) and thrombus volume calculated from Z-series images captured and analyzed using TCS SP2 software (Leica). To measure protein concentration, 50 μL Tris-buffered saline containing NP40 (1% vol/vol) was perfused through the capillaries, the lysates were collected, and Bradford Protein Assay was performed.
Tail bleeding assay
Mice were anesthetized using ketamine (80 mg/kg) and xylazine (5 mg/kg) administered via the intraperitoneal route, and placed on a heated mat. A total of 1 mm of tail tip was removed using a scalpel blade, and the tail tip was bathed in sterile saline at 37°C. The time to cessation of bleeding was measured up to 10 minutes, after which the assay was terminated.
Mouse model of thromboembolism
Blood was collected from terminally anesthetized donor mice by cardiac puncture as described and resuspended in modified Tyrode buffer and incubated with 1.8 MBq 111Indium oxine at room temperature for 5 minutes. Platelets were washed and resuspended in 200 μL Tyrode buffer per donor mouse. 111Indium oxine–labeled platelets were infused into the tail vein of the recipient mice and left to equilibrate for 20 minutes. Mice were anesthetized and injected intraperitoneally with vehicle control buffer (Tyrode-HEPES buffer) or the NK1 receptor antagonist L733060 (10 mg/kg) 5 minutes prior to administration of collagen (100 μg/kg) by intravenous injection into the femoral vein. Accumulation of platelets in the pulmonary vasculature was measured as changes in platelet-associated counts using a 1-cm single-point extended area radiation detector (eV Products, Saxonburg, PA) and recorded using custom software (Mumed Systems, London, United Kingdom) on a UCS-20 spectrometer (Spectrum Techniques, Oak Ridge, TN). A detailed description of this model is provided elsewhere (C. Tymvios, S.J., C. Moore, S. C. Pitchford, C. P. Page, M.E., manuscript submitted, July 2007).
Data were acquired as percentage change in basal counts and expressed as maximal percentage response where responses for vehicle control treated mice were taken as 100% response plus or minus SEM.
Statistical analysis
Aggregation traces are representative of at least 3 separate experiments from different donors. Numerical data are presented as means plus or minus SEM, and statistical significance was analyzed by t test.
Results
Peripheral tachykinins stimulate platelet activation
The potential role of peripheral tachykinins in the regulation of hemostasis was examined using a number of experimental approaches. While the effects of SP on platelets have been previously characterized,10 very little is known of the functional effects of the endokinins. Since TAC4 mRNAs are present in megakaryocytic cells,11 endokinins, in addition to SP, were incorporated into the present study. The processing of EKA/B is incompletely understood, but EKA is predicted to be 47 amino acids in length, and EKB is predicted to be 41 amino acids in length. As shown in Table 1, they share some sequence identity with SP at the C-terminus, which includes the tachykinin consensus motif required for receptor activation. In this study, the common C-terminus sequence of EKA/B, which has been pharmacologically characterized,11 was used.
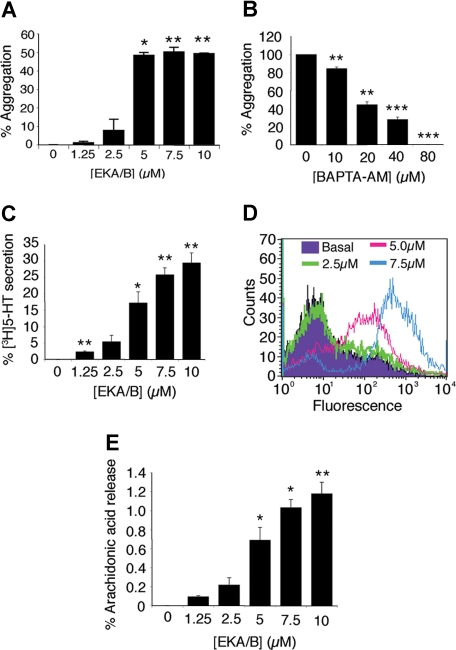
Initial experiments confirmed the ability of EKA/B to regulate human platelet function in vitro. EKA/B stimulated maximal aggregation, calculated as a percentage of light transmission through the buffer in which the platelets were suspended, using 5 μM EKA/B, which stimulated 48.4% (± 2.5%; Figure 1A). This was comparable with maximum aggregation produced by collagen and SP under similar conditions (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). EKC/D (Table 1), which do not bind to the known neurokinin receptors,11 were unable to stimulate platelet activation (data not shown).
Figure 1.
Peripheral tachykinins, EKA/B, stimulate functional responses in platelets. Washed platelets (A,D) or those loaded with the intracellular calcium chelator BAPTA-AM (B), [3H]5-HT (C), or [3H] arachidonic acid (E) were stimulated with vehicle alone (acetic acid 0.001%) or EKA/B. The results represent percentages of platelet aggregation (A,B), percentage of total [3H]-5HT (C), or [3H] arachidonic acid (E) taken up into the platelet and released, or surface fluorescence of PE-conjugated anti-CD62P antibody as a measure of alpha-granule secretion (D). n = 3 (A-C); n = 4 (E); mean plus or minus SEM. *P < .05; **P < .01; ***P < .001. Panel D is representative of 3 separate experiments.
To ensure that aggregation was not a consequence of peptide-induced agglutination but a result of receptor-mediated platelet activation, EKA/B-mediated activation was measured using platelets loaded with the intracellular calcium chelator BAPTA-AM. Consistent with the involvement of receptor-mediated mobilization of calcium from intracellular stores, BAPTA-AM reduced EKA/B-mediated (5 μM) aggregation in a dose dependent-manner by 15.5% (± 1.8%), 55.7% (± 3.2%), 72.1% (± 2.6%), and 100% compared with the vehicle control, at concentrations of 10, 20, 40, and 80 μM, respectively (Figure 1B). Dense granule secretion was examined using radiolabeled 5-hydroxytryptamine (5-HT). Platelets loaded with radiolabeled 5-HT were stimulated with EKA/B, and secreted radioactivity was measured. 5-HT secretion was observed in a dose-dependent manner (Figure 1C). Similarly, alpha-granule secretion was stimulated by EKA/B in a dose-dependent manner between concentrations of 2.5 and 7.5 μM as assessed by surface exposure of P-selectin (Figure 1D). A number of platelet agonists have been shown to increase TXA2 production by activation of phospholipase A2 (PLA2),24 which liberates arachidonic acid from phospholipid membranes that is converted rapidly to TXA2.23,25 To ascertain whether EKA/B could liberate arachidonic acid and therefore promote TXA2 synthesis, arachidonic acid release was measured from EKA/B-stimulated platelets preloaded with radiolabeled arachidonic acid. Arachidonic acid release from platelets was increased by 0.3% (± 0.2%), 0.71% (± 0.2%), 1.0% (± 0.3%), and 1.1% (± 0.3%) at concentrations of, 2.5, 5, 7.5, and 10 μM EKA/B, respectively (Figure 1E).
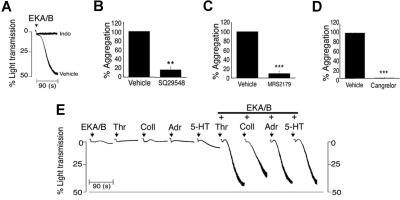
The platelet response observed with agonists such as collagen is often largely dependent on the release of secondary agonists from the platelet, predominantly ADP and TXA2. Similarly responses to EKA/B were dependent on the liberation of arachidonic acid and the subsequent synthesis and release of TXA2. Blocking the conversion of arachidonic acid to TXA2 using the cyclo-oxygenase inhibitor indomethacin (10 μM) abolished EKA/B-mediated aggregation (Figure 2A). Furthermore, antagonism of the TXA2 receptors TPα and β by SQ29548 (20 nM) inhibited EKA/B-mediated aggregation by 84.14% (± 6.8%; Figure 2B). Antagonism of the 2 purinergic receptors present on platelets demonstrated the requirement of signaling through both P2Y1 and P2Y12 receptors. P2Y1 antagonist MRS2179 (1 μM) and P2Y12 receptor antagonist cangrelor (AR-C69931MX; 0.1 μM) inhibited EKA/B-mediated aggregation by 90.5% (± 4.8%; Figure 2C) and 99.4% (± 0.6%; Figure 2D), respectively.
Figure 2.
EKA/B-mediated platelet aggregation is dependent on ADP and TXA2 release. Platelets were stimulated with EKA/B (5 μM) in the presence of indomethacin (10 μM) or vehicle control (Me2SO, A), SQ294890 or vehicle control (Tyrode-HEPES buffer, B), cangrelor (AR-C69931MX) or vehicle control (Tyrode-HEPES buffer, C), or MRS2179 or vehicle control (Tyrode-HEPES buffer, D), and aggregation was measured under constant stirring conditions at 37°C. Washed platelets were stimulated with subaggregatory concentrations of EKA/B (1.25 μM) alone, thrombin (Thr.; 0.005 U/mL), collagen (Coll.; 0.5 μg/mL), adrenaline (Adr.; 10 μM), or 5-HT (10 μM) in combination with EKA/B (1.25μM) or vehicle control (acetic acid 0.001%), and aggregation was measured at 37°C under constant stirring conditions (E). Individual aggregation traces are representative of 3 separate experiments (A,E). Numerical data represent means plus or minus SEM. (n = 3); **P < .01; ***P < .001.
EKA/B and SP therefore display typical characteristics of weak platelet agonists whose functional effects are dependent on secretion and synthesis of secondary mediators to elicit a full response.26,27 Consistent with this, EKA/B was found to synergise with a number of other agonists. Platelets stimulated with 1.25 μM EKA/B, 0.005 U/mL thrombin, 0.5 μg/mL collagen, 10 μM adrenaline, or 10 μM 5-HT displayed little or no evidence of aggregation. Combining each of the agonists with 1.25 μM EKA/B resulted in strong platelet aggregation, confirming that EKA/B can evoke synergistic responses (Figure 2E).
Peripheral tachykinins signal through the NK1 receptor on platelets
To establish whether the effects of EKA/B and SP on platelets are mediated by the NK1 receptor, aggregation assays were carried out in the presence of the NK1 antagonist L733060. Platelet aggregation stimulated by EKA/B (5 μM) was inhibited by 8.7% (± 3.2%), 22.2% (± 7.1%), 65.3% (± 14.5%), and 99.6% (± 0.4%) at concentrations of 5, 10, 20, and 30 μM L733030, respectively, compared with the vehicle control (Figure 3A). Similarly, SP (5 μM)–mediated platelet aggregation was inhibited by 17.6% (± 2.9%), 21.9% (± 1.0%), 70.2% (± 8.7%), and 85.3% (± 0.5%) by 5, 10, 20, and 30 μM concentrations of L733030, respectively (Figure 3B).
Figure 3.
Peripheral tachykinins signal through the NK1 receptor on platelets. Washed platelets were stimulated with EKA/B (5 μM) (A) or SP (5 μM) (B) in the presence of NK1 antagonist L733060 or vehicle control (Tyrode-HEPES buffer), and aggregation was measured using optical aggregometry. Data represent means plus or minus SEM. (n = 3); *P < .05; **P < .01; ***P < .001.
Platelet NK1 receptor signaling
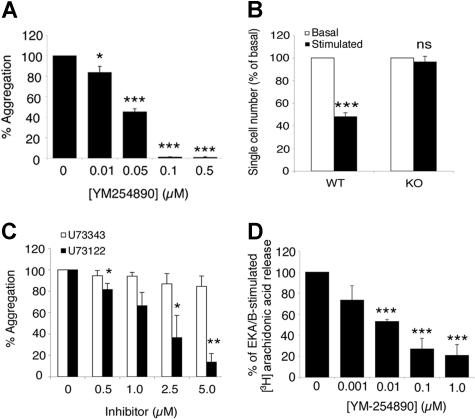
The NK1 receptor has been demonstrated in cell lines to couple to both Gq and Gs classes of G protein.28,29 Coupling to Gs would result in reduced platelet activity, and therefore we investigated the role of Gq in tachykinin-mediated platelet activation using the recently characterized Gq inhibitor YM-254890.30,31 Platelet aggregation evoked by EKA/B (5 μM) was inhibited in a dose-dependent manner by YM-254890 by 16.3% (± 5.9%), 54.8% (± 3%), 99.0% (± 0.3%), and 99.3% (± 0.7%) at concentrations of 0.01, 0.05, 0.1, and 0.5 μM, respectively (Figure 4A). Similarly, platelet aggregation stimulated by SP in mouse platelets, assessed by single-cell counts, was abolished in platelets obtained from Gq-deficient litter mates, confirming the importance of the Gq signaling pathway in tachykinin-mediated platelet aggregation (Figure 4B). This was further illustrated by the inhibition of phospholipase C (PLC), which lies downstream of Gq. The PLC inhibitor U73122 reduced EKA/B-mediated aggregation by 13.5% (± 5.6%), 27.5% (± 12.5%), 50.2% (± 20.7%), and 70.75% (± 7.9%) at concentrations of 0.5, 1.0, 2.5, and 5 μM, respectively, whereas the inactive analog U73343 had no effect (Figure 4C). Although these data confirm the involvement of the Gq/PLC signaling pathway in platelet aggregation stimulated by NK1 agonists, they do not demonstrate direct coupling of the NK1 receptor to Gq since EKA/B-mediated aggregation is dependent on released secondary agonists (Figure 2A-D). To determine whether the NK1 receptor couples directly to Gq or whether the inhibition of aggregation observed in Figure 4A-C was solely through inhibition of secondary agonists, NK1-mediated arachidonic acid mobilization was measured in the presence of YM-254890, as this occurs prior to TXA2 release and granule secretion (Figure S2). YM254890 inhibited arachidonic acid liberation stimulated by 10 μM EKA/B dose-dependently by 26.6% (± 13.6%), 46.9% (± 4.2%), 73% (± 2%), and 79.1% (± 9.5%), at concentrations of 0.001, 0.01, 0.1, and 1 μM, respectively, compared with vehicle control (Figure 4D). Moreover, increased arachidonic acid release stimulated by 10 μM SP was abolished in platelets taken from Gq-deficient mice (data not shown).
Figure 4.
Platelet NK1 receptors couple to Gq G proteins. Washed human platelets (A,C) or platelets loaded with [3H] arachidonic acid (D) were stimulated with EKA/B (5 μM), and mouse platelets were taken from Gαq-deficient mice or their litter-match controls and stimulated with SP (10 μM, B), and in the presence of the Gαq inhibitor YM-254890 (A,D) or PLC inhibitor U73122 or its inactive analog U73343 (C). The results represent platelet aggregation as a percent of the respective vehicle control (A,C), mouse platelet aggregation assessed by single-cell number is displayed as a percentage of basal cell number (B), and arachidonic acid release presented as the percentage of total [3H] arachidonic acid taken up into the platelet minus basal release (D). All data represent means plus or minus SEM. *P < .05; **P < .01; ***P < .001; n = 3 (A,C,D) or 6 (B).
EKA/B immunoreactivity is present in human platelets
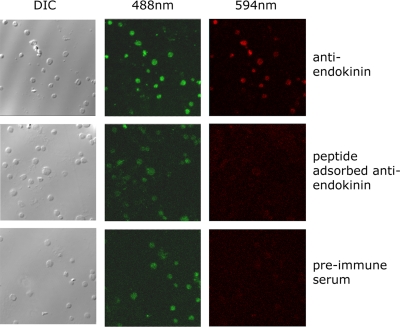
We have reported that platelets contain SP that is secreted during activation, and have proposed this to contribute to positive feedback regulation of platelet function. Given the presence of TAC4 mRNA in megakaryocytic cells, it is possible that platelets also contain endokinins, although cross-reactivity between available immunoassays for SP and EKA/B, due to sequence identity at the C-terminus, prevented confirmation of this. Antibodies were therefore raised against a peptide sequence that lies upstream of the common decapeptide (EKA/B) that is present in EKA and EKB. Human platelets were allowed to adhere to glass coverslips and probed using endokinin-selective antisera. Platelets were also prelabeled with DIOC6 and analyzed by confocal microscopy to detect platelets (488 nm) and endokinin (594 nm). Platelets stained positively for the presence of the endokinins (Figure 5). Antibody binding was not detected using preimmune serum or with antiendokinin antiserum preincubated with an excess of immunogen peptide. Similar results were obtained in the presence of saturating concentrations of mAb IV.3 (1 μg/mL) to block potential antibody binding of the platelet IgG receptor FcγRIIa (not shown). This is the first confirmation that endokinins are translated in tissues and that the human TAC4 gene is functional.
Figure 5.
EKA/B immunoreactivity is present in human platelets. Human platelets were labeled with the lipophilic fluorescent dye DIOC6, applied to glass coverslips, and fixed. Platelets were visualized by DIC microscopy and confocal microscopy at 488 nm. Endokinin expression in platelets was detected at 594 nm using an antiendokinin antibody and Texas red–conjugated secondary antibody. Parallel control experiments were performed using preimmune antiserum, and antiendokinin antiserum to which immunogen antibody had been previously added (peptide-adsorbed antiendokinin). Confocal fluorescence microscopic data represent composite images. Data are representative of 4 separate experiments.
NK1 is required for thrombus formation under arterial flow conditions
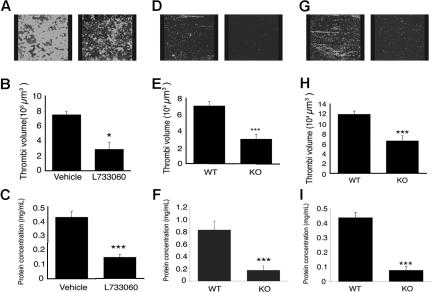
To establish whether NK1 agonists are involved in thrombus formation, human whole blood was perfused through collagen-coated microcapillary tubes at a shear (laminar flow) rate of 1000 s−1 in the presence of NK1 antagonist L733060 (10 μM) or vehicle control, and thrombus formation was measured. Thrombus volume, calculated as the mean of 5 randomly selected fields of view, was reduced by 57.2% (± 16.0%) in the presence of 10 μM L733060 compared with vehicle control (Figure 6A,B). To measure thrombus formation along the whole capillary, lysis buffer was passed through each capillary and protein concentration of the lysates measured as an indication of platelet number. Consistent with the thrombi volume data (Figure 6B), L733060 reduced protein concentration by 65.1% (± 2.7%) compared with thrombi formed in the presence of vehicle control (Figure 6C). The concentration of L733060 used was relatively high compared with Ki values derived from binding studies, although similar concentrations have been used to inhibit functional responses to SP in other peripheral cell types.32 To address this concern, thrombus formation was examined in blood from NK1 receptor–deficient mice.33 Blood from these mice produced thrombi 76% (± 7.8%) smaller than that formed from litter matched control blood (Figure 6D,E). Similarly, the protein concentration of thrombi formed from NK1 receptor–deficient mouse blood was 79.7% (± 5.5%) lower than that of thrombi formed from litter-matched control mouse blood (Figure 6F), indicating an important role for NK1 and substantiating data on human platelets using L733060.
Figure 6.
Tachykinins and their receptor are important for normal thrombus formation. Whole blood from drug-free donors (A-C) in the presence of NK1 antagonist, L733060 (10 μM), or blood obtained from NK1-deficient mice or litter-matched controls (D-F), or TAC1-deficient mice or control mice (G-I), was perfused through collagen coated capillaries at a sheer rate of 1000 s−1. Pictures of the thrombi were taken using confocal microscopy (A,D,G), the volume of the thrombi quantified (B,E,H), and protein concentration of the thrombi measured (C,F,I). Numeric data represent means plus or minus SEM. n = 3 (B,C), 8 (E), 6 (F), and 9 (H,I). *P < .05; **P < .01; ***P < .001.
To examine the involvement of SP in thrombus formation, blood obtained from mice lacking the TAC1 gene,18 which codes for SP, was perfused through collagen-coated microcapillaries, and thrombi formation was measured. Thrombi volume was 44% ± 9.5% smaller than in control blood (Figure 6G,H). A reduction of 80.9% (± 7.8%) in protein concentration was observed in lysates collected from TAC1-deficient thrombi compared with control thrombi (Figure 6I). NKA, which is also absent in TAC1-deficient mice, has no effect on platelets (G. J. Graham and J.M.G., unpublished data, 2002), suggesting the effects observed were a result of SP deficiency.
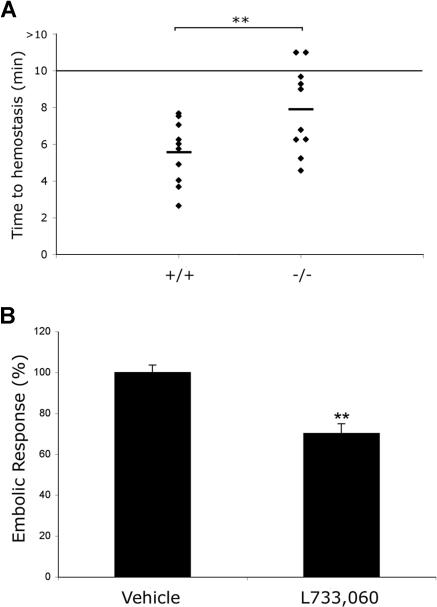
Increased bleeding and diminished thrombosis are observed in mice in the absence of NK1 receptor function
Having established a role for peripheral tachykinins in thrombus formation in vitro, experiments were performed to examine the significance of this for hemostasis using a mouse tail-bleeding assay. Bleeding time was found to be extended moderately in NK1-deficient mice33 in comparison with littermate controls (Figure 7A). The mean maximum duration for bleeding extended from 5.54 (± 0.51) minutes in control mice to 7.90 (± 0.71) minutes in NK1-deficient mice (P = .01; n = 10), with 2 NK1 mice bleeding beyond the 10-minute maximum duration of the assay. These experiments confirmed a role for tachykinin signaling in normal hemostasis.
Figure 7.
Increased bleeding and diminished thrombosis are observed in mice in the absence of NK1 receptor function. The time to cessation of bleeding following transection of the tail tip of NK1 receptor–deficient mice and littermate controls was measured. The assay was terminated after 10 minutes. Data represent individual mice (n = 10) with mean value indicated. **P < .01 (A). Collagen-induced thromboembolism was measured in Balb/c mice infused with 111Indium oxine–labeled platelets by measurement of the accumulation of radiolabeled platelets in the pulmonary region (B). Mice were treated with the NK1 receptor antagonist L733060 (10 mg/kg) or vehicle alone prior to stimulation of thromboembolism through intravenous administration of collagen (100 μg/kg). Data were acquired as percentage change in basal counts and expressed as maximal percentage response (embolic response, mean ± SEM) where measurements acquired for vehicle alone treated mice were taken as 100%.
To assess the potential impact of tachykinin and NK1 receptor signaling in thrombosis, experiments were performed to establish whether administration of the NK1 receptor antagonist L733060 provides protection against collagen-induced thromboembolism. Mice were injected with vehicle control or L733060, 5 minutes prior to administration of collagen (100 μg/kg), and platelet accumulation in the pulmonary vasculature measured. Results demonstrated a 30% (± 4.7%) reduction in maximal platelet accumulation in mice treated with L733060 compared with vehicle alone (Figure 7B). When performing these experiments, it was also noted that mice treated with the NK1 receptor antagonist experienced prolonged bleeding from the femoral vein after the collagen injection. These data further support the notion that tachykinins released from platelets play a role as secondary platelet agonists that are important for platelet recruitment to a growing thrombus through stimulation of the NK1 receptor.
Discussion
We have reported previously the presence of SP immunoreactivity in platelets, its secretion during activation, and its ability to stimulate platelet activation. We have therefore proposed SP to be a positive feedback regulator of platelet activation. The recently identified products of the human TAC4 gene, EKA/B share sequence identity with SP at C-termini, preferentially activate the NK1 receptor, and cross-react with available SP immunoassays.10 These data, together with the detection of TAC4 mRNA in megakaryocytic cells, indicate that EKA/B may also be present in platelets and contribute to tachykinin-mediated regulation of platelet function.
EKA/B, like SP,10 stimulated calcium-dependent platelet aggregation and alpha- and dense-granule secretion. It was also able to liberate arachidonic acid from platelet membranes, promoting the synthesis of the platelet agonist TXA2. Typical of other weak platelet agonists, EKA/B was capable of synergism with other agonists. It has been demonstrated that for platelet aggregation to occur, costimulation of both Gi and Gq signaling pathways is required.34 Purinergic receptors P2Y1 and P2Y12 are coupled to Gq and Gi signaling pathways, respectively. Inhibition of either of these abolishes aggregation, which can be recovered by stimulation of an alternative receptor coupled to the inhibited pathway. The range of agonists with which EKA/B (and SP10) synergize are coupled to a variety of signaling pathways. It is therefore likely that the synergy achieved by costimulation with this peptide was in part through the effects of secondary agonists. This correlates with the significant increase in dense granule secretion upon stimulation with 1.25 μM EKA/B observed in this study.
The involvement of NK1 receptors on platelets on the responses of these cells to SP and EKA/B was confirmed through the use of a selective NK1 receptor antagonist that was able to reduce substantially platelet aggregation. Signaling downstream of the NK1 receptor is potentially complex with possible contributions from a range of G proteins. Experiments carried out in this study illustrate that in platelets the NK1 receptor couples to Gq.
This study confirms the ability of NK1 receptor agonists SP and EKA/B to regulate platelet function. Furthermore, NK1 receptor–blocking antibodies inhibit platelet responses to collagen,10 indicating that NK1 agonists are released from activated platelets and act as paracrine agonists.
Due to the inability of existing antibodies or assays to distinguish between SP and EKA/B, it was initially unclear whether platelets secrete SP, EKA and EKB, or each of these. Antibodies were therefore raised to a peptide sequence that is present in endokinins A and B that lies upstream of the decapeptide that was used to stimulate platelet function and is therefore not shared with SP, and is unique to TAC4 gene products. Immunofluorescence experiments demonatrated the presence of TAC4-derived peptides in these cells. These are the first experiments that confirm that the human TAC4 gene is functional and that its products are translated in peripheral tissues. Furthermore, this indicates a potential role, along with SP, in the regulation of hemostasis. It is interesting to note that the TAC4 gene is not well conserved across species, which is suggestive that this gene is evolving rapidly in humans and perhaps is selected for platelet activation.
The importance of the peripheral tachykinins for thrombus formation was demonstrated on immobilized collagen under arterial flow conditions, which was reduced in the presence of NK1 receptor antagonist L733060. Moreover, whole blood taken from NK1-deficient mice33 produced markedly smaller thrombi compared with blood from control mice. These data illustrate a role for the NK1 receptor in thrombus formation and suggest that NK1 agonists are present in platelets and released upon activation, and at sufficiently high concentrations, act as positive feedback regulators.
Blood obtained from TAC1-deficient mice produced significantly smaller thrombi than blood obtained from litter-matched control mice. This reduction was comparable with that seen in blood obtained from NK1 receptor–deficient mice, suggesting that physiologically, SP is a major NK1 agonist responsible for amplifying the platelet response through the NK1 receptor. The relatively high concentrations of NK1 receptor agonists required to stimulate platelet signaling indicate that concentrations in the vicinity of a developing thrombus are likely to be similarly high, although our data suggest that synergism with other agonists is also critical. Given the presence of EKA/B in platelets, it is likely that they also contribute to thrombus formation through the stimulation of NK1 signaling, although the future development of endokinin-specific immunoassays will be necessary to measure the levels secreted from platelets.
To examine the contribution of peripheral tachykinins and NK1 receptor signaling to hemostasis, tail bleeding assays were performed. Bleeding was prolonged moderately in NK1 receptor–deficient mice33 in comparison with littermate controls, with some mice bleeding beyond the 10-minute duration of the assay. Similar assays were performed using TAC1-deficient mice,18 where no difference was observed in comparison with controls (data not shown). This is interesting, since it is indicative of redundancy, highlighting the potential involvement of TAC4 gene products (HK-1 in mice, EKA/B in humans).
Since these data implicate tachykinin signaling in the control of hemostasis, we have begun to assess contribution of the NK1 receptor to thrombosis using a thromboembolic model in mice. The administration of an NK1 receptor antagonist to mice resulted in a 30% reduction in the embolic response compared with infusion of vehicle alone. While it will be important in the future to examine the role of the NK1 receptor and the peripheral tachykinins in thrombosis using additional experimental models in vivo, the results of this study indicate that the inhibition of the actions of tachykinins may provide a new therapeutic approach for the prevention of thrombosis.
Acknowledgments
The authors would like to thank Francois Lanza (Strasbourg, France) and Stefan Offermans (Heidelberg, Germany) for Gq-deficient transgenic mice, Astellas (Tokyo, Japan) for Gq inhibitor YM254890, and The Medicines Company (Parsippany, NJ) for the P2Y12 antagonist cangrelor. We are also grateful to Hayley Mylroie for assistance with immunofluorescence experiments.
This work was supported by research grants from the British Heart Foundation and Wellcome Trust and National Centre for the Replacement, Refinement and Reduction of Animals in Research.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.J. designed and performed research, analyzed and interpreted data, and drafted the manuscript; K.L.T., T.S., W.J.K., and N.E.B. performed research and analyzed and interpreted data; P.J.L. analyzed and interpreted data and drafted the manuscript; A.Z. and S.P.H. contributed vital analytical tools and drafted the manuscript; M.E. designed research and interpreted data; and J.M.G. designed and performed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan M. Gibbins, School of Biological Sciences, University of Reading, Whiteknights, Reading, Berkshire RG6 6AJ, United Kingdom; e-mail: j.m.gibbins@reading.ac.uk.
References
- 1.Ruggeri ZM. Mechanisms initiating platelet thrombus formation. Thromb Haemost. 1997;78:611–616. [PubMed] [Google Scholar]
- 2.Gibbins JM. Adhesion receptor signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 3.Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- 4.Woulfe D, Yang J, Prevost N, et al. Signaling receptors on platelets and megakaryocyte. Methods Mol Biol. 2004;273:3–32. doi: 10.1385/1-59259-783-1:003. [DOI] [PubMed] [Google Scholar]
- 5.Angelillo-Scherrer A, de Frutos PG, Aparicio C, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 6.Andre P, Prasad KS, Denis CV, et al. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 7.Kashiwagi H, Shiraga M, Kato H, et al. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- 8.Zhu L, Bergmeier W, Wu J, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A. 2002;99:9219–9224. doi: 10.1073/pnas.142053899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham GJ, Stevens JM, Page NM, et al. Tachykinins regulate the function of platelets. Blood. 2004;104:1058–1065. doi: 10.1182/blood-2003-11-3979. [DOI] [PubMed] [Google Scholar]
- 11.Page NM, Bell NJ, Gardiner SM, et al. Characterization of the endokinins: human tachykinins with cardiovascular activity. Proc Natl Acad Sci U S A. 2003;100:6245–6250. doi: 10.1073/pnas.0931458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page NM, Woods RJ, Gardiner SM, et al. Excessive placental secretion of neurokinin B during the third trimester causes pre-eclampsia. Nature. 2000;405:797–800. doi: 10.1038/35015579. [DOI] [PubMed] [Google Scholar]
- 13.Rameshwar P, Gascon P. Hematopoietic modulation by the tachykinins. Acta Haematol. 1997;98:59–64. doi: 10.1159/000203593. [DOI] [PubMed] [Google Scholar]
- 14.Severini C, Improta G, Falconieri-Erspamer G, Salvadori S, Erspamer V. The tachykinin peptide family. Pharmacol Rev. 2002;54:285–322. doi: 10.1124/pr.54.2.285. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392–397. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- 16.Kurtz MM, Wang R, Clements MK, et al. Identification, localization and receptor characterization of novel mammalian substance P-like peptides. Gene. 2002;296:205–212. doi: 10.1016/s0378-1119(02)00861-2. [DOI] [PubMed] [Google Scholar]
- 17.De Felipe C, Herrero JF, O'Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer A, Zimmer AM, Baffi J, et al. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc Natl Acad Sci U S A. 1998;95:2630–2635. doi: 10.1073/pnas.95.5.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan PA, Stevens JM, Hubbard GP, et al. A role for the thiol isomerase ERP5 in platelet function. Blood. 2005;105:1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 20.Asselin J, Gibbins JM, Achison M, et al. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C gamma2 in platelets independent of the integrin alpha2beta1. Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- 21.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 22.Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. Platelet endothelial cell adhesion molecule-1 signaling inhibits the activation of human platelets. Blood. 2002;99:137–144. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- 23.Carey F, Menashi S, Crawford N. Localization of cyclo-oxygenase and thromboxane synthetase in human-platelet intracellular membranes. Biochem J. 1982;204:847–851. doi: 10.1042/bj2040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borsch-Haubold AG, Kramer RM, Watson SP. Cytosolic phospholipase A2 is phosphorylated in collagen- and thrombin-stimulated human platelets independent of protin kinase C and mitogen-activated protein kinase. J Biol Chem. 1995;270:25885–25892. doi: 10.1074/jbc.270.43.25885. [DOI] [PubMed] [Google Scholar]
- 25.Smith WL. The eicosanoids and their biochemical-mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offermanns S. The role of heterotrimeric G proteins in platelet activation. Biol Chem. 2000;381:389–396. doi: 10.1515/BC.2000.051. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed H, Saeed SA. Involvement of thromboxane A2 and tyrosine kinase in the synergistic interaction of platelet activating factor and calcium ionophore A23187 in human platelet aggregation. Exp Mol Med. 2004;36:220–225. doi: 10.1038/emm.2004.30. [DOI] [PubMed] [Google Scholar]
- 28.Khawaja AM, Rogers DF. Tachykinins: Receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 29.Maggi CA, Patacchini R, Rovero P, Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 30.Takasaki J, Saito T, Taniguchi M, et al. A novel G alpha(q/11)-selective inhibitor. J Biol Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi M, Nagai K, Arao N, et al. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp QS3666. J Antibiot. 2003;56:358–363. doi: 10.7164/antibiotics.56.358. [DOI] [PubMed] [Google Scholar]
- 32.Seabrook GR, Shepheard SL, Williamson DJ, et al. L-733,060, a novel tachykinin NK1 receptor antagonist; Effects in [Ca2+](i) mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol. 1996;317:129–135. doi: 10.1016/s0014-2999(96)00706-6. [DOI] [PubMed] [Google Scholar]
- 33.Bozic CR, Lu B, Hopken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- 34.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]