Abstract
Regulatory T (Tr) cells have the potential to treat immune-mediated disease, but cloning such cells for study from patients with autoimmune disease has proven difficult. Here, we describe autoantigen-specific, interleukin-10 (IL-10)–secreting Tr cell clones recovered ex vivo from a patient with autoimmune hemolytic anemia (AIHA) and characterize their phenotype, origin, and regulatory function. These IL-10+ Tr cells recognized a peptide, 72H-86L, derived from the Rh red blood cell autoantigen and shared phenotypic characteristics with both natural and inducible Tr cells. The clones also expressed different Tr markers depending on activation state: high levels of CD25 and LAG-3 when expanding nonspecifically, but FoxP3 after activation by the autoantigen they recognize. Despite a discrete Tr phenotype, these cells stably expressed the T helper 1 (Th1) signature transcription factor T-bet, suggesting they derive from Th1 T cells. Finally, the contribution of CTLA-4 in activating these IL-10+ Tr cells was confirmed by analyzing responses to transgenic B7.1-like molecules that preferentially bind either CD28 or CTLA-4. Overall, these Tr cells have a functional phenotype different from those described in previous studies of human Tr populations, which have not taken account of antigen specificity, and understanding their properties will enable them to be exploited therapeutically in AIHA.
Introduction
There is now compelling evidence from animal models that natural and inducible forms of CD4+ regulatory T (Tr) cells play an important role in immunologic tolerance and the control of immune-mediated pathology.1–4 It has been established that such cells also exist in humans,5,6 and the activation of autoreactive Tr cells in vivo therefore holds out the prospect of safe, effective treatments for clinical autoimmune disease. However, experimental approaches need to be devised to answer fundamental questions about the specificity and phenotype of human Tr cells. These questions could be addressed using cloned autoreactive Tr cells, but such cells, isolated ex vivo, are difficult to expand for further study, particularly when antigen-specific cells are sought.
Natural CD4+CD25+ Tr cells comprising approximately 5% of CD4+ T cells in humans, differentiate into suppressor cells during thymic development and are potent inhibitors of autoreactive effector T-cell responses.7,8 Regulatory cells with specificity for antigen can also develop in the periphery during the course of an immune response. These inducible Tr cells secrete regulatory cytokines (interleukin-10 [IL-10] and transforming growth factor β [TGF-β]) and can be induced from naive CD4+CD25− T cells in the presence of IL-10, vitamin D3, and dexamethasone or microbial proteins.9–13 Both natural and inducible Tr cells express increased levels of Foxp3, a transcription factor that seems to be a master regulator of Tr suppressive function. Mutation of FoxP3 leads to immune dysregulation in mice and humans, characterized by lymphoproliferation and autoimmune lesions.14,15 The normal immune system can therefore rely both on thymically derived Tr cells to broadly maintain immune homeostasis but can also recruit antigen-specific Tr cells to selectively inhibit active effector immune responses.
Autoimmune hemolytic anemia (AIHA) is a classic example of clinical autoimmunity in which to study Tr responses of pathogenic relevance, because the dominant target autoantigens have been well defined, and it was the first human disease in which autoantigen-specific Tr cells were identified. In most patients, pathogenic autoantibody and activated autoreactive CD4+ T helper 1 (Th1) cells that secrete interferon-γ (IFN-γ) are specific for the Rh proteins on the red blood cell (RBC) membrane.16 We have further shown that Tr cells specific for epitopes on the Rh proteins are also present in peripheral blood and spleens of patients with AIHA and that these cells are capable of inhibiting the Th1 effector responses in vitro by secretion of the suppressive cytokine IL-10. These findings, together with the observation that the Tr activity correlates with periods of remission, are consistent with the view that the autoimmune disease reflects an imbalance between pathogenic and regulatory responses.17
Although human Tr cells secreting the regulatory cytokine IL-10 have been induced in vitro through primary stimulation of peripheral blood T-cell cultures in the presence of exogenous IL-10 and other immunosuppressive components.9–13 Clones obtained in this way have been derived by biasing naive or uncommitted T cells toward a regulatory phenotype in vitro, and we wanted, instead, to study clones representative of autoreactive Tr cells present in patients in vivo. Cloning autoantigen-specific human Tr cells would allow us to address a number of unresolved questions. First, it will be important to determine the role of T-cell receptor (TCR) specificity in the effective stimulation of regulatory responses. It is generally accepted that Tr cells require activation by the TCR to be suppressive,18,19 but the nature of the ligands and strength of TCR interaction required need characterization. Second, we wanted to determine whether cells recovered ex vivo with specificity for an autoantigen are phenotypically characteristic of Tr cells, particularly for expression of the FoxP3 transcription factor. Examination of the phenotype could also offer clues to the origin and ontogeny of autoantigen-specific Tr cells. Are such cells induced and derived from genetically related effector T cells associated with autoimmune disease, or are they a discrete population of thymically derived natural Tr cells?
Finally, costimulatory requirements for Tr cell function are yet to be established and, in particular, the role of CTLA-4, an inhibitor of T-cell costimulation20 that is constitutively expressed in higher amounts on Tr cells compared with CD4+ T cells.9,21,22 Antibody blockade or gene deletion of CTLA-4 is associated with loss of tolerance in murine models,23 but it is not universally acknowledged that CTLA-4 ligation is critical for Tr function.24,25
Here, we describe ex vivo recovery and cloning of autoantigenic Rh peptide-specific Tr cells from a patient with AIHA. These Tr clones require cognate antigen, but not hyperstimulation to mount an immunosuppressive response and to maintain a phenotype characteristic of previously described Tr cells. We also provide evidence that, despite a distinct Tr phenotype, these cells are related to Th1 cells through expression of the Th1-associated T-bet transcription factor. Finally, we provide evidence that engagement of the T-cell costimulatory receptor, CTLA-4, is important for Tr- cell function.
Methods
Samples
T cells were obtained from a 71-year-old female patient who underwent splenectomy as part of treatment for AIHA. The patient was RhD positive, and the HLA-DR type was DRB1*0301/0401. Diagnosis was based on positive direct antiglobulin test and clinical evidence of hemolysis. At surgery the patient was not receiving any other form of treatment. The Grampian Health Board and the University of Aberdeen Ethical Committee approved the protocol for investigation, and was obtained in accordance with the Declaration of Helsinki informed consent.
Flow cytometry
FITC-labeled mouse anti-CD4 (clone 13B.2), and PC5-labeled mouse anti-CD25 (clone B1.49.9) antibodies were purchased from Beckman Coulter (High Wycombe, United Kingdom). Biotin-labeled mouse anti-CD152 (CTLA-4, clone BNI3) were purchased from BD Biosciences (Oxford, United Kingdom) and conjugated to Texas red-labeled streptavidin (Calbiochem, Nottingham, United Kingdom). An IL-10+ cell enrichment and detection kit was used to detect IL-10–secreting T cells (Miltenyi-Biotec, Bisley, United Kingdom). Because we were interested solely in cells actively secreting IL-10, monensin or Brefeldin A were not used to enrich intracellular IL-10. Four-color flow cytometry on stained cells used an EPICS XL cytometer (Beckman Coulter), and data were analyzed with Expo v2 software (Applied Cytometry Systems, York, United Kingdom). Anti–human T-bet–FITC (sc-21749) was purchased from Santa Cruz Biotechnology (Autogen Bioclear, Calne, United Kingdom). Anti–human LAG-3 (clone: 11E3) was obtained from Alexis Biochemicals (Nottingham, United Kingdom). An anti–human FoxP3-PE fix, permeabilization, and staining set was obtained from eBioscience (San Diego, CA).
Detection of FoxP3 transcription factor in Tr cells
Presence of FoxP3 transcription factor was determined by Western blot. Proteins from whole-cell lysates were fractionated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis and blotted overnight (Transphor; Hoeffer, San Francisco, CA) onto PVDF membranes. FoxP3 was detected with 1 μg/mL anti-FoxP3 antibody (Abcam Ltd, Cambridge, United Kingdom) and development using alkaline phosphatase–labeled anti–goat antibody and NBT/BCIP SIGMAFast substrate. Levels of FoxP3 were determined relative to actin by densitometry using a BioRad phosphoimager and Quantity 1 software (Hemel Hempstead, United Kingdom).
Cytokine secretion ELISA
Enzyme-linked immunoabsorbent assays (ELISAs) were based on previously published methods.17 The following antibody pairs were used: purified mouse anti–IFN-γ (clone NIB42) and biotinylated mouse anti–IFN-γ (clone 4S.B3), purified mouse anti–IL-10 (clone JES3–19F1) and biotinylated mouse anti–IL-10 (clone JES3–12G8), purified mouse anti–IL-4 (clone 8D4–8) and biotinylated mouse anti–IL-4 (clone MP4–25D2) (all from BD Biosciences). All recombinant human cytokines (IFN-γ, IL-4, IL-10, and IL-15) were from PeproTech EC Ltd (London, United Kingdom).
Proliferation assay
Cell proliferation was assessed by 3H thymidine incorporation, as previously described.11
Enrichment or depletion of CD4+ cells
Cells were fractionated with a Dynal CD4+ isolation kit, and nonspecific T-cell stimulation and expansion was driven by the Dynabeads CD3/CD28 T-cell expander reagent (Dynal Biotech, Wirral, United Kingdom).
Blockade of CTLA-4
As before,17 blockade was performed with mouse anti-CD152 F(ab′)2 fragment (clone ANC152.2/8H5) from Alexis Biochemicals. Choice of an F(ab′)2 precludes interference from the Fc region.
Antigens
A complete panel of 42 15-mer peptides, with 5–amino acid overlaps, was synthesized16 (Department of Biochemistry, University of Bristol, Bristol, United Kingdom), spanning the sequence of the 30-kDa Rh protein associated with expression of the D blood group antigen.26 To ensure purity, peptides were screened by high-performance liquid chromatography and amino acid analysis. Rh protein was purified from RBCs by immunoprecipitation using a monoclonal anti-D (T19; Scottish National Blood Transfusion Service), specific for RhD ep4.27 Rh protein was added to cultures at an estimated concentration of 5 μg/mL.
Generation of CD4+ Tr lines and clones
As previously described, peptides that induce IL-10 secretion were identified using ELISA to screen responses to the panel of Rh peptides.17 Splenocytes were obtained by cutting up 2-cm3 fractions of splenic tissue into smaller pieces and tamping tissue fragments through a 40μM cell strainer (BD Falcon, Oxford, United Kingdom) into a petri dish containing 10 mL ice-cold HBSS. These crude cell suspensions were collected in 50-mL Falcon tubes on ice, centrifuged (300g for 10 minutes), and resuspended in red cell lysis buffer (17mM Tris buffer, 144mM NH4Cl, pH 7.2). Cells were gently agitated for 2 minutes to destroy erythrocytes before 3 washes with cold HBSS. Splenocytes were frozen down in a mixture of 50% FCS, 40% HBSS, 10% DMSO, in aliquots of 1 × 108 cells/mL, before being put under liquid nitrogen. Splenocytes were cultured at 1 × 106 cells/well in complete medium containing peptide antigen (10 μg/mL) for 6 days at 37°C, 5% CO2. IL-10–secreting T cells were isolated with an IL-10+ cell enrichment and detection kit (Miltenyi Biotec). Typically, the yield of recovered IL-10+ Tr cells was low (≈ 4% of CD4+ T cells; in absolute numbers ≈ 20 000-40 000 cells). Isolated IL-10+ T cells were cloned by limiting dilution at 1 × 104/mL, 1 × 103/mL, and 1 × 102 cells/mL together with autologous, irradiated, CD4+ cell–depleted splenocytes at 1 × 106 cells/mL as antigen-presenting cells (APCs). Peptide (1-10 μg/mL), together with IL-15 (50 ng/mL)28 was added to cell suspensions, and 20-μL aliquots were dispensed into 384-well plates. After 6 to 14 days of incubation at 37°C, 5% CO2, potential Tr clones were transferred to round-bottom 96-well plates, and 50 μL of fresh CM containing both IL-15 (50 ng/mL) and 0.125 μL CD3/CD28 T-cell expander beads (expansion medium) were added. Together, these reagents allow CD4+ T cells to expand in the absence of APCs. Expanding cell cultures were divided and replenished with fresh expansion medium at 3-day intervals. After 40 days, several million cells were obtained, and their growth rate indicated a trebling in number every 2 days. Samples of DNA were taken from each potential peptide-specific Tr clone for TCR Vβ gene usage analysis as a check for clonality. Potential peptide-specific Tr clones were tested by incubation with Rh peptide (1 μg/mL) or purified Rh protein (≈ 5 μg/mL) and autologous, irradiated 30 Gy [3000 rad]), CD4+ cell–depleted splenocytes at 1 × 106 cells/mL as a source of APCs for 48 to 96 hours at 37°C, 5% CO2. Typically, 5000 to 100 000 cloned cells per well were used in experiments.
B7.1 shufflants
To study the role of CTLA-4 in Tr function we used an adherent human embryonic kidney cell line (HEK293) transfected with either wild-type B7.1 ligand (CD80; binds both CD28 and CTLA-4), CTLA-4 binding protein (CTLA-4BP; selectively binds CTLA-4), or CD28 binding protein (CD28BP; selectively binds CD28). These proteins, referred to here collectively as B7.1 shufflants, were kindly donated by Maxygen (Redwood City, CA) and are fully described by Lazetic et al.29 HEK293 is a fetal-derived cell line, commonly used as a vehicle for transfection of genes and gene fragments (no. CRL-1573; ATCC, Manassas, VA). These cells do not express MHC class II molecules, making them ideal for the experiments outlined here. The HEK293 cells were irradiated before use to prevent them from overwhelming cell cultures. Sole engagement with CD28BP/CD28 and CTLA-4BP/CTLA-4 was confirmed with soluble fragments of CD28 and CTLA-4 by flow cytometry. Washed, irradiated cells (50 Gy [5000 rad]; 400 000 per well) were added to 24-well plates and allowed to adhere for 24 hours before the addition of P8(1) clone (100 000 cells/mL). Clones were cultured in the presence or absence of anti-CD3 stimulation at 37°C, 5% CO2 for 96 hours before cytokine production was measured.
Results
Method for cloning human red cell autoantigen-specific CD4+ Tr clones
We developed a method for deriving human Tr clones from CD4+ populations that secrete IL-10 ex vivo, initially using a large sample of splenic T cells from a patient with AIHA. A key requirement of the technique was that it should not rely on the addition of exogenous IL-109–11 or immunosuppressive compounds12 in vitro because clones of such cells are not necessarily derived from, nor representative of, populations that secrete IL-10 in vivo.
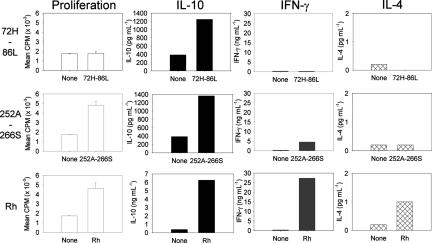
Previous mapping experiments with peptide panels spanning the sequences of the Rh protein autoantigen showed that particular peptides preferentially induced IL-10 responses by Tr cells from peripheral blood or spleen of patients.17 In 3 separate experiments on splenocytes and peripheral blood mononuclear cells from the current patient, we identified one such peptide, 72H-86L (sequence HSWSSVAFNLFMLAL), which consistently stimulated T-cell IL-10 secretion (Figure 1). Analysis of MHC-binding motifs within peptide 72H-86L, using ProPred prediction software,30 indicated the presence of a putative epitope within the C-terminal region of peptide 72H-86L, restricted by one of the HLA class II molecules expressed by the patient, DR4.
Figure 1.
Specific induction of IL-10 secretion by splenic T cells from a patient with AIHA in response to purified Rh protein or derived peptide. Splenocytes were stimulated in culture with purified Rh protein, peptides 72H-86L and 252A-266S, or no antigen, and proliferation and secretion of the cytokines IL-10, IL-4, and IFN-γ were measured. Data are presented as mean plus or minus standard error.
The IL-10–secreting cultures were used to derive CD4+ Tr clones specific for the peptide and purified Rh protein. First, cells were stimulated with peptide for 5 days, and then magnetic bead fractionation was used to isolate IL-10–producing cells, which were expanded and cloned by limiting dilution. To overcome problems of inducing Tr cells to proliferate in vitro under standard culture conditions, we developed a protocol to expand splenic Tr cells using IL-15.28 In preliminary experiments IL-15 alone (but not IL-2 or IL-7), at an optimum concentration of 50 ng/mL, preferentially expanded IL-10–secreting cells (data not shown). In 3 separate cloning experiments, we raised 5 Tr clones from a T-cell line specific for peptide 72H-86L, each with similar phenotypic characteristics. The frequency of Tr clones obtained by this method was approximately 0.001% of CD4+ T cells. Clonality was confirmed using polymerase chain reaction and flow cytometry analysis of TCR Vβ gene segment usage. Beads coated with IL-15 and anti-CD3/CD28 in the absence of APCs were used to maintain the cloned populations. Despite repeated attempts, we were unable to generate any Tr clones specific for a second peptide 252A-266S, which did not preferentially induce IL-10 secretion. In the absence of the step to select IL-10+ regulatory T cells, clones specific for control antigens, including PPD, generated from the peripheral blood of other persons exhibit classic Th effector phenotypes with no evidence of suppressive function. Availability of human autoreactive Tr clones specific for a target of pathogenic relevance in a patient with AIHA enabled, for the first time, a detailed characterization of such cells.
Tr phenotype, TCR antigen recognition, and regulatory activity
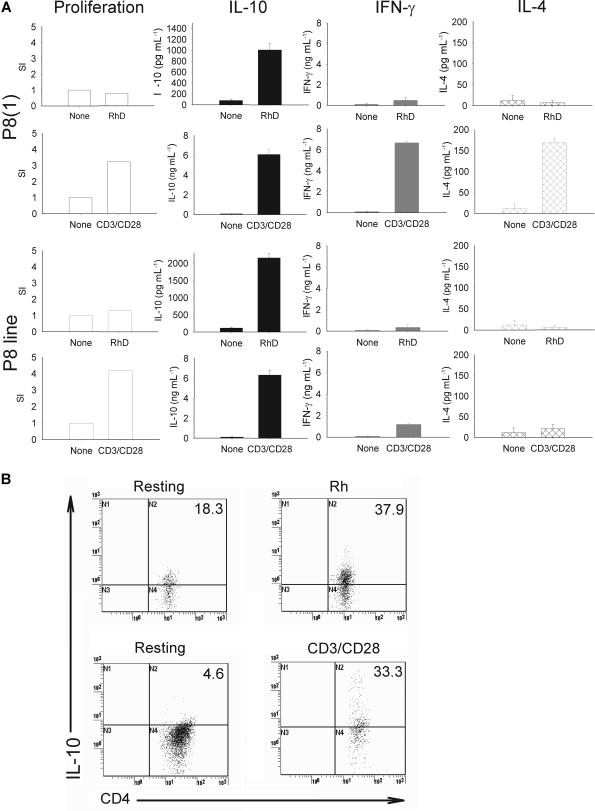
First, because the cloned Tr cells are of known specificity, it was possible to compare the effects of TCR ligation by antigen and nonspecific stimuli on their regulatory responses. ELISAs were used to confirm that recognition of cognate autoantigen induced predominant secretion of IL-10, but little Th1 cytokine IFN-γ and no Th2 cytokine IL-4 (Figure 2A), with no proliferation. However, clones activated with high-affinity anti-CD3/CD28 antibody bound on magnetic beads to allow cross-linking did not induce the Tr1-like cytokine phenotype, and the cells instead mounted a Th0 effector response, with proliferation and secretion of IFN-γ, IL-4, and IL-10. This characteristic, that the regulatory cytokine phenotype is dependent on antigen and masked by high-affinity nonspecific activation, was also exhibited by the other RBC-specific autoreactive Tr clones and lines studied (example shown in Figure 2A).
Figure 2.
IL-10–dominated regulatory responses are seen when autoreactive Tr cells are activated by cognate autoantigen but not high-avidity stimulation. Clones or cell lines (1 × 105/well) were incubated with irradiated (30 Gy [3000 rad]) autologous CD4-depleted antigen-presenting cells (1 × 106/well) for 96 hours in 1-mL cultures at 37°C, 5% CO2 in the presence or absence of antigen (Rh) or nonspecific stimulus (2 μg anti-CD3/CD28). Tr clone P8(1) and P8 Tr cell line (A) secrete predominantly IL-10 and little IFN-γ or IL-4, characteristic of a Tr response, when stimulated specifically by presentation of the Rh protein autoantigen. In contrast, the cells mount Th0-like effector responses, with secretion of IFN-γ and IL-4 in addition to IL-10, and proliferation, after nonspecific high-affinity activation with anti-CD3 and anti-CD28. Data are presented as mean plus or minus standard error. (B) Number of IL-10–secreting CD4+ T cells are increased on stimulation with Rh autoantigen and nonspecific stimulation with anti-CD3/CD28 T-cell stimulatory antibody. Experimental detail as above. CD4+ clones actively secreting IL-10 were detected on day 4 by flow cytometry using anti-CD4-FITC antibody with an IL-10 cell enrichment and detection kit (PE-label). Number of IL-10 positive cells are represented in upper right quadrant as a percentage of the total Cd4+ T-cell population. The results shown are representative of 3 separate experiments.
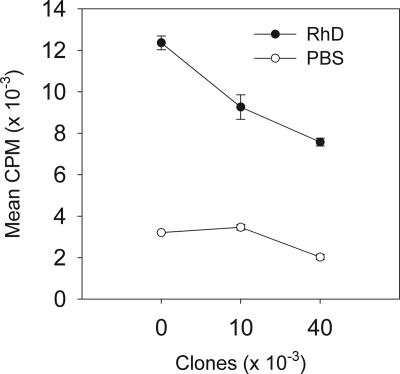
We also confirmed that cloned Tr cells retained their regulatory activity observed in the polyclonal population from which they were derived. Stimulation of clones by APC-presenting Rh protein led to increased numbers of cells actively secreting IL-10 compared with the resting cell population (Figure 2B top panels). The number of cells induced to secrete IL-10 by nonspecific activation (anti-CD3/CD28 antibody) were also increased (from 4.6% to 33.3%). In contrast, autologous purified CD4+ T cells stimulated with anti-CD3/CD28 antibody increased the numbers of IL-10–secreting cells from base levels of 0.1% to only 2.3% (data not shown). Further, cloned cells activated by Rh suppressed proliferative responses to the Rh protein mediated by autologous effector Th1 cells (Figure 3). Finally, when compared with other, conventional effector Th1/Th2 T-cell clones available in our laboratory, these cells were less able to proliferate when stimulated with anti-CD3 antibody. For example, there was approximately 100-fold less proliferation of clone P8(1) and 72H-86L–specific cell line compared with human Th1 and Th2 clones specific for Timothy grass allergen, after activation (data not shown).
Figure 3.
Regulatory activity of antigen-specific T-cell responses by autoantigen-specific Tr clones derived from a patient with AIHA. Tr clone P8(1) inhibits proliferation by unfractionated autologous CD4+ T cells responding to APCs presenting the Rh protein autoantigen. The ratio of clones to autologous CD4+ T cells was 1:50 (10 000) and 1:12.5 (40 000), respectively. Proliferation was measured after 96 hours at 37°C, 5% CO2. All data shown are representative of 4 separate experiments with clone P8(1), and similar results were obtained with the other lines obtained from the patient. Data are presented as mean plus or minus standard error.
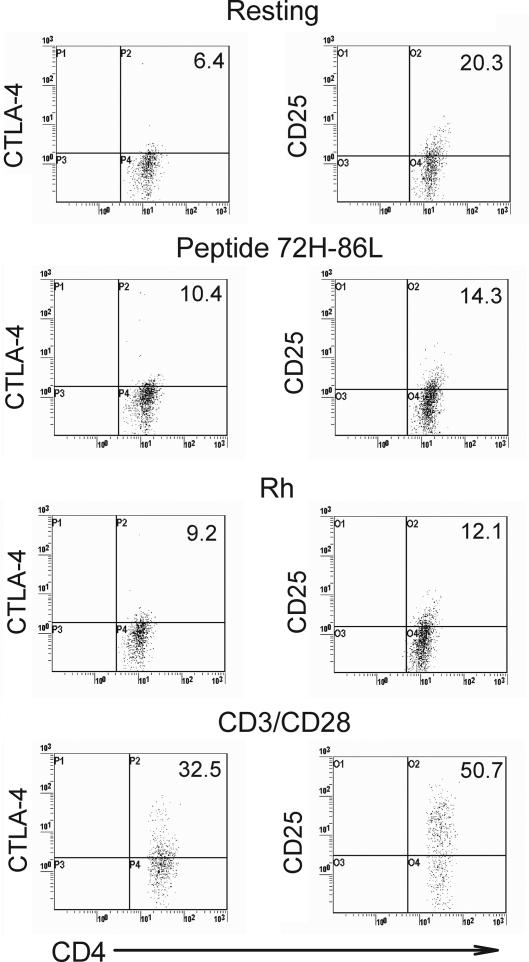
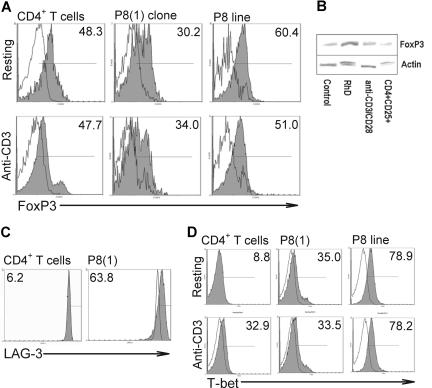
Differences in expression of particular markers are characteristic of both natural and induced forms of the Tr cell. Having cloned autoreactive human Tr cells, we wanted to determine whether they conformed to either of these currently recognized Tr types based on increased expression of associated phenotypic markers, including CTLA-4, CD25, FoxP3, and LAG-3. CTLA-4 is typically seen as inhibitory for effector cells, but its relevance to Tr function is controversial.25 Cell-surface expression of CTLA-4 was constitutively higher on the clones such as P8(1) than on autologous CD4+ T cells (6.1% vs 0.9%), and it was also higher when the clones were incubated either with cognate peptide 72H-86L, Rh autoantigen, or nonspecific stimulation with anti-CD3/CD28 antibody (Figure 4 left panels).
Figure 4.
Expression of CTLA-4 and CD25 as regulatory T-cell markers. Analysis of representative Tr clone P8(1) (left), showing CTLA-4 up-regulation when the clone is specifically stimulated with APCs presenting peptide 72H-86L, Rh protein autoantigen, or anti-CD3/CD28 stimulatory antibody. Conversely, CD25 is down-regulated by the clone after specific stimulation with APCs presenting cognate autoantigen in the form of peptide 72H-86L or Rh protein (right) but is up-regulated, as expected, by anti-CD3/CD28 stimulation. Number of IL-10 positive cells are represented in upper right quadrant as a percentage of the total CD4+ T-cell poplation. Experimental detail is described in Figure 2.
CD25 is expressed constitutively at high levels by the major category of natural Tr cells but is held to be expressed only as an activation marker on the induced form. The CD25 phenotype of clone P8(1) in different activation states was characterized (Figure 4 right panels). At rest, the clone constitutively expressed higher levels of CD25 than did autologous CD4+ T cells. As expected, expression of CD25 by P8(1) clone increased after nonspecific stimulation with anti-CD3/CD28 antibodies, but, surprisingly, CD25 levels decreased markedly when the cells were specifically activated with peptide 72H-86L or Rh protein. Thus, the Tr cells that we cloned express high levels of CD25 when expanding nonspecifically, but they down-regulate this marker when they mount a Tr IL-10 response to the autoantigen they recognize. Further analysis of CD25 on the P8(1) clone and 72H-86L Tr cell line by flow cytometry showed increased expression of the FoxP3 transcription factor in CD25bright cloned cells compared with CD25lo cells (Figure 5A). Thus, despite clonality the distribution pattern of FoxP3+ expression was similar to that of heterologous CD4+CD25+ T cells. Examination of FoxP3 expression by Western blot showed low but detectable expression in P8(1) clone when resting and after nonspecific anti-CD3/CD28 stimulation, but it was amplified after activation with the cognate antigen, Rh protein (Figure 5B). The semiquantitative Western blot analysis confirms the flow cytometric data showing that the Tr clones are FoxP3+. Overall, these results show that FoxP3, but not CD25, expression correlates with antigen-specific induction of Tr function.
Figure 5.
Analysis of phenotypic markers FoxP3, T-bet, and LAG-3. Heterologous CD4+ T cells (1 × 106/mL), P8(1) clones, or peptide 72H-86L specific cell lines (both 1 × 105/mL) were incubated in the presence or absence of anti-CD3 mAb for 24 hours at 37°C, 5% CO2 before analysis of FoxP3 expression by flow cytometry (A). Top (resting cells) and bottom (activated cells) panels illustrate number of FoxP3+ cells in CD3+CD4+CD25low (white histogram) and CD25bright (gray) T-cell populations from T cells, clone, and line, respectively. Analysis of FoxP3 expression by Western blot (B). Representative Western blot of cell extracts analyzed by SDS-PAGE and stained for FoxP3 or the control protein actin. The extracts were obtained from Tr clone P8(1) either resting, stimulated by presentation of Rh protein autoantigen, or activated nonspecifically by anti-CD3 and anti-CD28 for 96 hours at 37°C, 5% CO2. CD4+CD25+ peripheral blood cells isolated ex vivo are also included as a positive control for FoxP3 staining. Densitometric analysis normalized against actin levels confirmed increased FoxP3 expression by the Tr clone after specific Rh protein autoantigen stimulation (0.61) compared with nonstimulated control (0.43) or nonspecific stimulation (0.51) but similar to isolated CD4+CD25+ cells (0.59). Relative expression of the Th1 T-cell–associated transcription factor, T-bet (C). Cells were treated as described for panel A, and increases in number of T-bet+ clones or cell lines (gray histogram) at rest (top) or activated with anti-CD3 mAb (bottom) were compared with resting CD4+ T cells (white histogram). Comparison of LAG-3 analysis (D). CD4+ T cells shown in left panel (white and gray histograms are resting and activated cells, respectively) and clone, right panel, were costained with anti–LAG-3 antibody and compared for expression. Number of IL-10 positive cells are represented in upper right quadrant as a percentage of the total CD4+ T-cell population.
Lymphocyte activation gene-3 (LAG-3) is an MHC class II ligand that negatively regulates T-cell activation.31 Previous work has shown that expression of LAG-3 is increased on regulatory T-cell populations and could underpin cell-mediated inhibitory processes identified in these cells. Comparison of clonal cell-surface expression of LAG-3 with that of CD4+ T cells (Figure 5C) showed cell surface increases in the clonal population, indicating that enhanced LAG-3 production is characteristic of these cells.
Recent work has suggested that inducible Tr cells can arise from Th1 or Th2 CD4+ T cells expressing signature transcription factors such as T-bet and GATA-3.32,33 Because these clones were derived from a patient with active AIHA, we addressed the question of whether our cloned antigen-specific Tr cells were originally derived from a Th1 effector T-cell population by analyzing expression of the Th1-associated transcription factor T-bet (Figure 5D). As expected, expression levels of T-bet in resting heterologous CD4+ T cells was low but increased when cells were stimulated with anti-CD3 mAbs. In contrast, T-bet expression in both the P8(1) clone and the 72H-86L cell line was notably higher than activated heterologous CD4+ T cells.
Costimulation and regulatory activity
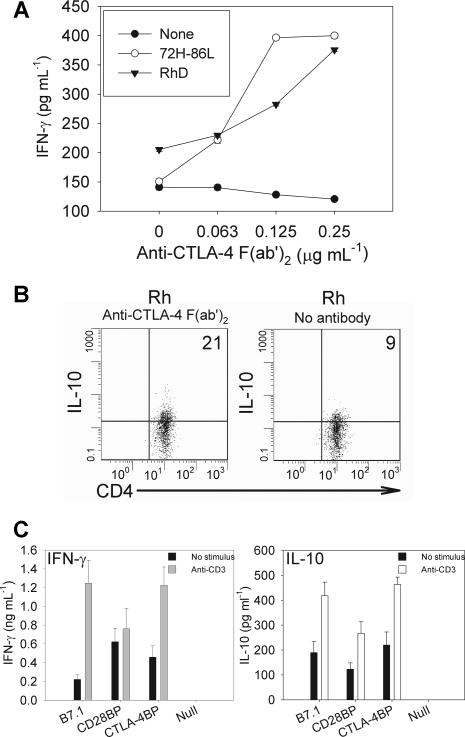
Because CTLA-4 expression was increased in clones, constitutively, and in response to antigen-driven stimulation, experiments were set up to determine the effects of CTLA-4 in the role of these Tr cells. Blockade of CTLA-4 with an anti-CTLA-4 F(a′)2 fragment deviated the cloned Tr cell response toward Th1, with proliferation and IFN-γ secretion (Figure 6A) and suppressed numbers of cells actively secreting IL-10 in response to the Rh autoantigen presented by autologous APCs (Figure 6B).
Figure 6.
Contribution of costimulatory molecule CTLA-4 to maintaining Tr clone cytokine phenotype. Tr clone cells (1 × 105/mL) were incubated with CD4 depleted, irradiated autologous antigen-presenting cells (1 × 106/mL) in the presence of stimuli for 4 days at 37°C, 5% CO2. (A) Blockade of CTLA-4 with F(ab′)2 antibody fragment deviates the response of autoreactive Tr clone P8(1) against presentation of either cognate peptide 72H-86L (○) or Rh protein autoantigen (▾) toward IFN-γ secretion, but not control cultures stimulated with irrelevant peptide (●). (B) Cytometric analysis showing that number of IL-10+ production by the Tr cells in response to presentation of Rh protein is reduced by anti-CTLA-4 F(ab′)2 antibody fragment blockade during cell culture. Number of IL-10 positive cells are represented in upper right quadrant as a percentage of the total CD4+ T-cell population. (C) Enhancement of IL-10 and IFN-γ by TR clone P8(1) after ligation of CTLA-4. Adherent HEK293 cells transfected with wild-type B7.1 (CD80), CD28BP (selectively binds CD28), CTLA-4BP (selectively binds CTLA-4), or empty vector (Null) were irradiated (50 Gy [5000 rad]), washed, and allowed to adhere to 24-well plates for 24 hours. P8(1) clones (1 × 105/mL per well) were incubated in plates containing transfected cells with no stimulus or anti-CD3 stimulatory antibody for 96 hours at 37°C, 5% CO2. Data are presented as mean plus or minus standard error.
To further assess the contribution of CTLA-4 to Tr activity we used HEK293 cell lines transfected with B7.1-like molecules (B7.1 shufflants) that selectively bind either to CD28 or CTLA-429 and compared responses with wild-type B7.1-transfected cell lines. In effect, these cell lines provide an additional signal either through CD28 (CD28BP), CTLA-4 (CTLA-4BP), or both (B7.1); thus, we were able to determine whether CTLA-4 engagement induced P8(1) to enhance its regulatory phenotype. When P8(1) clones were incubated with the B7.1 shufflants in the absence of a TCR stimulus, each induced low levels of IFN-γ and IL-10, with CD28BP and CTLA-4BP inducing more IFN-γ and IL-10, respectively (Figure 6C). On stimulation with anti-CD3 antibody, both B7.1 and CTLA-4BP induced small increases in IFN-γ and IL-10, whereas cytokine levels increased to a lesser extent when incubated with CD28BP. These data confirm that CTLA-4 contributes to secretion of IL-10 by inducible Tr clones function.
Discussion
We report here a method that enabled us to recover, expand, and characterize regulatory T-cell clones specific for a dominant autoantigen in a patient with autoimmune disease. The method differs from previously published approaches in that no exogenous IL-10 or immunosuppressive compounds, which can bias undifferentiated Th cells, were added to cultures, and the clones were derived from cells secreting IL-10 ex vivo. These clones therefore provide the first opportunity to characterize human Tr cells representative of regulatory populations in autoimmune disease. Their properties show the importance of antigen-specificity and costimulation in exposing the Tr phenotype.
The autoreactive Tr clones were derived from a patient with AIHA and are specific for a peptide corresponding to residues 72H-86L from the dominant Rh RBC protein autoantigen. The approach was developed using a large sample of splenocytes, but it has now been extended to derive Tr clones from peripheral blood and from other patients. We adapted the cloning method from a technique established for Th1/Th2 effector T cells,34 but we modified it first by acquiring only cells that secrete IL-10 during stimulation with self-peptide and then by adding IL-15 to favor Tr cell expansion.28 Both murine and human naive peripheral CD4+ T cells can be deviated into Tr1 cells if stimulated with antigen in the presence of IL-10 or dexamethasone/vitamin D.9–13 In contrast to these maneuvers, IL-15 allows expansion of IL-10–secreting Tr1 cells28 but does not promote de novo Tr cell differentiation. This approach enabled existing Tr cells, sampled from the periphery and relevant to disease, to be isolated, expanded, and characterized. A number of features of the clones are relevant to the role of Tr cells in self-tolerance and autoimmunity.
First, the ability to expand and clone suppressive Tr cells secreting IL-10, including clone P8(1), confirms the existence of such cells specific for autoantigens of pathogenic relevance in human patients. These cells were derived from an IL-10–secreting population, the activity of which correlates with remission from AIHA,17 consistent with the view that autoaggressive immune responses can be controlled in vivo by boosting their function or numbers.
Second, the regulatory phenotype, including predominant IL-10 secretion and increased FoxP3 expression, was shown by specific recognition of the Rh autoantigen but not higher avidity TCR stimulation. This shows the importance of studying autoreactive Tr cells in the context of known specificity, because polyclonal stimuli obscure their regulatory phenotype, but it also has implications for the understanding of regulation in vivo. Further studies will allow us to characterize such “tuning” of regulatory responses.35
Analysis of T-bet expression, a transcription factor associated with the Th1 T-cell phenotype, was stably increased in both antigen-specific clone and cell line, compared with resting T cells. This observation suggests that the IL-10 Tr cells we describe here are derived from Th1 T cells and may have arisen as a result of effector T-cell stimulation associated with the chronic autoimmune response underlying AIHA. This is also supported by the observation that inhibition of their regulatory activity and phenotype corresponds with increases in IFN-γ secretion.
Another important feature of Tr cells shown by this study is dependence on ligation of the costimulatory molecule CTLA-4 for regulatory function. The effects of CTLA-4 engagement in providing a negative costimulatory signal in effector T cells are well documented,20,23 but its role in suppression responses by Tr subsets is debatable. There is, however, both circumstantial and direct evidence that CTLA-4, expressed by regulatory cells, functions to suppress T cell–mediated inflammatory responses. CTLA-4 cell-surface expression is constitutively high on every regulatory T-cell subset so far described21,22 and, furthermore, blockade of CTLA-4 expressed by regulatory CD4+CD25+ T cells, but not CD4+CD25− T cells, leads to the development of autoimmune disease in mice.36 In other models of autoimmunity, blockade of CTLA-4 accelerates onset and exacerbates disease.24,37 This active suppression by CTLA-4–expressing Tr cells raises the possibility that CTLA-4 engagement may mediate a positive signal for regulatory function. Evidence from molecules phylogenetically related to the B7 ligand and engineered to selectively bind either CD28 or CTLA-4 (CD28BP and CTLA-4BP, respectively)29 showed that CTLA-4 engagement on T cells resulted in increased IL-10 secretion, whereas CD28 engagement prompted IFN-γ secretion and proliferation. The work we present here confirms this observation, because Rh-specific IL-10 secretion was abolished by CTLA-4 blockade of our autoreactive Tr clones in favor of increased interferon-γ production. This was further supported by our observation that engagement of CTLA-4 by CTLA-4BP enhanced activation of the clone and increased IL-10 production.
In summary, we have cloned human Tr cells associated with AIHA ex vivo. The clonal isolation of such autoantigen-specific Tr cells can now be extended to other diseases, and understanding their properties will be an essential step in the rational design of therapeutic strategies based on the selective stimulation of these cells.
Acknowledgment
This work was supported by The Wellcome Trust (United Kingdom) and Tenovus-Scotland.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.J.W. designed and performed research and wrote the paper; A.M.H. contributed new reagents; L.S.C. and A.S.L. performed research; S.J.U. contributed analytical tools; M.A.V. performed clinical assessments and design; and R.N.B. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frank J. Ward, Department of Medicine and Therapeutics, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen, AB25 2ZD United Kingdom; e-mail: mmd475@abdn.ac.uk.
References
- 1.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 5.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–6327. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 7.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- 8.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–1254. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 10.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall NA, Vickers MA, Barker RN. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J Immunol. 2003;170:6183–6189. doi: 10.4049/jimmunol.170.12.6183. [DOI] [PubMed] [Google Scholar]
- 12.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 15.Bacchetta R, Passerini L, Gambineri E, et al. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker RN, Hall AM, Standen GR, Jones J, Elson CJ. Identification of T-cell epitopes on the Rhesus polypeptides in autoimmune hemolytic anemia. Blood. 1997;90:2701–2715. [PubMed] [Google Scholar]
- 17.Hall AM, Ward FJ, Vickers MA, Stott LM, Urbaniak SJ, Barker RN. Interleukin-10-mediated regulatory T-cell responses to epitopes on a human red blood cell autoantigen. Blood. 2002;100:4529–4536. doi: 10.1182/blood-2002-05-1383. [DOI] [PubMed] [Google Scholar]
- 18.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 20.Walunas TL, Bluestone JA. CTLA-4 regulates tolerance induction and T cell differentiation in vivo. J Immunol. 1998;160:3855–3860. [PubMed] [Google Scholar]
- 21.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 24.Fowler S, Powrie F. CTLA-4 expression on antigen-specific cells but not IL-10 secretion is required for oral tolerance. Eur J Immunol. 2002;32:2997–3006. doi: 10.1002/1521-4141(2002010)32:10<2997::AID-IMMU2997>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 25.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 26.Arce MA, Thompson ES, Wagner S, Coyne KE, Ferdman BA, Lublin DM. Molecular cloning of RhD cDNA derived from a gene present in RhD-positive, but not RhD-negative individuals. Blood. 1993;82:651–655. [PubMed] [Google Scholar]
- 27.Scott ML, Voak D, Jones JW, et al. A model for RhD: the relationship of 30 serologically defined epitopes to structure. Biotest Bulletin. 1997;5:459–466. [Google Scholar]
- 28.Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol. 2002;32:2237–2245. doi: 10.1002/1521-4141(200208)32:8<2237::AID-IMMU2237>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Lazetic S, Leong SR, Chang JC, Ong R, Dawes G, Punnonen J. Chimeric co-stimulatory molecules that selectively act through CD28 or CTLA-4 on human T cells. J Biol Chem. 2002;277:38660–38668. doi: 10.1074/jbc.M205808200. [DOI] [PubMed] [Google Scholar]
- 30.Singh H, Raghava GP. ProPred: prediction of HLA-DR binding sites. Bioinformatics. 2001;17:1236–1237. doi: 10.1093/bioinformatics/17.12.1236. [DOI] [PubMed] [Google Scholar]
- 31.Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–622. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Akbari O, Freeman GJ, Meyer EH, et al. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 33.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 34.Levine BL, Bernstein WB, Connors M, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 35.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J Exp Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips JM, Parish NM, Drage M, Cooke A. Cutting edge: interactions through the IL-10 receptor regulate autoimmune diabetes. J Immunol. 2001;167:6087–6091. doi: 10.4049/jimmunol.167.11.6087. [DOI] [PubMed] [Google Scholar]