Abstract
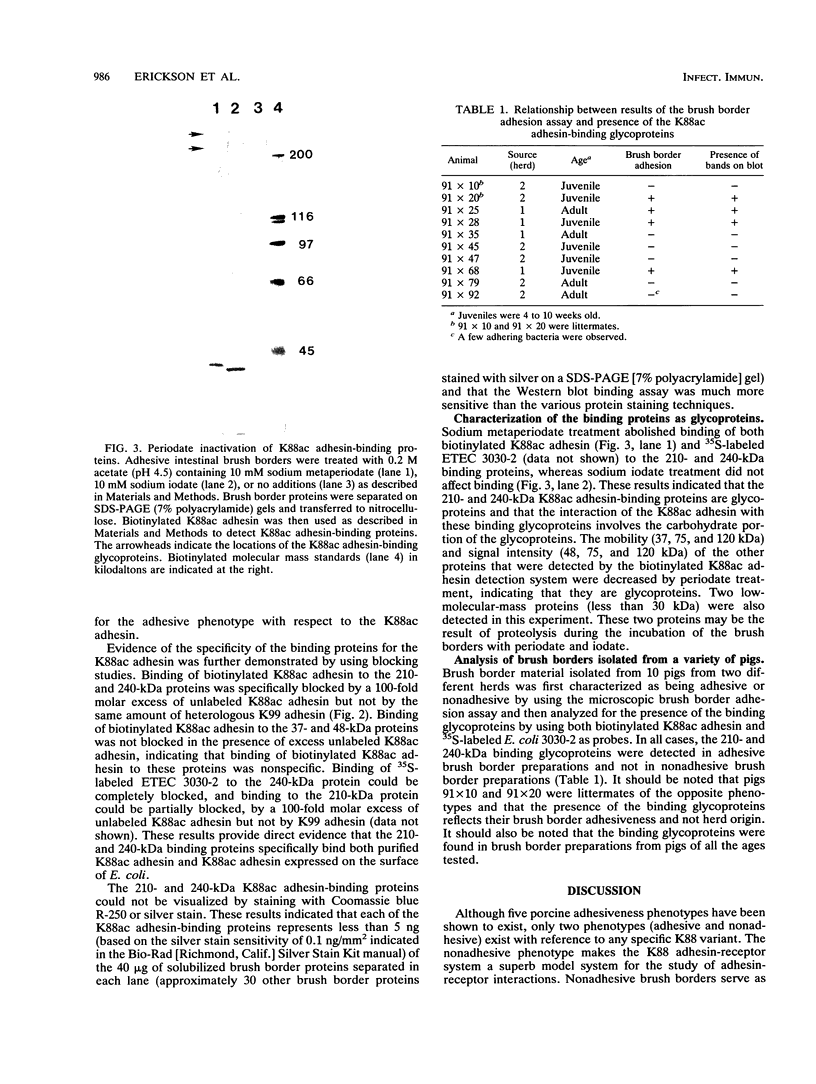
In this study, we identified two brush border glycoproteins (210 and 240 kDa) that bind both K88ac+ Escherichia coli and purified K88ac adhesin. The specificity of these binding glycoproteins for the K88ac adhesin was demonstrated in studies in which the binding of 35S-labeled K88ac+ E. coli and biotinylated K88ac adhesin to these glycoproteins was blocked in the presence of a 100-fold molar excess of unlabeled K88ac adhesin but not in the presence of the K99 adhesin. Pretreatment of adhesive brush borders with sodium metaperiodate destroyed both binding activities, indicating that the interaction between the K88ac adhesin and the binding glycoproteins requires the glycoprotein carbohydrate moiety. It was demonstrated previously that K88ac+ E. coli binds to adhesive brush borders but not to nonadhesive brush borders (R. Sellwood, R. A. Gibbons, G. W. Jones, and J. M. Rutter, J. Med. Microbiol. 8:405-411, 1975). In the present study, brush borders isolated from 10 different pigs were tested first for brush border adhesiveness and then for the presence of the binding glycoproteins. In all cases, the binding glycoproteins were detected only in the adhesive brush border preparations. These two binding glycoproteins may be the receptors used by K88ac+ ETEC to adhere to intestinal brush border cells. Their presence on adhesive brush borders and absence on nonadhesive brush borders may be the basis for resistance and susceptibility of pigs to K88ac+ ETEC infections.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Whitehead J. S., Kim Y. S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980 Sep;29(3):897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlsma I. G., de Nijs A., van der Meer C., Frik J. F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982 Sep;37(3):891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway P. L., Welin A., Cohen P. S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990 Oct;58(10):3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A. Comparison of receptors for 987P pili of enterotoxigenic Escherichia coli in the small intestines of neonatal and older pig. Infect Immun. 1990 Dec;58(12):4030–4035. doi: 10.1128/iai.58.12.4030-4035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Isaacson R. E. Purification and characterization of a receptor for the 987P pilus of Escherichia coli. Infect Immun. 1985 Jan;47(1):98–105. doi: 10.1128/iai.47.1.98-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D. H., Ryan C. J., Fritzemeier J. D. Effect of sodium acetate on expression of K99 pili by Escherichia coli. Infect Immun. 1983 Sep;41(3):1368–1369. doi: 10.1128/iai.41.3.1368-1369.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979 Mar;23(3):700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. A., Roosendaal B., van Breemen J. F., de Graaf F. K. Role of phenylalanine 150 in the receptor-binding domain of the K88 fibrillar subunit. J Bacteriol. 1987 Nov;169(11):4907–4911. doi: 10.1128/jb.169.11.4907-4911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., WITTIG W. K ANTIGENS K88AB(L) AND K88AC(L) IN E. COLI. A NEW O ANTIGEN: 0147 AND A NEW K ANTIGEN: K89(B). Acta Pathol Microbiol Scand. 1964;62:439–447. doi: 10.1111/apm.1964.62.3.439. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Sarmiento J. I., Casey T. A., Moon H. W. Postweaning diarrhea in swine: experimental model of enterotoxigenic Escherichia coli infection. Am J Vet Res. 1988 Jul;49(7):1154–1159. [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980 Oct 1;632(2):326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Wilson I. B. Soluble pig intestinal cell membrane components with affinities for E. coli K88+ antigen. Mol Cell Biochem. 1983;52(2):177–189. doi: 10.1007/BF00224926. [DOI] [PubMed] [Google Scholar]
- Teneberg S., Willemsen P., de Graaf F. K., Karlsson K. A. Receptor-active glycolipids of epithelial cells of the small intestine of young and adult pigs in relation to susceptibility to infection with Escherichia coli K99. FEBS Lett. 1990 Apr 9;263(1):10–14. doi: 10.1016/0014-5793(90)80693-d. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Valpotić I., Runnels P. L., Moon H. W. In vitro adhesion of K88ac+ Escherichia coli to Peyer's patch and peripheral blood lymphocytes, buccal and rectal epithelial cells or intestinal epithelial brush borders of weaned pigs. Vet Microbiol. 1989 Aug;20(4):357–368. doi: 10.1016/0378-1135(89)90060-6. [DOI] [PubMed] [Google Scholar]
- Westerman R. B., Mills K. W., Phillips R. M., Fortner G. W., Greenwood J. M. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 1988 Jan;26(1):149–150. doi: 10.1128/jcm.26.1.149-150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. B., Staley T. E., Bush L. J., Gilliland S. E. Recovery of intestinal membrane binding sites for K88 E. coli from pig mucosal organ cultures. Mol Cell Biochem. 1984 Apr;62(1):57–65. doi: 10.1007/BF00230078. [DOI] [PubMed] [Google Scholar]