Abstract
Purpose:
Constitutive activation of the Wnt signaling pathway is a hallmark of many cancers and has been associated with familial and sporadic desmoid tumors. The aim of the present study is to assess the therapeutic potential of oncolytic adenoviruses selectively replicating in cells in which the Wnt signaling pathway is active on primary cells from desmoid tumors.
Experimental design:
Primary cells extracted from familial (n=3) or sporadic (n=3) desmoid tumors were cultured short term. Cancer cell survival and viral replication were measured in vitro upon infection with two different oncolytic adenoviruses targeting a constitutive activation of the Wnt signaling pathway. Adenoviral infectivity was also assessed.
Results:
Although cells extracted from one sporadic desmoid tumor responded very well to the oncolytic action of the adenoviruses (less than 20% of viable cells upon infection at a multiplicity of infection of 10), cells from two tumor samples were totally resistant to the viral action. Cells from the remaining samples showed intermediate sensitivity to the oncolytic viruses. These effects were correlated to the level of infectivity of the cells. Finally, in responder cells, evidences of viral replication was observed.
Conclusions:
Our experimental data suggest that the response of desmoid tumor cells to oncolytic adenovirus is neither correlated to the type of mutation activating the Wnt signaling pathway nor to the familial or sporadic nature of the tumor. In addition, they highlight the variability of infectivity of individual tumors and predict a great variability in the response to oncolytic adenoviruses.
Keywords: desmoid tumors, gene therapy, oncolytic adenovirus, Wnt signaling, Na/I symporter
INTRODUCTION
Desmoid tumors are rare, benign, sometimes aggressive tumors resulting from unregulated proliferation of fibroblast-like cells (1, 2). Their natural history is one of slow growth with progressive infiltration of surrounding soft tissues leading in some cases to strangulation of nerves and blood vessels. Abdominal desmoid tumors are the most common and can be classified into sporadic and familial forms (2). In the latter case, inherited desmoid tumors are most frequently associated with Familial Adenomatous Polyposis (FAP). At the molecular level, the development of desmoid tumors has been associated with a constitutive activation of the Wnt signaling pathway (3-7). FAP-associated desmoid tumors are usually caused by germline adenomatous polyposis coli (APC) mutations followed by somatic inactivation of the wild-type APC allele (3-5) while sporadic desmoids are usually characterized by oncogenic mutations in the β-catenin gene, both identical molecular alterations to those found in the vast majority of colorectal cancers (3, 4).
The management of patients is difficult and these tumors are prone to local recurrence. Surgery is an obvious option but the development of 10 to 30% of sporadic abdominal wall desmoid tumors and 68 to 86% of FAP associated intra-abdominal tumors has been associated with surgical trauma (8). As a result, there is currently no satisfactory treatment for these tumors, especially in the context of FAP-related desmoid tumors for which they represent a major cause of morbidity and mortality (1, 9). Therefore, there is a need for alternative or complementary therapies that could delay surgery if not eradicate the tumor. In this context, gene therapy through direct injection of gene delivery vectors in the tumor mass has already been suggested through pre-clinical studies involving non-viral and replication-deficient adenoviral vectors (10, 11).
Oncolytic adenoviruses are a class of adenoviruses genetically modified to selectively replicate in cancer cells (12, 13). This selectivity can be achieved through deletions in the viral genome (14-16) or replacement of the viral endogenous promoters by cancer-selective promoters (17-19). The resulting viruses have the unique property of tumor-dependent self-amplification. In addition, initial clinical trials have demonstrated that these agents are very safe and well tolerated (20). Recently, adenoviruses with Tcf binding sites in the early promoters were generated and these oncolytic adenoviruses showed up to 100 000-fold selectivity for cells with activated Wnt signaling (17, 21). As a constitutive activation of the Wnt signaling pathway is a common molecular defect in sporadic or familial desmoid tumors, the aim of the present study is to assess the efficacy of Wnt-selective oncolytic adenoviruses in these cells. In addition and as molecular imaging of gene transfer using the Na/I symporter (NIS) as a reporter gene could provide crucial information on the extent of gene transfer in vivo through PET imaging (18, 22, 23), we have generated a Wnt-selective oncolytic adenoviruses encoding hNIS. The efficacy of these viruses was assessed in vitro on primary tumor cells extracted from familial (n=3) or sporadic (n=3) desmoid tumors.
MATERIALS AND METHODS
Establishment of primary cultures of desmoid cells, cell lines
Desmoid tumors samples were obtained following surgical resection. The protocol was used in conjunction with ethical approval from the Harrow or Leuven Research Ethics Committee. The tumors were macerated in small pieces with a scalpel and digested overnight at 37°C in RPMI containing 20% FBS FBS (AutogenBioclear, Ltd, Calne, UK), 100 units/mL penicillin, 100 μg/mL streptomycin, 0.2 μg/mL Butyl-p-hydroxybenzoate supplemented with 1 mg/mL of Collagenase D (Roche Diagnostic GmbH, Lewes, UK). The digest was then filtered through a 100μm nylon cell strainer and centrifuged twice at 1000rpm. Cell pellets were washed twice with PBS, resuspended in RPMI 20%FBS containing antibiotics (see above), and seeded. Primary cultures were maintained in a 5% CO2 atmosphere at 37°C. Erythrocytes and cell debris were washed away with PBS 24 hours later. Germline and somatic mutations were identified: Desmoid 1: β-catenin substitution TTT for TCT resulting is a Ser to Phe change at codon 45; B-Cat substitution TTT for TCT resulting is a Ser to Phe change at codon 45. Desmoid 3: Mutations in APC or β-catenin not detected; Desmoid 4: APC: exon 15 c.3927_3931delAAAGA (p.Glu1309AspfsX4); Desmoid 5: APC: exon 13 c.1495C>T (p.Arg499X); Desmoid 5.2: germline exon 6 c.646 C > T (p.R216X), somatic exon 15H: c.4385delA.
SKBR3, MCF7, HPAF, SUIT-2 and DLD-1 cells were cultured as previously described (11, 24, 25).
Generation and production of a Wnt-selective adenovirus encoding NIS
The plasmid vpKH1 encodes the genome of a Wnt-specific adenovirus in which TCF-4 binding sites are present in E1A, E1B and E4 of the viral genome (21). Insertion of the NIS cDNA in the gp19k of this adenovirus was performed as previously described (18), using homologous recombination in yeast (26). Virus were produced and titrated as previously described (27).
Adenovirus nomenclature
AdWt : Wild-type adenovirus type 5.
Ad-LacZ : Replication-deficient adenovirus type 5 encoding the β-galactosidase gene.
Ad-hNIS : Replication-deficient adenovirus type 5 encoding the NIS coding sequence (23).
AdKH1: Adenovirus selectively replicating in cells in which the Wnt signalling pathway is constitutively activated (21).
AdIP2: Adenovirus selectively replicating in cells in which the Wnt signalling pathway is constitutively activated and encoding NIS.
Infections with adenoviruses
Cells were seeded at a cell density of 104 cells/cm2. The following day they were infected with adenoviruses at different multiplicity of infection (MOI) in serum-free medium for 30 to 45 minutes. Culture medium was then added to reach a serum concentration up to 20%.
Biochemical assays
Cell survival assays, measurement of GFP-positive cells and assessment of in vitro viral replication and E1A expression were performed as previously described (28), (29), (19) and (18), respectively.
Coxsackievirus and Adenovirus Receptor (CAR) staining
Cells for staining were collected by trypsinisation, and washed. The pellet was re-suspended and incubated with the anti-CAR primary monoclonal mouse antibody (30) 1:500 in FACS buffer containing 5% normal goat serum (Dako Cytonation, Denmark) for 90min on ice. Cells were then washed three times and incubated for 30min at room temperature in the dark with secondary antibody goat anti-mouse FITC 1:30 in FACS buffer. The cells were washed three times and the final pellet was re-suspended in 400μl FACS buffer. Measurement of the number of CAR-positive cells was performed by FACS on at least 5 105 cells.
RESULTS
Effect of Wnt-selective oncolytic adenoviruses on desmoid tumor cells
To determine whether the insertion of the NIS cDNA affects the selectivity of the Wnt-selective virus, DLD-1 colon cancer cells carrying an APC mutation that results in constitutively high β-catenin signalling (31) and cell lines in which the Wnt signalling pathway is not activated in normal culture conditions (MCF7, SKBR3) were infected with AdIP2 and a wild-type adenovirus type 5 as positive control. Table 1 demonstrates that the EC50 of AdIP2 and AdWt are in the same range in DLD-1 target cells, while AdWt is more than two log potent than AdIP2 on non-target MCF7 or SKBR3 cells. These results demonstrate that AdIP2 can selectively kill cells in which the Wnt signalling pathway is constitutively activated.
Table 1.
| MCF7 | SKBR3 | DLD-1 | |
|---|---|---|---|
| AdWt | 8 | 0.63 | 1.33 |
| AdIP2 | >100 | >100 | 4.77 |
DLD-1 colon cancer cells carrying an APC mutation that results in constitutively high β-catenin signalling and cell lines in which the Wnt signalling pathway is not activated in normal culture conditions (MCF7, SKBR3) were infected with AdIP2 and a wild-type adenovirus type 5 as positive control at different multiplicity of infection. Six days later, the number of viable cells was determined using a MTT assay. Dose–response curves were generated and concentrations killing 50% of cells (EC50-values) were determined using untreated cells as reference and are presented in pfu.
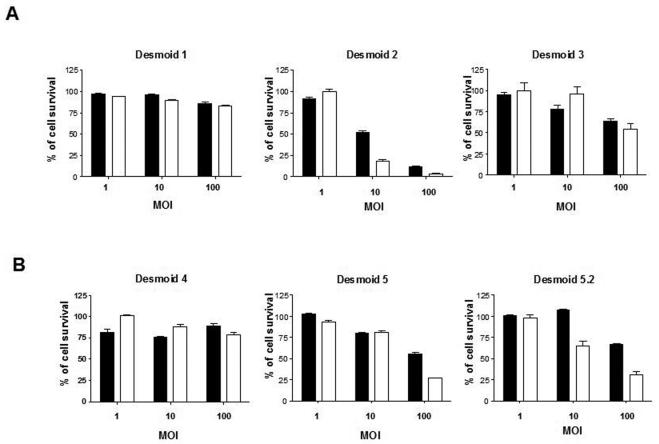
To determine the effect of adenoviral infection on primary desmoid tumor cells, cells from sporadic (fig 1A) or FAP-related (fig 1B) desmoid tumors were infected at different multiplicity of infection (MOI) with AdKH1 or AdIP2. Different levels of sensitivity to oncolytic adenoviral treatment were observed with the following range of sensitivity: desmoid 2 (sporadic) > desmoid 5 (familial) ; desmoid 5.2 (familial) > desmoid 3 (sporadic) > desmoid 1 (sporadic); desmoid 4 (familial). Statistical analysis showed a highly significant difference (P<0.0001) between uninfected cells and cell infected with AdKH1 and AdIP2 at MOI 100 for desmoids 2, 3, 5 and 5.2. These results demonstrated that the sensitivity to oncolytic adenoviral treatment is not related to the type of mutation in the Wnt signaling pathway or the sporadic or familial nature of desmoid tumors.
Figure 1.
Effect of Wnt-selective oncolytic adenoviruses on desmoid tumor cell survival. Primary cells from (A) sporadic or (B) familial desmoid tumors were infected at different multiplicities of infection (MOI) with AdKH1 (black bars) or AdIP2 (white bars). Six days later, the number of surviving cells was assessed using an MTT assay. 100% correspond to the number of cells in control, non-infected cells. Two-way ANOVA statistical analysis was performed. Results are mean +/− SEM of 6 experimental points. The data presented are representative of 3 independent experiments.
Correlation between infectivity and sensitivity to adenoviral vectors
The infectivity of primary cells extracted from the different desmoid tumors was determined by infection with a replication-deficient adenovirus (MOI 10) encoding the green fluorescent protein (GFP) and measurement of GFP-positive cells by FACS. Fig 2 shows that the range of infectivity is similar to that obtained for the sensitivity to adenoviral infection (fig 1). The coxsackievirus and adenovirus receptor (CAR) has been shown to act as a primary receptor to adenovirus and to play a critical role in viral entry in the cell (32). Surprisingly, CAR staining was negative in all desmoid cell samples (not shown). This is in sharp contrast to highly positive (>95%) A549 cells used as positive control (not shown) and known to express high levels of CAR (33). In an attempt to further demonstrate infection in the absence of CAR expression, E1A expression was monitored upon AdIP2 infection of Desmoid 3. Quantitative RT-PCR revealed that E1A expression was weak and hardly detectable twenty-four hours after infection but increased very significantly four days later (Supplementary data), demonstrating that incubation of Desmoid 3 with AdIP2 lead to E1A expression resulting from cell infection, independently of the presence or CAR. Altogether, these results suggest that sensitivity to adenoviral agents and infectivity are tightly correlated and that a CAR-independent mechanism is involved in adenoviral infection of primary cells from desmoid tumors 2, 3, 5 and 5.2.
Figure 2.
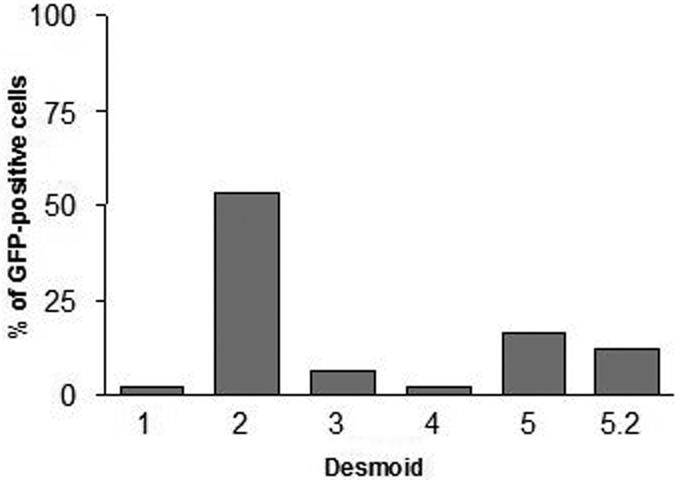
Infectivity of primary desmoid tumor cells. Adherent cells were infected with a replication-deficient recombinant adenovirus encoding GFP. Forty-eight hours later, the cells were trypsinized and the number of GFP-positive cells was determined by FACS. The data presented corresponds to an acquisition of at least 50 000 events and are representative of three independent experiments. Data were analyzed using the χ2 test. In all conditions, comparisons of the uninfected versus the infected cells showed P values <0.0001.
Replication of adenovectors in primary desmoid tumor cells sensitive to the viruses
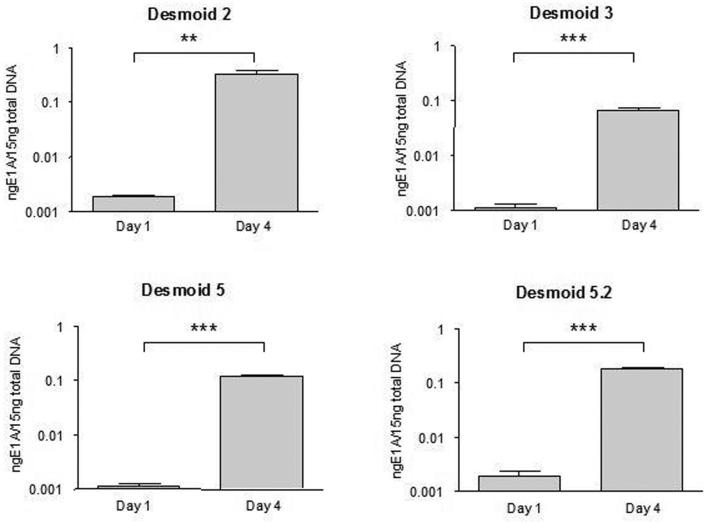
To determine whether the reduced cell viability observed upon adenoviral infection of cells from desmoid 2, 3, 5 and 5.2 was associated with viral replication, DNA from cells infected with AdIP2 was collected one day or four days after infection. This DNA was subjected to quantitative PCR to titrate the amount of adenoviral DNA per culture well. Fig 3 shows that in all cases, viral amplification of the adenoviral genome was observed between day 1 and 4, suggesting replication of the oncolytic virus in these cells.
Figure 3.
Evidence of adenoviral replication in primary desmoid tumor cells infected with AdIP2. Cells were infected at low MOI (0.01) and total DNA was extracted on day one and four after infection. Adenoviral DNA was titrated by quantitative PCR. Statistical analysis was performed using a two-tailed student t test. P values are 0.0052 and 0.0008 for desmoids 2 and 3 respectively and < 0.0001 for desmoids 5 and 5.2. The data presented are the mean +/− SEM of triplicate and are representative of three experiments.
Additional toxic effect of the NIS transgene
In addition, at MOI 100, AdIP2, the NIS-positive virus is statistically more efficient (P<0.0001 for the three tumors) than AdKH1 (NIS-negative) in cells from desmoids 2, 5 and 5.2 (fig 1). To determine whether the presence of NIS in the viral genome and its expression is toxic for desmoid cells, independently from the oncolytic action of the virus, primary cells from the desmoid tumors 2, 5 and 5.2 were infected at a MOI of 100 with a replication-deficient recombinant adenovirus encoding either the β-galactosidase gene (Ad-LacZ) or NIS (Ad-hNIS) under the control of the immediate-early CMV promoter. Fig 4 demonstrate that the toxicity of Ad-hNIS is not observed with Ad-LacZ, suggesting a toxicity of the NIS gene product in desmoid tumor cells. By contrast, this hNIS cytotoxicity is not observed with established cell lines such as HPAF or SUIT-2 cells (Figure 4).
Figure 4.
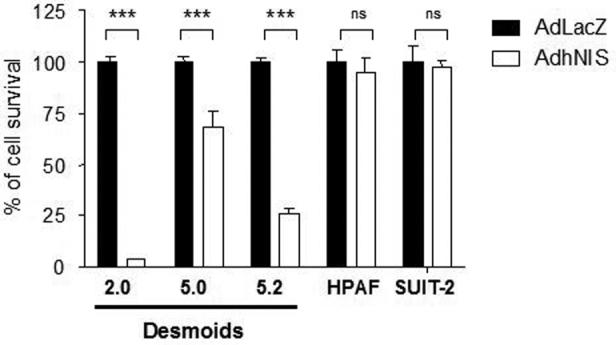
Effect of replication incompetent adenoviruses encoding LacZ or NIS on desmoid tumor cell survival. Primary cells from desmoid tumors 2, 5 and 5.2 and cell lines HPAF and SUIt-2 were infected at MOI of 100 with a replication incompetent adenoviruses encoding LacZ (black bars) or NIS (white bars). Six days later, the number of surviving cells was assessed using an MTT assay. 100% correspond to the number of cells in control, non-infected cells. Statistical analysis was performed using a two-tailed student t test. P values are < 0.0001 in all the conditions compared involving the desmoid tumors and non-significant (ns, P > 0.05) with the cell lines HPAF and SUIT-2. The data presented are the mean +/− SEM of four experimental points and are representative of three independent experiments.
DISCUSSION
The Wnt signaling pathway is considered as the key molecular defect in sporadic or familial desmoid tumors (3-5) and therapeutic strategies targeting this pathway are expected to provide therapeutic benefits with minimal side-effects in patients affected with this pathology. In this context, an oncolytic adenovirus, replicating selectively in cells in which the Wnt signaling pathway is constitutively activated is particularly relevant.
Replication-selective oncolytic adenoviruses represent a novel class of therapeutic agents. Pre-clinical and clinical studies have demonstrated the safety and feasibility of the approach and their suitability for association with more classical cancer treatments such as chemotherapy has been demonstrated (34-36). However, a clear limitation of adenoviral vectors is that only a minimal proportion of the injected dose reaches the tumors after systemic administration. As a result, intratumoral injections are advised. Considering the lack of metastasis and the size of the tumors observed in patients with desmoids (1), local injection of oncolytic adenovirus represents a practical approach that could even be implemented in conjunction with chemotherapy. Therefore, desmoid tumors are a relevant indication for adenoviral vectors.
Our data clearly demonstrate that primary cells from some desmoid tumors allow replication and are sensitive to the action of oncolytic adenoviruses. However, in two cases, the cells were completely refractory and the key difference between responder- and non-responders-cells is the infectivity of the cells. Low or no CAR expression has already been reported in desmoids (37) and we confirm that CAR was absent from all the cellular samples tested, whether infectable with adenovirus or not. Taken together, these data suggest an alternative, CAR-independent mechanism of infection. Such mechanisms have been suggested (38) and may involve heparan sulfate proteoglycans (39) or coagulation factors (40, 41). Further studies will be required to address the mechanism of infection in these cells.
One of the particularity of desmoid tumors is their abundance in extracellular matrix that could prevent the diffusion of the virus in the tumour mass. In this context, pre-treatment or co-administration of the oncolytic virus with enzymes capable of dissociating this matrix, could enhance vector diffusion. A proof of principle has already been demonstrated using hyaluronidase that was shown to improve adeno-associated virus-mediated gene transfer in the muscle (42) and this type of approach could be applied to the context of adenovirus gene therapy and desmoid tumors.
The fate of gene therapy vectors encoding NIS can be visualised after administration into a subject using positron emission tomography (PET) (22, 23) or single-photon emission computed tomography (SPECT) (18). Both imaging methodologies are potentially applicable to humans and can provide unique information on the anatomical distribution of gene transfer. In addition, NIS can also be used as a therapeutic transgene through the administration of 131I and the accumulation of radioactivity in tumor deposits transduced by the gene therapy vector (43, 44). In this report, we demonstrate for the first time a direct effect of NIS expression on the viability cells. This effect is unique to primary desmoid cells as many different types of tumor cell lines can be transduced with the NIS gene without apparent toxicity and therapeutic doses of 131I (45-47) or high doses of non radioactive iodide (48) are necessary to trigger cell death.
This report demonstrates that gene therapy for desmoid tumor is feasible and the efficacy of the treatment is highly dependent on the infectivity of the cells. In the clinical setting, direct injection of the oncolytic adenovirus into desmoids under radiological guidance could be envisaged. This gene therapy approach would probably aim at debulking the tumor before attempting surgical resection and in the case of a tumor susceptible to adenoviral infection, even a relatively modest effect could have an important clinical implication by making a previously unoperable intra-abdominal tumor amenable to surgery. Imaging of NIS gene transfer could also be performed to provide additional information on the extent of gene transfer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr Jhiang for providing the human NIS cDNA. This work is supported by grants from Cancer Research UK, the Medical Research Council, INSERM, la Ligue Nationale Contre le Cancer and by grant 0607 - 3D1615 - 66 / AO INSERM from the French National Cancer Institute (INCa). ST is a Senior Clinical Investigator of the Fund for Scientific Research-Flanders(Belgium) (Fonds Wetenschappelijk Onderzoek-Vlaanderen). This project was funded by the Belgian Federation against Cancer, and the Fund for Scientific Research Flanders (FWO). The publication of this article was possible thanks to financial support brought by Association Française pour l'Etude du Foie and BMS laboratories.
Footnotes
Statement of clinical relevance
This study investigates the potential of replication-selective oncolytic adenoviruses in the treatment of desmoid tumors. The results demonstrate that infectivity of desmoid tumor cells is variable and is the key factor in determining whether oncolytic adenoviruses are likely to be efficient against this type of tumor. Therefore, this factor should be assessed before embarking into a clinical trial.
REFERENCES
- 1.Sturt NJ, Clark SK. Current ideas in desmoid tumours. Fam Cancer. 2006;5:275–85. doi: 10.1007/s10689-005-5675-1. [DOI] [PubMed] [Google Scholar]
- 2.Sakorafas GH, Nissotakis C, Peros G. Abdominal desmoid tumors. Surg Oncol. 2007;16:131–42. doi: 10.1016/j.suronc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Tejpar S, Michils G, Denys H, et al. Analysis of Wnt/Beta catenin signalling in desmoid tumors. Acta Gastroenterol Belg. 2005;68:5–9. [PubMed] [Google Scholar]
- 4.Signoroni S, Frattini M, Negri T, et al. Cyclooxygenase-2 and platelet-derived growth factor receptors as potential targets in treating aggressive fibromatosis. Clin Cancer Res. 2007;13:5034–40. doi: 10.1158/1078-0432.CCR-07-0336. [DOI] [PubMed] [Google Scholar]
- 5.Sturt NJ, Gallagher MC, Bassett P, et al. Evidence for genetic predisposition to desmoid tumours in familial adenomatous polyposis independent of the germline APC mutation. Gut. 2004;53:1832–6. doi: 10.1136/gut.2004.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wunder JS, Nielsen TO, Maki RG, O'Sullivan B, Alman BA. Opportunities for improving the therapeutic ratio for patients with sarcoma. Lancet Oncol. 2007;8:513–24. doi: 10.1016/S1470-2045(07)70169-9. [DOI] [PubMed] [Google Scholar]
- 7.Kotiligam D, Lazar AJ, Pollock RE, Lev D. Desmoid tumor: a disease opportune for molecular insights. Histol Histopathol. 2008;23:117–26. doi: 10.14670/HH-23.117. [DOI] [PubMed] [Google Scholar]
- 8.Tolan S, Shanks JH, Loh MY, Taylor B, Wylie JP. Fibromatosis: benign by name but not necessarily by nature. Clin Oncol (R Coll Radiol) 2007;19:319–26. doi: 10.1016/j.clon.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Latchford AR, Sturt NJ, Neale K, Rogers PA, Phillips RK. A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br J Surg. 2006;93:1258–64. doi: 10.1002/bjs.5425. [DOI] [PubMed] [Google Scholar]
- 10.Bright-Thomas RM, Agrawal A, Hargest R. Preclinical studies of gene transfer for the treatment of desmoid disease in familial adenomatous polyposis. Br J Surg. 2002;89:1563–9. doi: 10.1046/j.1365-2168.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- 11.Martinico SC, Jezzard S, Sturt NJ, et al. Assessment of endostatin gene therapy for familial adenomatous polyposis-related desmoid tumors. Cancer Res. 2006;66:8233–40. doi: 10.1158/0008-5472.CAN-06-1209. [DOI] [PubMed] [Google Scholar]
- 12.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 13.Alemany R. Cancer selective adenoviruses. Mol Aspects Med. 2007;28:42–58. doi: 10.1016/j.mam.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Manzano C, Alonso MM, Yung WK, et al. Delta-24 increases the expression and activity of topoisomerase I and enhances the antiglioma effect of irinotecan. Clin Cancer Res. 2006;12:556–62. doi: 10.1158/1078-0432.CCR-05-1892. [DOI] [PubMed] [Google Scholar]
- 16.Cascallo M, Gros A, Bayo N, Serrano T, Capella G, Alemany R. Deletion of VAI and VAII RNA genes in the design of oncolytic adenoviruses. Hum Gene Ther. 2006;17:929–40. doi: 10.1089/hum.2006.17.929. [DOI] [PubMed] [Google Scholar]
- 17.Fuerer C, Iggo R. Adenoviruses with Tcf binding sites in multiple early promoters show enhanced selectivity for tumour cells with constitutive activation of the wnt signalling pathway. Gene Ther. 2002;9:270–81. doi: 10.1038/sj.gt.3301651. [DOI] [PubMed] [Google Scholar]
- 18.Merron A, Peerlinck I, Martin-Duque P, et al. SPECT/CT imaging of oncolytic adenovirus propagation in tumours in vivo using the Na/I symporter as a reporter gene. Gene Ther. 2007;14:1731–8. doi: 10.1038/sj.gt.3303043. [DOI] [PubMed] [Google Scholar]
- 19.Bilsland AE, Merron A, Vassaux G, Keith WN. Modulation of telomerase promoter tumor selectivity in the context of oncolytic adenoviruses. Cancer Res. 2007;67:1299–1307. doi: 10.1158/0008-5472.CAN-06-3000. [DOI] [PubMed] [Google Scholar]
- 20.Hermiston T. A demand for next-generation oncolytic adenoviruses. Curr Opin Mol Ther. 2006;8:322–30. [PubMed] [Google Scholar]
- 21.Homicsko K, Lukashev A, Iggo RD. RAD001 (everolimus) improves the efficacy of replicating adenoviruses that target colon cancer. Cancer Res. 2005;65:6882–90. doi: 10.1158/0008-5472.CAN-05-0309. [DOI] [PubMed] [Google Scholar]
- 22.Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Keith WN, Vassaux G. Noninvasive imaging of the transcriptional activities of human telomerase promoter fragments in mice. Cancer Res. 2004;64:4906–11. doi: 10.1158/0008-5472.CAN-04-0426. [DOI] [PubMed] [Google Scholar]
- 23.Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Reader AJ, Vassaux G. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther. 2004;9:436–42. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Vassaux G, Hurst HC, Lemoine NR. Insulation of a conditionally expressed transgene in an adenoviral vector. Gene Ther. 1999;6:1192–97. doi: 10.1038/sj.gt.3300910. [DOI] [PubMed] [Google Scholar]
- 25.Groot-Wassink T, Aboagye EO, Glaser M, Lemoine NR, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13:1723–35. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- 26.Gagnebin J, Brunori M, Otter M, Juillerat-Jeanneret L, Monnier P, Iggo R. A photosensitising adenovirus for photodynamic therapy. Gene Ther. 1999;6:1742–50. doi: 10.1038/sj.gt.3300992. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Duque P, Jezzard S, Kaftansis L, Vassaux G. Direct comparison of the insulating properties of two genetic elements in an adenoviral vector containing two different expression cassettes. Hum Gene Ther. 2004;15:995–1002. doi: 10.1089/hum.2004.15.995. [DOI] [PubMed] [Google Scholar]
- 28.Critchley RJ, Jezzard S, Radford KJ, et al. Potential therapeutic applications of recombinant, invasive E. coli. Gene Ther. 2004;11:1224–33. doi: 10.1038/sj.gt.3302281. [DOI] [PubMed] [Google Scholar]
- 29.Critchley-Thorne RJ, Stagg AJ, Vassaux G. Recombinant Escherichia coli expressing invasin targets the Peyer's patches: the basis for a bacterial formulation for oral vaccination. Mol Ther. 2006;14:183–91. doi: 10.1016/j.ymthe.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Thorne S, Hannock J, et al. A novel assay to assess primary human cancer infectibility by replication-selective oncolytic adenoviruses. Clin Cancer Res. 2005;11:351–60. [PubMed] [Google Scholar]
- 31.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 32.Bergelson JM. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol. 1999;57:975–9. doi: 10.1016/s0006-2952(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 33.Vincent T, Pettersson RF, Crystal RG, Leopold PL. Cytokine-mediated down-regulation of coxsackievirus-adenovirus receptor in endothelial cells. J Virol. 2004;78:8047–58. doi: 10.1128/JVI.78.15.8047-8058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 35.Alonso MM, Gomez-Manzano C, Jiang H, et al. Combination of the oncolytic adenovirus ICOVIR-5 with chemotherapy provides enhanced anti-glioma effect in vivo. Cancer Gene Ther. 2007;14:756–61. doi: 10.1038/sj.cgt.7701067. [DOI] [PubMed] [Google Scholar]
- 36.Cheong SC, Wang Y, Meng JH, et al. E1A-expressing adenoviral E3B mutants act synergistically with chemotherapeutics in immunocompetent tumor models. Cancer Gene Ther. 2008;15:40–50. doi: 10.1038/sj.cgt.7701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu W, Ogose A, Kawashima H, et al. High-level expression of the coxsackievirus and adenovirus receptor messenger RNA in osteosarcoma, Ewing's sarcoma, and benign neurogenic tumors among musculoskeletal tumors. Clin Cancer Res. 2004;10:3831–8. doi: 10.1158/1078-0432.CCR-03-0345. [DOI] [PubMed] [Google Scholar]
- 38.Nicklin SA, Wu E, Nemerow GR, Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005;12:384–93. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, Cabrini G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol. 2001;75:8772–80. doi: 10.1128/JVI.75.18.8772-8780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waddington SN, Parker AL, Havenga M, et al. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J Virol. 81:9568–71. doi: 10.1128/JVI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Favre D, Cherel Y, Provost N, et al. Hyaluronidase enhances recombinant adeno-associated virus (rAAV)-mediated gene transfer in the rat skeletal muscle. Gene Ther. 2000;7:1417–20. doi: 10.1038/sj.gt.3301256. [DOI] [PubMed] [Google Scholar]
- 43.Boelaert K, Franklyn JA. Sodium iodide symporter: a novel strategy to target breast, prostate, and other cancers? Lancet. 2003;361:796–7. doi: 10.1016/s0140-6736(03)12720-1. [DOI] [PubMed] [Google Scholar]
- 44.Groot-Wassink T, Barthel H, Lemoine NR, Vassaux G. Sodium iodide symporter: a new strategy to target cancer? Lancet. 2003;361:1905–6. doi: 10.1016/S0140-6736(03)13510-6. [DOI] [PubMed] [Google Scholar]
- 45.Boland A, Ricard M, Opolon P, et al. Adenovirus-mediated transfer of the thyroid sodium/iodide symporter gene into tumors for a targeted radiotherapy. Cancer Res. 2000;60:3484–92. [PubMed] [Google Scholar]
- 46.Cho JY, Shen DH, Yang W, et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002;9:1139–45. doi: 10.1038/sj.gt.3301787. [DOI] [PubMed] [Google Scholar]
- 47.Spitzweg C, Baker CH, Bergert ER, O'Connor MK, Morris JC. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum Gene Ther. 2007;18:916–24. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Sharma S, Zhu LX, et al. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res. 2003;63:5065–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.