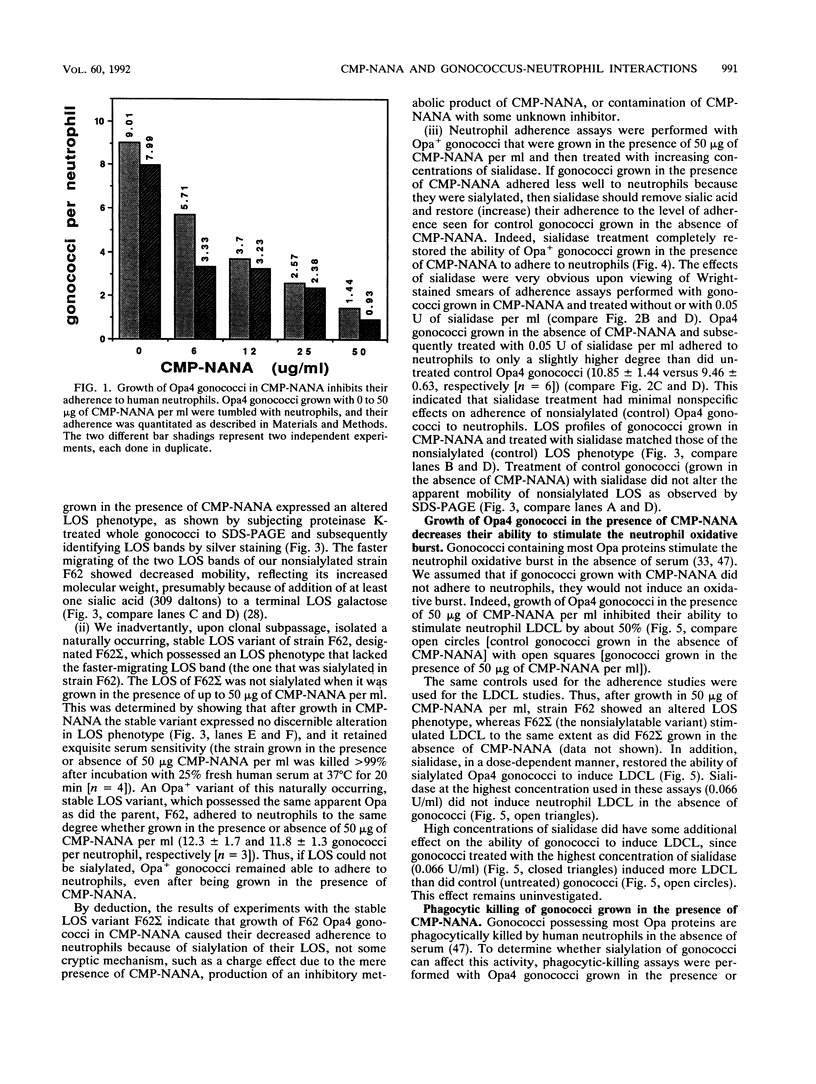
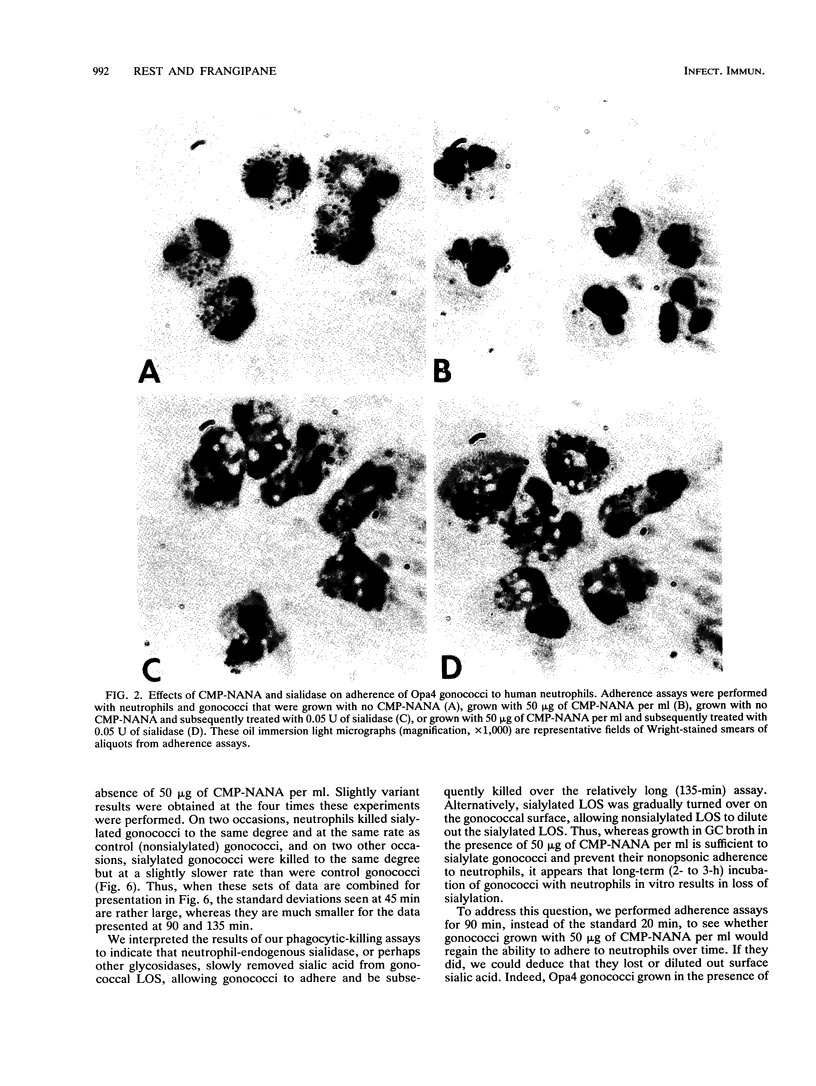
Abstract
Gonococci possessing certain opacity-associated (Opa) outer membrane proteins adhere to and are phagocytosed by human neutrophils in the absence of serum. Recently, it has been shown that serum-sensitive strains of Neisseria gonorrhoeae possessing the appropriate lipooligosaccharide phenotype become serum resistant when grown in the presence of CMP-N-acetylneuraminic acid (CMP-NANA) because of sialylation of their lipooligosaccharide. We investigated whether such sialylation affects nonopsonic (antibody- and complement-independent) interactions of gonococci with human neutrophils in vitro. We grew Opa+ gonococci in the presence of up to 50 micrograms of CMP-NANA per ml, incubated them with neutrophils in vitro, and measured their abilities to adhere to neutrophils, stimulate neutrophil luminol-dependent chemiluminescence (LDCL), and be phagocytically killed by neutrophils. Growth in CMP-NANA dramatically inhibited (in a dose-dependent manner) the ability of Opa+ gonococci to adhere to neutrophils and stimulate neutrophil LDCL. Growth of Opa+ gonococci in 50 micrograms of CMP-NANA per ml appeared to delay, but did not inhibit, their killing by neutrophils. Sialidase treatment of sialylated Opa+ gonococci, i.e., gonococci grown with CMP-NANA, totally restored their abilities to adhere to neutrophils and stimulate neutrophil LDCL. Opa- gonococci grown in the presence of 50 micrograms of CMP-NANA per ml and opsonized with fresh human serum bound to neutrophils only about 30% less efficiently than did Opa- gonococci grown without CMP-NANA and opsonized. The results of our studies show that sialylated Opa+ gonococci have dramatically reduced nonopsonic interactions with neutrophils. Some gonococcal strains may resist killing by human neutrophils in vivo by such a mechanism.
Full text
PDF

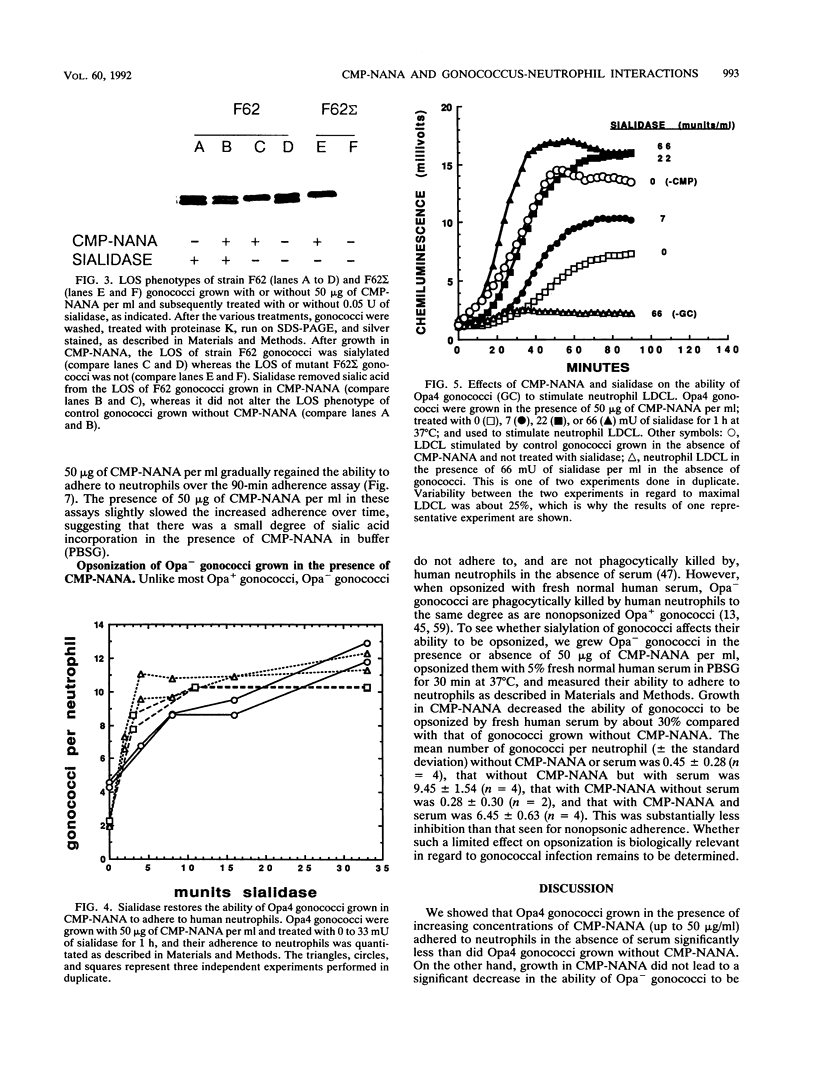
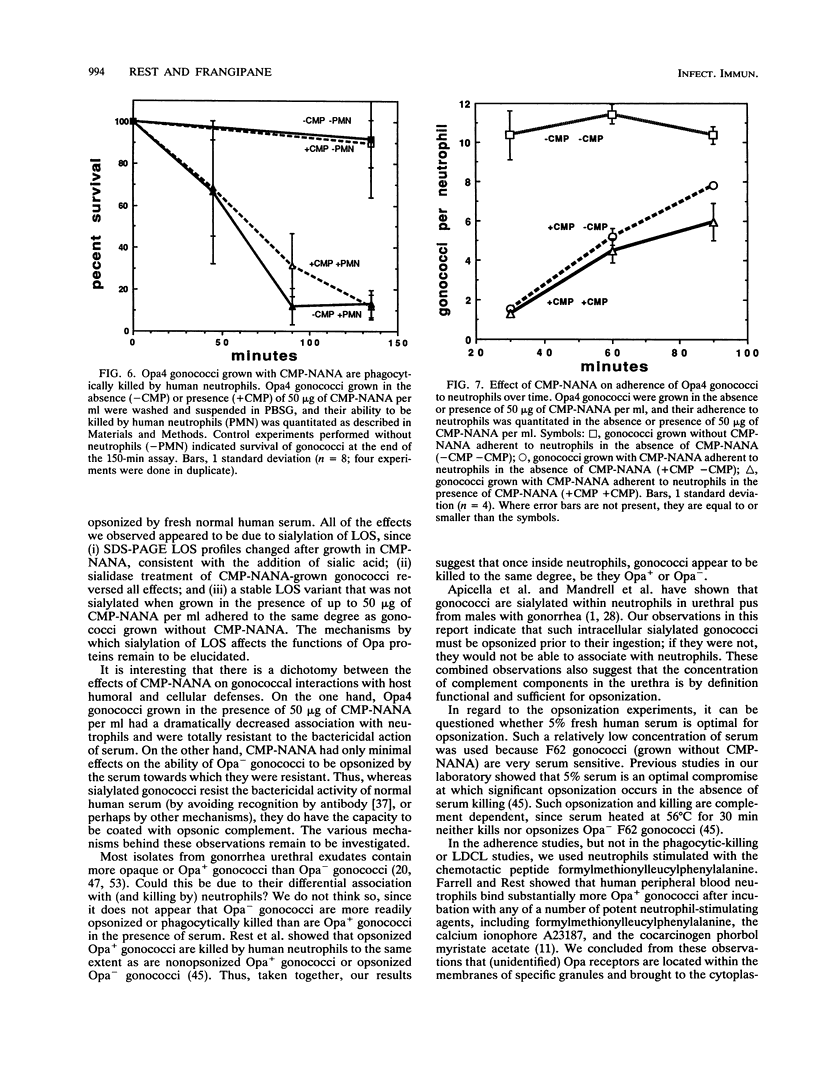



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Mandrell R. E., Shero M., Wilson M. E., Griffiss J. M., Brooks G. F., Lammel C., Breen J. F., Rice P. A. Modification by sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J Infect Dis. 1990 Aug;162(2):506–512. doi: 10.1093/infdis/162.2.506. [DOI] [PubMed] [Google Scholar]
- Barritt D. S., Schwalbe R. S., Klapper D. G., Cannon J. G. Antigenic and structural differences among six proteins II expressed by a single strain of Neisseria gonorrhoeae. Infect Immun. 1987 Sep;55(9):2026–2031. doi: 10.1128/iai.55.9.2026-2031.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W. J., Schwalbe R. S., Nachamkin I., Cannon J. G. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984 Aug;45(2):453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Casey S. G., Veale D. R., Smith H. Demonstration of intracellular growth of gonococci in human phagocytes using spectinomycin to kill extracellular organisms. J Gen Microbiol. 1979 Aug;113(2):395–398. doi: 10.1099/00221287-113-2-395. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Connell T. D., Shaffer D., Cannon J. G. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990 Mar;4(3):439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Elkins C., Rest R. F. Monoclonal antibodies to outer membrane protein PII block interactions of Neisseria gonorrhoeae with human neutrophils. Infect Immun. 1990 Apr;58(4):1078–1084. doi: 10.1128/iai.58.4.1078-1084.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell C. F., Rest R. F. Up-regulation of human neutrophil receptors for Neisseria gonorrhoeae expressing PII outer membrane proteins. Infect Immun. 1990 Sep;58(9):2777–2784. doi: 10.1128/iai.58.9.2777-2784.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs C., Haas R., Meyer T. F. Structural and functional modulation of gonococcal surface proteins. Microb Pathog. 1988 Jun;4(6):393–399. doi: 10.1016/0882-4010(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Heckels J. E., Blackett B., Everson J. S., Ward M. E. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976 Oct;96(2):359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J Bacteriol. 1981 Feb;145(2):736–742. doi: 10.1128/jb.145.2.736-742.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978 Jan;19(1):332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G., James J. F., Swanson J. Studies on gonococcus infection. XI. Comparison of in vivo and vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis. 1978 Jan;137(1):38–43. doi: 10.1093/infdis/137.1.38. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., James L. T., Watt P. J. Variations in surface protein composition associated with virulence properties in opacity types of Neisseria gonorrhoeae. J Gen Microbiol. 1979 Oct;114(2):305–312. doi: 10.1099/00221287-114-2-305. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Campagnari A. A., Bittner W. E., Apicella M. A. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990 Jan 24;126(1):109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. M., Patel P. V., Parsons N. J., Smith H. Induction in gonococci of phenotypic resistance to killing by human serum by human genital secretions. Br J Vener Dis. 1982 Dec;58(6):363–365. doi: 10.1136/sti.58.6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. M., Patel P. V., Parsons N. J., Smith H. Induction of phenotypically determined resistance of Neisseria gonorrhoeae to human serum by factors in human serum. J Gen Microbiol. 1981 Nov;127(1):213–217. doi: 10.1099/00221287-127-1-213. [DOI] [PubMed] [Google Scholar]
- Martin P. M., Patel P. V., Parsons N. J., Smith H. Induction of phenotypically determined resistance of neisseria gonorrhoeae to human serum by sera from patients with gonorrhoea. Br J Vener Dis. 1982 Oct;58(5):302–304. doi: 10.1136/sti.58.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W. Rates in vitro changes of gonococcal colony opacity phenotypes. Infect Immun. 1982 Aug;37(2):481–485. doi: 10.1128/iai.37.2.481-485.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naids F. L., Rest R. F. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect Immun. 1991 Dec;59(12):4383–4390. doi: 10.1128/iai.59.12.4383-4390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. A., Cole J. A., Patel P. V., Parsons N. J., Fox J. E., Smith H. Cytidine 5'-monophospho-N-acetylneuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988 Dec;134(12):3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Electron microscope studies of gonococci in the urethral secretions of patients with gonorrhoea. Br J Vener Dis. 1971 Dec;47(6):419–439. doi: 10.1136/sti.47.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons N. J., Andrade J. R., Patel P. V., Cole J. A., Smith H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989 Jul;7(1):63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Cole J. A., Smith H. Resistance to human serum of gonococci in urethral exudates is reduced by neuraminidase. Proc Biol Sci. 1990 Jul 23;241(1300):3–5. doi: 10.1098/rspb.1990.0056. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Patel P. V., Martin P. M., Smith H. Association of resistance of Neisseria gonorrhoeae to killing by human phagocytes with outer-membrane proteins of about 20 kilodaltons. J Gen Microbiol. 1985 Mar;131(3):601–610. doi: 10.1099/00221287-131-3-601. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Patel P. V., Nairn C. A., Smith H. A determinant of resistance of Neisseria gonorrhoeae to killing by human phagocytes: an outer membrane lipoprotein of about 20 kDa with a high content of glutamic acid. J Gen Microbiol. 1986 Dec;132(12):3277–3287. doi: 10.1099/00221287-132-12-3277. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Perera V. Y., Patel P. V., Martin P. M., Smith H. Outer membrane proteins of Neisseria gonorrhoeae associated with survival within human polymorphonuclear phagocytes. J Gen Microbiol. 1982 Dec;128(12):3077–3081. doi: 10.1099/00221287-128-12-3077. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Kwaasi A. A., Turner J. A., Veale D. R., Perera V. Y., Penn C. W., Smith H. Investigation of the determinants of the survival of Neisseria gonorrhoeae within human polymorphonuclear phagocytes. J Gen Microbiol. 1981 Nov;127(1):103–112. doi: 10.1099/00221287-127-1-103. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Patel P. V., Tan E. L., Andrade J. R., Nairn C. A., Goldner M., Cole J. A., Smith H. Cytidine 5'-monophospho-N-acetyl neuraminic acid and a low molecular weight factor from human blood cells induce lipopolysaccharide alteration in gonococci when conferring resistance to killing by human serum. Microb Pathog. 1988 Oct;5(4):303–309. doi: 10.1016/0882-4010(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Patel P. V., Martin P. M., Goldner M., Parsons N. J., Smith H. Red blood cells, a source of factors which induce Neisseria gonorrhoeae to resistance to complement-mediated killing by human serum. J Gen Microbiol. 1984 Nov;130(11):2767–2770. doi: 10.1099/00221287-130-11-2767. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Fischer S. H., Ingham Z. Z., Jones J. F. Interactions of Neisseria gonorrhoeae with human neutrophils: effects of serum and gonococcal opacity on phagocyte killing and chemiluminescence. Infect Immun. 1982 May;36(2):737–744. doi: 10.1128/iai.36.2.737-744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J. P., Rest R. F. Rapid damage to membranes of Neisseria gonorrhoeae caused by human neutrophil granule extracts. J Gen Microbiol. 1988 Feb;134(2):509–519. doi: 10.1099/00221287-134-2-509. [DOI] [PubMed] [Google Scholar]
- Shafer W. M., Rest R. F. Interactions of gonococci with phagocytic cells. Annu Rev Microbiol. 1989;43:121–145. doi: 10.1146/annurev.mi.43.100189.001005. [DOI] [PubMed] [Google Scholar]
- Smith H. Pathogenicity and the microbe in vivo. The 1989 Fred Griffith Review Lecture. J Gen Microbiol. 1990 Mar;136(3):377–393. doi: 10.1099/00221287-136-3-377. [DOI] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Swanson J. Colony opacity and protein II compositions of gonococci. Infect Immun. 1982 Jul;37(1):359–368. doi: 10.1128/iai.37.1.359-368.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978 Jan;19(1):320–331. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. L., Patel P. V., Parsons N. J., Martin P. M., Smith H. Lipopolysaccharide alteration is associated with induced resistance of Neisseria gonorrhoeae to killing by human serum. J Gen Microbiol. 1986 May;132(5):1407–1413. doi: 10.1099/00221287-132-5-1407. [DOI] [PubMed] [Google Scholar]
- Trust T. J., Lambden P. R., Watt P. J. The cohesive properties of variants of Neisseria gonorrhoeae strain P9: specific pilus-mediated and non-specific interactions. J Gen Microbiol. 1980 Jul;119(1):179–187. doi: 10.1099/00221287-119-1-179. [DOI] [PubMed] [Google Scholar]
- Veale D. R., Goldner M., Penn C. W., Ward J., Smith H. The intracellular survival and growth of gonococci in human phagocytes. J Gen Microbiol. 1979 Aug;113(2):383–393. doi: 10.1099/00221287-113-2-383. [DOI] [PubMed] [Google Scholar]
- Veale D. R., Penn C. W., Smith H. Factors affecting the induction of phenotypically determined serum resistance of Neisseria gonorrhoeae grown in media containing serum or its diffusible components. J Gen Microbiol. 1981 Feb;122(2):235–245. doi: 10.1099/00221287-122-2-235. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The effect of protein II and pili on the interaction of Neisseria gonorrhoeae with human polymorphonuclear leucocytes. J Gen Microbiol. 1986 Feb;132(2):503–512. doi: 10.1099/00221287-132-2-503. [DOI] [PubMed] [Google Scholar]
- Walstad D. L., Guymon L. F., Sparling P. F. Altered outer membrane protein in different colonial types of Neisseria gonorrhoeae. J Bacteriol. 1977 Mar;129(3):1623–1627. doi: 10.1128/jb.129.3.1623-1627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Glynn A. A., Watt P. J. The fate of gonococci in polymorphonuclear leucocytes: an electron microscopic study of the natural disease. Br J Exp Pathol. 1972 Jun;53(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J. Adherence of Neisseria gonorrhoeae to urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972 Dec;126(6):601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Glynn A. A. Gonococci in urethral exudates possess a virulence factor lost on subculture. Nature. 1970 Jul 25;227(5256):382–384. doi: 10.1038/227382a0. [DOI] [PubMed] [Google Scholar]
- Witt K., Veale D. R., Finch H., Penn C. W., Sen D., Smith H. Resistance of Neisseria gonorrhoeae grown in vivo to ingestion and digestion by phagocytes of human blood. J Gen Microbiol. 1976 Oct;96(2):341–350. doi: 10.1099/00221287-96-2-341. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]