Abstract
Few studies have examined the relationship between naturally rewarding behaviors and ethanol drinking behaviors in mice. Although natural and drug reinforcers activate similar brain circuitry, there is behavioral evidence suggesting food and drug rewards differ in perceived value (Bickel et al., 1995; DeGrandpre et al., 1993; Petry and Heyman, 1995). The primary goal of the present study was to investigate the relationships between naturally reinforcing stimuli and consumption of ethanol in ethanol preferring C57BL/6J mice. Mouse behaviors were observed after the following environmental manipulations: standard or enhanced environment, accessible or inaccessible wheel, and presence or absence of ethanol. Using a high resolution volumetric drinking monitor and wheel running monitor, we evaluated whether alternating access to wheel running modified ethanol-related behaviors and whether alternating access to ethanol modified wheel running or subsequent ethanol-related behaviors. We found that ethanol consumption remains stable with alternating periods of wheel running. Wheel running increases in the absence of ethanol and decreases upon reintroduction of ethanol. Upon reintroduction of ethanol, an alcohol deprivation effect was seen. Collectively, the results support theories of hedonic substitution and suggest that female C57BL/6J mice express ethanol seeking and craving under these specific conditions.
Keywords: Alcohol or Ethanol Consumption, Enhanced environment, Wheel Running, Alcohol Deprivation Effect, Hedonic Substitution
Introduction
The brain’s mesocorticolimbic reward pathway is a focus for addiction science research and dysregulation of this reward pathway is thought to underlie addiction (Kalivas and Volkow, 2005). Regions of the mesocorticolimbic system are activated in response to reinforcing stimuli such as food, sexual encounters, wheel running, enriched environments, and drugs of abuse (Hyman et al., 2006; Kelley and Berridge, 2002; Kolb et al., 2003; Vargas-Perez et al., 2003; Werme et al., 2002). Although natural and drug reinforcers activate similar brain circuitry, there is behavioral evidence that natural and drug rewards differ in perceived value (Bickel et al., 1995; DeGrandpre et al., 1993; Petry and Heyman, 1995). Hyman et al. (2006) suggest that the value of addictive drugs increases at the expense of natural rewards without serving to increase health. Koob and Le Moal (2005) propose that drug seeking is not only associated with reward neurocircuitry, but that drug addiction involves a decrease in the function of normal reward-related circuitry and chronic activation of anti-reward circuitry. According to the Diagnostic and Statistical Manual for Mental Disorders, a symptom of dependence is the substitution of natural rewards in favor of drug rewards (DSM-IV, 2004).
Enhanced environments, ethanol consumption and wheel running are reinforcing stimuli when presented individually to rodents (Nowak et al., 2000; Middaugh and Kelley, 1999; Samson et al., 2000; Werme et al., 2002). Several rodent models of ethanol self-administration have been developed to characterize specific aspects of alcohol drinking, such as initiation, binge drinking, maintenance/chronic, withdrawal, and craving/relapse (Lovinger and Crabbe, 2005). We are interested in investigating the behaviors of mice that have chronically consumed alcohol, as it has been shown that C57BL/6J mice display signs of ethanol dependence after three weeks of continuous drinking (Phillips et al., 1994). The primary goal of the present study was to investigate the relationship between wheel running and consumption of ethanol in ethanol preferring C57BL/6J mice. Wheel running has been shown to be rewarding and anti-depressive in several mouse strains (Brene et al., 2007). Wheel running activates brain reward pathways known to be responsive to drugs of abuse (Nestler, 2005; Vargas-Perez et al., 2003). After conducting a pilot experiment we noted lower than expected ethanol consumption in housing required for use of a wheel and wheel monitor, making it evident that we needed to consider housing as an additional variable. Housing required for wheel use and monitoring was larger and more complex than standard housing, and is referred to here as an enhanced environment. Therefore, mouse behaviors were observed after the following environmental manipulations: standard or enhanced environment, accessible or inaccessible wheel, and presence or absence of ethanol.
Few studies have examined the relationship between naturally rewarding behaviors and ethanol drinking behaviors in mice. Crews et al. (2004) reported that ethanol consumption does not differ for mice with or without access to a running wheel, however these mice had not chronically consumed ethanol prior to wheel running. For rats that had ethanol consumption experience, it has been shown that wheel running during alcohol deprivation increases subsequent ethanol consumption in rats (Werme et al., 2002). We hypothesize that C57BL/6J mice will maintain ethanol consumption in a standard housing environment (for several weeks of drinking). We hypothesized: 1) that ethanol consumption would be lower in the enhanced environment than in the standard housing, regardless of wheel access, 2) patterns of ethanol consumption would change during wheel access, 3) that mice would increase wheel running during ethanol deprivation and decrease wheel running upon reintroduction of ethanol, and 4) mice would increase ethanol consumption after a deprivation period. In alcohol dependent mice there may be a decrease in the function of normal reward-related circuitry and chronic activation of anti-reward circuitry. Increased ethanol consumption could represent hedonic allostasis, a deviation in the natural reward and anti-reward processes in order to maintain a deceptive stability.
Materials and Methods
Animals and Experimental Design
C57BL/6J breeding stocks were obtained from Jackson Laboratories (Bar Harbor, ME) and mice were bred in house to generate the mice used in this study. C57BL/6J female mice were chosen for this experiment because they consistently consume higher amounts of alcohol and display higher levels of wheel running as compared with C57BL/6J males (Belknap et al., 1993; de Visser et al., 2007). Fifteen female mice were group housed three or four to a standard mouse cage (19.05 × 31.75 × 12 cm). The temperature and humidity of the room were maintained at 20°C and 50%, respectively. All experiments were approved by the Institutional Animal Care and Use Committee and adhered to NIH Guidelines. The University of Texas facility is Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited.
Behavioral testing began when the mice were at least 70 days old. Food, water, and 10% (v/v) ethanol were available ad libitum for one month while the mice were acclimated to a phase advance in the 12:12 hour light/dark cycle (with lights off at 3:00 PM). After this initial alcohol exposure (termed Habituation period), the mice were housed individually in standard mouse cages and their fluid intake was measured daily for a period of six days. Ethanol was offered in a two bottle choice paradigm in which one bottle contained a 10% ethanol solution in tap water and the second identical bottle contained tap water. The positions of the bottles were alternated on a daily basis just before the offset of lights. Data from this condition is represented by the term “Standard”. The mice were then housed in a larger (23.62 × 35.3 × 19.56 cm) cage containing a 12.7 cm diameter running wheel (Automated Wheel Monitor, LaFayette Instruments, LaFayette, IN), feeder, and plexiglass drinking platform (18.5 × 9.5 × 2.5 cm) the remainder of the experiment. Although ethanol was still offered in a choice paradigm, fluid access was provided using Precision Drinking Monitors (Columbia Instruments, Columbus, Ohio). The mice experienced a period in which the running wheel was blocked mechanically followed by a period of free access to wheel running. Data from these conditions are represented by the terms “Baseline 1” and “Wheel 1”, respectively. This cycle was repeated three times followed by a fourth wheel access period in which ethanol was not offered (termed “Wheel 4”) and a fifth wheel access period where ethanol was again offered (termed “Wheel 5”). Each period lasted six days. During the baseline periods the wheel was mechanically blocked by inserting a metal rod through the wheel apparatus to prevent it from moving. The metal rod was removed during wheel periods to allow wheel running. Mice were weighed at the beginning of each period and at the end of the experiment (every 6 days). As per University of Texas at Austin animal research facility guidelines (at the time the experiment was conducted) cages were changed once every two weeks. Measured ethanol-related behaviors included ethanol preference ratio [calculated as (mL of 10% ethanol solution consumed)/( mL of 10% ethanol solution consumed + mL of water consumed)], pure ethanol consumption (g pure ethanol consumed/kg body weight/day), number of ethanol bouts, and amount (in g/kg) of pure ethanol consumed per bout. We defined a bout as consumption of a fluid at any time, lasting 1–60 seconds, during a one minute interval followed by consumption of the same fluid at any time during subsequent one minute interval(s) (lasting 1–60 seconds). Refer to Table 1 for timetable of experimental design.
Table 1.
Experiment Timetable
| Period | Days | Housing | 10% Ethanol | Wheel Access |
|---|---|---|---|---|
| Habituation | 1–30 | Group/SE | Available | Not Available |
| Standard | 31–37 | Individual/SE | Available | Not Available |
| Baseline 1 | 38–44 | Individual/EE | Available | Blocked |
| Wheel 1 | 45–51 | Individual/EE | Available | Accessible |
| Baseline 2 | 52–58 | Individual/EE | Available | Blocked |
| Wheel 2 | 59–65 | Individual/EE | Available | Accessible |
| Baseline 3 | 66–72 | Individual/EE | Available | Blocked |
| Wheel 3 | 73–79 | Individual/EE | Available | Accessible |
| Wheel 4 | 80–86 | Individual/EE | Not Available | Accessible |
| Wheel 5 | 87–93 | Individual/EE | Available | Accessible |
Abbreviations: SE (Standard Environment), EE (Enhanced Environment)
Data from each baseline and wheel period was collected simultaneously at one minute intervals independently from the Precision Drinking Monitors and Automated Wheel Monitors. The Precision Drinking Monitors interfaced with a desktop PC (Windows operating system) and the Volumetric Drinking Monitor program recorded fluid consumption at one minute intervals during lights off (3:00 PM to 3:00 AM). One interface monitored consumption of water and the other interface monitored 10% ethanol consumption. Fluids were available for consumption only during measured periods; therefore fluid intake was restricted to lights off periods when the Precision Drinking Monitor was used. Fluid restriction was a technical consequence of limiting volumetric drinking monitor data collection to lights off. Measuring fluid consumption every minute of the day seemed excessive, so we restricted data collection to lights off, when mice are know to be active and consume most of their fluids and food (Gill et al., 1986). When a mouse removed one drop from her sipper, the next was being delivered from a fluid reservoir to individual dispensers by a pump. The Precision Drinking Monitor measures liquid consumption with 20 microliter resolution.
The Automated Wheel Monitor interfaced with another desktop PC (Windows operating system) and the Wheel Activity Monitor program recorded wheel activity at one minute intervals during lights off (3:00 PM to 3:00 AM). Although activity was only measured for 12 hours during periods Wheel 1, 2, 3, 4, and 5, the wheel was accessible 24 hours a day. During Baseline periods (Baseline 1, 2, and 3), the wheel was mechanically blocked 24 hours a day and no activity was recorded by the monitor. The Wheel Activity Monitor program recorded wheel rotations per minute (RPM), distance traveled on the wheel (in km/minute and km/12 hours), and time spent on the running wheel (in minutes/12 hours).
Statistical Procedures
Data from each period is presented as the mean ± SEM. Student’s t-test (paired, two-tailed) was used to compare ethanol-related behaviors (Baseline 1 vs. Wheel 1, and Wheel 3 vs. Wheel 5) and wheel running behaviors (Wheel 3 vs. Wheel 4, and Wheel 4 vs. Wheel 5). We tested the effect of changing environmental conditions (Baseline 1 and Wheel 1 periods) on fluid consumption using Student’s t-test (paired, two-tailed). We tested the effect of alternating periods of wheel access (Baseline 1, Wheel 1, Baseline 2, Wheel 2, Baseline 3, and Wheel 3 periods) on ethanol-related behaviors using repeated measures two-way ANOVA. Repeated measures one-way ANOVA was used to test whether alternating periods of wheel access (Wheel 1, Wheel 2, and Wheel 3 periods) in the presence of ethanol had an effect on running wheel behaviors. An outlier was observed when analyzing the ethanol-related behaviors from period Wheel 5. Data for this mouse was greater than ±2σ from the mean, therefore it was considered to be an outlier. Data from this mouse was removed from all analyses, resulting in an n=14 mice for this study. Statistics were performed using Statistica version 6 (StatSoft, Tulsa, Oklahoma, USA) and GraphPad Prism version 4.00 (GraphPad Software, San Diego, California, USA).
Results
Remarks on the effects of limiting fluid access and modifying environments on fluid consumption
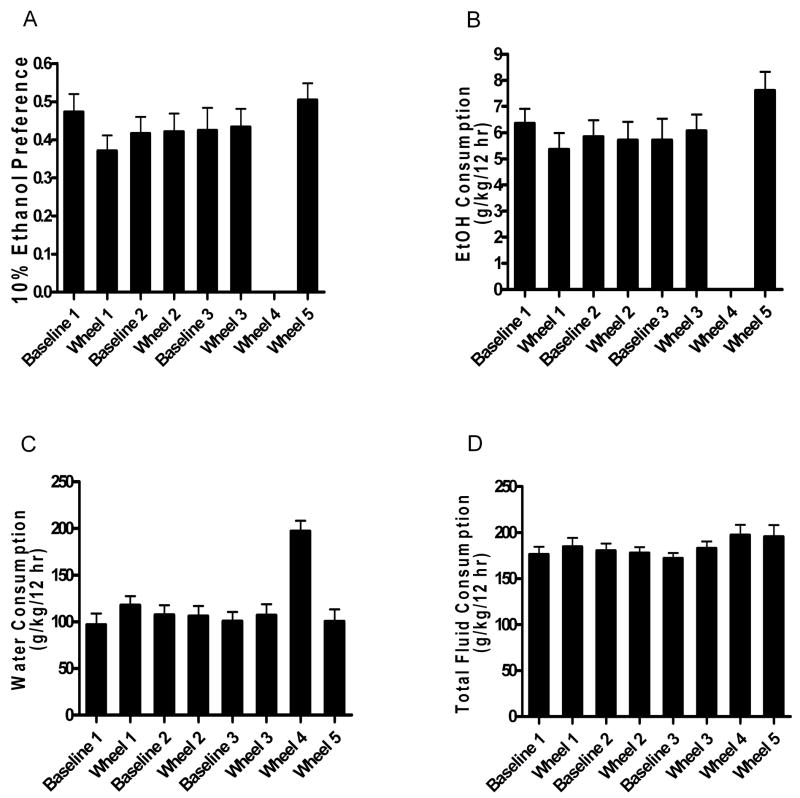
The purpose of the first phase of the experiment was to assess the effect of changing environments on ethanol drinking behaviors. Use of the Automated Wheel Monitor (AWM) system created a more complex, enhanced housing condition; therefore, we measured ethanol drinking over three changing conditions (1) standard housing, (2) enhanced AWM cage with a blocked wheel, and (3) enhanced AWM cage with wheel access. During the Standard period mice were subjected to standard individual housing conditions for the two bottle choice ethanol preference test where they consumed 10.8 ± 0.7 g/kg/24 hours pure ethanol and 74 ± 11 g/kg/24 hours water with an ethanol preference ratio of 0.66 ± 0.05. During the Baseline 1 period, fluids were accessible only during lights off (3:00 PM to 3:00 AM) and mice were subjected to enhanced environment housing conditions with a mechanically blocked wheel. Changing the environment and restricting fluid access resulted in 6.4 ± 0.6 g/kg/12 hours pure ethanol consumption and a water consumption of 97 ± 12 g/kg/12 hours, with an ethanol preference ratio of 0.47 ± 0.05. Unblocking the wheel, thus allowing access to wheel running (Wheel 1 period) did not produce a change in 10% ethanol solution consumption (67 ± 14 g/kg/12 hours, with 5.4 ± 0.6 g/kg/12 hours pure ethanol), but did increase water consumption (118 ± 10 g/kg/12 hours) without a change in total fluid consumption [Baseline 1 vs. Wheel 1 comparison, paired Student’s t-test; water consumption, P <.01] (Figures 1a, b, c). Accompanying these results was a significant decrease in ethanol preference ratio (0.37 ± 0.04) [Baseline 1 vs. Wheel 1 comparison, paired Student’s t-test; ethanol preference, P <.05].
Figure 1.
Voluntary fluid consumption. (A) 10% ethanol preference is presented as a ratio of the amount of ethanol solution consumed divided by total fluid consumption. (B) Ethanol consumption (g/kg/12 hr). (C) Water consumption (g/kg/12 hr). (D) Total fluid consumption (g/kg/12 hr).
Alternating periods of wheel access modifies ethanol drinking patterns but not wheel running
During the second phase of the experiment, mice were subjected to six alternating periods in which the running wheel was blocked (Baseline 1, 2, and 3) or accessible (Wheel 1, 2, and 3) to assess the effects of wheel running on ethanol-related behaviors. We hypothesized that wheel access would not change ethanol preference or consumption, but would compress normal drinking patterns, resulting in fewer ethanol bouts and a greater amount of ethanol consumed per bout. Data for ethanol preference, ethanol consumption, water consumption, and total fluid consumption from each period are presented in Figure 1, while ethanol-related behaviors characterizing patterns of drinking from each period are presented in Table 2.
Table 2.
Ethanol-related behavioral data
| Period | No.EtOH Bouts | Amount/Bout (g/kg) | Time Drinking EtOH (min) |
|---|---|---|---|
| Baseline 1 | 21 ± 2 | 0.34 ± 0.04 | 24 ± 3 |
| Wheel 1 | 21 ± 3 | 0.26 ± 0.02 | 24 ± 4 |
| Baseline 2 | 22 ± 3 | 0.29 ± 0.02 | 26 ± 4 |
| Wheel 2 | 24 ± 3 | 0.26 ± 0.03 | 28 ± 4 |
| Baseline 3 | 22 ± 3 | 0.26 ± 0.01 | 26 ± 4 |
| Wheel 3 | 28 ± 4 | 0.23 ± 0.02 | 32 ± 4 |
| Wheel 4 | N/A | N/A | N/A |
| Wheel 5 | 31 ± 4 | 0.28 ± 0.05 | 36 ± 4 |
Measured ethanol-related behaviors included number of ethanol bouts (where a bout is defined by consummatory behavior lasting equal to or greater than one minute without disruption during 12 hour period), amount of ethanol (in g/kg) consumed per bout (calculated by dividing ethanol consumption by number of ethanol bouts for individual mice), and time spent drinking (calculated from the number of minutes with a value greater than zero for amount of ethanol consumption per minute during 12 hour period).
Wheel access had no significant effect on ethanol consumption, ethanol preference or total fluid consumption, but wheel access changed ethanol drinking patterns. Wheel access resulted in an increased number of ethanol bouts [main effect of wheel access - F(1,13) = 5.28, P <.05], an increased time spent drinking ethanol [main effect of wheel access - F(1,13) = 4.65, P <.05], and a decreased amount of ethanol consumed per bout [main effect of wheel access - F(1,13) = 4.81, P <.05, P <.05; Table 2].
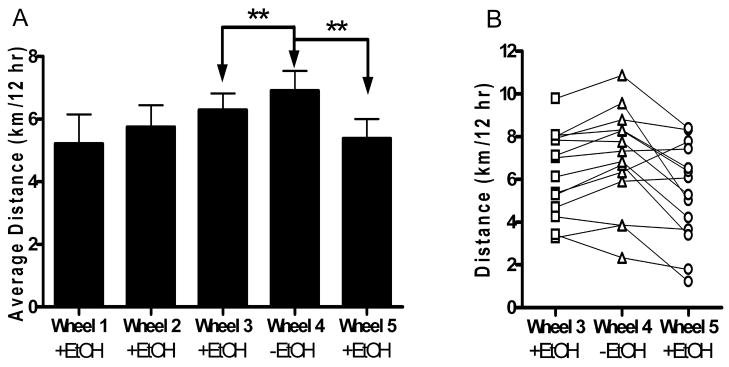
Changes in drinking patterns due to wheel access can be summarized as an increased number of ethanol bouts, time spent drinking ethanol, and a reduced amount of ethanol consumed per bout. Alternating periods of wheel access (periods Wheel 1, 2, and 3) in the presence of ethanol did not significantly change subsequent wheel running distance, time spent running, or average RPM (Figure 2a and Table 3).
Figure 2.
Average distance travelled on the running wheel. (A) Wheel running distance (km/12 hours) data for each period. **-P <.01 (paired Student’s t-test). (B) Wheel running distance (km/12 hours) data for individual mice for Wheel 3, Wheel 4, and Wheel 5.
Table 3.
Measurement of wheel activity
| Period | Time Running (min/12 hr) | Average RPM |
|---|---|---|
| Wheel 1 | 319 ± 43 | 34 ± 5 |
| Wheel 2 | 332 ± 24 | 40 ± 4 |
| Wheel 3 | 349 ± 17 | 44 ± 3 |
| Wheel 4 | 363 ± 16 | 47 ± 3 |
| Wheel 5 | 282 ± 19 | 44 ± 3 |
For each period, the average time spent running (minutes/12 hours) was calculated from the number of minutes with a value greater than zero for wheel rotations per minute. Average wheel rotations per minute (RPM) when running on the wheel were calculated from RPM values greater than zero per 12 hours for each period.
Effect of alternating ethanol availability on wheel running and ethanol consumption
The third phase of the experiment began with period Wheel 3, followed by a fourth wheel access period in which ethanol was not offered (Wheel 4) and a fifth wheel access period where ethanol was again offered (Wheel 5). Forced ethanol abstinence resulted in increased wheel running time and distance, and this effect was reversed upon the reintroduction of ethanol (Figure 2a, b). We measured distance traveled, time spent running, and average wheel RPM when running as wheel activity parameters (Figure 2a, 2b and Table 3). In the third phase of the experiment, mice ran 6.9 ± 0.6 km/day during period Wheel 4 (in the absence of ethanol), a significantly greater distance than during Wheel 3 (in the presence of ethanol), where mice ran a distance of 6.3 ± 0.5 km/12 hours [paired Student’s t-test, P <.01]. This distance decreased to 5.4 ± 0.6 km/12 hours during Wheel 5, when ethanol was reintroduced [paired Student’s t-test, P <.01]. In addition to running distance, the amount of time spent running varied with ethanol accessibility. The amount of time spent running was significantly decreased from 363 ± 16 minutes during Wheel 4 to 282 ± 19 minutes during Wheel 5, when ethanol was reintroduced [paired Student’s t-test, P <.0001]. The average wheel RPM was not significantly changed by ethanol deprivation [paired Student’s t-test to compare Wheel 3 and 4, P >.05; paired Student’s t-test to compare Wheel 4 and 5, P >.05].
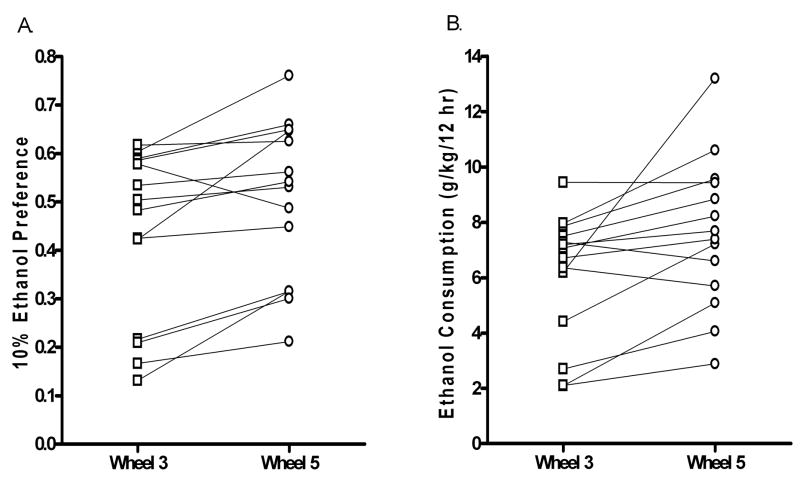
Wheel running in the absence of ethanol resulted in an increase in alcohol consumption, suggesting an ethanol deprivation effect (Figures 1a, 3a). In addition to ethanol consumption, ethanol preference and the time spent drinking ethanol were increased in Wheel 5 as compared with Wheel 3 [paired Student’s t-tests - ethanol consumption, P <.05; ethanol preference, P <.01; time spent drinking ethanol]. There were no observed changes in total fluid consumption, number of ethanol bouts, or amount of ethanol consumed per bout.
Figure 3.
Ethanol deprivation effect: Individual means for Wheel 3 and Wheel 5 periods. (A) 10% ethanol preference is presented as a ratio of the amount of ethanol consumed divided by total fluid consumption. (B) Ethanol consumption (g/kg/12 hr).
Discussion
Natural and drug reinforcers activate similar brain circuitry (Carrelli and Wondolowski, 2006; Kelley and Berridge, 2002). Few studies have examined the relationship between naturally rewarding behaviors and ethanol drinking behaviors. In our study, we examined the relationship between wheel running and ethanol-related behaviors. Specifically, we evaluated whether alternating access to wheel running modified ethanol-related behaviors and whether alternating access to ethanol modified wheel running or subsequent ethanol-related behaviors. Given that changes in environment, consumption of ethanol, and wheel running can induce neuroadaptive responses (with each stimulus having an independent response timeline), it is important to point out that results might be different under different temporal testing periods.
Comments of the effect of fluid restriction, enhanced environment, and first access to a running wheel on ethanol-related behaviors
In the first phase of the experiment, we were interested in assessing the effect of increasingly complex environments on ethanol drinking behaviors. Two limitations of our experimental design that hinder our ability to interpret the effects of enhanced environment on ethanol-related behaviors are 1) fluids were available for 24 hours in the standard cage, but restricted to 12 hours in the AWM cage, and 2) the order of treatments was not counterbalanced. Future studies will attempt to correct for this by measuring ethanol-related behaviors under limited access conditions throughout the experiment and measuring ethanol-related behaviors in a standard cage after the last wheel access period. Based on previous data (unpublished observation, A.R. Ozburn, 2005), we hypothesize that returning mice to their standard cage would increase ethanol consumption and preference as compared with the enhanced environment AWM cage. To our knowledge, no study has examined the effect of enhanced environmental conditions on ethanol consumption in mice, but previous studies have shown that under specific conditions, complex environments can increase ethanol preference and consumption in rats (Ellison et al., 1983; Fernandez-Teruel et al., 2002; Rockman et al., 1989). However, direct comparisons of our results to others are not possible due to differences in experimental paradigms (such as prolonged or limited access to enriched housing and the use of more complex environments including multiple rats of same and opposite gender, running wheels, and toys).
Ethanol preference decreased during the first wheel access period as compared with Baseline 1 period. This decrease is a result of increased water consumption without a change in ethanol consumption.
Wheel access modifies ethanol drinking patterns
In the second phase of the experiment we assessed the effect of alternating periods of wheel access on ethanol-related behaviors. Ethanol preference and consumption were maintained regardless of wheel access. These results support earlier findings by Crews et al. (2004) that ethanol consumption does not differ for mice with or without access to running wheels. It has been previously reported that voluntary wheel running reduces the effects of acute ethanol (delivered via i.p. injections) in C57BL/6 mice, suggesting development of cross-tolerance (Mollenauer et al., 1991). Since ethanol consumption and wheel running distances were maintained during this experimental phase, our data do not support earlier findings describing the development of cross-tolerance.
Although wheel access did not impose a change in ethanol intake, it did modify ethanol drinking patterns. When the wheel was accessible mice had an increased number of ethanol bouts, but they consumed less ethanol per bout. Under these conditions, wheel running resulted in several sipping bouts or a dilute drinking pattern rather than the hypothesized condensed bouts of consumption or binge type drinking pattern.
We also assessed whether wheel running behaviors changed over time. In the present study running distance, average RPM, and time spent running did not change over wheel periods 1, 2, and 3, indicating that the running behavior is stable over time and with intermittent wheel access. These results are in agreement with data reported by Jung et al. (2006), indicating that wheel running in (distance and duration) is stable for at least 15 weeks in C57BL/6J mice. It has been shown that intermittent wheel availability or periods of alternating access do not induce a rebound effect on running distance in C57BL/J mice (de Visser et al., 2007).
Wheel running increases during ethanol deprivation and decreases upon reintroduction of ethanol
In the third phase of the experiment, we assessed the effects of forced ethanol abstinence and subsequent reintroduction on wheel running. We found that running distance increased during abstinence and decreased upon reintroduction of ethanol. Koob and Le Moal (2005) have proposed that drug seeking is not only associated with reward neurocircuitry, but that drug addiction involves a decrease in the function of normal reward-related circuitry and chronic activation of anti-reward circuitry. The increased running distance during ethanol deprivation and decreased running distance upon reintroduction of ethanol may represent hedonic substitution in order to maintain stability in the reward and anti-reward processes. Upon reintroduction of ethanol, it is unlikely that the decreased running distance is due to the sedative effects of ethanol since mice are not consuming sufficient alcohol to produce sedation at any given time.
It is of interest to point out that Crews et al. (2004) reported no difference in wheel running distance between ethanol drinking and water drinking mice, indicating a similar stability in normal reward-related processes. Without the experience of chronic self-administration followed by forced deprivation, hedonic substitution processes are not recruited. The reinforcing value of running does not appear to change during ethanol abstinence, given that mice do not run more vigorously (as demonstrated by the unchanged average wheel RPM). There have been reports of correlations between high running in ethanol preferring animals and low running in ethanol non-preferring animals (Belknap et al., 1993; deVissar et al., 2007; Festing, 1977; Werme et al., 1999). Specifically, Hofstetter et al., (2003) reported marked differences in the circadian activities of two selected lines of mice, high alcohol preferring (HAP) and low alcohol preferring (LAP). The circadian period of wheel running activity in ethanol naïve HAP and LAP mice was assessed over five weeks in the dark. Although running-wheel counts were not different between the two selected lines, the HAP mice ran more vigorously in the wheel and had a shorter circadian period of wheel running than LAP mice.
Effect of wheel running during ethanol deprivation on subsequent ethanol consumption
In the third phase of the experiment, we also assessed the effects of wheel running during forced ethanol abstinence and subsequent reintroduction on ethanol-related behaviors. Wheel running in the absence of ethanol stimulated an ethanol deprivation effect, resulting in a subsequent increase in alcohol consumption, ethanol preference and the time spent drinking. During this time, ethanol consumption was below the metabolic capacity for mice. Since we did not measure blood alcohol levels during this experiment, the reported ethanol consumption may not reflect a pharmacological effect of alcohol. However, Phillips et al., (1994) reported signs of physical dependence (ethanol withdrawal measured by handling-induced convulsions) when blood alcohol levels were not detectable after consumption of a sweetened ethanol solution (average 10 g/kg/day) for three weeks. There are notable methodological differences in the present study and that of Phillips et al., (2004): 1. Phillips reported ethanol consumption as g/kg/day and the present study measured ethanol consumption as g/kg/12 hours; and 2. Phillips reported withdrawal severity after 21 days of (saccharin sweetened) ethanol consumption, while mice in the present study consumed (unsweetened) ethanol consumption for 72 days.
The ethanol deprivation effect is a model of alcohol craving expressed as relapse-type drinking (Li TK, 2000). This effect has been reported for rodents of specific genetic backgrounds under precise conditions (Melendez et al., 2006; Rodd-Henricks et al., 2000). These results are consistent with Koob’s theory on the plasticity of reward neurocircuitry and the dark side of addiction (Koob and LaMoal, 2005). Specifically, that dependence involves a decrease in the function of normal reward-related circuitry (expressed here as a decrease in wheel running) and chronic activation of anti-reward circuitry (expressed here as increased ethanol seeking and consumption). The increased ethanol consumption may represent hedonic allostasis, a deviation in the natural reward and anti-reward processes in order to maintain a deceptive stability. However, without additional studies, we cannot determine whether increases in drinking are reflective of cross-sensitization, a decrease in function of normal reward-related circuitry, both or neither.
We conclude that ethanol consumption is maintained during periods of wheel running. Wheel running is increased in the absence of ethanol and decreased upon reintroduction of ethanol. Upon reintroduction of ethanol, an alcohol deprivation effect is seen. Collectively, the results support theories of hedonic allostasis and suggest that female C57BL/6J mice express ethanol seeking under these specific conditions.
Acknowledgments
We would like to thank Marni Martinez for her assistance in data collection and Dr. Igor Ponomarev for his comments on early versions of this manuscript. This research was supported by the Integrative Neuroscience Initiative on Alcoholism Consortium Grant AA13520, and NIH Grants AA06399-S and AA16424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology. 1995;118:250–259. doi: 10.1007/BF02245952. [DOI] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Aberg E, Mathé AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007;10:136–140. doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J. Anatomic distribution of reinforcer selective cell firing in the core and shell of the nucleus accumbens. Synapse. 2006;59:69–73. doi: 10.1002/syn.20217. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK, Hughes JR, Layng MP, Badger G. Unit price as a useful metric in analyzing effects of reinforcer magnitude. J Exp Anal Behav. 1993;60:641–666. doi: 10.1901/jeab.1993.60-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Stoker AK, Kas MJ, Spruijt BM. Effects of genetic background and environmental novelty on wheel running as a rewarding behaviour in mice. Behav Brain Res. 2007;27:290–297. doi: 10.1016/j.bbr.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Ellison G, Levy A, Lorant N. Alcohol-preferring rats in colonies show withdrawal, inactivity, and lowered dominance. Pharmacol Biochem Behav. 1983;18:565–570. doi: 10.1016/0091-3057(83)90237-x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teruel A, Driscoll P, Gil L, Aguilar R, Tobena A, Escorihuela RM. Enduring effects of environmental enrichment on novelty seeking, saccharin and ethanol intake in two rat lines (RHA/Verh and RLA/Verh) differing in incentive-seeking behavior. Pharmacol Biochem Behav. 2002;73:225–231. doi: 10.1016/s0091-3057(02)00784-0. [DOI] [PubMed] [Google Scholar]
- Festing MF. Wheel activity in 26 strains of mouse. Lab Anim. 1977;11:257–258. doi: 10.1258/002367777780936530. [DOI] [PubMed] [Google Scholar]
- Gill K, France C, Amit Z. Voluntary ethanol consumption in rats: an examination of blood/brain ethanol levels and behavior. Alcohol Clin Exp Res. 1986;10:457–462. doi: 10.1111/j.1530-0277.1986.tb05124.x. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Grahame NJ, Mayeda AR. Circadian activity rhythms in high alcohol-preferring and low alcohol-preferring mice. Alcohol. 2003;30:81–85. doi: 10.1016/s0741-8329(03)00095-8. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jung AP, Curtis TS, Turner MJ, Lightfoot JT. Influence of age of exposure to a running wheel on activity in inbred mice. Med Sci Sports Exerc. 2006;38:51–56. doi: 10.1249/01.mss.0000181157.87366.f6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Soderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Li TK. Clinical perspectives for the study of craving and relapse in animal models. Addiction. 2000;95:S55–60. doi: 10.1080/09652140050111645. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Middaugh LD, Kalivas PW. Development of an alcohol deprivation and escalation effect in C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:2017–2025. doi: 10.1111/j.1530-0277.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Mollenauer S, Bryson R, Speck C, Chamberlin JR. Voluntary wheel running reduced the effects of acute ethanol on activity and avoidance in C57BL/6J mice. Pharmacol Biochem Behav. 1991;39:821–824. doi: 10.1016/0091-3057(91)90173-y. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Ingraham CM, Mckinzie DL, Mcbride WJ, Lumeng L, Li TK, Murphy JM. An assessment of novelty-seeking behavior in alcohol-preferring and nonpreferring rats. Pharmacol Biochem Behav. 2000;66:113–121. doi: 10.1016/s0091-3057(00)00206-9. [DOI] [PubMed] [Google Scholar]
- Petry NM, Heyman GM. Behavioral economics of concurrent ethanol-sucrose and sucrose reinforcement in the rat: effects of altering variable-ratio requirements. J Exp Anal Behav. 1995;64:331–359. doi: 10.1901/jeab.1995.64-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Rockman GE, Gibson JE, Benarroch A. Effects of environmental enrichment on voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1989;34:487–490. doi: 10.1016/0091-3057(89)90545-5. [DOI] [PubMed] [Google Scholar]
- Rodd-Hernricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcohol Clin Exp Res. 2000;24:747–753. [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Slawecki CJ. A new assessment of the ability of oral ethanol to function as a reinforcing stimulus. Alcohol Clin Exp Res. 2000;24:766–773. [PubMed] [Google Scholar]
- Vargas-Perez H, Mena-Segovia J, Giordano M, Diaz JL. Induction of c-fos in nucleus accumbens in naive male Balb/c mice after wheel running. Neurosci Lett. 2003;352:81–84. doi: 10.1016/j.neulet.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002;133:301–308. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Werme M, Thoren P, Olson L, Brene S. Addiction-prone Lewis but not Fischer rats develop compulsive running that coincides with downregulation of nerve growth factor inducible-B and neuron derived orphan receptor 1. J Neurosci. 1999;19:6169–6174. doi: 10.1523/JNEUROSCI.19-14-06169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]