Abstract
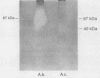
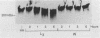
During skin penetration, infective hookworm larvae encounter hyaluronic acid as they migrate between epidermal keratinocytes and through the ground substance of the dermis. A hyaluronidase would facilitate passage through the epidermis and dermis during larval invasion. Zoonotic hookworm larvae of the genus Ancylostoma were shown to contain a hyaluronidase activity that migrated on modified sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) hyaluronic acid gels with an apparent Mr of 49,000. A second form with an Mr of 87,000 was also identified. The major etiologic agent of cutaneous larva migrans, A. braziliense, was shown to have the greatest enzyme activity, hydrolyzing up to 3.3 micrograms of hyaluronic acid per h per micrograms of total parasite protein at pH 6.0, whereas A. caninum and A. tubaeforme each had much less enzyme activity. The differences in enzyme activities between species correlated with differences in the intensities of the lytic zones at 49 and 87 kDa on SDS-PAGE hyaluronic acid gels. Hookworm hyaluronidase activity exhibited a broad pH optimum between 6.0 and 8.0 and did not hydrolyze chondroitin sulfate, two features that suggest that the hookworm enzyme is more like the invertebrate leech hyaluronidase than mammalian testicular or lysosomal hyaluronidase. Larvae of A. braziliense were shown to release hyaluronidase activity and degrade radiolabeled hyaluronic acid in vitro. Gold sodium thiomalate was identified as an enzyme inhibitor. The hyaluronidase is the second major virulence factor that we have identified from infective hookworm larvae.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alho A. M., Underhill C. B. The hyaluronate receptor is preferentially expressed on proliferating epithelial cells. J Cell Biol. 1989 Apr;108(4):1557–1565. doi: 10.1083/jcb.108.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allalouf D., Ber A., Ishay J. Hyaluronidase activity of extracts of venom sacs of a number of Vespinae (Hymenoptera). Comp Biochem Physiol B. 1972 Sep 15;43(1):119–123. doi: 10.1016/0305-0491(72)90207-6. [DOI] [PubMed] [Google Scholar]
- BARKER S. A., BAYYUK S. I., BRIMACOMBE J. S., PALMER D. J. CHARACTERIZATION OF THE PRODUCTS OF THE ACTION OF BEE VENOM HYALURONIDASE. Nature. 1963 Aug 17;199:693–694. doi: 10.1038/199693a0. [DOI] [PubMed] [Google Scholar]
- Benchetrit L. C., Gray E. D., Edstrom R. D., Wannamaker L. W. Purification and characterization of a hyaluronidase associated with a temperate bacteriophage of group A, type 49 streptococci. J Bacteriol. 1978 Apr;134(1):221–228. doi: 10.1128/jb.134.1.221-228.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchetrit L. C., Pahuja S. L., Gray E. D., Edstrom R. D. A sensitive method for the assay of hyaluronidase activity. Anal Biochem. 1977 May 1;79(1-2):431–437. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- DI FERRANTE N. Turbidimetric measurement of acid mucopolysaccharides and hyaluronidase activity. J Biol Chem. 1956 May;220(1):303–306. [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A. The hyaluronidase associated with Treponema pallidum facilitates treponemal dissemination. Infect Immun. 1987 May;55(5):1023–1028. doi: 10.1128/iai.55.5.1023-1028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles H. M. Selective primary health care: strategies for control of disease in the developing world. XVII. Hookworm infection and anemia. Rev Infect Dis. 1985 Jan-Feb;7(1):111–118. doi: 10.1093/clinids/7.1.111. [DOI] [PubMed] [Google Scholar]
- Harrison R. A. Hyaluronidase in ram semen. Quantitative determination, and isolation of multiple forms. Biochem J. 1988 Jun 15;252(3):865–874. doi: 10.1042/bj2520865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A. Preliminary characterization of the multiple forms of ram sperm hyaluronidase. Biochem J. 1988 Jun 15;252(3):875–882. doi: 10.1042/bj2520875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P. J. Hookworm disease in children. Pediatr Infect Dis J. 1989 Aug;8(8):516–520. doi: 10.1097/00006454-198908000-00009. [DOI] [PubMed] [Google Scholar]
- Hotez P., Haggerty J., Hawdon J., Milstone L., Gamble H. R., Schad G., Richards F. Metalloproteases of infective Ancylostoma hookworm larvae and their possible functions in tissue invasion and ecdysis. Infect Immun. 1990 Dec;58(12):3883–3892. doi: 10.1128/iai.58.12.3883-3892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes W. L., Ferretti J. J. Sequence analysis and expression in Escherichia coli of the hyaluronidase gene of Streptococcus pyogenes bacteriophage H4489A. Infect Immun. 1989 Feb;57(2):533–539. doi: 10.1128/iai.57.2.533-539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE C. L., LEWERT R. M. Studies on the presence of mucopolysaccharidase in penetrating helminth larvae. J Infect Dis. 1957 Nov-Dec;101(3):287–294. doi: 10.1093/infdis/101.3.287. [DOI] [PubMed] [Google Scholar]
- LEWERT R. M., LEE C. L. Studies on the passage of helminth larvae through host tissues. I. Histochemical studies on the extracellular changes caused by penetrating larvae. II. Enzymatic activity of larvae in vitro and in vivo. J Infect Dis. 1954 Jul-Aug;95(1):13–51. doi: 10.1093/infdis/95.1.13. [DOI] [PubMed] [Google Scholar]
- LINCICOME D. R. A streptococcal decapsulation test for detection of hyaluronidase activity in animal parasites. Exp Parasitol. 1953 Oct;2(4):333–340. doi: 10.1016/0014-4894(53)90018-6. [DOI] [PubMed] [Google Scholar]
- Lamberg S. I., Yuspa S. H., Hascall V. C. Synthesis of hyaluronic acid is decreased and synthesis of proteoglycans is increased when cultured mouse epidermal cells differentiate. J Invest Dermatol. 1986 Jun;86(6):659–667. doi: 10.1111/1523-1747.ep12275707. [DOI] [PubMed] [Google Scholar]
- Matthews B. E. Mechanism of skin penetration by Ancylostoma tubaeforme larvae. Parasitology. 1975 Feb;70(1):25–38. doi: 10.1017/s0031182000048836. [DOI] [PubMed] [Google Scholar]
- Miller T. A. Hookworm infection in man. Adv Parasitol. 1979;17:315–384. doi: 10.1016/s0065-308x(08)60552-7. [DOI] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault S., Zaneveld L. J., Rogers B. J. Inhibition of fertilization in the hamster by sodium aurothiomalate, a hyaluronidase inhibitor. J Reprod Fertil. 1980 Nov;60(2):461–467. doi: 10.1530/jrf.0.0600461. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Schad G. A. Ancylostoma duodenale: maintenance through six generations in helminth-native pups. Exp Parasitol. 1979 Apr;47(2):246–253. doi: 10.1016/0014-4894(79)90077-8. [DOI] [PubMed] [Google Scholar]
- Tammi R., Ripellino J. A., Margolis R. U., Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate binding region of cartilage proteoglycan as a specific probe. J Invest Dermatol. 1988 Mar;90(3):412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- Unsworth P. F. Hyaluronidase production in Streptococcus milleri in relation to infection. J Clin Pathol. 1989 May;42(5):506–510. doi: 10.1136/jcp.42.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUKI H., FISHMAN W. H. Purification and characterization of leech hyaluronic acid-endo-beta-glucuronidase. J Biol Chem. 1963 May;238:1877–1879. [PubMed] [Google Scholar]