Abstract
Cell adhesion molecules of the Immunoglobulin superfamily (IgCAMs) play diverse functions during neural development. Previously, we have identified SYG-1/Neph1 and SYG-2/Nephrin, IgCAMs necessary for synaptic specificity in Caenorhabditis elegans. Here, we conduct an in vivo structure-function analysis of SYG-1 and SYG-2 to identify domains of SYG-1 and SYG-2 necessary for heterophilic binding as well as synaptic specificity. We find the first Ig domain of SYG-1 and the first 5 Ig domains of SYG-2 are necessary and sufficient for their binding in vivo, as well as for synapse formation. We also find the SYG-2 cytoplasmic domain is required for SYG-2 subcellular trafficking, while the intracellular region of SYG-1 is required for synaptic function at earlier developmental stages, but is dispensable for later stages. This study defines the domain requirements for SYG-1/SYG-2 heterophilic binding and suggests that unknown SYG-1 extracellular interactors may play a role in SYG-1-mediated synaptic specificity.
Keywords: IgSF, synaptogenesis, Neph1, Nephrin, structure-function, synaptic specificity, syg-1, syg-2
Introduction
The formation of neural circuits requires the coordination of multiple developmental events such as cell migration, axon and dendrite outgrowth and guidance, followed by target recognition and synapse assembly. Although it is has been documented that synaptic connections in the brain are precise and stereotyped, relatively little is known about the molecular mechanisms by which neurons select their correct synaptic partners to initiate synaptic assembly while rejecting other contacting cells in the same target field. It has been proposed that cell adhesion molecules found on pre-and postsynaptic cells may be used to mediate cell recognition and initiate synaptogenesis.
Recently, a number of cell adhesion molecules of the Immunoglobulin domain family (IgCAMs) have been implicated in synapse formation. SynCAMs, homophilic IgCAMs, have been shown to promote synaptogenesis in vitro (Biederer et al., 2002). Sidekicks, Ig domain containing proteins, have been implicated in the laminar choices within the inner plexiform layer of the vertebrate retina (Yamagata et al., 2002). Recently, another well recognized IgCAM, UNC-40/DCC, was shown to be enriched at presynaptic sites and to be essential for normal synaptogenesis in the AIY neuron of Caenorhabditis elegans (Colon-Ramos et al., 2007). In addition, we have previously shown that heterophilic interactions between SYG-1 and SYG-2, a pair of IgSF proteins, are required for specification of synapses in the HSNL neuron of C. elegans (Shen and Bargmann, 2003; Shen et al., 2004).
SYG-1, SYG-2, and their homologs have been demonstrated to be cell adhesion molecules that play diverse roles during development. In Drosophila, there are two SYG-1 homologs, Irregular chiasm C-roughest (IrreC-Rst) and Kin of IrreC/DumbFounded (Kirre or Duf), and two SYG-2 homologs, Sticks and Stones (Sns) and Hibris. IrreC-Rst and Hibris are required for proper patterning of the Drosophila eye (Ramos et al., 1993). During ommatidial development, heterophilic interaction between IrreC-Rst, expressed on the interommantidial precursor cells (IPCs) and Hibris, expressed on the primary pigment cells, is necessary for proper IPC cell sorting and remodeling of adhesive contacts, which then leads to apoptotic death of surplus IPCs (Bao and Cagan, 2005; Carthew, 2007). Additionally, heterophilic interactions between IrreC-Rst and Sns, as well as between Kirre and SNS have been shown to be important for Drosophila myoblast fusion. IrreC-Rst and Kirre are expressed on muscle founder cells while SNS and Hibris are expressed on fusion competent myoblasts. IrreC-Rst and Kirre act redundantly to bind SNS, while Hibris is thought modify SNS activity (Chen et al., 2007; Dworak and Sink, 2002). While weak homophilic interactions of IrreC-Rst and Kirre have been shown in cell culture, heterophilic interactions between SNS and IrreC-Rst as well as between SNS and Kirre are thought to be most important for myoblast fusion. Additionally, a zebrafish Kirre-like molecule has been shown to be required for myoblast fusion, suggesting that this pathway may be conserved in vertebrates (Srinivas et al., 2007).
In vertebrates, Neph1/Kirrel1 and Nephrin, orthologs of SYG-1 and SYG-2 respectively, play essential roles in kidney development. There are three homologs of SYG-1 in vertebrates: Neph1/Kirrel1, Neph2/ Kirrel3, and Neph3/Kirrel2, and a single homolog of SYG-2, nephrin (for simplicity, SYG-1 homologs will be called neph1, neph2 and neph3 in this paper). Neph1 and Nephrin have been implicated in glomerular slit diaphragm formation, the permeable membrane which allows for filtration of solutes in the kidney. In either humans with inherited mutations or mice with targeted deletions, loss of either Neph1 or Nephrin function leads to failure of glomerular slit membrane formation and lethal proteineuria (Donoviel et al., 2001; Kestila et al., 1998). In cell culture experiments it has been shown that both Neph1 and Nephrin exhibit homotypic as well as heterotypic interactions, but which of these interactions are of functional importance is unclear (Gerke et al., 2003; Khoshnoodi et al., 2003; Liu et al., 2003). In addition, Neph1 and Neph2 have been shown to be expressed at synaptic sites in the brain, and Neph1 and Neph2 physically associate with CASK, a synaptic scaffolding protein, suggesting that neph proteins may play a role in synapse formation in the vertebrate CNS (Gerke et al., 2006). In addition, Neph2 and Neph3 are expressed in olfactory glomeruli, and gain-of-function experiments suggest that SYG-1 orthologs may be involved in olfactory axon sorting and targeting (Serizawa et al., 2006).
SYG-1 and SYG-2 are Immunoglobulin (Ig) domain containing transmembrane proteins. Based on alignments with Drosophila and vertebrate homologs, the SYG-1 cDNA is predicted to encode a signal sequence, 5 Immunoglobulin-like domains in its extracellular region, a transmembrane domain, and a short intracellular domain ending with a consensus type 1 PDZ binding motif (Fig. 1D). SYG-2 is predicted to encode a signal sequence, 9 Imunoglobulin-like domains in its extracellular region, a transmembrane domain, and a consensus type 1 PDZ binding motif (Fig. 4A).
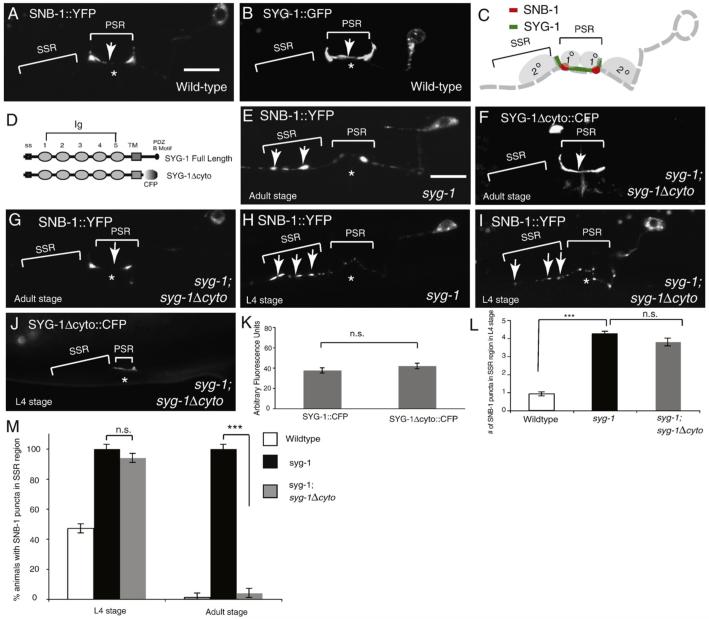
Fig. 1.
The SYG-1 Extracellular domain is sufficient to rescue the syg-1 phenotype in adults. (A) Representative wild-type animals expressing the synaptic vesicle marker SNB-1::YFP in HSNL. Asterisk marks position of the vulva. Note that SNB-1 expression (arrow) is concentrated around the vulva at the primary synaptic region (PSR) (bracket). Secondary synaptic region (SSR) is defined as region anterior to the vulva (bracket) Scale bar is 10 um. (B) Wild-type animal expressing SYG-1::GFP in HSNL. Note that SYG-1 is localized to the PSR (arrow). (C) Schematic of synaptobrevin and SYG-1 localization in HSNL. 1° and 2° vulval epithelial cells are shown in light grey. SYG-1 is localized by SYG-2 expressed in 1° epithelial cells. (D) Schematic of domain structure of SYG-1 and the SYG-1Δcyto construct. SYG-1 contains a signal sequence, 5 Ig domains, a transmembrane domain and a PDZ binding motif. SYG-1Δcyto is SYG-1 trunctated after the transmembrane domain and replaced with CFP. (E) Representative syg-1 adult expressing SNB-1::YFP. Ectopic SNB-1 clusters are present anteriorly at the secondary synaptic region (SSR)(arrows). (F) Localization of SYG-1Δcyto::CFP construct. SYG-1Δcyto::CFP localizes to the vulva in an identical fashion as the full-length SYG-1 protein (arrow). (G) syg-1 adult expressing the SYG-1Δcyto construct in HSNL. SNB-1 is no longer at the SSR but is localized at the vulva in the PSR, similar to the wild-type animals. (H) syg-1 animal expressing SNB-1::YFP in the L4 stage. Ectopic SNB-1 clusters are found anteriorly in the SSR(arrows). (I) syg-1 animal in the L4 stage expressing the SYG-1Δcyto construct in HSNL. Ectopic SNB-1 clusters are present anteriorly in the SSR at this stage (arrows). (J) syg-1 animal expressing SYG-1Δcyto::CFP construct in L4 stage. SYG-1Δcyto::CFP is localized to the PSR in L4 stage (bracket). (K) Comparison of average intensity of signal in the PSR between SYG-1Δcyto::CFP and SYG-1::CFP in L4 stage. No statistically significant difference is observed. (L) Quantification of number of SSR puncta between wild-type, syg-1, and syg-1 animals expressing the SYG-1Δcyto::CFP construct in the L4 stage. Error bars, standard error. n>50. ***p<0.001, student's t-test. (M) Quantification of the adult and L4 stage phenotypes. Error bars, standard error of proportion. n>50. ***p<0.001, Chi-squared test.
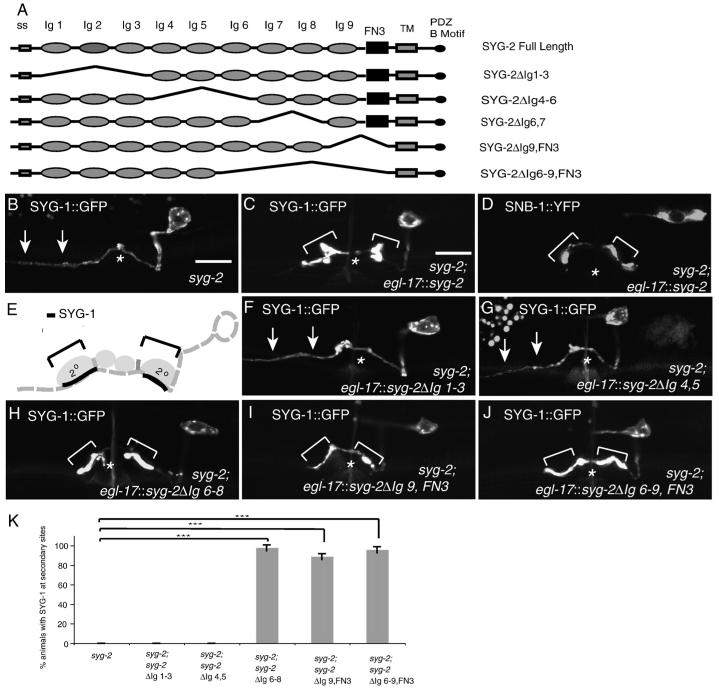
Fig. 4.
The 1st 5 Ig domains of SYG-2 are necessary and sufficient to localize SYG-1. (A) Schematic of SYG-2 and SYG-2 Ig truncation constructs. SYG-2 contains a signal sequence, 9 Ig domains, a fibronectin III domain, a transmembrane domain, and a PDZ binding motif. (B) SYG-1::GFP localization in a syg-2 animal. SYG-1 is diffuse along the entire axon (arrows). (C) SYG-1::GFP localization in a syg-2 animal expressing SYG-2 in the 2° vulval epithelial cells. SYG-1 is redistributed along the regions of contact between HSNL and the 2° vulval epithelial cells (brackets). (D) SNB-1::YFP localization in a syg-2 animal expressing SYG-2 in the 2° vulval epithelial cells. Note that synaptic vesicles are relocalized near secondary epithelial cells (brackets). (E) Schematic of SYG-1 expression when SYG-2 is expressed in the 2° vulval epithelial cells. (F, G) SYG-1::GFP localization in a syg-2 animal expressing SYG-2ΔIg1-3(F) or SYG-2ΔIg4,5 (G) in the 20 vulval epithelial cells. SYG-1 is still diffuse along the axon (arrows). (H-J) SYG-1::GFP localization in a syg-2 animal expressing SYG-2ΔIg6-8(H) or SYG-2ΔIg9,FN3 (I) or SYG-2ΔIg6-9, FN3 (J) in the 2° vulval epithelial cells. SYG-1 is redistributed along the border of the 2° vulval epithelial cells (brackets). (K) Quantification of the SYG-2 truncation constructs. Error bars, standard error of proportion. n>50 in each group. ***p<0.001, Chi-squared test.
Studies of SYG-1 and SYG-2 homologs have begun to yield insights into which domains may be necessary for adhesion and function. It was observed that the transmembrane and cytoplasmic domains of SNS are dispensable for adhesion to Kirre/Duf or IrreC-rst in a cell culture assay, but these domains are necessary for myobolast fusion in vivo (Galletta et al., 2004). A study on the binding interactions between Neph1 and Nephrin suggested that binding between Neph1 and Neprhin is glycoslyation dependent, and that the ability of Neph1 to physically associate with Nephrin in vitro is not confined to a single Ig domain in Neph1 (Gerke et al., 2003). As mentioned before, Neph1 and Neph2 have been shown to physically associate with the PDZ domain of CASK, an established synaptic scaffolding molecule, suggesting that Neph1 and Neph2 might recruit synaptic proteins through interactions with their PDZ domain binding motif (Gerke et al., 2006).
To gain insight into how SYG-1 and SYG-2 act to specify synapses in HSNL, we performed an in vivo structure-function analysis of these proteins to determine which domains were required for their function. Surprisingly, we found that the cytoplasmic domain was dispensable for SYG-1 function in adults, and a single Ig domain of SYG-1 was necessary and sufficient to provide the function of the entire extracellular domain. For SYG-2, the first 5 Igs are necessary and sufficient to localize SYG-1, and the SYG-2 cytoplasmic domain, not including the PDZ binding motif, is required for proper subcellular trafficking of SYG-2. These results define the domains required for heterophilic binding between SYG-1 and SYG-2 in vivo, and suggest that there may be extracellular interactors with SYG-1 that play an important role in SYG-1 mediated synaptic specificity.
Results
The SYG-1 extracellular domain is sufficient to rescue the syg-1 phenotype in adult animals
HSNL, a cholinergic motor neuron, forms synapses with two postsynaptic targets: VC neurons and vulval muscles. The synapses are located in a stereotyped region near the vulva which we have called the primary synaptic region (PSR) (Figs. 1A and C). This synaptic distribution pattern can be visualized in vivo using a SNB-1::YFP fusion protein, which labels synaptic vesicles. In syg-1 and syg-2 mutants, ectopic SNB-1 clusters are formed in a region just anterior to the PSR, which we have termed the secondary synaptic region (SSR) (Figs. 1C and E) (Ding et al., 2007; Shen and Bargmann, 2003; Shen et al., 2004). SYG-1 localizes exclusively to the PSR (Fig. 1B) and acts cell autonomously in HSNL, while SYG-2 acts in the primary vulval epithelial cells to localize SYG-1 (Fig. 1C). SYG-1 and SYG-2 were shown to heterophilically interact using cell culture aggregation assays, and SYG-2 is necessary and sufficient to localize SYG-1 (Shen et al., 2004). These results led to the hypothesis that interaction between the extracellular domains of SYG-1 and SYG-2 triggers signal transduction mediated by the intracellular domain of SYG-1 and eventually causes synapse elimination in the SSR and synapse buildup in the PSR.
To understand which domains of SYG-1 are necessary for SYG-1 function, we created various SYG-1 truncation constructs fused to CFP and used the unc-86 promoter to express these transgenes in the HSN neurons in syg-1(ky652) null mutant animals. We previously showed that the localization of SYG-1 to the PSR is strictly dependent on its interaction with SYG-2; SYG-1 becomes diffusely localized in the absence of SYG-2 (Shen et al., 2004). Hence, we assessed the ability of these SYG-1 truncation constructs to interact with SYG-2 by examining their subcellular localization to the PSR. The full-length SYG-1 is normally localized at the PSR, at the points of contact between the primary vulval epithelial cells expressing SYG-2 and the HSNL axon at the vulva (Figs. 1B and C). SYG-1 contains 5 Immunglobulin-like domains in its extracellular region, a transmembrane domain, and a cytoplasmic tail containing a PDZ binding motif at its C terminus (Fig. 1D). We first asked whether the cytoplasmic domain of SYG-1 was required for its localization to the PSR. Interestingly, a SYG-1Δcyto::CFP construct in HSNL localized at the PSR in a manner indistinguishable from the full-length SYG-1 protein at both L4 and adult stages, indicating that the cytoplasmic domain is dispensable for the subcellular localization of SYG-1 (Figs. 1F and J), and consistent with the notion that SYG-1 is recruited to PSR through its interaction with the SYG-2 extracellular domain.
The function of the SYG-1 deletion constructs can be assayed by their ability to rescue the synaptic phenotype in syg-1 mutants. Surprisingly, the SYG-1Δcyto::CFP construct was able to rescue the syg-1 phenotype robustly in adult animals, as evidenced by the lack of SNB-1 puncta in the SSR (Fig. 1G). Previously, we have shown that HSNL forms synapses both in SSR and in PSR at the L4 stage and the SSR synapses were selectively eliminated when the animals matured. Our molecular genetic analysis indicated that an SCF ubiquitin complex was involved in this synapse elimination (Ding et al., 2007). Inhibition of the SCF activity resulted in more SSR synapses in the L4 stage. Furthermore, the cytoplasmic domain of SYG-1 binds to SKR-1 and inhibits the assembly of the SCF complex, which perturbs synapse elimination at PSR (Ding et al., 2007). This SYG-1 cytosolic domain and SCF-mediated mechanism appears to be effective in the L4 stage since loss-of-function phenotype of either SKR-1 or CUL-1 is more dramatic at the L4 stage, compared to the adult stage (Figs. 3B and C from Ding et al., 2007). Interestingly, both in the skr-1 and cul-1 RNAi animals, synapse elimination still occurs in the adult stage. To address whether the cytoplasmic domain and extracellular domain of SYG-1 act at different time stages, we examined L4 stage syg-1 mutant animals expressing SYG-1Δcyto::CFP. We found that despite the fact that the SYG-1Δcyto::CFP was localized to the PSR in the L4 stage (Fig. 1J), it was unable to rescue the syg-1 phenotype (Figs. 1I, J, L, and M). Previously, we have reported that the levels of SYG-1 have an effect on the number of SSR puncta in the L4 stage. One possibility for our lack of rescue is that the SYG-1Δcyto::CFP is expressed at lower levels than a full-length SYG-1 construct. To explore this possibility, we compared the levels of SYG-1Δcyto::CFP with a SYG-1::CFP construct at L4 stages. Both of these constructs were able to robustly rescue the syg-1 phenotype in adults (data not shown). We found no signi fi cant difference in intensity between these two constructs, suggesting that SYG-1Δcyto::CFP is expressed at comparable levels to full-length SYG-1 (Fig. 1K). Taken together, this data suggests that SYG-1 may have both cytoplasmic tail dependent as well as cytoplasmic tail independent functions that act at different stages of development to specify synapses in HSNL.
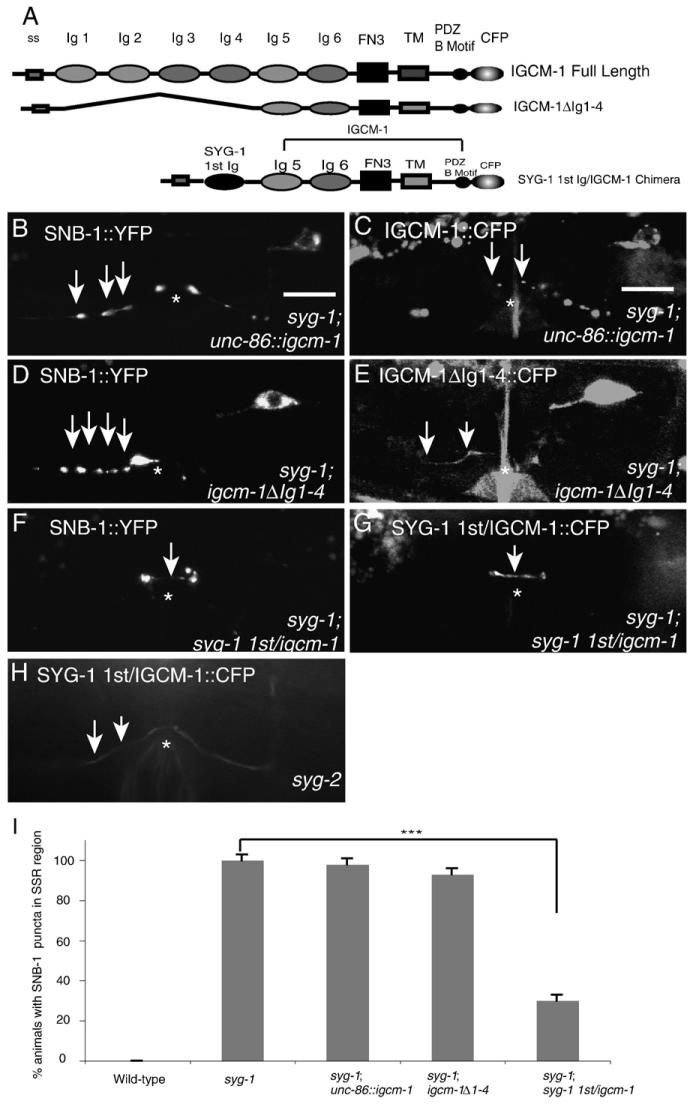
Fig. 3.
The SYG-1 1st Ig domain is sufficient for SYG-1 function in adults. (A) Schematic of SYG-1 sufficiency constructs. (top) Domain structure of IGCM-1. IGCM-1 contains a signal sequence, 6 Ig domains, a fibronectin III domain (FN3), a transmembrane domain, and a PDZ binding motif. (middle) IGCM-1ΔIg1-4 schematic. (bottom) SYG-1 1st/Igcm-1 chimera. The signal sequence and 1st Ig of SYG-1 were fused to the IGCM-protein with the 1st 4 Ig domains deleted (IGCM-1ΔIg1-4). (B) SNB-1::YFP localization in a syg-1 animal expressing IGCM-1::CFP. Ectopic anterior SNB-1 clusters are still present at the SSR (arrows). (C) IGCM-1::CFP localization in HSNL. CFP is found in punctate vesicular structures (arrows). (D) SNB-1::YFP localization in a syg-1 mutant expressing IGCM-1ΔIg1-4 in HSNL. Ectopic anterior SNB-1 clusters are still present at the SSR (arrows). (E) Localization of IGCM-1ΔIg1-4::CFP in HSNL. CFP is mainly trapped in the cell body, with some faint diffuse staining in the axon. (F) SNB-1 localization in syg-1 mutants expressing the SYG-1 1st/IGCM-1 chimera in HSNL. Ectopic SNB-1 clusters are no longer present at the SSR and SNB-1 is localized at the vulva in the PSR (arrow). (G) Localization of SYG-1 1st/IGCM-1::CFP. CFP is localized to the vulva in an identical fashion to SYG-1::GFP (arrow). (H) SYG-1 1st/IGCM-1::CFP localization in a syg-2 mutant. CFP is now diffusely spread out in the axon (arrows). (I) Quantification of the phenotypes. Error bars, standard error of proportion. n>50 in each group. ***p<0.001, Chi-squared test.
The first Ig domain of SYG-1 is necessary for SYG-1 function
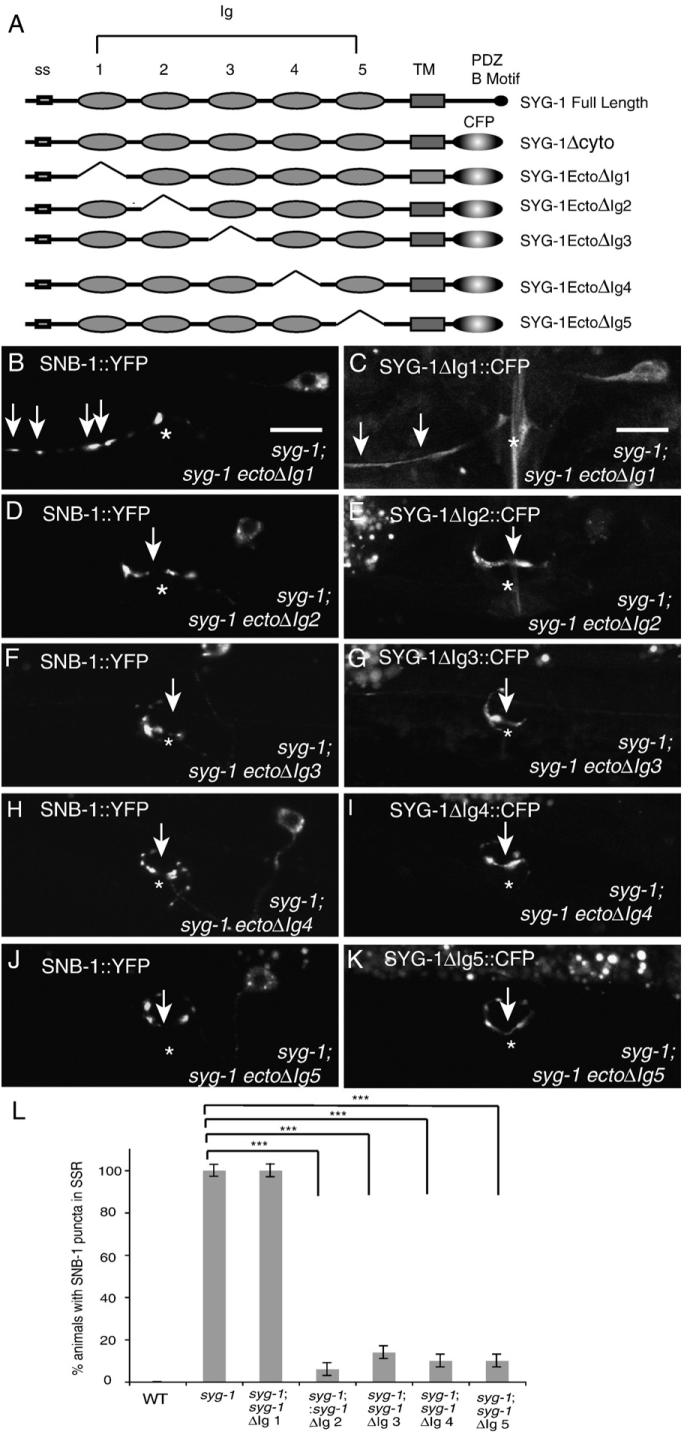
Next, we wished to determine which of the extracellular Immunoglobulin-like domains were required for SYG-1's binding to SYG-2 and for SYG-1's function. SYG-1 extracellular domain truncation constructs deleting each of the Ig domains were created (Fig 2A). A SYG-1 Ecto truncation construct deleting the first Ig domain did not localize to the PSR, but was instead diffusely spread out throughout the axon (Fig. 2C). This construct was also unable to rescue the syg-1 mutant phenotype, as ectopic SNB-1 clusters were still present at the SSR at all stages (Figs. 2B and L). Conversely, deletion constructs for the 2nd, 3rd, 4th and 5th Ig domains of SYG-1 were able to localize to the synaptic region in a manner identical to full-length SYG-1 protein, and were able to robustly rescue the syg-1 phenotype in adult animals (Figs 2D-K and L). Thus, the first Ig domain is required for SYG-1 function, while the rest of the Ig domains are dispensable.
Fig. 2.
The 1st Ig domain of SYG-1 is required for SYG-1 function. (A) Schematic of SYG-1 full length and Ig deletion constructs. (B) SNB-1::YFP expression in a syg-1 animal expressing the SYG-1 Ecto ΔIg 1 construct in HSNL. Ectopic SNB-1 clusters are still present in the SSR (arrows). Asterisk marks the vulva in all images. (C) Localization of SYG-1 EctoΔIg 1 ::CFP. CFP is diffuse throughout the entire axon (arrows). (D, F, H, J) SNB-1::YFP expression in a syg-1 animal expressing the SYG-1 Ecto ΔIg 2 (D), SYG-1 Ecto ΔIg 3 (F), SYG-1 Ecto ΔIg 4 (H), SYG-1 Ecto ΔIg 5 (J) in HSNL. Ectopic SNB-1 clusters are no longer present in the SSR and SNB-1 is localized at the vulva in the PSR(arrow). (E, G, I, K) Localization of SYG-1 Ecto ΔIg 2::CFP (E), SYG-1 Ecto ΔIg 3::CFP (G), SYG-1 Ecto ΔIg 4::CFP (I), SYG-1 Ecto ΔIg 5::CFP (K) protein in HSNL. CFP is localized to the vulva in an identical fashion as SYG-1::GFP. (L) Quantification of rescue in syg-1 truncation constructs. Error bars, standard error of proportion. n>50 in each group. ***p<0.001, Chi-squared test.
The first SYG-1 Ig domain is sufficient for SYG-1 function
After determining that the first Ig is required for SYG-1 function, we wished to determine whether the first Ig domain alone is sufficient for SYG-1 function. A construct expressing only the first Ig domain and the transmembrane domain of SYG-1 did not localize or rescue the syg-1 phenotype (data not shown). One explanation for this result is that this construct is much shorter than the endogenous SYG-1 protein, and the first Ig domain alone might be too short to reach SYG-2 molecules expressed on the guidepost cells. We therefore decided to make a chimeric protein containing the SYG-1 first Ig domain fused to an unrelated Ig protein, in order to mimic the distance from the membrane found in the endogenous SYG-1 1st Ig domain. Ideally, this chimeric protein would have a similar structure to the endogenous SYG-1 protein, yet would neither bind SYG-2 nor have any activity in HSNL. We chose IGCM-1, a C. elegans Ig protein with 6 predicted Ig domains and a Fibronectin III domain in its extracellular region, and a PDZ binding motif at its C terminus (Fig. 3A). We constructed a chimeric protein between the first Ig of SYG-1 and an IGCM-1 fragment lacking the first 4 Igs because the size of this construct most closely mimicked the domain structure of SYG-1 (Fig. 3A).
We first determined whether IGCM-1 had any function in HSNL. Igcm-1(ok711) loss-of-function mutant animals, which are predicted to contain a frame shift mutation leading to a premature stop codon after the first 3 Igs, do not have any morphological synaptic phenotype in HSNL (data not shown), suggesting that igcm-1 is not required for synapse formation in HSNL. We next wished to determine whether full-length IGCM-1 could rescue the syg-1 phenotype or localize to the synapse. Expression of a full-length IGCM-1 protein in HSNL localized to punctate vesicular structures in HSNL (Fig. 3C), and was not able to rescue the syg-1 phenotype (Fig. 3B), suggesting that IGCM-1 could not localize in a manner similar to SYG-1 and could not replace SYG-1 function in HSNL. Next, we wished to determine whether the fragment of IGCM-1 lacking the 1st 4 Ig domains could localize to the synapse or rescue the syg-1 phenotype. IGCM-1Δ1-4 Ig:CFP was mainly trapped in the cell body, with some faint axonal staining (Fig. 3E), and IGCM-1Δ1-4 Ig:CFP was also unable to rescue the syg-1 phenotype (Figs. 3D and H). However, a chimeric protein consisting of the signal sequence and first Ig domain of SYG-1 fused to the IGCM-1Δ1-4 fragment, termed SYG-1 1st Ig/IGCM-1, was able to localize to the synaptic region in a manner identical to the full-length SYG-1::GFP (Fig. 3G). This construct was also able to robustly rescue the syg-1 phenotype in adults (Figs. 3F and J). Additionally, this protein was mislocalized in syg-2 mutants similar to SYG-1 protein (Fig. 3H). Thus, the first Ig domain is sufficient for SYG-1 function in adult animals.
The first 5 Igs of SYG-2 are necessary and sufficient for localizing SYG-1
To assess which domains of SYG-2 are important for function, we created various truncation constructs of SYG-2 and assessed their ability to localize SYG-1 in syg-2(ky671) animals, a null allele. In syg-2(ky671) animals, SYG-1::GFP is diffuse along the entire axon (Fig. 4B). Expression of full-length SYG-2 in the secondary vulval epithelial cells with an egl-17 promoter was sufficient to localize SYG-1 near the secondary cells (Fig. 4C). The subcellular distribution pattern of SYG-1::GFP in these egl-17::syg-2 animals precisely delineates the segment of HSN contacting the secondary cells, strongly suggesting that full-length SYG-2 is sufficient to localize SYG-1 (Fig. 4C and E). In addition, we have previously shown that relocalization of SYG-2 is sufficient to relocalize synaptic vesicles around the secondary epithelial cells ((Shen et al., 2004), Fig. 4D). To address which domains in SYG-2 are required to interact with SYG-1, we expressed the SYG-2 truncation constructs under the egl-17 promoter. We found that expression of SYG-2 constructs deleting the 1st 3 Igs (SYG-2ΔIg1-3) or the 4th and 5th Igs (SYG-2ΔIg4-5) were unable to localize SYG-1 to the secondary cells (Figs. 4F and G), as SYG-1::GFP was still diffuse along the axon. A SYG-2 construct deleting Igs 6-8 (SYG-2ΔIg6-8) was sufficient to localize SYG-1 to the secondary epithelial cells, as was deleting SYG-2 Ig9 and the FNIII domain (Figs. 4H, I and K). This suggests that the 1st 5 Ig domains of SYG-2 are necessary for SYG-2 function.
Finally, we determined whether the 1st 5 Igs of SYG-2 were sufficient for localizing SYG-1. A SYG-2 construct deleting Igs 6-9 as well as the FN3 domain of SYG-2 (SYG-2ΔIg6-9, FN3) was sufficient to relocalize SYG-2 near the secondary epithelial cells (Figs. 4J and K). Thus, Igs 6-8 and the FN3 domain are dispensable for SYG-2 function, and the 1st 5 Igs are the extracellular components of SYG-2 used for localizing SYG-1.
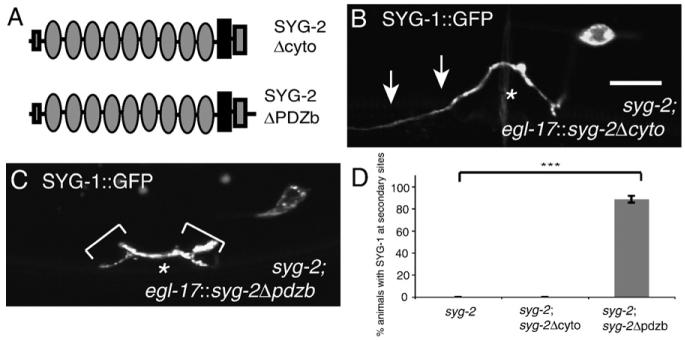
The cytoplasmic domain of SYG-2, but not the PDZ binding motif is required to localize SYG-1
To determine what components of the cytoplasmic domain of SYG-2 are necessary for SYG-2 function, we created truncation constructs of SYG-2 deleting the cytoplasmic tail as well as the PDZ binding motif and expressed them in the vulval secondary epithelial cells using the egl-17 promoter (Fig. 5A). A SYG-2 construct deleting the cytoplasmic domain was not sufficient to localize SYG-1 to the secondary vulval epithelial cells (Figs. 5B and D). This suggests that the SYG-2 cytoplasmic domain is required for localizing SYG-1. To determine what components of the cytoplasmic domain might be important for SYG-2 function, we created a SYG-2 construct that deleted the PDZ binding motif, which is the only well conserved cytoplasmic sequence among SYG-2 and its homologs. A SYG-2 construct deleting the PDZ binding motif was sufficient to localize SYG-1 to secondary cells, suggesting that the PDZ binding motif is dispensable for the ability of SYG-2 to localize SYG-1 (Figs. 5C and D).
Fig. 5.
The cytoplasmic domain, but not the PDZ binding motif, is required for SYG-2 function. (A) Schematic of SYG-2Δcyto and SYG-2ΔPDZ binding motif constructs. (B) SYG-1::GFP localization in a syg-2 animal expressing SYG-2Δcyto in the 2° vulval epithelial cells. SYG-1 is still diffuse along the axon (arrows). (C) SYG-1::GFP localization in a syg-2 animal expressing SYG-2ΔPDZ binding motif in the 2° vulval epithelial cells. SYG-1 is redistributed along the borders of the 2° vulval epithelial cells (brackets). (D) Quantification of SYG-2 truncation phenotypes. Error bars, standard error of proportion n>50 in each group. ***p<0.001, Chi-squared test.
The SYG-2 cytoplasmic domain is required for proper localization and endocytosis
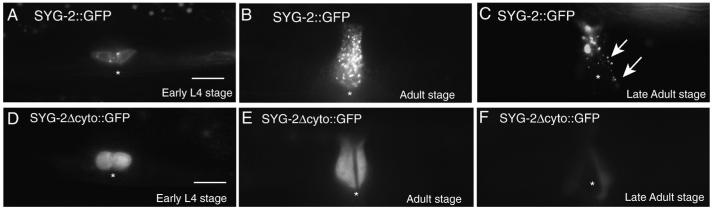
To better understand the role of the cytoplasmic domain in SYG-2 function, we asked whether the cytoplasmic domain of SYG-2 was necessary for the proper trafficking and subcellular localization of SYG-2. Using a functional SYG-2::GFP transgene, we examined the localization of SYG-2 in several different developmental stages. In the early L4 stage, SYG-2 is expressed on the primary vulval epithelial cells, where it is nucleus excluded, with a few puncta near the cortical areas of the cells (Fig. 6A). In the young adult stages, SYG-2 is sequestered in a large number of punctate structures (Fig. 6B). In later adult stages, SYG-2 expression is downregulated and a few punctate structures remain (Fig. 6C). In contrast, a SYG-2Δcyto::GFP construct displayed homogenous staining in early L4 stage (Fig. 6D), and continued to exhibit homogenous staining pattern in the adult stage (Fig. 6E), and then was downregulated (Fig. 6F). This suggests that the cytoplasmic domain of SYG-2 is necessary for its proper subcellular localization.
Fig. 6.
The SYG-2 cytoplasmic domain is required for surface expression on epithelial cells as well as for endocytosis. (A) SYG-2::GFP localization in 1° vulval epithelial cells at early L4 stage. SYG-2 is expressed on the primary vulval epithelial cells, where it is nuclear excluded, with some puncta near the cortical areas of the cells. (B) SYG-2::GFP localization at the young adult stage. SYG-2 is sequestered into puncta. (C) SYG-2::GFP expression at the late adult stage. SYG-2 expression is downregulated and a few puncta remain (arrows). (D) SYG-2Δcyto::GFP at the early L4 stage. SYG-2 is homogeneous throughout the cell. (E) SYG-2Δcyto::GFP at the young adult stage. SYG-2 is still homogeneous throughout the cell and not sequestered as in (B). (F) SYG-2Δcyto::GFP at the late adult stage. SYG-2Δcyto::GFP expression is downregulated and still homogenous throughout the cell.
Discussion
We have conducted a systematic functional domain analysis of SYG-1 and SYG-2 in vivo in order to define domains that are necessary for their function. We found that the SYG-1 Ig1 and SYG-2 Ig1-5 are the critical domains that mediate the interactions between these two adhesion molecules in vivo. Interestingly, the cytosolic domain of SYG-2 is required for its subcellular distribution and function, while the cytosolic domain of SYG-1 is required for SYG-1's function in the L4 stage but dispensable for its function in later stages.
The SYG-1 cytoplasmic domain is dispensable for SYG-1 function in adults
Our in vivo structure-function analysis demonstrates that the cytoplasmic domain is dispensable for SYG-1 function in adults. This suggests that interactions with the extracellular domain of SYG-1 and an unknown “co-receptor” for SYG-1 may be necessary for SYG-1 mediated specification of synapses. This result is surprising in light of our previous results demonstrating the importance of the cytoplasmic tail of SYG-1 in preventing synapse elimination by inhibiting SCF ubiquitin complex activity at the PSR. Previously, we found that the cytoplasmic domain of SYG-1 physically associates with SKR-1, a component of the SCF ubiquitin complex, and loss of function of either skr-1 or sel-10, two components of the SCF ubiquitin complex, yields phenotypes similar to syg-1. Interestingly, phenotypic analysis of skr-1 and sel-10 loss of function demonstrates a strong effect in the L4 stage, with a relatively small effect at the adult stage on the elimination of SSR puncta. The SYG-1Δcyto construct can fully rescue the syg-1 phenotype in adults but has no rescuing activity in the L4 stage, suggesting that cytoplasmic domain-dependent and -independent functions of SYG-1 may be temporally separated. These results are consistent with a model where the functions of SYG-1 in synaptic target selection are carried out by two downstream pathways, a cytoplasmic domain-dependent pathway that regulates synapse elimination by inhibiting SCF ubiquitin complex activity at the L4 stage, and a cytoplasmic domain-independent pathway at the adult stage. Because the SYG-1 extracellular domain alone is capable of rescuing the terminal phenotype of syg-1 in adults, it is plausible that the cytoplasmic domain-independent function of SYG-1 is the most critical process for SYG-1 function. One caveat of such an analysis is that we have used the exogenous unc-86 promoter to drive expression of SYG-1 truncation constructs. It is conceivable that the endogenous level of SYG-1 is much lower than the unc-86::syg-1 transgenic animals, under which conditions the cytosolic domain-dependent pathway may play a more important function. One possible mechanism for the cytosolic independent pathway is that the extracellular domain of SYG-1 recruits a “co-receptor” of SYG-1, which has a redundant function with the SYG-1 cytoplasmic tail in preventing synapse elimination, or it may act in a completely parallel molecular pathway.
This result is also surprising in light of the fact that all other SYG-1 homologs require their cytoplasmic tails for function. In particular the PDZ binding motif at the C terminus of SYG-1 is highly conserved among all its homologs and has been demonstrated to have physical interactions of functional importance. The mammalian homologs of SYG-1, Neph1 have been shown to bind the PDZ proteins ZO-1 and CASK (Gerke et al., 2006; Huber et al., 2003) and Drosophila IrreC-Rst has been shown to bind the PDZ protein Dmint (Vishnu et al., 2006). It is possible that different domains of SYG-1 are required for different functions of SYG-1 throughout evolution. One precedent for such diverging evolution has been shown for lin-18, a Ryk Wnt receptor, whose conserved intracellular domain is dispensable for polarization of P7.p cells in C. elegans (Inoue et al., 2004), although the Drosophila homolog of lin-18 Derailed requires the intracellular domain for axon repulsion (Yoshikawa et al., 2003).
Heterophilic binding requirements between SYG-1 and SYG-2
Our data suggest that the first Ig domain of SYG-1 and the first 5 Ig domains of SYG-2 are necessary and sufficient for localization and function in vivo. The requirement for a single Ig domain and 5 Ig domains for heterophilic interaction has not been reported among Ig heterophilic interactions studied. The requirement for the most N terminal Ig domains being sufficient for binding specificity is consistent with other structure-function studies done on Ig domain proteins. However, multiple Ig domains are usually required for binding. For instance, the 1st 3 Igs of NCAM are sufficient for homophilic binding, while the 1st 4 Ig domains are sufficient for binding between F11 and NgCAM, and the 1st 4 Ig domains are sufficient for binding of Axonin-1 to NgCAM (Brummendorf and Rathjen, 1996). The requirement for a single N terminal Ig domain sufficient for binding specificity is most similar to the protein tyrosine phosphatase RPTPµ (Brummendorf and Rathjen, 1996). The sufficiency for a single N terminal domain is also reported for the cadherin superfamily of molecules, where the most N terminal EC1 domains of cadherins provide cadherin binding specificity. (Pokutta and Weis, 2007). It is interesting to note that our results seems to differ from experiments done with the mammalian homologs of SYG-1, as at least two Ig domains of Neph1 seem to be necessary and sufficient for binding to Nephrin in culture (Gerke et al., 2003). The requirement of 5 Ig domains of SYG-2 for binding suggests simple head to head binding to SYG-1 is not occurring, but there is instead a more complex interaction. Future work will lead to a better understanding of the structural basis of heterophilic interaction between SYG-1 and SYG-2.
The cytoplasmic domain of SYG-2 is required for its subcellular localization
We demonstrate that the SYG-2 cytoplasmic domain, but not its PDZ binding motif, is necessary for SYG-2 function. We also demonstrate that a truncated form of SYG-2 protein deleting the cytoplasmic domain has an altered subcellular localization compared to full-length SYG-2. We propose that the cytoplasmic tail of SYG-2 is necessary for subcellular localization as well as sequestering of SYG-2. This is consistent with the fact that the cytosolic tail of Sns, is required in vivo for its function (Galletta et al., 2004).
Taken together, this study has defined domains of SYG-1 and SYG-2 necessary for heterophilic interaction as well as synapse function. Future work will allow us to better understand the structural basis of heterophilic interactions between IgCAMs, as well as better understand the molecular pathways by which SYG-1 and SYG-2 mediate synaptic specificity.
Experimental methods
Strains and genetics
Strains were grown at either 20 °C or 25 °C and maintained as described. Mutant strains syg-1(ky652), and syg-2(ky671) were used.
Mutants
LGX, syg-1(ky652); syg-2(ky671.
Molecular biology
Deletions in SYG-1 were created by PCR using overlapping fragments that flank the deletion area. The domain structure of SYG-1 was predicted based on alignments of SYG-1 with IrreC-Rst and Neph1. We have determined that the SYG-1 domain structure contains a signal sequence, 5 Immunoglobulin domains, a transmembrane domain, and a PDZ binding motif. Domain boundaries were predicted using the SMART program (http://smart.embl-heidelberg.de/) and alignments with IrreC-Rst and Neph1. SYG-1 deletion fragments were then ligated between Nhe1 and Kpn1 in the C-Terminal CFP pSM vector containng the unc-86 promoter. SYG-1 deletion fragments were then sequenced for mutations.
SYG-1Δcyto (Amino acids 1-574 of SYG-1);
SYG-1 Ecto Δ1st Ig (Amino acids 1-574 of SYG-1 with AA 35-111 deleted)
SYG-1 Ecto Δ2nd Ig (Amino acids 1-574 of SYG-1 with AA 131-278 deleted)
SYG-1 Ecto Δ3rd Ig (Amino acids 1-574 of SYG-1 with AA 283-343 deleted)
SYG-1 Ecto Δ 4th Ig (Amino acids 1-574 of SYG-1 1-574 with AA 369-427 deleted)
SYG-1 EctoΔ 5th Ig (Amino acids 1-574 of SYG-1 with AA 453-526 deleted)
Chimeric proteins between IGCM-1 and SYG-1 were created using overlapping PCR fragments. The domain structure of IGCM-1 was predicted using the SMART program. Chimeric protein fragments were then cloned into pENTR TOPO vector (Invitrogen) and recombined into a C-terminal CFP pSM Gateway Destination vector with LR Clonase (Invitrogen).
SYG-1 1st Ig/Igcm-1-(Amino acids 1-130 of SYG-1 and Amino acids 503-1074 of IGCM-1)
SYG-1 2nd Ig/Igcm-1 (Amino acids 1-36 and 134-284 of SYG-1 and Amino acids 503-1074 of IGCM-1)
IGCM-1Δ1-4 Ig (Amino acids 1-1074 of IGCM-1 with Amino acids 34-502 deleted)
IGCM-1 (Amino acids 1-1074 of IGCM-1)
Deletions in SYG-2 DNA were created through overlapping PCR fragments which flank the deletion. The domain structure of SYG-2 was predicted based on alignments with Sns, Hibris, and Nephrin. We have concluded that SYG-2 consists of a signal sequence, 9 Immunoglobulin domains, a transmembrane domain, and a PDZ binding motif. Domain boundaries were predicted using the SMART program and alignments of SYG-2 with Sns, Hibris, and Nephrin. SYG-2 deletion fragments were then cloned into the pCR8 GW TOPO vector. (Invitrogen), and then recombined into a C-terminal CFP pSM Gateway Destination vector with LR Clonase (Invitrogen). SYG-2 deletions were then sequenced to make sure there were no mutations.
SYG-2 Δ1-3 Ig (Amino acids 1-1270 with AA 31-315 deleted)
SYG-2Δ4,5Ig (Amino acids 1-1270 with AA 316-582 deleted)
SYG-2Δ6-8 Ig (Amino acids 1-1270 with AA 597-857 deleted)
SYG-2Δ9 Ig, FN3 (Amino acids 1-1270 with AA 886-1052 deleted)
SYG-2Δ cyto (Amino acids 1-1106 of SYG-2)
SYG-2Δ PDZ (Amino acids 1-1268 of SYG-2)
SYG-2 ΔIg6-9 Ig, FN3 (Amino acids 1-1270 with AA597-1052 deleted)
Intensity measurements
20 images were taken of each genotype using a Zeiss Axioplan 2 compound microscope. Average intensity of PSR was then determined using Image J.
Trangenic lines and fluorescence microscopy
Germline transformation was performed by injecting DNA plasmids into the gonads of adult worms at concentrations of 0.1-0.5 µg/µl for syg-1 deletion constructs and 20 ng/µl for Egl-17 syg-2 deletion constructs, with 10-20 µg/µl of odr-1 GFP as a co-injection marker. Multiple transgenic lines were examined for localization and rescue, and a representative transgenic line was then quantified. Fluorescence images were obtained using a Zeiss LSM 510 META laser scanning confocal imaging system or a Zeiss Axioplan 2 compound microscope.
Acknowledgments
We acknowledge Y. Kohara for the syg-1 and syg-2 cDNAs, the CGC Knockout Consortium for the Igcm-1(ok711) allele, the Shen lab for helpful discussions, and T. Cutforth for the critical reading of the manuscript.
References
- Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Developmental Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Current Opinion in Neurobiology. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Carthew RW. Pattern formation in the Drosophila eye. Current Opinion in Genetics & Development. 2007;17:309–313. doi: 10.1016/j.gde.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Letters. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. (New York, N.Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951. doi: 10.1126/science.1145727. (New York, N.Y. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, Mathur BN, Turner CA, Geske R, Montgomery CA, Starbuck M, Brandt M, Gupta A, Ramirez-Solis R, Zambrowicz BP, Powell DR. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol. Cell. Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworak HA, Sink H. Myoblast fusion in Drosophila. Bioessays. 2002;24:591–601. doi: 10.1002/bies.10115. [DOI] [PubMed] [Google Scholar]
- Galletta BJ, Chakravarti M, Banerjee R, Abmayr SM. SNS: adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mechanisms of Development. 2004;121:1455–1468. doi: 10.1016/j.mod.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Gerke P, Huber TB, Sellin L, Benzing T, Walz G. Homodimerization and heterodimerization of the glomerular podocyte proteins nephrin and NEPH1. J. Am. Soc. Nephrol. 2003;14:918–926. doi: 10.1097/01.asn.0000057853.05686.89. [DOI] [PubMed] [Google Scholar]
- Gerke P, Benzing T, Hohne M, Kispert A, Frotscher M, Walz G, Kretz O. Neuronal expression and interaction with the synaptic protein CASK suggest a role for Neph1 and Neph2 in synaptogenesis. J. Comp. Neurol. 2006;498:466–475. doi: 10.1002/cne.21064. [DOI] [PubMed] [Google Scholar]
- Huber TB, Schmidts M, Gerke P, Schermer B, Zahn A, Hartleben B, Sellin L, Walz G, Benzing T. The carboxyl terminus of Neph family members binds to the PDZ domain protein zonula occludens-1. J. Biol. Chem. 2003;278:13417–13421. doi: 10.1074/jbc.C200678200. [DOI] [PubMed] [Google Scholar]
- Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, Katz WS, Sternberg PW. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Molecular Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, Tryggvason K. Nephrin promotes cell-cell adhesion through homophilic interactions. Am. J. Pathol. 2003;163:2337–2346. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. Journal of Clinical Investigation. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annual Review of Cell and Developmental biology. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- Ramos RG, Igloi GL, Lichte B, Baumann U, Maier D, Schneider T, Brandstatter JH, Frohlich A, Fischbach KF. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes & Development. 1993;7:2533–2547. doi: 10.1101/gad.7.12b.2533. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Takeuchi H, Yamagishi Y, Suzuki M, Sakano H. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127:1057–1069. doi: 10.1016/j.cell.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Srinivas BP, Woo J, Leong WY, Roy S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nature Genetics. 2007;39:781–786. doi: 10.1038/ng2055. [DOI] [PubMed] [Google Scholar]
- Vishnu S, Hertenstein A, Betschinger J, Knoblich JA, Gert de Couet H, Fischbach KF. The adaptor protein X11Lalpha/Dmint1 interacts with the PDZ-binding domain of the cell recognition protein Rst in Drosophila. Developmental Biology. 2006;289:296–307. doi: 10.1016/j.ydbio.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]