Abstract
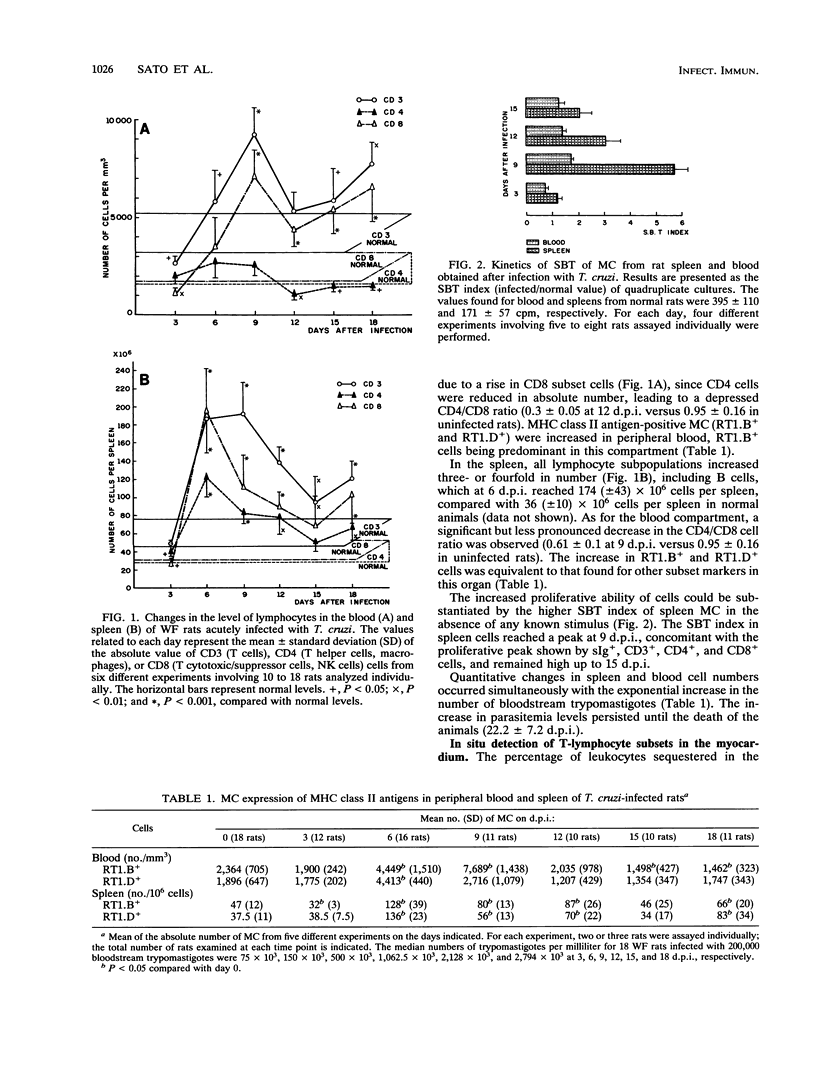
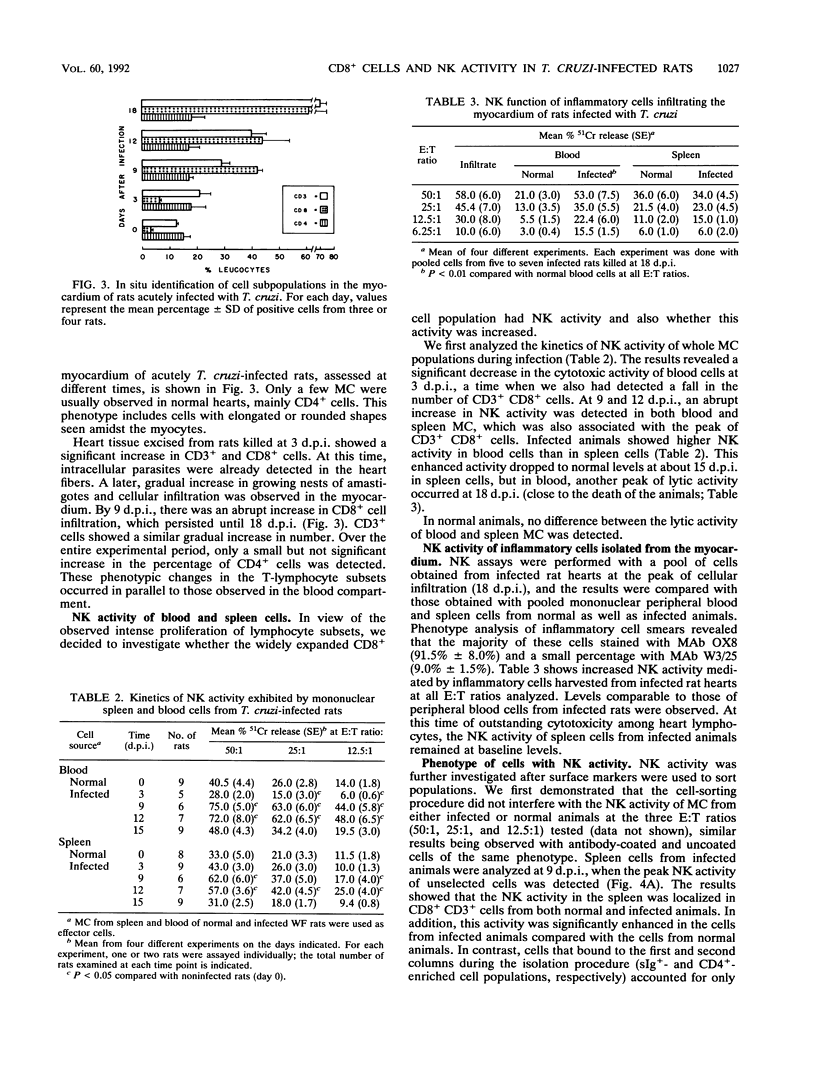
The infection developed by Wistar Furth rats inoculated with the Y strain of Trypanosoma cruzi was the experimental model used in our study. The results showed that this infection altered considerably the CD4/CD8 lymphocyte subset ratio and the natural cytotoxic activity of mononuclear cells in the spleen, blood, and myocardial tissue. Concomitantly, an expansion of the number of cells expressing major histocompatibility complex (MHC) class II antigens was observed, as well as spontaneous development of high levels of blast cells, mainly in the spleen. The inflammatory infiltration of the myocardium, made up essentially of CD8+ cells (cytotoxic/suppressor T cells, natural killer cells), was initially found at 9 days postinfection, spread continuously, and was observed until the death of the animals at about 18 days postinfection. T. cruzi infection also enhanced the natural killer activity of mononuclear cells in the blood, spleen, and myocardium. Sorting these cells by affinity columns showed that the natural killer function was performed exclusively by the CD8+ population, which did not express MHC class II antigens. It was shown that the polyclonal T-lymphocyte activation induced by T. cruzi infection results in a wide distribution of CD8+ cells with enhanced natural cytotoxic activity in the spleen, blood, and cardiac tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Hatcher F. M., Albright J. F. Interaction between murine natural killer cells and trypanosomes of different species. Infect Immun. 1984 May;44(2):315–319. doi: 10.1128/iai.44.2.315-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P., Scala G., Djeu J. Y., Procopio A. D., Oppenheim J. J., Herberman R. B., Ortaldo J. R. Production of multiple cytokines by clones of human large granular lymphocytes. Cancer Immunol Immunother. 1985;19(2):121–126. doi: 10.1007/BF00199719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Younès-Chennoufi A., Said G., Eisen H., Durand A., Hontebeyrie-Joskowicz M. Cellular immunity to Trypanosoma cruzi is mediated by helper T cells (CD4+). Trans R Soc Trop Med Hyg. 1988;82(1):84–89. doi: 10.1016/0035-9203(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Flannery G. R., Cantrell D. A., Gray J. D., Robins R. A., Baldwin R. W. Rat NK cells active against lymphoma and sarcoma tumor cells are probably identical. J Immunol. 1982 Feb;128(2):913–919. [PubMed] [Google Scholar]
- Cantrell D. A., Robins R. A., Brooks C. G., Baldwin R. W. Phenotype of rat natural killer cells defined by monoclonal antibodies marking rat lymphocyte subsets. Immunology. 1982 Jan;45(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- Duarte A. J., Carpenter C. B., Strom T. B. Expression of T cell differentiation antigens and Ia on rat cytotoxic T lymphocytes. J Immunol. 1982 Feb;128(2):580–584. [PubMed] [Google Scholar]
- Goldberg S. S., Cordeiro M. N., Silva Pereira A. A., Mares-Guia M. L. Release of lipopolysaccharide (LPS) from cell surface of Trypanosoma cruzi by EDTA. Int J Parasitol. 1983 Feb;13(1):11–18. doi: 10.1016/s0020-7519(83)80062-9. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher F. M., Kuhn R. E. Destruction of Trypanosoma cruzi by Natural killer cells. Science. 1982 Oct 15;218(4569):295–296. doi: 10.1126/science.6812218. [DOI] [PubMed] [Google Scholar]
- Hidore M. R., Murphy J. W. Murine natural killer cell interactions with a fungal target, Cryptococcus neoformans. Infect Immun. 1989 Jul;57(7):1990–1997. doi: 10.1128/iai.57.7.1990-1997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson-Parra A., Forsum U., Klareskog L., Sjöberg O. A simple immunoenzyme batch staining method for the enumeration of peripheral human T lymphocyte subsets. J Immunol Methods. 1983 Nov 11;64(1-2):85–90. doi: 10.1016/0022-1759(83)90386-1. [DOI] [PubMed] [Google Scholar]
- Kierszenbaum F., Gharpure H. M. Killing of circulating forms of Trypanosoma cruzi by lymphoid cells from acutely and chronically infected mice. Int J Parasitol. 1983 Aug;13(4):377–381. doi: 10.1016/s0020-7519(83)80044-7. [DOI] [PubMed] [Google Scholar]
- Kobayakawa T., Louis J., Izui S., Lambert P. H. Autoimmune response to DNA, red blood cells, and thymocyte antigens in association with polyclonal antibody synthesis during experimental African trypanosomiasis. J Immunol. 1979 Jan;122(1):296–301. [PubMed] [Google Scholar]
- Minoprio P., Burlen O., Pereira P., Guilbert B., Andrade L., Hontebeyrie-Joskowicz M., Coutinho A. Most B cells in acute Trypanosoma cruzi infection lack parasite specificity. Scand J Immunol. 1988 Nov;28(5):553–561. doi: 10.1111/j.1365-3083.1988.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Minoprio P., Itohara S., Heusser C., Tonegawa S., Coutinho A. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol Rev. 1989 Dec;112:183–207. doi: 10.1111/j.1600-065x.1989.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz L., Parks D. E., Rodriguez M., Weigle W. O. Polyclonal B lymphocyte activation during Trypanosoma cruzi infection. J Immunol. 1980 Jan;124(1):121–126. [PubMed] [Google Scholar]
- Peters P. M., Ortaldo J. R., Shalaby M. R., Svedersky L. P., Nedwin G. E., Bringman T. S., Hass P. E., Aggarwal B. B., Herberman R. B., Goeddel D. V. Natural killer-sensitive targets stimulate production of TNF-alpha but not TNF-beta (lymphotoxin) by highly purified human peripheral blood large granular lymphocytes. J Immunol. 1986 Oct 15;137(8):2592–2598. [PubMed] [Google Scholar]
- Sanderson C. J., de Souza W. A morphological study of the interaction between Trypanosoma cruzi and rat eosinophils, neutrophils and macrophages in vitro. J Cell Sci. 1979 Jun;37:275–286. doi: 10.1242/jcs.37.1.275. [DOI] [PubMed] [Google Scholar]
- Sato M. N., Yamashiro-Kanashiro E. H., Tanji M. M., Kaneno R., Iqueoka R. Y., Duarte A. J. Immunomodulatory effect of cimetidine on the proliferative responses of splenocytes from T. cruzi-infected rats. Rev Inst Med Trop Sao Paulo. 1991 May-Jun;33(3):187–192. doi: 10.1590/s0036-46651991000300004. [DOI] [PubMed] [Google Scholar]
- Steiniger B., Klempnauer J., Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. doi: 10.1097/00007890-198408000-00016. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta F., Kierszenbaum F. Role of inflammatory cells in Chagas' disease. I. Uptake and mechanism of destruction of intracellular (amastigote) forms of Trypanosoma cruzi by human eosinophils. J Immunol. 1984 Apr;132(4):2053–2058. [PubMed] [Google Scholar]
- d'Imperio Lima M. R., Eisen H., Minoprio P., Joskowicz M., Coutinho A. Persistence of polyclonal B cell activation with undetectable parasitemia in late stages of experimental Chagas' disease. J Immunol. 1986 Jul 1;137(1):353–356. [PubMed] [Google Scholar]



