Abstract
Stable isotope tracer experiments of human muscle amino acid and protein kinetics often involve a sequential design, with the same subject studied at baseline and during an intervention. However, prolonged fasting and sequential muscle biopsies from the same area could theoretically affect muscle protein metabolism. The purpose of this study was to determine if sequential muscle biopsies and extended fasting significantly affect parameters of muscle protein and amino acid kinetics in six human subjects. After a 12-h overnight fast, a primed continuous infusion of l-[ring-2H5]phenylalanine was started. After 120 min, we took the first of a series of five hourly muscle biopsies from the same vastus lateralis to measure mixed muscle protein fractional synthetic rate. Furthermore, between 150–180, 210–240, and 330–360 min, we measured leg phenylalanine kinetics using the two-pool and the three-pool arteriovenous balance models. Tracer enrichments were at steady state, and muscle protein FSR and phenylalanine kinetics did not change throughout the experiment (P = not significant). We conclude that a 6-h tracer infusion during extended fasting (up to 18 h) with five sequential muscle biopsies from the same muscle do not affect basal mixed muscle protein synthesis and muscle phenylalanine kinetics in human subjects. Thus, when using a sequential study design over this period of time, it is unnecessary to include a saline only control group to account for these variables.
Keywords: stable isotopes, muscle protein turnover, tracer kinetics, muscle biopsies
stable isotope studies of human muscle amino acid and protein kinetics commonly involve a sequential study design, with the same subject being studied at baseline and during an intervention within the same session. This design allows to control for day-to-day individual metabolic variability and accounts for individual differences in amino acid kinetics, thereby increasing statistical power (for example, see Refs. 1, 2, 7, 12, 13, 20, 21, 23, 24, 26, 27). However, prolonged fasting and sequential muscle biopsies from the same area could theoretically affect muscle protein metabolism.
The experimental design typically involves an overnight fast followed by a primed-continuous tracer infusion extended over several hours during which the subjects may not receive nutrients. Prolonged fasting may impact muscle protein kinetics due to metabolic and hormonal adaptations that occur to maintain an adequate energy and amino acid supply to other tissues and organs, particularly brain and heart (10). Furthermore, when biopsies are taken sequentially from the same muscle area, it is theoretically possible that the earlier biopsies may produce an inflammatory or stress response in the surrounding tissue, which in turn can affect the turnover rate of the muscle tissue removed by later biopsies. Although a recently published paper indicates that mRNA expression is not influenced by repeated muscle biopsies (18), this does not exclude that amino acid turnover, including release from breakdown and transport across the cell membrane, is influenced by the biopsy procedure.
One possible approach to this problem is to include in each experiment a control group receiving no treatment (e.g., saline infusion) throughout the entire tracer infusion. However, aside from being costly and time consuming, muscle protein kinetics experiments are fairly invasive, since they involve, at the very least, muscle biopsies and oftentimes femoral or forearm arteriovenous catheterization.
Therefore, we tested whether extended fasting (beyond the physiological 12-h overnight fast) and sequential muscle biopsies can significantly alter skeletal muscle protein or amino acid kinetics to establish the necessity of including a control group in muscle protein kinetics experiments with sequential design.
SUBJECTS AND METHODS
Subjects.
We studied six young subjects (1 woman and 5 men) from the Houston/Galveston metropolitan area. All subjects were healthy and physically active, but they were not engaged in a formal exercise training program. The female subject was studied during the follicular phase. Screening of subjects was performed with clinical history, physical exam, and laboratory tests. Only subjects with normal screening results were included in the experiment. All subjects gave informed written consent before participating in the study, which was approved by the Institutional Review Board of the University of Texas Medical Branch (Galveston, TX).
Study design.
The night before the study, each subject was admitted to the General Clinical Research Center of the University of Texas Medical Branch. At admission, a pregnancy test was done in the female subject. Subjects were fed a standard dinner at 1900 (12 kcal/kg, 60% carbohydrate, 20% protein, 20% fat), after which they were allowed only water ad libitum until the end of the experiment, at 1300 the next day. The total length of fasting by the end of the experiment was 18 h.
The morning of the study, polyethylene catheters were inserted in a forearm vein for tracer infusion, in a contralateral hand or wrist vein for arterialized blood sampling, and in the femoral artery and vein of one leg for blood sampling. The femoral arterial catheter was also used to infuse indocyanine green (ICG; Akorn, Buffalo Grove, IL) for measurement of leg blood flow.
At 0700, after drawing a blood sample for the measurement of background amino acid enrichments and ICG concentration, a primed continuous infusion of l-[ring-2H5]phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was started and maintained at a constant rate until the end of experiment. The priming dose was 2 μmol/kg, and the infusion rate was 0.05 μmol·kg−1·min−1. After 120 min, a first muscle biopsy was taken from the lateral portion of the vastus lateralis of the leg with the femoral catheters, ∼20 cm above the knee, using a 5-mm Bergström biopsy needle, sterile procedure, and local anesthesia with 1% Lidocaine injected subcutaneously and on the fascia. The muscle sample, ∼100 mg, was rinsed with ice-cold saline and blotted. Any visible fat or connective tissue was quickly removed, immediately frozen in liquid nitrogen, and stored at −80°C until analysis. Four sequential hourly biopsies were repeated at 180, 240, 300, and 360 min. The first three biopsies were taken from the same incision, with the biopsy needle inserted at a different angle each time to remove the muscle tissue samples from areas at least 2–3 cm apart. The last two biopsies were taken from another incision, ∼5 cm above the first incision, with the biopsy needle inserted at a different angle as described above. Each biopsy was taken with a single needle pass.
Blood samples were collected from femoral artery and vein and hand/wrist vein during three periods (150–180, 210–240, and 330–360 min) to measure phenylalanine enrichments and concentrations and ICG concentration.
Analyses.
Serum ICG concentration for the determination of leg blood flow was measured spectrophotometrically (Beckman Coulter, Fullerton, CA) at λ = 805 nm (16, 17).
Concentrations and enrichments of blood and intracellular free phenylalanine were determined by gas chromatography mass-spectrometry (GCMS, 6890 Plus GC, 5973N MSD/DS, 7683 autosampler; Agilent Technologies, Palo Alto, CA) as previously described (28).
Mixed muscle protein-bound phenylalanine enrichment was determined by GCMS after protein hydrolysis and amino acid extraction using the external standard curve approach (4).
Calculations.
The muscle phenylalanine kinetic parameters were calculated using two different methods: the two-pool model (28) and the three-pool model (3).
The two- and the three-pool model shared the following parameters:
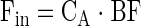 |
(1) |
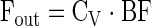 |
(2) |
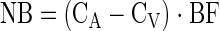 |
(3) |
where Eq. 1 is used to calculate delivery to the leg, Eq. 2 release from the leg, and Eq. 3 leg net balance. Fin and Fout are delivery to the leg and release from the leg, respectively. CA and CV are the plasma amino acid concentrations in the femoral artery and vein, respectively. NB is leg net balance, and BF is leg blood flow as calculated from the steady-state ICG concentration values in the femoral and wrist veins, as previously described (16, 17).
The other kinetic parameters of the two-pool method were calculated as follows. Total leg rate of appearance (Ra) was calculated as:
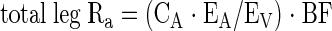 |
(4) |
where EA and EV are the amino acid enrichments, expressed as tracer-to-tracee ratio, in the femoral arterial and venous plasma, respectively.
Release in the vein from proteolysis is equal to leg Ra, which is the flux of phenylalanine deriving from muscle protein breakdown released in the venous blood, and is calculated as
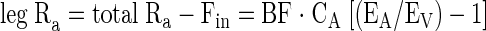 |
(5) |
Disappearance from arterial blood is equal to leg rate of disappearance (Rd), which is the rate at which phenylalanine is taken up from the blood and used for muscle protein synthesis, and is calculated as
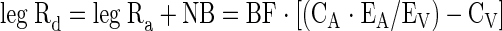 |
(6) |
Data are expressed per 100 ml of leg volume as measured with an anthropometric method (15).
Next, the specific parameters of the three-pool model were calculated. Tissue inward transport was calculated as:
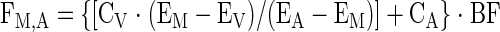 |
(7) |
where EM is the amino acid enrichment, expressed as the tracer-to-tracee ratio, in the muscle.
Tissue outward transport, release from proteolysis, and utilization for protein synthesis were calculated using Eqs. 8, 9, and 10, respectively, as follows:
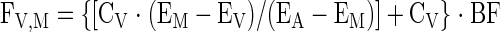 |
(8) |
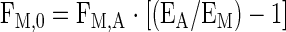 |
(9) |
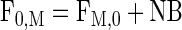 |
(10) |
where FM,0 is the amount of amino acids that appear in the muscle tissue from protein breakdown, and F0,M is the total amount of amino acids utilized for muscle protein synthesis.
We also calculated the fractional synthetic rate (FSR) of mixed muscle proteins using the precursor-product model (25):
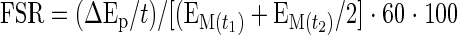 |
(11) |
where ΔEp is the increment in protein-bound phenylalanine enrichment between two sequential biopsies, t is the time between the two biopsies, 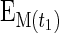 and
and 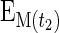 are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
are the phenylalanine enrichments in the free intracellular pool in the two sequential biopsies. Data are expressed as percent per hour.
Statistical analysis.
All values are expressed as means ± SE. Comparisons were performed using both analysis of variance with repeated measures, the effects being subject and time, and the Dunnett's test, using the first measure as control. Significance was set at P < 0.05. One possible concern with taking repeated biopsies is that the calculated or actual synthesis and breakdown rates might change over time as a result of the biopsies. To determine whether there was a systemic change in synthesis or breakdown over time, we performed for each subject a linear regression of F0,M, FM,0, net balance, and FSR vs. time. We then calculated the resulting average slope between subjects and tested whether this slope was different from zero via F-test. Finally, using the actual variability, we calculated the sample size needed to detect a time effect with α = 0.05 and β = 0.80.
RESULTS
Subjects' characteristics.
The subjects' characteristics were as follows: age 31 ± 3 yr; height 178 ± 4 cm; weight 87 ± 5 kg; body mass index 27.6 ± 2.5 kg/m2; leg volume 12.4 ± 0.7 liters.
Blood flow.
There were no differences (P = 0.89) in blood flow as measured during the three hourly periods of the infusion study (Table 1). Within-subject coefficient of variation for repeated blood flow measures was 9 ± 1%.
Table 1.
Blood flow, phenylalanine concentrations, and kinetics in healthy subjects undergoing a 6-h labeled phenylalanine infusion with five sequential muscle biopsies and extended fasting
|
Sampling Period |
|||
|---|---|---|---|
| 150–180 min | 210–240 min | 330–360 min | |
| Blood flow, ml·min−1·100 ml leg−1 | 4.08±0.20 | 4.31±0.42 | 4.29±0.45 |
| Arterial concentration, μmol/l | 63±3 | 65±3 | 65±2 |
| Venous concentration, μmol/l | 67±3 | 69±3 | 69±2 |
| Tissue free concentration, μmol/l | 85±6 | 81±6 | 88±6 |
| Delivery to the leg, nmol·min−1·100 ml leg−1 | 258±17 | 282±33 | 283±36 |
| Release from the leg, nmol·min−1 ·100 ml leg−1 | 275±17 | 300±35 | 299±38 |
| Leg net balance, nmol·min−1 ·100 ml leg−1 | −17±3 | −18±4 | −17±3 |
| Leg Ra, nmol·min−1·100 ml leg−1 | 83±5 | 83±10 | 75±9 |
| Leg Rd, nmol·min−1 ·100 ml leg−1 | 66±3 | 65±8 | 59±7 |
| Tissue inward transport, nmol·min−1·100 ml leg−1 | 197±16 | 258±40 | 218±21 |
| Tissue outward transport, nmol·min−1·100 ml leg−1 | 214±17 | 276±43 | 235±24 |
| Release from proteolysis, nmol·min−1·100 ml leg−1 | 89±5 | 86±9 | 80±10 |
| Utilization for protein synthesis, nmol·min−1·100 ml leg−1 | 73±3 | 67±7 | 64±9 |
Data are means ± SE. Ra, rate of appearance; Rd, rate of disappearance. Data were analyzed with ANOVA with repeated measures and Dunnett's test. No significant time effect was found for any of the parameters.
Phenylalanine enrichments and concentrations.
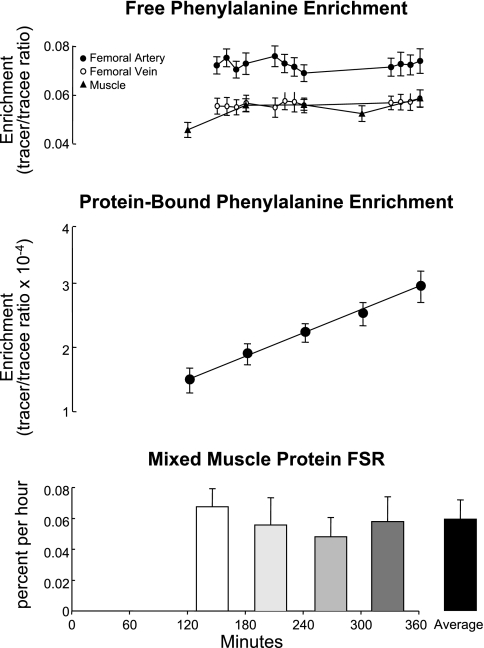
Phenylalanine enrichments in the femoral artery and vein in the muscle free pool and in the mixed muscle proteins (protein-bound pool) are shown in Fig. 1. Phenylalanine enrichments in the femoral artery and vein and in the muscle free pool did not change significantly with time (P > 0.66), suggestive of isotopic steady state. The within-subject coefficient of variation was 5 ± 1, 3 ± 1, and 9 ± 1% for phenylalanine arterial, venous, and intracellular enrichment, respectively. Similarly, phenylalanine concentrations in the femoral artery and vein, and in the muscle free pool, did not significantly change over time (P > 0.56) (data reported in Table 1). The protein-bound phenylalanine enrichment increased linearly over time and fitted a straight line in all subjects (r2 > 0.95 in all subjects).
Fig. 1.
Phenylalanine enrichments and mixed muscle protein fractional synthetic rate (FSR). Free phenylalanine enrichments in femoral arterial and venous blood and in the intracellular pool (top) did not change significantly with time. Protein-bound phenylalanine enrichment (middle) increased linearly in each subject (r2 = 0.98 ± 0.01) over time. Mixed muscle protein FSR (bottom) did not change significantly over time.
Muscle phenylalanine kinetics.
Leg and muscle phenylalanine kinetics (expressed/100 ml leg volume) are shown in Table 1. Delivery of phenylalanine to the leg (Fin) and release from the leg (Fout) were not different between hourly periods (P > 0.80). Phenylalanine net balance was negative throughout the study, with no significant differences in phenylalanine NB between periods (P = 0.95). With the use of the two-pool model, phenylalanine Ra in blood from proteolysis (Leg Ra) and the phenylalanine Rd from blood for protein synthesis (Leg Rd) were not different between periods (P > 0.67). Similarly, none of the three-pool model parameters, including phenylalanine transport in the muscle (FM,A) and out of the muscle (FV,M), intracellular release from breakdown (FM,0) and intracellular utilization for protein synthesis (F0,M) changed significantly over time (P > 0.32, Table 1). The slope of linear regression of the individual values for F0,M, FM,0, and net balance over time were −5 ± 5, −4 ± 5, and 0 ± 1, respectively, which were not different from zero (F0,M: P = 0.40; FM,0: P = 0.44; net balance: P = 0.96). The sample size necessary to demonstrate that these slopes are different from zero with a power of β of 80% and P = 0.05 are as follows: 57 for F0,M, 70 for FM,0, and 21,473 for net balance.
Mixed muscle FSR.
Mixed muscle protein FSR (Fig. 1) did not change significantly over time (P = 0.83). When linear regressions were performed on the FSR values over time for each subject, we found a slope of −0.006 ± 0.006%/h, which was not significantly different from zero (P = 0.30). This slope could be determined to be different from zero with a power of 80% at the P = 0.05 level only if 45 subjects were studied.
DISCUSSION
The primary finding from this work is that mixed muscle protein synthesis, breakdown, and phenylalanine kinetics appear to be unaffected by extended fasting and five sequential muscle biopsies from the same muscle. To our knowledge, this is the first study that specifically addresses this issue and indicates that a saline-only control group is not necessary when performing acute muscle protein metabolism experiments with stable isotope tracers and a pre-post sequential study design. Our data also provide important power information for muscle protein turnover experiments involving a sequential design in humans.
The main reasons for using a sequential study design in muscle protein metabolism are: 1) to control for individual and day-to-day metabolic variability and 2) to reduce the number of experimental subjects, thereby reducing risks and costs. However, such a design may expose to criticism because, in the absence of a control group, it is not possible to account for the potential confounding effects of extended fasting and repeated muscle biopsies.
Specifically, our data show that the free phenylalanine enrichments in blood and muscle were in steady state, the protein-bound phenylalanine enrichment increased linearly as expected, and the calculated phenylalanine kinetics across the leg were unaffected throughout the experimental period. Furthermore, mixed muscle protein synthesis (FSR) and the calculated rates of muscle protein synthesis and breakdown using both arteriovenous models of phenylalanine kinetics also showed no effect of the experimental procedures. It is important to highlight, however, that we studied only a relatively small number of subjects and that our power calculations indicate that, with a much larger samples size, we may be able to detect a significant time effect. However, it is important to put these findings in a perspective relative to the size of the changes observed in this study and those expected when anabolic or catabolic stimuli are sequentially applied to the muscle. The size of physiologically meaningful changes from baseline observed in muscle protein metabolism experiments involving a sequential study design is typically 30–50% or larger (up to 200%) (1, 2, 7, 12, 13, 20, 21, 23, 24, 26, 27), i.e., much larger than that observed for the main parameters measured in the current experiment (<10%). Using this experiment's data, we calculated that, to detect a 30% change in any of the main muscle protein metabolism parameters, we would need a sample size of 10 subjects, while we would need 7 subjects to detect a 50% change, and 5 subjects to detect a 100% change with a power of 80% and P = 0.05.
Prolonged fasting in humans lasting for 2–4 days (i.e., short-term starvation) has demonstrable effects on protein metabolism, as shown by an increase in whole body leucine oxidation and protein breakdown (5, 9, 10, 14, 19). The rate of appearance of phenylalanine and leucine from protein breakdown across the forearm also increases significantly by 40–80% following 2.5 days of starvation (10), and mixed muscle protein synthesis has also been shown to decrease by 13% following 3 days of starvation (8). Overall, these studies indicate that prolonged fasting or starvation over several days can influence protein metabolism and therefore must be accounted for. On the other hand, our data show that extended fasting for up to 18 h has negligible effects on measures of muscle protein and amino acid metabolism.
We are unaware of previous studies designed to address if repeated needle biopsies from the same muscle area can affect muscle amino acid and protein metabolism. One recent paper reports that sequential muscle biopsies do not influence mRNA expression (18), which is consistent with our current data indicating that repeated biopsies over the course of a 6-h isotope infusion do not affect mixed muscle protein synthesis or muscle phenylalanine kinetics. In a recent paper, we have also shown that sequential biopsies taken 1 h apart from the same incision at baseline do not alter the phosphorylation of several key signals involved in the regulation of translation initiation (7, 11). However, it is important to underscore that our results were obtained by carefully taking the biopsies at least 2–3 cm apart from each other, which was achieved by inclining the Bergström needle at different angles while using the same skin and fascia incision (see subjects and methods). It is plausible that if several additional biopsies are serially taken from the same exact area inflammation, swelling, or blood clotting may alter amino acid and protein kinetics in the sampled tissue. However, if inflammation occurred, we would expect an increase in muscle protein synthesis and breakdown over time due to the rapid turnover of inflammatory cells and proteins. Our data suggest that if there was a trend for change it was toward a decrease rather than an increase in muscle protein turnover. Thus, despite the lack of direct measures of inflammation, we feel confident that inflammation did not play a significant role in this experiment. Nonetheless, while these considerations are valid for the bulk of muscle proteins (i.e., contractile proteins), we cannot exclude that some less abundant protein was influenced by the study procedures, since we did not measure the turnover rates of individual proteins.
Finally, tracer recycling can be a concern in prolonged isotope infusion experiments. However, the impact of intracellular tracer recycling on our experimental results is negligible and undetectable with the current study design, since it has been reported to occur at a rate of 1%/h, i.e., well below the within-subject blood and muscle enrichment variability (20).
We conclude that extended fasting and up to five carefully spaced sequential muscle biopsies from the same muscle do not appear to affect basal mixed muscle protein and phenylalanine kinetics in human subjects. Thus it is unnecessary to include a control group to account for these variables when using a sequential design to measure mixed muscle protein synthesis and muscle phenylalanine kinetics in human subjects.
GRANTS
This study was supported by National Institutes of Health Grants P30 AG-024832, R01 AG-18311, R01 AR-049877, S10 RR-16650, and M01 RR-00073.
Acknowledgments
We thank the study volunteers for their patience and dedication, all nurses and personnel of the General Clinical Research Center of the University of Texas Medical Branch for help with the conduct of the clinical portion of this study, and Ming Zheng for superb technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1–13C]leucine. Clin Sci 76: 447–454, 1989. [DOI] [PubMed] [Google Scholar]
- 2.Biolo G, Declan Fleming RY, Wolfe RR. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J Clin Invest 95: 811–819, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biolo G, Fleming RY, Maggi SP, Wolfe RR. Transmembrane transport and intracellular kinetics of amino acids in human skeletal muscle. Am J Physiol Endocrinol Metab 268: E75–E84, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–009 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Sp 6: 421–424, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Carlson MG, Snead WL, Campbell PJ. Fuel and energy metabolism in fasting humans. Am J Clin Nutr 60: 29–36, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Carraro F, Rosenblatt J, Wolfe RR. Isotopic determination of fibronectin synthesis in humans. Metabolism 40: 553–561, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol (Lond) 576: 2–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essen P, McNurlan MA, Wernerman J, Milne E, Vinnars E, Garlick PJ. Short-term starvation decreases skeletal muscle protein synthesis rate in man. Clin Physiol 12: 287–299, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Frexes-Steed M, Warner ML, Bulus N, Flakoll PJ, Abumrad NN. Role of insulin and branched-chain amino acids in regulating protein metabolism during fasting. Am J Physiol Endocrinol Metab 258: E907–E917, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Fryburg DA, Barrett EJ, Louard RJ, Gelfand RA. Effect of starvation on human muscle protein metabolism and its response to insulin. Am J Physiol Endocrinol Metab 259: E75–E82, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol (Lond) 582: 2–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab 291: E745–E754, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab 278: E620–E626, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MD, Miles JM, Gerich JE, Cryer PE, Haymond MW. Preservation of insulin effects on glucose production and proteolysis during fasting. Am J Physiol Endocrinol Metab 254: E700–E707, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Jones PRM, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 16.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism 27: 97–106, 1978. [DOI] [PubMed] [Google Scholar]
- 17.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci 41: 459–473, 1971. [DOI] [PubMed] [Google Scholar]
- 18.Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD, Pilegaard H. Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol 95: 351–360, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Nair KS, Woolf PD, Welle SL, Matthews DE. Leucine, glucose, and energy metabolism after 3 days of fasting in healthy human subjects. Am J Clin Nutr 46: 557–562, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273: E99–E107, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J 20: 768–769, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwenk WF, Tsalikian E, Beaufrere B, Haymond MW. Recycling of an amino acid label with prolonged isotope infusion: implications for kinetic studies. Am J Physiol Endocrinol Metab 248: E482–E487, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab 287: E513–E522, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest 88: 27–33, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toffolo G, Foster DM, Cobelli C. Estimation of protein fractional synthetic rate from tracer data. Am J Physiol Endocrinol Metab 264: E128–E135, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest 101: 2000–2007, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpi E, Kobayashi H, Mittendorfer B, Sheffield-Moore M, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 78: 250–258, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolfe RR Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 1992.
- 29.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. Hoboken, NJ: Wiley, 2004.