Abstract
The mechanism by which human immunodeficiency virus (HIV)-1 infection in humans leads to the erosion of lean body mass is poorly defined. Therefore, the purpose of the present study was to determine whether transgenic (Tg) rats that constitutively overexpress HIV-1 viral proteins exhibit muscle wasting and to elucidate putative mechanisms. Over 7 mo, Tg rats gained less body weight than pair-fed controls exclusively as a result of a proportional reduction in lean, not fat, mass. Fast- and slow-twitch muscle atrophy in Tg rats did not result from a reduction in the in vivo-determined rate of protein synthesis. In contrast, urinary excretion of 3-methylhistidine, as well as the content of atrogin-1 and the 14-kDa actin fragment, was elevated in gastrocnemius of Tg rats, suggesting increased muscle proteolysis. Similarly, Tg rats had reduced cardiac mass, which was independent of a change in protein synthesis. This decreased cardiac mass was associated with a reduction in stroke volume, but cardiac output was maintained by a compensatory increase in heart rate. The HIV-induced muscle atrophy was associated with increased whole body energy expenditure, which was not due to an elevated body temperature or secondary bacterial infection. Furthermore, the atrophic response could not be attributed to the development of insulin resistance, decreased levels of circulating amino acids, or increased tissue cytokines. However, skeletal muscle and, to a lesser extent, circulating insulin-like growth factor I was reduced in Tg rats. Although hepatic injury was implicated by increased plasma levels of aspartate and alanine aminotransferases, hepatic protein synthesis was not different between control and Tg rats. Hence, HIV-1 Tg rats develop atrophy of cardiac and skeletal muscle, the latter of which results primarily from an increased protein degradation and may be related to the marked reduction in muscle insulin-like growth factor I.
Keywords: protein synthesis, protein degradation, 3-methylhistidine, insulin-like growth factor I, cytokines, human immunodeficiency virus
the loss of body weight, in general, and lean body mass (LBM), in particular, is a hallmark of human immunodeficiency virus (HIV)-1 infection. Although altered body composition is most pronounced in later phases of the disease and represents an acquired immunodeficiency syndrome (AIDS)-related condition when sufficiently severe, such a change may also be observed in weight-stable patients (36). Furthermore, whereas the introduction of highly active antiretroviral therapy (HAART) has limited involuntary weight loss, a significant number of AIDS patients still manifest muscle wasting (46, 56), especially as the severity of the disease progresses (5, 53). However, investigation of the effects of HIV-1 infection under controlled conditions in humans is complicated by recurrent opportunistic bacterial infections and the pharmacological side effects of HAART, each of which can negatively impact muscle protein balance (15, 24). Although the pathogenesis of HIV-induced wasting is multifactorial, where a reduction in energy intake and/or malabsorption is clearly contributory (12, 30), the etiology of the metabolic defect is poorly characterized. Regardless of the mechanism, this atrophic response increases the risk of morbidity and mortality in this patient population (17, 45).
To circumvent the above-mentioned caveats in human studies, several animal models have been developed (45). For example, the experimental simian immunodeficiency virus infection of macaque monkeys shows a decrease in body weight gain; however, although it allows for better control of nutritional variables than human studies, its use is limited by its expense (32, 33). In contrast, several lines of transgenic (Tg) rodents, both mice and rats, that are noninfectious and express one or more of the HIV-1 viral envelope proteins have been developed (39, 41). Although these types of small animal models are not without their own limitations (48), they offer the opportunity to define the etiology of muscle wasting under well-controlled nutritional and therapeutic conditions and, potentially, to optimize interventions designed to limit the loss of LBM. In this regard, the HIV-1 Tg mouse and rat have a smaller body size than wild-type (WT) littermates, and both have been reported to have a decrease in cross-sectional area in fast-twitch skeletal muscle (35, 41). However, neither study controlled the nutritional state of the WT animals (e.g., WT animals were not pair-fed), and neither study systematically investigated possible cellular or physiological mechanisms for the decreased muscle mass.
Therefore, to address these deficiencies, we used a noninfectious HIV-1 rat model containing a gag/pol-deleted provirus that expresses seven of nine HIV-1 genes (38, 39, 57). On the basis of the background presented above, the present studies were designed to address the hypothesis that HIV-1 protein expression will decrease skeletal muscle mass as a result of changes in rates of muscle protein synthesis and degradation and that these changes will persist, even when the control rats are pair-fed to match the caloric intake of the HIV-1 Tg animals. Furthermore, we hypothesized that this HIV-induced muscle atrophy is associated with an increase in catabolic mediators (e.g., inflammatory cytokines) and a decrease in the prevailing concentration of selected anabolic mediators [e.g., insulin and insulin-like growth factor I (IGF-I)].
METHODS AND MATERIALS
Animal preparation and experimental protocols.
Male HIV-1 Tg (4 wk) and age-matched parental WT inbred Fischer 344/Hsd non-Tg control rats were purchased from Harlan (Indianapolis, IN). Specific pathogen-free HIV-1 Tg and non-Tg control rats were housed under pathogen-free conditions in microisolator cages on a high-efficiency particulate air (HEPA)-filtered ventilated rack. Rats were housed in such cages to minimize the opportunity for secondary bacterial infections, which might confound data interpretation in these animals, which are known to have impaired immune responses (38, 39, 57). Rats were housed in a light-controlled room (12:12-h light-dark cycle) under constant temperature. Water and commercial rat chow (no. 2018 18% protein rodent diet, Harlan Teklad, Madison, WI) were provided. Within the first 2 wk, it became obvious that the HIV-1 Tg rats consumed less food than ad libitum-fed control animals (data not shown). Therefore, the majority of the control animals were subsequently pair-fed to match the food consumption of the HIV-1 Tg group, and a cohort of control animals was permitted food ad libitum. Hence, three groups of rats were used in the study: ad libitum-fed HIV-1 Tg rats (n = 14), ad libitum-fed control rats (n = 6), and pair-fed control rats (n = 12). Body weight and food consumption were measured weekly for 7 mo, and all additional parameters were assessed during the subsequent week. This 7-mo time point was selected, because 1) HIV-1 Tg rats demonstrated a consistent lower body weight than pair-fed control animals, 2) the body weight of HIV-1 Tg rats appeared to have reached a plateau, and 3) there was no evidence of skin lesions or hindlimb paralysis, which are characteristic long-term changes noted in these animals (39). All experiments were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine and adhered to National Institutes of Health guidelines for use of experimental animals.
Indirect calorimetry, temperature, and body composition.
During the final week of the study, whole body O2 consumption was assessed using indirect calorimetry (Oxymax, Columbus Instruments, Columbus, OH) at 15-min intervals during a complete 12:12-h light-dark cycle, during which food was available. Energy expenditure was obtained using an energy equivalent of 20.27 J/ml O2 consumed. Body fat and LBM were also measured using an NMR system (model LF90 Minispec, Bruker, Woodlands, TX). The description of these two methods has been previously reported by our laboratory (42). Rectal temperature was determined using a thermometer (model 5111, Fluke) with a mouse thermocouple probe (Harvard Apparatus).
Tissue protein synthesis.
On the morning of the experiment, fed rats were anesthetized with pentobarbital sodium (50 mg/kg), and a catheter was inserted into the carotid artery. Rats were injected with l-[2,3,4,5,6-3H]phenylalanine (Phe, 150 mM, 30 μCi/ml, 1 ml/100 g body wt) via percutaneous puncture of the jugular vein, and arterial blood samples were collected into heparinized syringes at 2, 6, and 10 min for measurement of plasma Phe-specific radioactivity. Thereafter, various tissues (i.e., gastrocnemius, soleus, heart, and liver) were excised and freeze-clamped. Blood was centrifuged, and plasma was collected. All tissue and plasma samples were stored at −70°C until they were analyzed. Muscle was powdered under liquid nitrogen, and a portion was used to estimate the rate of incorporation of [3H]Phe into protein, exactly as described previously (18, 23, 25, 49, 51, 52). Additionally, the dry-to-wet weight ratio of gastrocnemius and liver was determined in tissues that had been dried at 80°C for 48 h.
Estimates of protein degradation.
For estimation of muscle protein breakdown, rats were housed for 24 h in standard metabolic cages, which prevented fecal contamination, and 3-methylhistidine (3-MH) was determined by HPLC in an aliquot of the urine collected (31). In addition, in vitro proteasome activity was assessed by quantification of the chymotryptic-like peptidase activity in gastrocnemius. Briefly, muscle was homogenized in buffer containing 50 μM Tris·HCl (pH 7.4), 5 mM MgCl2, 250 mM sucrose, 2 mM ATP, and 1 mM DTT. The homogenate was clarified by sequential centrifugation steps to isolate the 20S and 26S proteasomes (54). After resuspension, proteasome chymotryptic-like activity was determined as the release of 7-amino-4-methylcoumarin (AMC) from the fluorogenic peptide substrate LLVY-AMC (Chemicon, Temecula, CA). Finally, the amount of the 14-kDa actin fragment in gastrocnemius was determined by Western blotting (anti-actin, catalog no. A2066, Sigma, St. Louis, MO) and is indicative of activated caspase-3 (54).
Plasma determinations.
The concentrations of various substances were determined on blood collected from fed rats in all three groups between 9 and 11 AM and immediately before injection of [3H]Phe. Plasma was used for determination of insulin (Linco Research, St. Charles, MO) and total IGF-I (Immunodiagnostic Systems, Fountain Hills, AZ), as well as testosterone and leptin (R & D Systems, Minneapolis, MN), by enzyme-linked immunosorbent assay. Plasma branched-chain amino acids were determined using reverse-phase HPLC after precolumn derivatization of amino acids with phenylisothiocyanate. Glucose, lactate, and triglycerides were determined using an Analox analyzer (Lunenburg, MA). Concentrations of urea nitrogen (blood urea nitrogen), creatinine, albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were determined using the Vitros Chemistry System (models DT60 II and DTSCII, Rochester, NY). The same system was used to determine the creatinine concentration in 24-h urine samples collected for 3-MH. The creatinine clearance [(urine volume × urine creatinine concentration) ÷ plasma creatinine concentration] provided an estimate of the glomerular filtration rate. Similarly, the urinary protein excretion was the product of the urine volume and urinary protein concentration. Finally, total red and white blood cell (RBC and WBC) counts, as well as the WBC differential, were determined using standard methods. Although many of the hematologic and blood chemistry end points described above are not directly related to the stated aim of the investigation, they were nonetheless deemed important to better characterize the model and place the protein metabolic derangements in perspective of the overall pathophysiological presentation of the animals.
RNase protection assays and Northern blot analyses.
Total RNA was extracted from tissues in a mixture of phenol and guanidine thiocyanate (TRI Reagent, Molecular Research Center, Cincinnati, OH) using the manufacturer's protocol. RNA was separated from protein and DNA and quantified, and 10 μg of RNA were used for each assay. Riboprobes were synthesized from a multiprobe rat cytokine template set (catalog no. rCK-1, Pharmingen, San Diego, CA) using an in vitro transcription kit (Pharmingen). Primer sequences for other mRNAs, i.e., IGF-I, regulated in development and DNA damage responses (REDD-1), atrogin-1, and muscle RING finger (MuRF1), have been reported by our laboratory (18, 25, 26, 49). The labeled riboprobe was hybridized with RNA overnight using an RNase protection assay. Protected RNAs were separated using a 5% acrylamide gel, and dried gels were exposed to a PhosphorImager screen (Molecular Dynamics, Sunnyvale, CA). The resulting data were quantified using ImageQuant software and normalized to the rat ribosomal protein L32 and GAPDH mRNA signal in each lane (18, 24, 25, 49).
Western blot analysis.
Fresh muscle was homogenized at a 1:5 ratio in ice-cold homogenization buffer (pH 7.4) composed of (in mM) 20 HEPES, 2 EGTA, 50 NaF, 100 KCl, 0.2 EDTA, 50 β-glycerophosphate, 1 DTT, 0.1 PMSF, 1 benzamidine, and 0.5 sodium vanadate and clarified by centrifugation (23, 25, 51, 52). Equal amounts of protein per sample were subjected to standard SDS-PAGE for total and phosphorylated (Thr389) ribosomal protein S6 kinase-1 (Cell Signaling, Beverly, MA), total and phosphorylated (Ser235/Ser236) ribosomal protein S6 (Cell Signaling), and total and phosphorylated (Thr37/46) 4E-binding protein-1 (Bethyl Laboratories, Montgomery, TX) (22, 23, 25). In addition, to determine activation of eukaryotic elongation factor (eEF)-2, we determined total and Thr56 phosphorylated eEF2 (Cell Signaling) (18, 19, 23, 25, 51). The blots were washed with 1× TBS + 0.1% Tween 20 and incubated with secondary antibody (horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit) at room temperature. Blots were developed with enhanced chemiluminescence Western blotting reagents according to the manufacturer's (Amersham) instructions and then exposed to X-ray film in a cassette equipped with an intensifying screen (Lightning Plus, DuPont). After development, the film was scanned (Microtek ScanMaker IV) and analyzed using National Institutes of Health Image 1.6 software.
Echocardiography.
Heart function was assessed by echocardiography using the Sequoia C256 Echocardiography System (Siemens Medical Solutions, Mountain View, CA) equipped with a 7.5-MHz transducer, as previously described (51). Rats were lightly anesthetized by intraperitoneal injection of ketamine (40 mg/kg) + acepromazine (1 mg/kg), and body temperature was maintained during the procedure by placement of rats on a heating pad. The transducer was placed on the thorax, and M-mode recordings were performed by direction of the ultrasound beam at the midpapillary muscle level. End points were obtained after well-defined, continuous interfaces of the septal and posterior walls were visualized (51). The operator was blinded to the treatment group of the experimental animal, and measurements from three to four consecutive cardiac cycles were averaged for all animals. Intraobserver variability was <3% for the echocardiographic parameters (data not shown).
Statistical analysis.
Experimental data for each condition are summarized as means ± SE. Unless otherwise indicated, statistical evaluation of the data was performed using two-way ANOVA with post hoc Student-Newman-Keuls test when the interaction was significant. Differences between the groups were considered significant when P < 0.05.
RESULTS
Body weight, composition, temperature, and energy expenditure.
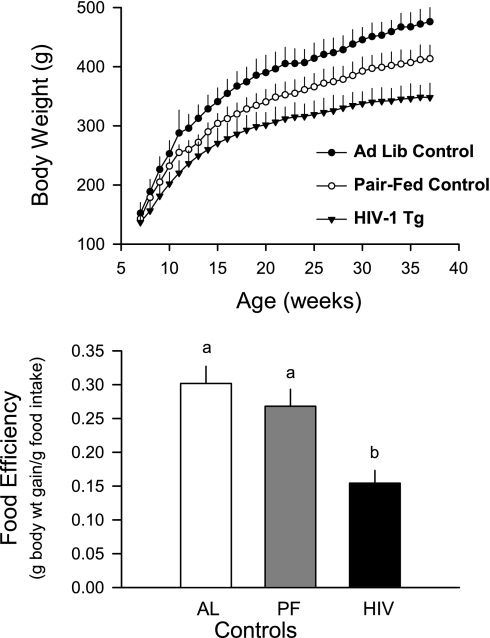
Initially, body weight and food consumption were determined in control and HIV-1 Tg rats allowed ad libitum access to food. However, after 2 wk, it was clear that the HIV-1 Tg rats consumed 23% less food than ad libitum-fed control animals (15.1 ± 0.2 vs. 19.7 ± 0.2 g/day, P < 0.05). Thereafter, the control group was divided into rats that were fed ad libitum and those that were pair-fed to match the food consumption of the HIV-1 Tg rats. As illustrated in Fig. 1, growth rate was slower in HIV-1 Tg rats than in either of the control groups, and a significant portion of this growth failure could be attributed to the reduction in food consumption. However, it is also evident that growth was slower for the HIV-1 Tg rats than control animals, even when food intake was closely matched. As a result, the incremental increase in body weight over the 7-mo period was 17% less in HIV-1 Tg rats than in pair-fed controls (219 ± 10 vs. 264 ± 11 g body wt, P < 0.05). Finally, food efficiency, expressed as the ratio of body weight gained to food consumed, was reduced 42% in HIV-1 Tg rats compared with either group of control animals (Fig. 1).
Fig. 1.
Body weight and food efficiency in control and human immunodeficiency virus (HIV)-1 transgenic (Tg) rats. Control rats were fed ad libitum (Ad Lib, AL) or pair-fed (PF) to match food consumption by HIV-1 Tg rats. Values are means ± SE of 6 AL, 12 PF, and 14 HIV-1 Tg rats. Means without a common letter (a, b) are significantly different (P < 0.05).
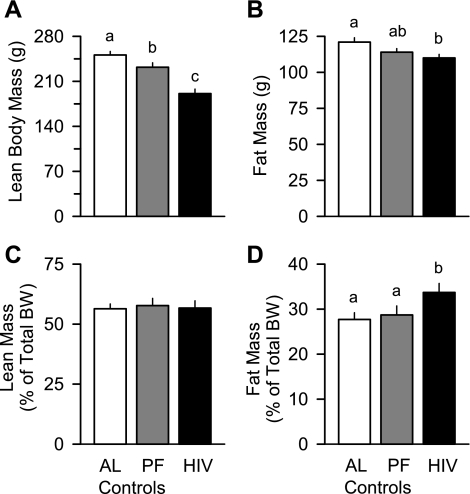
NMR determination of body composition detected differences in fat mass and LBM between control and HIV-1 Tg rats. A reduction in the absolute amount of LBM (Fig. 2A), not fat mass (Fig. 2B), accounted for the difference in body weight between the ad libitum- and pair-fed control groups. Moreover, the LBM of HIV-1 Tg rats was reduced compared with that of the pair-fed controls, whereas the fat mass was relatively preserved in HIV-1 Tg rats. As a consequence of these changes, the calculated percentage of total body weight contributed by LBM was unchanged among the three groups (Fig. 2C), whereas HIV-1 Tg rats showed a significant 17% increase in fat mass when normalized to body weight (Fig. 2D).
Fig. 2.
Lean body mass (LBM) and fat mass in ad libitum- and pair-fed control and HIV-1 Tg rats. Body composition was determined by NMR spectroscopy before determination of in vivo protein synthesis. BW, body weight. Values are means ± SE of 6 AL, 12 PF, and 14 HIV-1 Tg rats. Means without a common letter (a, b, c) are significantly different (P < 0.05).
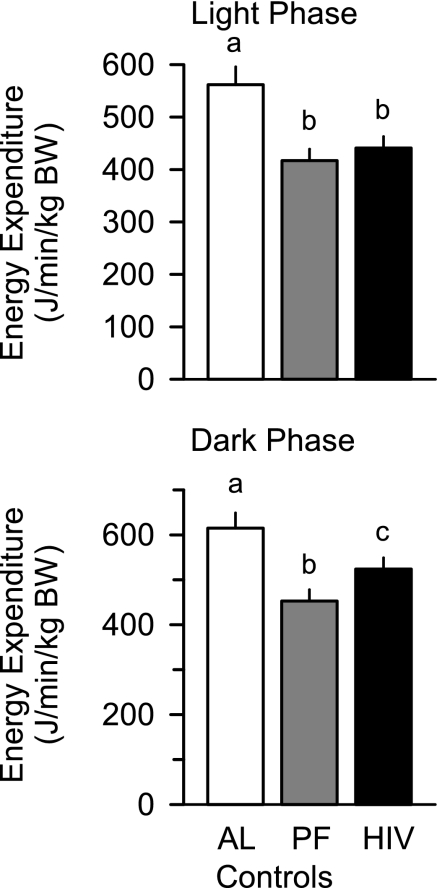
Energy expenditure in the light and the dark phase was higher in the ad libitum-fed control animals than in the pair-control and HIV-1 Tg rats. Although energy expenditure was not different between the pair-fed control and HIV-1 Tg rats during the light phase, it was increased 15% in HIV-1 Tg rats during the dark phase (Fig. 3). Comparable changes where observed when energy expenditure was normalized to total body weight or LBM (data not shown). Differences in energy expenditure could not be accounted for by a difference in body temperature determined 4 h after the start of the dark cycle [38.2 ± 0.3 and 38.4 ± 0.2°C in ad libitum- and pair-fed controls, respectively, and 38.2 ± 0.3°C in Tg rats, P = not significant (NS)] or 4 h after the start of the light cycle (data not shown).
Fig. 3.
Whole body resting energy expenditure during light and dark phases in ad libitum- and pair-fed control and HIV-1 Tg rats. Values are means ± SE of 6 AL, 12 PF, and 14 HIV-1 Tg rats. Means without a common letter (a, b, c) are significantly different (P < 0.05).
Tissue weights.
The wet weights of the gastrocnemius and soleus were decreased in HIV-1 Tg rats (26% and 17%, respectively) compared with time-matched pair-fed control values (Table 1). Both muscles tended to weigh less in pair-fed than in ad libitum-fed controls, but these changes did not achieve statistical significance. The dry-to-wet weight ratio for gastrocnemius was not different among the three groups (0.28 ± 0.02 and 0.28 ± 0.01 in ad libitum- and pair-fed controls, respectively, and 0.27 ± 0.01 in Tg rats, P = NS). Similarly, cardiac atrophy was also detected, with the ventricular weight being reduced by 12% in the HIV-1 Tg rats compared with pair-fed controls. Cardiac weight was also reduced in pair-fed controls vs. ad libitum fed-controls. In general, these decreases in striated muscle mass in HIV-1 Tg rats were proportional to the above-mentioned decreases in body weight and LBM; therefore, there were no differences between groups in gastrocnemius, soleus, or heart weights when normalized to total body weight (Table 1). The absolute weights of the liver, kidney, and spleen were not different among the three groups of rats (Table 1). However, when normalized to body weight, liver weight increased 22% (e.g., hepatomegelia) in HIV-1 Tg rats compared with either control group. Finally, there was no difference in the dry-to-wet weight ratio for the liver among the three groups (data not shown).
Table 1.
Organ weights of ad libitum- and pair-fed control and HIV-1 Tg rats
|
Control |
HIV-1 Tg | ||
|---|---|---|---|
| Ad Libitum-Fed | Pair-Fed | ||
| Gastrocnemius | |||
| g | 4.17±0.12* | 3.99±0.13* | 2.96±0.08† |
| g/kg body wt | 8.76±0.29 | 8.89±0.18 | 8.75±0.22 |
| Soleus | |||
| g | 0.26±0.01* | 0.23±0.01* | 0.19±0.01† |
| g/kg body wt | 0.55±0.04 | 0.56±0.02 | 0.56±0.03 |
| Heart | |||
| g | 1.09±0.06* | 0.94±0.02† | 0.83±0.02‡ |
| g/kg body wt | 2.28±0.15 | 2.29±0.11 | 2.45±0.12 |
| Liver | |||
| g | 12.03±0.27 | 11.09±0.14 | 11.11±0.35 |
| g/kg body wt | 25.27±1.38* | 26.79±1.48* | 32.86±1.91† |
| Kidney | |||
| g | 1.38±0.09 | 1.27±0.05 | 1.14±0.04 |
| g/kg body wt | 2.89±0.31 | 3.06±0.22 | 3.37±0.17 |
| Spleen | |||
| g | 1.23±0.07 | 1.15±0.03 | 1.00±0.04 |
| g/kg body wt | 2.60±0.28 | 2.78±0.19 | 2.95±0.14 |
Values are means ± SE of 6 ad libitum-fed control, 12 pair-fed control, and 14 human immunodeficiency virus 1 (HIV-1) transgenic (Tg) rats. Weight of gastrocnemius and soleus represents combined weight of muscles from right and left hindlimbs; kidney weight is for a single organ. Means without a common symbol (*, †, ‡) are significantly different (P < 0.05).
Hematologic profile.
Hematologic data from ad libitum- and pair-fed controls and HIV-1 Tg rats are presented in Table 2. There was no statistical difference in total number of RBCs, hemoglobin concentration, or hematocrit among the groups. There were no differences in total WBC count or number of neutrophils, lymphocytes, monocytes, eosinophils, or platelets (absolute numbers or percentage of total WBCs) among the groups.
Table 2.
Hematologic profile of ad libitum- and pair-fed control and HIV Tg rats
|
Control |
HIV-1 Tg | ||
|---|---|---|---|
| Ad Libitum-Fed | Pair-Fed | ||
| Total RBCs, ×106/μl | 8.84±0.27 | 8.80±0.22 | 9.30±0.23 |
| Hematocrit, % | 40±1 | 41±1 | 42±1 |
| Hemoglobin, g/dl | 14.8±0.3 | 14.9±0.2 | 15.4±0.3 |
| Total WBCs, ×103/μl | 4.16±0.30 | 4.03±0.34 | 4.26±0.20 |
| Neutrophils | |||
| cells/μl | 1,197±142 | 1,120±101 | 1,248±85 |
| %WBCs | 29±3 | 28±3 | 29±2 |
| Lymphocytes | |||
| cells/μl | 2,808±144 | 2,796±102 | 2,905±89 |
| %WBCs | 68±4 | 69±3 | 68±2 |
| Monocytes | |||
| cells/μl | 47±11 | 54±11 | 69±10 |
| %WBCs | 1±1 | 2±1 | 2±1 |
| Eosinophils | |||
| cells/μl | 37±8 | 34±9 | 42±10 |
| %WBCs | 1±1 | 1±1 | 1±1 |
| Platelets, ×103/μl | 660±58 | 597±25 | 659±26 |
Values are means ± SE of 6 ad libitum-fed control, 12 pair-fed control, and 14 HIV-1 Tg rats.There were no statistical differences among the 3 groups for any hematologic parameter.
Plasma concentration of substrates and hormones.
There was no difference in the plasma concentrations of glucose and insulin among the three experimental groups; therefore, there was no difference in homeostasis model assessment of insulin resistance, which provides an estimate of insulin resistance (Table 3). In contrast, the plasma lactate concentration was increased 65% in HIV-1 Tg rats compared with ad libitum- or pair-fed control values, which were not different. The plasma testosterone concentration was not significantly different among the three groups. In contrast, the IGF-I concentration was slightly, albeit statistically, reduced in HIV-1 Tg rats (10%) compared with ad libitum- or pair-fed control values. The ALT and AST concentrations in the plasma were twofold greater in HIV-1 Tg rats than in either control group. Although these latter data suggest some degree of hepatic damage in the HIV-1 Tg rats, there was no difference in the circulating albumin concentration, which is the primary secretory protein synthesized by the liver. Finally, although there was no difference in the plasma triglyceride and leptin concentrations between ad libitum-fed control and HIV-1 Tg rats, the concentrations of these substances in the pair-fed control animals were reduced 25–35%.
Table 3.
Plasma concentrations of substrates and hormones in ad libitum- and pair-fed control and HIV Tg rats
|
Control |
HIV-1 Tg | ||
|---|---|---|---|
| Ad Libitum-Fed | Pair-Fed | ||
| Glucose, mM | 9.2±0.3 | 9.1±0.3 | 8.9±0.4 |
| Lactate, mM | 1.5±0.7* | 1.4±0.2* | 2.3±0.2† |
| Triglycerides, mg/dl | 107±12* | 71±5† | 98±9* |
| BUN, mg/dl | 12.4±0.6 | 11.9±0.5 | 12.3±0.5 |
| Creatinine, mg/dl | 0.78±0.04 | 0.74±0.02 | 0.72±0.08 |
| Albumin, g/dl | 3.0±0.2 | 2.8±0.1 | 2.6±0.1 |
| ALT, U/l | 45±4* | 49±2* | 93±9† |
| AST, U/l | 68±10* | 83±5* | 141±15† |
| Insulin, pmol/l | 604±52 | 588±43 | 556±39 |
| IGF-I, ng/ml | 1,642±52* | 1,641±48* | 1,473±28† |
| Testosterone, ng/ml | 4.6±0.3 | 4.7±0.5 | 4.1±0.4 |
| Leptin, ng/ml | 3.84±0.33* | 2.88±0.16† | 3.64±0.21* |
| HOMA-IR | 8.4±0.8 | 8.1±0.9 | 7.7±0.8 |
Values are means ± SE of 6 ad libitum-fed control, 12 pair-fed control, and 14 HIV-1 Tg rats. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; IGF-I, insulin-like growth factor I. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as reported edsewhere (34). Means without a common symbol (*, †) are significantly different (P < 0.05).
Renal function.
On the basis of the blood urea nitrogen and the plasma creatinine concentration, renal function does not appear to differ among the three experimental groups (Table 3). However, the calculated creatinine clearance, which provides an estimate of glomerular filtration rate and renal function, was reduced in the HIV-1 Tg rats. Although there was no difference in the urine output between the pair-fed control and HIV-1 Tg rats (840 ± 49 and 789 ± 52 μl/day, respectively, P = NS), the urinary creatinine concentration was 30% less in the HIV-1 Tg rats (489 ± 68 vs. 333 ± 36 mg/dl, P < 0.05). As a result, creatinine clearance was reduced 33% in HIV-1 Tg rats (390 ± 50 vs. 270 ± 35 μl/min). There was a similar trend for creatinine clearance to be reduced when normalized to LBM (1.63 ± 0.25 and 1.31 ± 0.27 ml·min−1·kg LBM−1 in pair-fed controls and Tg rats, respectively), but this difference did not achieve statistical significance. Finally, HIV-1 Tg rats also exhibited proteinuria at the time of study (10.9 ± 1.9 and 18.2 ± 2.1 mg/day in pair-fed controls and HIV Tg rats, respectively, P < 0.05). The renal excretions of creatinine and protein in ad libitum-fed controls were not determined because of technical problems with urine collection.
Tissue protein synthesis.
In vivo rates of protein synthesis were determined in selected tissues using the flooding-dose method in fed animals. There was no significant difference between the pair-fed control and HIV-1 Tg rats in protein synthesis for the gastrocnemius (1.57 ± 0.06 and 1.41 ± 0.06 nmol Phe·mg protein−1·h−1, respectively), soleus (1.87 ± 0.06 and 1.72 ± 0.05 nmol Phe·mg protein−1·h−1, respectively), heart (2.96 ± 0.09 and 2.81 ± 0.11 nmol Phe·mg protein−1·h−1, respectively), or liver (18.05 ± 0.78 and 18.92 ± 0.64 nmol Phe·mg protein−1·h−1, respectively). Moreover, there was no statistical difference in the rates of protein synthesis between the ad libitum- and pair-fed control animals for any of the tissues examined (data not shown). To confirm that muscle protein synthesis was not altered in HIV-1 Tg rats, Western blot analysis of proteins known to be important in the translational control of protein synthesis was carried out on homogenates of gastrocnemius. There was no difference in the total or phosphorylation state of ribosomal protein S6 kinase-1, ribosomal protein S6, or 4E-binding protein-1 between control and HIV-1 Tg rats (data not shown). Moreover, there was no difference in the total amount or phosphorylation of the major protein regulating translation elongation, eEF2 (data not shown).
Myofibrillar breakdown and plasma amino acid concentrations.
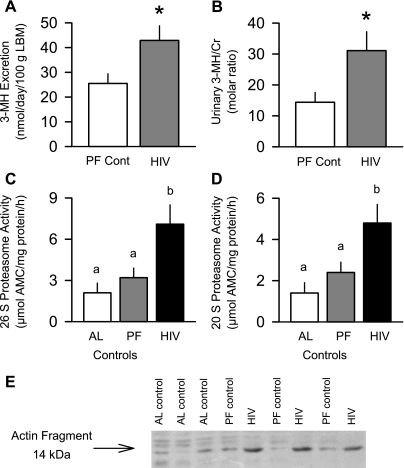
As illustrated in Fig. 4, urinary 3-MH excretion was increased 68% in HIV-1 Tg rats compared with pair-fed controls. Moreover, this HIV-induced increase was even more accentuated when the urinary 3-MH-to-creatinine ratio was calculated. This increased proteolysis appears to result, at least in part, from a stimulation of the ubiquitin proteasome pathway in skeletal muscle, because proteasome chymotryptic-like peptidase activity was increased more than twofold in gastrocnemius of HIV-1 Tg rats compared with ad libitum- or pair-fed controls (Fig. 4, C and D). Proteasome activity was not statistically different between ad libitum- and pair-fed controls, although it tended to be increased in the latter group. Finally, the abundance of the 14-kDa actin fragment was increased by 80% in HIV-1 Tg rats compared with ad libitum- or pair-fed controls, which were not different (Fig. 4E; 1.00 ± 0.5, 1.09 ± 0.06, and 2.31 ± 0.36 AU in ad libitum-fed, pair-fed, and Tg rats, respectively, P < 0.05).
Fig. 4.
Estimates of whole body and muscle proteolysis in control and HIV-1 Tg rats. A and B: whole body proteolysis estimated from excretion of 3-methylhistidine (3-MH) in a 24-h urine sample normalized to LBM or urinary creatinine (Cr) concentration. Values are means ± SE of 12 PF control (PF Cont) and 14 HIV Tg rats. *P < 0.05 vs. PF Cont. C and D: in vitro-determined 26S and 20S proteasome activity of muscle (gastrocnemius) from ad libitum- and pair-fed control and HIV-1 Tg rats. Values are means ± SE of 6 AL, 12 PF, and 14 HIV-1 Tg rats. Means without a common letter (a, b) are significantly different (P < 0.05). E: representative Western blot showing increased abundance of 14-kDa actin fragment in gastrocnemius from HIV-1 Tg vs. control (AL and PF) rats.
Plasma free amino acid concentrations were also determined in all animals. As presented in Table 4, although the total amino acid concentration was not different among the three groups, changes in the circulating concentrations of individual amino acids were observed. For example, the concentrations of taurine (25%), valine (20%), isoleucine (18%), leucine (16%), and phenylalanine (31%) were increased in HIV-1 Tg rats compared with pair-fed controls. In contrast, the plasma concentrations of glutamate (24%) and glycine (21%) were reduced in HIV-1 Tg rats compared with pair-fed controls. There was no significant difference for any plasma amino acid between ad libitum- and pair-fed controls.
Table 4.
Plasma amino acid concentrations in ad libitum- and pair-fed control and HIV-1 Tg rats
|
Control |
HIV Tg | ||
|---|---|---|---|
| Ad Libitum-Fed | Pair-Fed | ||
| Taurine | 91±5* | 89±6* | 112±5† |
| Aspartic acid | 10±2 | 10±1 | 9±1 |
| Hydroxyproline | 18±2 | 19±2 | 18±2 |
| Threonine | 211±12 | 206±10 | 223±7 |
| Serine | 208±10 | 206±8 | 191±11 |
| Asparagine | 44±5 | 41±2 | 51±3 |
| Glutamic acid | 92±11* | 85±3* | 66±3† |
| Glutamine | 643±31 | 638±23 | 653±20 |
| Proline | 169±10 | 171±8 | 173±6 |
| Glycine | 191±21* | 183±14* | 143±6† |
| Alanine | 394±24 | 391±16 | 440±12 |
| Citrulline | 61±4 | 59±3 | 56±2 |
| Valine | 122±20* | 144±6* | 174±7† |
| Methionine | 38±3 | 38±2 | 38±2 |
| Isoleucine | 61±7* | 69±3* | 81±3† |
| Leucine | 131±10* | 140±6* | 162±5† |
| Tyrosine | 70±4 | 63±2 | 70±2 |
| Phenylalanine | 43±4* | 48±2* | 63±3† |
| Ethanolamine | 9±2 | 11±1 | 11±1 |
| Ornithine | 63±6 | 56±4 | 66±5 |
| Tryptophan | 77±10 | 71±4 | 61±4 |
| Lysine | 245±20 | 237±14 | 262±14 |
| Histidine | 64±7 | 60±3 | 70±2 |
| Arginine | 64±6 | 70±3 | 65±6 |
| Total AAs | 3,132±105 | 3,107±87 | 3,252±92 |
Values are means ± SE expressed in μM; n = 6 ad libitum-fed control, 9 pair-fed control, and 14 HIV-1 Tg rats. Means without a common symbol (*, †) are significantly different (P < 0.05).
Cardiac structure and function.
Because of the decrease in heart weight, we also examined selected aspects of heart structure and function in vivo using M-mode echocardiography (Table 5). There were no statistical differences for any of the values between the ad libitum- and pair-fed controls. However, HIV-1 Tg rats demonstrated a 40% reduction in the left ventricle (LV) wall dimension and a 15% reduction in the intraventricular septal dimension compared with pair-fed controls. Although the LV diastolic dimension and the LV posterior wall thickness also tended to be decreased in HIV-1 Tg rats, these changes were of much smaller magnitude and did not achieve statistical significance. In HIV-1 Tg rats, the LV stroke volume was significantly reduced by 25% compared with control values. However, because of the compensatory tachycardia (+20%) in HIV-1 Tg rats, the calculated cardiac output was not different from control (ad libitum- or pair-fed) values when expressed as milliliters per minute or normalized to gram of heart weight.
Table 5.
Myocardial structure and function in ad libitum- and pair-fed control and HIV Tg rats
|
Control |
HIV-1 Tg | ||
|---|---|---|---|
| Ad Libitum-Fed | Pair-Fed | ||
| LV systolic dimension, mm | 1.41±0.18* | 1.43±0.22* | 0.86±0.11† |
| LV diastolic dimension, mm | 6.44±40 | 6.29±0.39 | 5.91±0.22 |
| LVPW thickness, mm | 2.20±0.11 | 2.21±0.16 | 2.15±0.18 |
| IVS dimension, mm | 1.90±0.05* | 1.89±0.06* | 1.61±0.06† |
| IVS/LVPW | 0.85±0.04 | 0.87±0.05 | 0.79±0.06 |
| LV stroke volume, ml | 0.71±0.06* | 0.66±0.05* | 0.49±0.05† |
| Heart rate, beats/min | 335±23* | 334±21* | 398±10† |
| Cardiac output | |||
| ml/min | 234±26 | 214±28 | 198±21 |
| ml·min−1·g heart−1 | 217±32 | 228±31 | 240±29 |
Values are means ± SE of 6 ad libitum-fed control, 12 pair-fed control, and 14 HIV-1 Tg rats. LV, left ventricle; LVPW, LV posterior wall; IVS, intraventricular septum. Means without a common symbol ( *, †) are significantly different (P < 0.05).
Potential modulators of tissue protein balance.
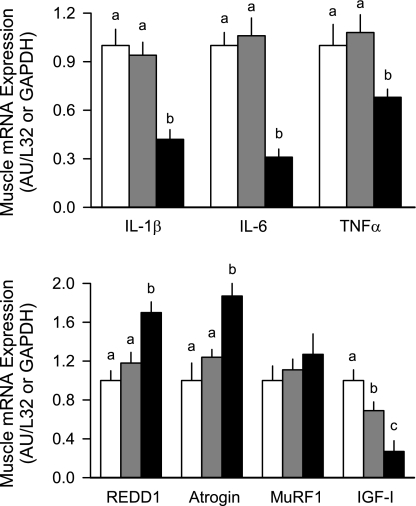
Many circulating and tissue factors have the potential to regulate rates of protein synthesis and/or degradation, and those selected in the present study have been reported to be important under other catabolic conditions (24). For example, inflammatory cytokines have been consistently reported to increase protein degradation. Contrary to expectations, the mRNA content for three representative inflammatory cytokines, IL-1β, IL-6, and TNF-α, in skeletal muscle from HIV-1 Tg rats was reduced compared with ad libitum- or pair-fed control values (∼40%, 70%, and 40%, respectively; Fig. 5). Increases in the ubiquitin E3 ligases atrogin-1 and MuRF1, as well as REDD-1, are also considered part of the atrophic gene response (3, 4). In this regard, skeletal muscle from HIV-1 Tg rats showed a significant increase in REDD-1 (55%) and atrogin-1 (70%), but not MuRF-1, mRNA content compared with ad libitum- or pair-fed control values (Fig. 5). In contradistinction, there was no significant difference in the content of any of these mRNAs in liver from the three groups (data not shown).
Fig. 5.
Muscle (gastrocnemius) mRNA expression of potential regulators of tissue protein balance in ad libitum-fed control (open bars), pair-fed control (gray bars), and HIV-1 Tg (solid bars) rats. Tissue mRNA contents were determined by RNase protection assays, and data are shown as arbitrary densitometry units (AU), where values for ad libitum-fed control rats were set at 1.0 AU. REDD1, regulated in development and DNA damage responses; MuRF1, muscle RING finger; IGF-I, insulin-like growth factor I. Values are means ± SE of 6 AL, 12 PF, and 14 HIV-1 Tg rats. Means without a common letter (a, b, c) are significantly different (P < 0.05).
Finally, the content of IGF-I in tissues, as well as the circulation, is a critical positive regulator of tissue protein accretion (21). There was a small, albeit statistically significant, 10% reduction in the plasma IGF-I concentration in HIV-1 Tg rats (Table 3) that was not due to food restriction, since there was no difference in the IGF-I of ad libitum- and pair-fed controls. This change was associated with a ∼70% reduction in IGF-I mRNA in muscle compared with values from ad libitum-fed control rats (Fig. 5). A portion of this reduction could be attributed to the decreased food consumption. In contrast, there was no significant difference for IGF-I mRNA in liver among the three experimental groups (data not shown).
DISCUSSION
The HIV-1 Tg rats demonstrated a consistently reduced rate of body weight gain over the 7-mo experimental period. The reduced growth in HIV-1 Tg rats was attributable, in part, to a lower spontaneous caloric intake, which is similar to the anorexia often reported in AIDS patients (13). Many of the metabolic hormones can potentially regulate appetite (11). In this regard, we determined the plasma concentration of the adipocyte-derived hormone leptin, a known satiety signal (10). Plasma leptin levels were elevated in HIV-1 Tg rats compared with pair-fed controls, consistent with the reduced food intake in these rats. The HIV-induced increase in leptin could not be explained by differences in total fat mass between HIV-1 Tg rats and pair-fed controls or an increase in inflammatory cytokines, which are known to upregulate leptin production (43). In addition, a portion of the growth failure in HIV-1 Tg rats was also independent of food intake and appears to be due, in part, to an increased whole body energy expenditure. The magnitude of this increased expenditure in HIV-1 Tg rats compared with pair-fed controls would be sufficient to completely account for the loss of LBM if it persisted throughout much of the study period, on the basis of the same calculations used to assess wasting in HIV-infected patients by Suttmann et al. (44). These results are consistent with a recent meta-analysis of the available clinical data that reported a significant increase in resting energy expenditure in HIV sero-positive individuals (2). The mechanism for the elevated energy expenditure in the HIV-1 Tg rats is unclear but was associated with the hyperleptinemia, which can increase whole body energy expenditure (8), and was independent of an increase in body temperature and muscle/liver cytokines. Alternatively, the mechanism may be related to the disproportionate increase in energy expenditure observed in HIV-infected patients in response to caloric intake (37) or by enhanced substrate cycling, as recently demonstrated in another animal model of wasting (42).
A reduction in gastrocnemius, soleus, and plantaris muscle mass of HIV-1 Tg rats has been reported (35) and differs from the preferential reduction in fast-twitch muscles produced by other catabolic stimuli, including bacterial infection and acute alcohol intoxication (27, 50). Moreover, the cross-sectional area of fast-twitch skeletal muscle is reduced in HIV-1 Tg rats and mice (35, 41). Our data confirm the generalized atrophic response in fast- and slow-twitch muscle in HIV-1 Tg rats and extend this pathological alteration to cardiac muscle. Although not directly determined in this or previous studies, the reduced mass would be anticipated to decrease force generation by the affected skeletal muscles. Using NMR spectroscopy, we determined that this reduction in hindlimb skeletal muscle mass was generalized to the entire animal. Hence, the decrement in body weight exhibited by HIV-1 Tg rats resulted essentially exclusively from a reduction in LBM, with little or no change in the absolute amount of fat. As a result, there was an increased proportion of total body weight that was fat in HIV-1 Tg rats, a change reminiscent of that seen in HIV-infected individuals in the absence of antiretroviral therapy (1, 17, 55).
Muscle protein content represents a balance between protein synthesis and degradation, and changes in one or both of these metabolic processes can account for the reduction in LBM. In humans, changes in muscle protein synthesis produced by HIV infection appear variable. One study reported no change in muscle protein synthesis in HIV-positive individuals, in those with AIDS and no muscle wasting, and even in those with AIDS and reduced LBM, compared with sero-negative controls (31). In contrast, muscle protein synthesis was reported to be increased in HIV-positive asymptomatic individuals but decreased in those with AIDS wasting (59). Finally, increased muscle protein synthesis was observed in HIV-infected patients after HAART, which lowered HIV RNA content in blood to near nondetectable levels (58). In our present study, there was no detectable change in the rate of protein synthesis in HIV-1 Tg rats in fast- or slow-twitch muscle compared with pair-fed control values. Collectively, these data suggest that HIV-1 proteins per se do not decrease global muscle protein synthesis and that differences in the prevailing nutritive status, drug treatment regimens, and/or presence of underlying pathology or secondary complications may explain the change in synthetic rate observed in humans.
HIV-1 infection and AIDS also increase the rate of whole body proteolysis (31, 58, 59), which results in part from an increase in protein degradation in skeletal muscle (58). Consistent with this observation is the increased urinary excretion of 3-MH, as well as the elevated plasma concentrations of all branched-chain amino acids (leucine, isoleucine, and valine) detected in our HIV-1 Tg rats compared with ad libitum- or pair-fed controls. Finally, the increased mRNA content of the E3 ubiquitin ligase atrogin-1 in skeletal muscle observed by us and others (35) is suggestive of enhanced proteolysis. Although many atrophic conditions concomitantly increase atrogin-1 and MuRF1 (3), the increase in the former “atrogene” appears relatively specific in skeletal muscle from HIV-1 Tg rats. Enhanced muscle proteolysis in HIV-1 Tg rats was further confirmed using two complementary approaches. 1) In HIV-1 Tg rats, we detected an increase in the 14-kDa actin degradation fragment, which is believed to result from increased caspase-3-mediated protein cleavage and may represent a rate-controlling step in muscle protein loss (54). 2) Proteasomal chymotryptic-like peptidase activity was also increased in muscle from HIV-1 Tg rats. The mechanism for this activation of the ubiquitin-proteasome pathway and the resultant increased muscle proteolysis remain to be elucidated but cannot be explained by differences in food intake or the circulating concentration of testosterone or insulin (9, 21).
The etiology of AIDS-induced cardiomyopathy in humans is poorly understood (28) but often characterized by LV hypertrophy with biventricular or four-chamber dilation (14, 40). Whereas some mouse Tg models also demonstrate cardiomyopathy [e.g., impaired contractility and relaxation (29)], other models have an increased heart weight with no change in cardiac dimension (6, 7). Our data indicate the presence of cardiomyopathy in the HIV-1 Tg rat, as evidenced by the reduction in myocardial mass, which could not be attributed to the decreased food intake or a reduction in the rate of cardiac protein synthesis. Therefore, these data suggest an enhanced rate of protein degradation in the heart, which is consistent with the previously reported increased mRNA expression of atrogin-1 and MuRF1 in HIV-1 Tg rats (35). Moreover, echocardiography revealed that the loss of cardiac mass in HIV-1 Tg rats was associated with changes in cardiac structure and function. Specifically, the systolic LV chamber and the intraventricular septal dimensions were reduced in HIV-1 Tg rats, and again these changes could not be attributed to the reduced caloric intake in HIV-1 Tg rats. The reduction in LV systolic dimension and the accompanying reduction in stoke volume in HIV-1 Tg rats are suggestive of an increased wall stiffness and reduced LV compliance. However, there was no diminution of cardiac output because of the compensatory tachycardia. Therefore, the observed changes in the metabolism of peripheral tissues in the HIV-1 Tg rats do not appear to result from a frank reduction in organ perfusion or secondary to overt heart failure.
The plasma concentrations of the enzymes AST and ALT were increased in HIV-1 Tg rats compared with ad libitum- or pair-fed controls, indicating hepatic cellular damage. Although such damage was not manifested by a change in the rate of global protein synthesis in the HIV-1 Tg rats, we cannot exclude the possibility that the synthetic rate of select individual proteins was altered. Nevertheless, the circulating concentration of several proteins appears reasonably well maintained. For example, the plasma albumin concentration was not different between either control group and the HIV-1 Tg rats, despite the increased urinary excretion of albumin in the latter group. The absence of hypoalbuminemia and the small decrease in plasma IGF-I suggest that the HIV-1 Tg rats received adequate nutrition for the continued hepatic synthesis of secretory proteins (47).
Multiple immunologic defects, such as depletion of splenic T cells, impaired T cell interferon-γ production, and increased T cell apoptosis, have been reported in HIV-1 Tg rats (38, 39, 57). The resulting immune suppression would be expected to increase the susceptibility of these animals to secondary viral and bacterial infections, which might decrease muscle protein synthesis indirectly (22, 24). Several lines of evidence suggest that HIV-1 Tg animals did not have a secondary bacterial infection. 1) All rats were maintained in a barrier facility in a HEPA-filtered ventilated rack, and no animals showed external signs (e.g., piloerection, ocular discharge, and diarrhea) of illness. 2) The differential WBC count was not different between control and HIV-1 Tg rats (e.g., no leukocytosis, leucopenia, or leftward shift in the WBC differential), and there was a lack of febrile response, which might be expected in response to bacterial infection (20). 3) Data from the present study indicate no increase in the mRNA content of inflammatory cytokines in liver or skeletal muscle, and data from other studies indicate no increase in circulating nitric oxide in HIV-1 Tg rats (16). Hence, the atrophic response detected in HIV-1 Tg rats appears to result from the production of one or more of the HIV-1 viral proteins.
In conclusion, our results demonstrate a sustained growth failure in HIV-1 Tg rats over many months that cannot be solely explained by a reduction in caloric intake. The decreased body weight in HIV-1 Tg rats results from a reduction in LBM produced by an increase in muscle protein degradation without an accompanying change in protein synthesis. Enhanced proteolysis was evidenced by increased excretion of 3-MH, as well as the concomitant increase of the ubiquitin E3 ligase atrogin-1, the 14-kDa actin fragment, and in vitro proteasome activity. The change in muscle protein balance was not associated with increased inflammatory cytokines in liver or muscle. In contrast, muscle and, to a lesser extent, circulating IGF-I were reduced in HIV-1 Tg rats, and the reduction in this growth factor represents a putative physiological mechanism for the atrophic response in skeletal muscle. However, we cannot exclude the possibility that one or more of the HIV-1 viral proteins directly upregulates gene transcription for components of the ubiquitin-proteasome pathway independent of a change in IGF-I. Finally, it is noteworthy that the present studies were performed in fed rats, and additional studies should be performed in the fasted condition to assess whether the changes in protein balance and hormone levels seen in the HIV-1 Tg rats differ during periods of fasting. Despite this caveat, the HIV-1 Tg rat mimics many of the metabolic disturbances seen in humans with active HIV-1 infection and should permit future studies to further investigate the mechanism for the reduction in caloric intake and muscle wasting as well as to test potential therapeutic interventions.
GRANTS
This work was supported in part by National Institutes of Health Grants DK-072909, AA-11290, AA-12814 and GM-39722.
Acknowledgments
We thank Danuta Huber, Jay Nystrom, and Gina Deiter for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bacchetti P, Gripshover B, Grunfeld C, Heymsfield S, McCreath H, Osmond D, Saag M, Scherzer R, Shlipak M, Tien P. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr 40: 121–131, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batterham MJ Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. Am J Clin Nutr 81: 702–713, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Chan IS, Neaton JD, Saravolatz LD, Crane LR, Osterberger J. Frequencies of opportunistic diseases prior to death among HIV-infected persons. Community Programs for Clinical Research on AIDS. AIDS 9: 1145–1151, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Chaves AA, Baliga RS, Mihm MJ, Schanbacher BL, Basuray A, Liu C, Cook AC, Ayers LW, Bauer JA. Bacterial lipopolysaccharide enhances cardiac dysfunction but not retroviral replication in murine AIDS: roles of macrophage infiltration and toll-like receptor 4 expression. Am J Pathol 168: 727–735, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaves AA, Mihm MJ, Schanbacher BL, Basuray A, Liu C, Ayers LW, Bauer JA. Cardiomyopathy in a murine model of AIDS: evidence of reactive nitrogen species and corroboration in human HIV/AIDS cardiac tissues. Cardiovasc Res 60: 108–118, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int J Obes Relat Metab Disord 22: 83–88, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature 395: 763–770, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gao Q, Horvath TL. Cross-talk between estrogen and leptin signaling in the hypothalamus. Am J Physiol Endocrinol Metab 294: E817–E826, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Grunfeld C, Pang M, Shimizu L, Shigenaga JK, Jensen P, Feingold KR. Resting energy expenditure, caloric intake, and short-term weight change in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Am J Clin Nutr 55: 455–460, 1992. [DOI] [PubMed] [Google Scholar]
- 13.Hecker LM, Kotler DP. Malnutrition in patients with AIDS. Nutr Rev 48: 393–401, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Himelman RB, Chung WS, Chernoff DN, Schiller NB, Hollander H. Cardiac manifestations of human immunodeficiency virus infection: a two-dimensional echocardiographic study. J Am Coll Cardiol 13: 1030–1036, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Hong-Brown LQ, Pruznak AM, Frost RA, Vary TC, Lang CH. Indinavir alters regulators of protein anabolism and catabolism in skeletal muscle. Am J Physiol Endocrinol Metab 289: E382–E390, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kline ER, Kleinhenz DJ, Liang B, Dikalov S, Guidot DM, Hart CM, Jones DP, Sutliff RL. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. Am J Physiol Heart Circ Physiol 294: H2792–H2804, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotler DP, Tierney AR, Wang J, Pierson RN Jr. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr 50: 444–447, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289: E969–E980, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292: E1555–E1567, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Lang CH, Bagby GJ, Dobrescu C, Nelson S, Spitzer JJ. Effect of granulocyte colony-stimulating factor on sepsis-induced changes in neutrophil accumulation and organ glucose uptake. J Infect Dis 166: 336–343, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA. Role of growth hormone, insulin-like growth factor-I, and insulin-like growth factor binding proteins in the catabolic response to injury and infection. Curr Opin Clin Nutr Metab Care 5: 271–279, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Lang CH, Frost RA. Glucocorticoids and TNFα interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med 12: 291–299, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 286: E916–E926, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res 32: 796–805, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Lang CH, Nystrom G, Frost RA. β-Adrenergic blockade exacerbates sepsis-induced changes in tumor necrosis factor-α and interleukin-6 in skeletal muscle and is associated with impaired translation initiation. J Trauma 64: 477–486, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Lewis W Cardiomyopathy in AIDS: a pathophysiological perspective. Prog Cardiovasc Dis 43: 151–170, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM, Bruggeman L, Klotman P. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest 80: 187–197, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Macallan DC, Noble C, Baldwin C, Jebb SA, Prentice AM, Coward WA, Sawyer MB, McManus TJ, Griffin GE. Energy expenditure and wasting in human immunodeficiency virus infection. N Engl J Med 333: 83–88, 1995. [DOI] [PubMed] [Google Scholar]
- 31.McNurlan MA, Garlick PJ, Steigbigel RT, Decristofaro KA, Frost RA, Lang CH, Johnson RW, Santasier AM, Cabahug CJ, Fuhrer J, Gelato MC. Responsiveness of muscle protein synthesis to growth hormone administration in HIV-infected individuals declines with severity of disease. J Clin Invest 100: 2125–2132, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32: 138–147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res 30: 2065–2078, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Otis JS, Ashikhmin YI, Brown LA, Guidot DM. Effect of HIV-1-related protein expression on cardiac and skeletal muscles from transgenic rats. AIDS Res Ther 5: 8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ott M, Lembcke B, Fischer H, Jager R, Polat H, Geier H, Rech M, Staszeswki S, Helm EB, Caspary WF. Early changes of body composition in human immunodeficiency virus-infected patients: tetrapolar body impedance analysis indicates significant malnutrition. Am J Clin Nutr 57: 15–19, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Poizot-Martin I, Benourine K, Philibert P, Boulet JM, Badetti C, Tramier M, Vollot F, Dalmas AM, Manelli JC, Gastaut JA. Diet-induced thermogenesis in HIV infection. AIDS 8: 501–504, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Reid W, Abdelwahab S, Sadowska M, Huso D, Neal A, Ahearn A, Bryant J, Gallo RC, Lewis GK, Reitz M. HIV-1 transgenic rats develop T cell abnormalities. Virology 321: 111–119, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA 98: 9271–9276, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roldan EO, Moskowitz L, Hensley GT. Pathology of the heart in acquired immunodeficiency syndrome. Arch Pathol Lab Med 111: 943–946, 1987. [PubMed] [Google Scholar]
- 41.Serrano AL, Jardi M, Suelves M, Klotman PE, Munoz-Canoves P. HIV-1 transgenic expression in mice induces selective atrophy of fast-glycolytic skeletal muscle fibers. Front Biosci 13: 2797–2805, 2008. [DOI] [PubMed] [Google Scholar]
- 42.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steiner AA, Romanovsky AA. Leptin: at the crossroads of energy balance and systemic inflammation. Prog Lipid Res 46: 89–107, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suttmann U, Ockenga J, Hoogestraat L, Selberg O, Schedel I, Deicher H, Muller MJ. Resting energy expenditure and weight loss in human immunodeficiency virus-infected patients. Metabolism 42: 1173–1179, 1993. [DOI] [PubMed] [Google Scholar]
- 45.Suttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Muller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. J Acquir Immune Defic Syndr Hum Retrovirol 8: 239–246, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Tang AM, Jacobson DL, Spiegelman D, Knox TA, Wanke C. Increasing risk of 5% or greater unintentional weight loss in a cohort of HIV-infected patients, 1995 to 2003. J Acquir Immune Defic Syndr 40: 70–76, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Thissen JP, Underwood LE, Ketelslegers JM. Regulation of insulin-like growth factor-I in starvation and injury. Nutr Rev 57: 167–176, 1999. [DOI] [PubMed] [Google Scholar]
- 48.van Maanen M, Sutton RE. Rodent models for HIV-1 infection and disease. Curr HIV Res 1: 121–130, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R1777–R1789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vary TC, Kimball SR. Sepsis-induced changes in protein synthesis: differential effects on fast- and slow-twitch muscles. Am J Physiol Cell Physiol 262: C1513–C1519, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Vary TC, Kimball SR, Sumner A. Sex-dependent differences in the regulation of myocardial protein synthesis following long-term ethanol consumption. Am J Physiol Regul Integr Comp Physiol 292: R778–R787, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Vary TC, Lang CH. Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol 447: 343–355, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Visnegarwala F, Shlay JC, Barry V, Gibert CL, Xiang Y, Wang J, Kotler D, Raghavan S, El-Sadr WM. Effects of HIV infection on body composition changes among men of different racial/ethnic origins. HIV Clin Trials 8: 145–154, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Heo M, Lee RC, Kotler DP, Withers RT, Heymsfield SB. Muscularity in adult humans: proportion of adipose tissue-free body mass as skeletal muscle. Am J Hum Biol 13: 612–619, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis 31: 803–805, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Yadav A, Pati S, Nyugen A, Barabitskaja O, Mondal P, Anderson M, Gallo RC, Huso DL, Reid W. HIV-1 transgenic rat CD4+ T cells develop decreased CD28 responsiveness and suboptimal Lck tyrosine dephosphorylation following activation. Virology 353: 357–365, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Yarasheski KE, Smith SR, Powderly WG. Reducing plasma HIV RNA improves muscle amino acid metabolism. Am J Physiol Endocrinol Metab 288: E278–E284, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yarasheski KE, Zachwieja JJ, Gischler J, Crowley J, Horgan MM, Powderly WG. Increased plasma Gln and Leu Ra and inappropriately low muscle protein synthesis rate in AIDS wasting. Am J Physiol Endocrinol Metab 275: E577–E583, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]