Abstract
Cloned mouse embryos display a marked preference for glucose-containing culture medium, with enhanced development to the blastocyst stage in glucose-containing medium attributable mainly to an early beneficial effect during the first cell cycle. This early beneficial effect of glucose is not displayed by parthenogenetic, fertilized, or tetraploid nuclear transfer control embryos, indicating that it is specific to diploid clones. Precocious localization of the glucose transporter SLC2A1 to the cell surface, as well as increased expression of glucose transporters and increased uptake of glucose at the one- and two-cell stages, is also seen in cloned embryos. To examine the role of glucose in early cloned embryo development, we examined glucose metabolism and associated metabolites, as well as mitochondrial ultrastructure, distribution, and number. Clones prepared with cumulus cell nuclei displayed significantly enhanced glucose metabolism at the two-cell stage relative to parthenogenetic controls. Despite the increase in metabolism, ATP content was reduced in clones relative to parthenotes and fertilized controls. Clones at both stages displayed elevated concentrations of glycogen compared with parthenogenetic controls. There was no difference in the number of mitochondria, but clone mitochondria displayed ultrastructural alterations. Interestingly, glucose availability positively affected mitochondrial structure and localization. We conclude that cloned embryos may be severely compromised in terms of ATP-dependent processes during the first two cell cycles and that glucose may exert its early beneficial effects via positive effects on the mitochondria.
Keywords: glucose metabolism, mitochondrial ultrastructure, somatic cell nuclear transfer, cumulus cell nuclei, pentose phosphate pathway
the successful development of cloned embryos produced by the technique of somatic cell nuclear transfer (SCNT) requires that the donor cell genome be reprogrammed by the oocyte. Although the specific molecular mechanisms of reprogramming remain largely undefined, the process should, in principle, involve some degree of silencing of the donor cell gene expression program followed by activation of the embryonic gene expression program. We recently reported that although many genes apparently are reprogrammed successfully within the first two cell cycles, >2,000 mRNAs are differentially expressed between clones and control embryos at the two-cell stage, including 880 mRNAs that are overexpressed in clones in a transcription-dependent manner and another 302 transcribed mRNAs that are underexpressed. The affected mRNAs span a variety of functional categories, most notably transcription factors, oxidoreductase activity, and transporter functions (43). These observations revealed a substantial amount of difference in gene expression between clones and control embryos that may render the two very different phenotypically.
Preimplantation-stage cloned mouse embryos display a number of characteristics very different from control embryos and, in particular, manifest a number of somatic cell characteristics. These include differences in culture medium preference, expression of the somatic form of the DNA methyltransferase DNMT1, expression of molecular markers of donor cells (e.g., glucose transporter SLC2A4, also known as GLUT-4), and precocious localization of GLUT-1 to the cell surface (11, 12, 15, 16). These observations indicate that the somatic cell donor nucleus is expressed and modifies cloned embryo phenotype, in some cases even before the first cleavage division. In combination with the DNA microarray data, these observations indicate that nuclear reprogramming is slow and/or incomplete, such that when the cloned embryo becomes transcriptionally competent, it expresses a broadened array of transcripts (12, 15). The extent to which this broadened array of transcripts alters cloned embryo physiology, metabolism, and viability has not been examined in detail, but changes in culture medium preference suggest that the alterations may be substantial.
SCNT embryos differ from fertilized embryos with respect to carbon substrate requirements and energy production. These characteristics greatly distinguish somatic cells from normal fertilized embryos. During the first few cell cycles, normal embryos use predominantly small sugars, such as pyruvate and lactate, as carbon substrates and can complete early cleavage divisions without glucose (5, 6, 18, 22, 25, 27, 28). Early embryos also differ from somatic cells in their repertoire of glucose transporters (1, 7, 8, 10, 15, 21, 26, 30, 32, 34, 35). By the blastocyst stage, embryonic requirements are similar to those of somatic cells (5, 6, 18, 22, 25, 27, 28). The mitochondria of mature oocytes and early mouse embryos also differ greatly from those of somatic cells (20, 29, 37, 38, 39). Specifically, the mitochondria display a dense matrix and few archlike, circular, or transverse cristae until about the eight-cell stage. By the blastocyst stage, the mitochondria have less dense matrices and, mostly, transverse cristae. Total mitochondrial volume increases during preimplantation development. These changes correlate with an increased expression of mRNAs encoding cytochrome c oxidases IV, Vb, and VIIc and H+-ATPase subunit 9 (P1) (40). These observations reveal that the conversion of mitochondrial architecture and the shift of energy substrate utilization are under coordinated, developmentally programmed control.
We observed a significant preference of cumulus cell cloned embryos for glucose during the one-cell stage (11). SCNT embryos prepared with cumulus cell nuclei undergo the first cleavage division twice as frequently in the presence of glucose as in culture without glucose. Development to the blastocyst stage is enhanced for clones cultured in glucose-containing medium, primarily because of this early effect on progression to the two-cell stage (11). This early beneficial effect of glucose is not seen with parthenogenetic, fertilized, or tetraploid nuclear transfer control embryos, indicating that diploid cloned constructs have a unique early requirement for glucose (11). We also observed an increase in glucose uptake in cumulus and myoblast cloned embryos, aberrant expression of glucose transporter SLC2A4 (GLUT-4; typical of muscle cells) in myoblast clones, and precocious localization of SLC2A1 (GLUT-1) to the cell surface (15).
To discover the role of glucose in promoting early cloned embryo development, we examined glucose metabolism and associated metabolites, as well as mitochondrial ultrastructure, distribution, and number, in SCNT and control embryos. We report that cloned embryos prepared with cumulus cell nuclei exhibit significant differences in some, but not all, parameters, most notably a higher rate of glucose metabolism, particularly through the pentose phosphate pathway at the two-cell stage, reduced ATP content, increased glycogen deposition, and aberrant mitochondrial ultrastructure and localization, which are sensitive to the availability of glucose. These results indicate unique responses of clones to glucose in the culture medium and a possible uncoupling of glucose metabolism from ATP production in cloned embryos, which may compromise nuclear reprogramming and development.
MATERIALS AND METHODS
Oocyte collection.
Adult female (B6D2) F1 mice (8–12 wk of age) were superovulated by administration of 5 IU of equine chorionic gonadotropin (Calbiochem, San Diego, CA) followed 48 h later by 5 IU of human chorionic gonadotropin (Sigma-Aldrich, St. Louis, MO) 48 h apart. The oocytes were isolated at ∼14–15 h after human chorionic gonadotropin injection, and cumulus cells were removed by hyaluronidase treatment in HEPES-buffered M2 medium and gentle pipetting, as described elsewhere (11). All studies adhered to procedures consistent with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Temple University.
SCNT and embryo culture.
Oocytes were cultured in CZB medium supplemented with 5.5 mM glucose, as described previously (11, 12, 14, 15, 17). Spindle-chromosome complexes (SCCs) were removed using a narrow-bore pipette attached to a piezo pipette driver as described elsewhere (44). Removal of the SCCs was performed in HEPES-buffered CZB medium (HCZB) supplemented with 5.5 mM glucose and 2.5 μg/ml cytochalasin B (Sigma-Aldrich) (11). Cloned embryos were prepared by introduction of cumulus cell nuclei into SCC-depleted oocytes. This was accomplished using piezo-mediated microinjection of nuclei after rupture of the donor cell membrane with gentle trituration for removal of the plasma membrane and most of the cytoplasm. The donor nuclei free of most of the cytoplasm were then injected into the recipient oocytes with use of a single piezo pulse to penetrate the plasma membrane without cell rupture. Parthenogenetic embryos were obtained from the same pools of oocytes used to make cloned embryos. The parthenogenetic and cloned embryos were activated simultaneously by 5.5 h of culture in Ca2+-free CZB medium supplemented with 10 mM Sr2+ and cytochalasin B, as described elsewhere (44). Dimethylsulfoxide was not employed at any step, to avoid the documented uncontrolled effects on cloned embryo characteristics (11). Fertilized embryos were obtained by mating superovulated (B6D2)F1 females to (B6D2)F1 males, with isolation at the one-cell stage, as described previously (11). All embryos were cultured in Whitten's medium (WM) (11). WM was selected, because parthenotes, fertilized embryos, and clones prepared with eggs from (B6D2)F1 females develop efficiently beyond the two-cell stage in this medium (11), whereas culture medium more highly optimized for normal embryos produces arrested development in clones and, thus, cannot be employed.
Analysis of glucose metabolism.
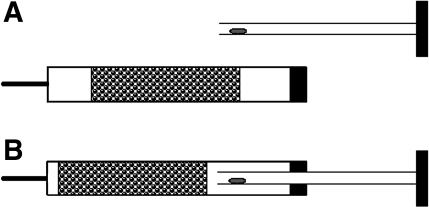
For analysis of glucose metabolism, embryos were cultured overnight in WM until the late one-cell or the middle two-cell stage. To investigate glucose metabolism, we used [1-14C]glucose (7.4 MBq/ml, 200 μCi/ml), [6-14C]glucose (7.4 MBq/ml, 200 μCi/ml), or [5-3H]glucose (37 MBq/ml, 1 mCi/ml; Amersham Bioscience or PerkinElmer) (13, 24). With [1-14C]glucose, 14CO2 is released via metabolism through the pentose phosphate pathway or glycolysis followed by the tricarboxylic acid (TCA) cycle. When [6-14C]glucose is employed, 14CO2 is released exclusively via glycolysis followed by the TCA cycle. When [5-3H]glucose is employed, 3H2O is released exclusively via glycolysis. 14CO2 and 3H2O are captured in a trap (see below) and then quantified by scintillation counting. By comparison of the rates of metabolism of the three different isotopically labeled compounds, it is possible to determine the rates of metabolism via the different pathways (13, 24). For preparation of the metabolic measurement medium, the appropriate quantities of [1-14C]glucose, [6-14C]glucose, and [5-3H]glucose were lyophilized under vacuum and then resuspended in the glucose-free HCZB. Cold nonradioactive glucose was added to bring the final glucose concentration to 4 mM. Embryos were placed in groups of 5–20 embryos (depending on the sensitivity of detecting metabolism of a given isotope) on the internal walls of the hollow plungers of 1-ml insulin syringes (Becton-Dickinson, Franklin Lakes, NJ) in 5 μl of labeling medium (Fig. 1). (This syringe is designed with a plunger that is open at the end that is inserted into the syringe but closed and airtight at the other end.) The syringes were loaded with 600 μl of 0.1 M NaOH to trap the released 14CO2 and 3H2O. The plunger was then reinserted into the syringe, and the needle cap was reapplied. No difference in sensitivity or detection was observed between this syringe system and the Eppendorf hanging-drop system previously employed (13, 24), but we found that this system is much less prone to accidental transfer of labeling medium to the trap and also permits quick and efficient collection of the trap. After 3 h of incubation at 37°C, the NaOH trap solutions were carefully expelled into scintillation vials, and radioactivity was measured by scintillation counting for 1 h. For each experiment, four blanks (drops without embryos) were incubated under the same condition that revealed background due to glucose degradation. Additionally, 5 μl of HCZB were added directly into the four traps in each experiment for use in subsequent calculations. The rates of glucose metabolism were calculated as described elsewhere (13) and expressed as picomoles per embryo per hour. Each experiment was repeated at least twice, with each experiment containing three to six samples of embryos.
Fig. 1.
Syringe system employed for glucose metabolism studies. Embryos are placed inside the hollow plunger, which is then inserted into the syringe with 0.6 ml of NaOH trap solution. This airtight system traps metabolites in the NaOH solution.
Enzyme and metabolite assays.
For the enzyme and metabolite assays, embryos were freeze-dried, as described elsewhere (9). Individual embryos were then extracted under oil and subjected to microanalytic enzymatic cycling assays. ATP was measured in each embryo individually as described for embryos (9). Similarly, intraembryonic glycogen levels were measured in single embryos, as reported elsewhere (31). Adenyl kinase and glycogen synthase assays have been described previously (36). All metabolite measurements were expressed as millimoles per kilogram wet weight with the value of 160 ng or 160 pl per embryo as measured (2). Each experiment was performed at least three times, with a total of 10–20 individual embryos assayed for each type of embryo.
Mitochondrial copy number.
Mitochondrial DNA (mtDNA) content was determined by comparison of the ratio of mtDNA [NADH-ubiquinone oxidoreductase subunit 1 (ND1): 5′-CAG GAT GAG CCT CAA ACT CC-3′ (forward primer) and 5′-GGT CAG GCT GGC AGA AGT AA-3′ (reverse primer)] to nuclear DNA [nDNA; 18S ribosomal RNA: 5′ TCG AGG CCC TGT AAT TGG AA-3′ (forward primer) and 5′ CCC TCC AAT GGA TCC TCG TT-3′ (reverse primer)] (42) measured by real-time quantitative PCR using LightCycler FastStart DNA Master SYBR Green I reagents (Roche). Each embryo was transferred separately into 0.2-ml PCR tubes containing 1 μl of 17 μM SDS and 2 μl of 125 μg/ml proteinase K (45) and incubated at 37°C overnight and then for 15 min at 95°C to inactivate the enzyme. DNA was amplified in a 20-μl-volume reaction mixture containing 4 μl of SYBR Green 5× PCR Master Mix, which contains FastStart Taq DNA polymerase, reaction buffer, dNTP mix, SYBR Green I dye, and MgCl2 and 0.5 μmol of the forward or reverse primer. PCR conditions were as follows: 95°C for 10 min followed by 45 cycles at 95°C for 10 s, 60°C for 5 s, and 72°C for 8 s. Immediately after amplification, samples were heated to 95°C immediately followed by 65°C for 15 s and then heated to 95°C immediately followed by cooling to 40°C for 30 s to create a melting curve for PCR product identification. The experiment was repeated three times, with 10–30 samples of each type of embryo.
Transmission electron microscopy/mitochondrial ultrastructure.
Five embryos from each treatment group were fixed in a mixture of 2% paraformaldehyde and 0.6% glutaraldehyde and postfixed with 1% osmium tetroxide in a sodium cacodylate buffer, dehydrated by an ascending ethanol series, and embedded in PolyBed 812 resin. Ultrathin sections were prepared using a microtome (Ultracut, Leica), placed on formwar-coated copper grids, stained with uranyl acetate and lead citrate, and examined under a Jeol 1200 EX microscope. At least three embryos from each group were evaluated subjectively for mitochondrial morphology and assigned a grade of good, fair, or poor (see results and Table 7).
Table 7.
Ultrastructural analysis of mitochondrial morphology in fertilized, cloned, and parthenogenetically activated mouse embryos
| Prevailing Mitochondrial Shape | Prevailing Central Vacuole Type | Cristae | Other Abnormalities | Mitochondrial Grade | |||||
|---|---|---|---|---|---|---|---|---|---|
| Late 1-cell stage | |||||||||
| Cloned | |||||||||
| WM+ | Round | Irregular | Circular | Few abnormalities | + | ||||
| WM− | Oval | Oval; filled with membranous material | Missing | Concentric membrane; inclusions in mitochondrial matrix; associated MVBs | − | ||||
| Fertilized | |||||||||
| WM+ | Oval | Small, eccentrically positioned | Circular, transverse | Debris inside mitochondrial vacuole | + | ||||
| WM− | Round | Irregular | Circular | Membranes inside central vacuole of some mitochondria | + | ||||
| Parthenogenetic | |||||||||
| WM− | Round | Irregular; very large | Circular; a few cristae | Cytoplasmic vacuoles | ± | ||||
| 2-Cell stage | |||||||||
| Cloned | |||||||||
| WM+ | Oval, irregular | Irregular; small | Circular, transverse | Unusually large numbers of mitochondria, evenly distributed | + | ||||
| WM− | Round, oval | Round; with concentric membranes inside | Missing, circular, transverse; often no cristae | Clustered mitochondria; cytoplasmic vacuoles | − | ||||
| Fertilized | |||||||||
| WM+ | Irregular | Irregular | Missing | Many degenerated mitochondria; debris in mitochondrial matrix and oocyte cytoplasm | − | ||||
| WM− | Round | Irregular | Missing | Few mitochondria | − | ||||
| WM+ | Round | Irregular; large | Circular; often no cristae | Clustering of mitochondria in cytoplasm | ± | ||||
| Parthenogenetic | |||||||||
| WM− | Oval | Irregular; filled with granular material/debris | Circular/missing | Cytoplasmic vacuoles; degenerated mitochondria | − | ||||
MVBs, multivesiculated bodies; +, good; ±, fair; −, poor.
MitoTracker staining and analysis.
For visualization of active mitochondria, embryos were incubated with 500 nM chloromethyltetramethylrosamine (MitoTracker; Molecular Probes, Eugene, OR) for 30 min in WM without glucose (WM−) or WM with glucose (WM+). MitoTracker is a vital, fixable fluorescent probe taken up specifically by live, active mitochondria. The samples were washed in medium for 60 min and then fixed for 30 min with 4% paraformaldehyde. Staining was visualized and embryos were photographed by a microscope (Eclipse 800; Nikon Instruments, Melville, NY) equipped with epifluorescence and differential interference contrast optics. Images were acquired with standard settings at ×60 lens magnification, focusing on the largest, equatorial diameter of each embryo, using a Cool Snap camera (Roper Scientific, Tucson, AZ) and MetaMorph 7.1.0.0 software (Universal Imaging, Downington, PA). Morphometry was performed using the morphometric tool package in MetaMorph. Each embryo (5 embryos per treatment per stage) was traced manually along the oolemma to select the entire area of the embryo, and the relative pixel intensity value (raw value) was recorded and imported into Microsoft Excel for calculation of averages for each stage and treatment.
Statistical analysis.
All experiments were performed at least twice, and most of the experiments were performed three times. Values are means ± SE. Results were analyzed using Student's t-test, and P < 0.05 was considered significant.
RESULTS
The overall experimental design of these studies was comparison of the glucose metabolic activities and related parameters of diploid SCNT and control embryos. We employed two kinds of control embryos, parthenogenetic and fertilized. Parthenogenetic (B6D2)F1 embryos develop in WM to the two-cell stage and beyond with an efficiency comparable to that of fertilized embryos (11, 16). In contrast to fertilized embryos, parthenogenetic embryos can be isolated, cultured, and activated using identical procedures, simultaneously, and from the same pool of oocytes as SCNT embryos. Additionally, previous studies revealed significant differences in glucose uptake between cloned and parthenogenetic embryos, thereby providing an ideal opportunity for examination of the basis for increased glucose uptake and the subsequent fates of glucose in the different types of embryos. In vivo fertilized embryos were also isolated, cultured, and analyzed. Fertilized embryos provide less-than-ideal controls for evaluating changes in cloned embryo properties. Direct comparisons between cloned and fertilized embryos are complicated by the lack of synchrony among the fertilized cohorts and the difference in relative developmental staging between the two groups. Additionally, fertilized embryos are activated by sperm factors and possess a sperm-derived genome and, thus, differ significantly from cloned embryos. Comparisons between cloned and fertilized embryos may be skewed by these differences, whereas comparisons between clones and parthenotes avoid these differences. Nevertheless, it is valuable to compare results for SCNT and parthenogenetic embryos with data from fertilized embryos, inasmuch as this comparison provides an indication of the relative ranges of measurements among the three types of embryos. Consequently, cloned embryo properties are compared with parthenogenetic and fertilized embryo properties for assessment of alterations that are characteristic of cloned embryos.
Glucose metabolism.
Glucose metabolism by late one-cell-stage embryos was measured 14 h after activation (Table 1). Measurements with the three different forms of radiolabeled glucose showed no significant difference between cloned and parthenogenetic embryos. Glucose metabolism by middle two-cell-stage embryos was measured 25 h after activation (Table 1). Measurement of [1-14C]glucose and [5-3H]glucose showed significantly higher glucose metabolism in cloned two-cell embryos than in control two-cell parthenogenetic embryos: a 62% higher rate through the pentose phosphate pathway ([1-14C]glucose − [6-14C]glucose) and an 89% higher rate through glycolysis ([5-3H]glucose). Metabolism through the TCA cycle did not appear increased in clones compared with parthenotes. Comparison of the results obtained for clones and parthenotes with rates displayed by fertilized embryos revealed lower overall rates of metabolism in the clones and parthenotes, except with [6-14C]glucose, for which we obtained values that were lower than expected on the basis of published data for other strains of fertilized embryos (36). Thus the most pronounced difference in cloned embryos relative to parthenogenetic controls and fertilized embryos is the increase in metabolism via the pentose phosphate pathway.
Table 1.
Glucose metabolism of cloned, parthenogenetic, and fertilized embryos at 1- and 2-cell stages
| Glucose Metabolized | Cloned Embryos | Parthenogenetic Embryos | Fertilized Embryos | ||
|---|---|---|---|---|---|
| 1-Cell stage | |||||
| [1-14C]glucose | 0.091±0.009 | 0.083±0.004 | |||
| [6-14C]glucose | 0.051±0.005 | 0.046±0.002 | |||
| [5-3H]glucose | 0.01±0.001 | 0.01±0.0006 | |||
| [1-14C] − [6-14C] | 0.04 | 0.037 | |||
| 2-Cell stage | |||||
| [1-14C]glucose | 0.084±0.006* | 0.054±0.005† | 0.217±0.031‡ | ||
| [6-14C]glucose | 0.008±0.002† | 0.007±0.001† | 0.002±0.0003 | ||
| [5-3H]glucose | 0.051±0.003*† | 0.027±0.002† | 0.101±0.005‡ | ||
| [1-14C]/[6-14C] | 0.076*† | 0.047† | 0.215 | ||
Values are means ± SE. [1-14C] − [6-14C], [1-14C]glucose − [6-14C]glucose.
Significantly different from parthenogenetic embryos (P < 0.05).
Significantly different from fertilized embryos (P < 0.05).
Measurements employed radiolabeled compound from PerkinElmer, instead of Amersham.
ATP content.
We next determined whether the increased rate of glucose uptake and metabolism in clones led to an increase in the ATP content, maintenance of a normal amount of ATP, or an ATP deficit. We applied a sensitive assay applicable at the level of single embryos to detect and quantify ATP (9) and collected embryos in a total of three independent series of experiments each for one- and two-cell stages: two cultured with WM+ and one with WM−. Clones and parthenotes displayed no significant differences at the one-cell stage, but both contained significantly less ATP than the fertilized embryos (Table 2). At the two-cell stage, clones displayed significant reductions in ATP content in all three experimental groups (range 9–21% reduction) compared with parthenotes, regardless of the presence or absence of glucose. Once again, clones and parthenotes contained less ATP than fertilized embryos.
Table 2.
ATP content of parthenogenetic, cloned, and fertilized embryos
|
Cloned Embryos |
Parthenogenetic Embryos
|
Fertilized Embryos
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WM− | WM+ | WM− | WM+ | WM− | WM+ | ||||||
| 1-Cell assay | |||||||||||
| Expt 1 | 3.87±0.14† (15) | 4.07±0.11† (14) | |||||||||
| Expt 2 | 3.89±0.07† (14) | 3.98±0.08† (20) | 3.81±0.09† (20) | 3.79±0.06† (19) | |||||||
| Expt 3 | 4.59±0.08 (15) | 4.51±0.12 (15) | |||||||||
| 2-Cell assay | |||||||||||
| Expt 1 | 2.74±0.05*† (15) | 3.01±0.08† (15) | |||||||||
| Expt 2 | 2.58±0.06*† (20) | 2.48±0.08*† (18) | 3.08±0.05† (19) | 3.14±0.05† (20) | |||||||
| Expt 3 | 3.60±0.09 (15) | 3.62±0.09 (15) | |||||||||
Values are means ± SE of number of measurements in parentheses. WM−, Whitten's medium (WM) without glucose; WM+, WM with glucose.
Significantly different from parthenogenetic embryos within the same culture medium (P < 0.05).
Significantly different from fertilized embryos within the same culture medium (P < 0.05).
Adenylate kinase activity.
Next, we sought to determine whether the ATP deficiency could be the result of a deficiency in adenylate kinase activity. There was no significant difference in activity between clones and parthenotes or between cloned and fertilized embryos at the one- or two-cell stage (Table 3). Parthenotes displayed slightly less adenylate kinase activity than fertilized embryos at the one-cell stage. Activity remained virtually constant between the two stages for all three types of embryos.
Table 3.
Adenylate kinase assays of parthenogenetic, cloned, and fertilized embryos
| Cloned Embryos (n = 10) | Parthenogenetic Embryos (n = 10) | Fertilized Embryos (n = 15) | |||
|---|---|---|---|---|---|
| 1-Cell assay | |||||
| Expt 1 | 163.0±3.63 | 160.3±4.01† | |||
| Expt 2 | 169.9±2.27 | ||||
| 2-Cell assay | |||||
| Expt 1 | 156.6±3.94 | 163.5±2.28 | |||
| Expt 2 | 164.1±1.7 | ||||
Values are means ± SE.
Significantly different from fertilized embryos within the same culture medium (P < 0.05).
Glycogen content.
The above-described results fail to account for the previously observed preference of cloned embryos for glucose-containing medium during the one-cell stage (15). In addition to the metabolism of glucose through the pentose phosphate pathway, glycolysis, and the TCA cycle, embryos can also convert glucose to glycogen, which could affect glucose availability for metabolic processes. Therefore, we examined glycogen content in one- and two-cell embryos (Table 4). We observed no consistent difference between clones and parthenotes at the one-cell stage in WM+ or WM−; however, clones and parthenotes contained less glycogen than the fertilized one-cell embryos. We observed at the two-cell stage that glycogen content was generally higher in WM+ for clones and parthenotes. At the two-cell stage, we also found that clones consistently displayed increased glycogen contents relative to parthenotes, indicating that the cloned embryos respond to glucose with an enhanced accumulation of glycogen relative to the parthenogenetic control embryos at the two-cell stage, but not at the one-cell stage. Fertilized two-cell embryos contained more glycogen than cloned or parthenogenetic embryos at either stage.
Table 4.
Glycogen assays of parthenogenetic, cloned, and fertilized embryos
|
Cloned Embryos |
Parthenogenetic Embryos
|
Fertilized Embryos
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WM− | WM+ | WM− | WM+ | WM− | WM+ | ||||||
| 1-Cell assay | |||||||||||
| Expt 1 | 6.15±0.29† (15) | 6.55±0.39† (14) | |||||||||
| Expt 2 | 4.52±0.18† (13) | 6.71±0.32† (19) | 4.39±0.15† (18) | 6.47±0.21† (19) | |||||||
| Expt 3 | 7.40±0.37‡ (15) | 10.29±0.48 (15) | |||||||||
| 2-Cell assay | |||||||||||
| Expt 1 | 12.76±0.55*† (14) | 8.79±0.29† (14) | |||||||||
| Expt 2 | 5.78±0.24*†‡ (20) | 12.05±0.58*† (18) | 5.00±0.23†‡ (17) | 9.80±0.33† (20) | |||||||
| Expt 3 | 9.37±0.49‡ (15) | 40.02±1.42 (15) | |||||||||
Values are means ± SE of number of measurements in parentheses.
Significantly different from parthenogenetic embryos within the same culture medium (P < 0.05).
Significantly different from fertilized embryos within the same culture medium (P < 0.05).
Significantly different from WM+ within embryo type (P < 0.05).
Glycogen synthase activity.
Because glycogen accumulation was increased in two-cell clones, we measured glycogen synthase activity at the two stages (Table 5). There was no significant difference in glycogen synthase activity among the three types of embryos at either stage.
Table 5.
Glycogen synthase assays of parthenogenetic, cloned, and fertilized embryos
| Cloned Embryos (n = 10) | Parthenogenetic Embryos (n = 10) | Fertilized Embryos (n = 15) | |||
|---|---|---|---|---|---|
| 1-Cell assay | |||||
| Expt 1 | 6.41±0.2 | 6.61±0.18 | |||
| Expt 2 | 6.81±0.2 | ||||
| 2-Cell assay | |||||
| Expt 1 | 7.39±0.14 | 7.78±0.24 | |||
| Expt 2 | 7.58±0.26 | ||||
Values are means ± SE.
Mitochondrial copy number.
To test whether a difference in mitochondrial copy number could underlie the ATP deficiency, we determined the relative mtDNA copy number by comparing the amount of the mitochondrial ND1 gene DNA with the nuclear encoded ribosomal 18S gene DNA (mtDNA-to-nDNA ratio). There was no significant difference in the mtDNA-to-nDNA ratio between clones and parthenotes at the one- or two-cell stage. Clones and parthenotes had significantly lower mtDNA-to-nDNA ratios than the fertilized embryos at the two-cell stage, but only for WM+ cultures (Table 6).
Table 6.
Mitochondrial copy numbers of parthenogenetic, cloned, and fertilized embryos
|
Cloned Embryos |
Parthenogenetic Embryos
|
Fertilized Embryos
|
||||
|---|---|---|---|---|---|---|
| WM− | WM+ | WM− | WM+ | WM− | WM+ | |
| 1-Cell stage | 100.84±2.59 | 99.47±1.96 | 98.99±1.84 | 100.35±1.56 | 103.20±1.48 | 104.18±2.73 |
| 2-Cell stage | 80.54±1.18 | 79.09±2.67† | 79.87±1.39 | 76.69±2.61† | 81.54±2.33 | 86.98±1.43 |
Values are means ± SE expressed as ratio of mitochondrial to nuclear DNA.
Significantly different from fertilized embryos within the same culture medium (P < 0.05).
Analysis of mitochondrial ultrastructure.
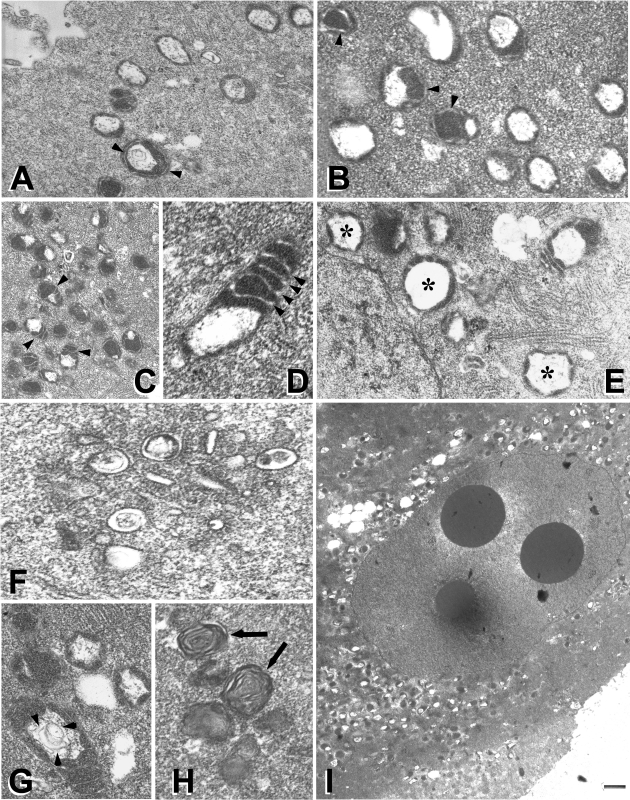
The fertilized, cloned, and parthenogenetic embryos cultured in WM+ were compared with the cloned and parthenogenetically activated embryos cultured in WM− at late one-cell and two-cell stages of embryo development in vitro. Normal mitochondrial morphology was seen in fertilized embryos (Fig. 2A) and WM+ cloned embryos (Fig. 2B), characterized by a round shape and circular cristae at the one-cell stage (Fig. 2, A and B) or traverse cristae at the two-cell stage (Fig. 2, C and D; 2-cell WM), a regularly shaped central vacuole, uniform size of mitochondria, regular round or elongated mitochondrion shape, and an even distribution of mitochondria throughout the cytoplasm with no clustering or cortical localization.
Fig. 2.
A: normal mitochondrial morphology in a 1-cell in vivo embryo. B: typical mitochondrial morphology in a 1-cell clone cultured in Whitten's medium (WM) with glucose (WM+). Note round shape and circular cristae (arrowheads in A and B). C and D: traverse cristae (arrowheads) in 2-cell WM+ embryos with good mitochondrial morphology. E: abnormally large mitochondria with a large central vacuole (*) in a 2-cell parthenote cultured in WM without glucose (WM−). F: cluster of deteriorated mitochondrial membranes in a fertilized 2-cell WM+ embryo. G: mitochondrion with an irregular central vacuole filled with concentric lamellar membranes (arrowheads) in a 2-cell fertilized WM− embryo. H: deteriorated mitochondria with concentric membrane rings in a 2-cell fertilized WM− embryo (arrows). I: clustering of mitochondria around blastomere nucleus in a 2-cell WM+ clone.
The most common anomalies contributing to poor mitochondrial morphology include size variation, irregular shape, very large mitochondria with a large central vacuole (Fig. 2E; WM+ parthenotes at the 2-cell stage), lack of cristae or disorganized cristae, decaying mitochondria without cristae displaying signs of breakdown such as the interrupted outer mitochondrial membrane and electron-dense clumps or nuages inside or adjacent to the mitochondria (Fig. 2F; WM+ fertilized embryos at the 2-cell stage), and irregular central vacuole with cristae or lamellar membranes in the lumen (Fig. 2G; WM− fertilized embryos at the 2-cell stage). Concentric membrane ring structures near mitochondria (Fig. 2H; WM+ fertilized embryos at the 2-cell stage) and mitochondrial clustering in the cytoplasm or around the nuclei (Fig. 2I; WM+ cloned embryos at the 2-cell stage) were also observed. (For additional examples of the most common mitochondrial anomalies, see supplemental Figs. S1–S3 in the online version of this article.)
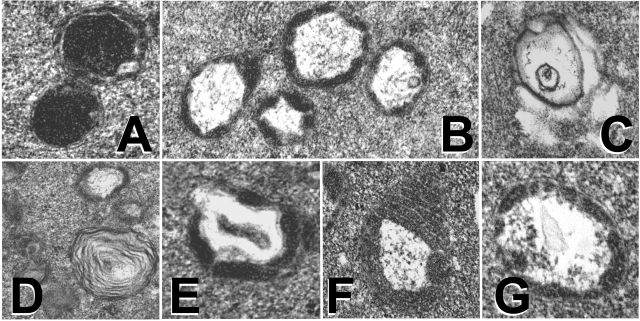
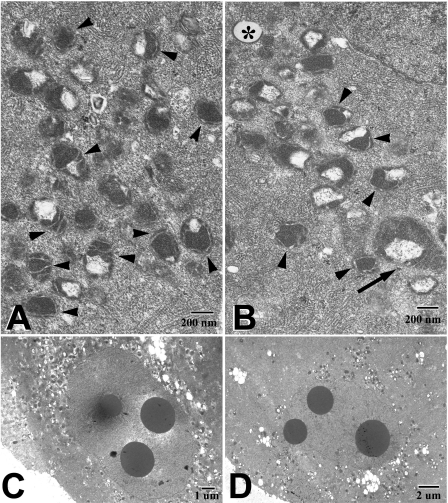
Mitochondrial morphology was rated subjectively as good, fair, or poor on the basis of prevailing size, shape, and ultrastructural characteristics of the mitochondria (Fig. 3). Data summarized in Table 7 show that the best mitochondrial morphology consistent with previously described mitochondrial ultrastructure and distribution of mouse oocytes and zygotes (33, 41) was observed in the fertilized embryos at the one-cell stage and in the reconstructed/activated WM+ embryos. The cloned WM+ embryos had a better mitochondrial morphology than the parthenogenetically activated WM+ embryos. Surprisingly, the fertilized embryos cultured up to the two-cell stage showed “poor” mitochondrial morphology whether they were cultured in WM+ or WM−. The cloned and parthenogenetically activated embryos cultured in WM− showed the poorest mitochondrial morphology (3 of 4 groups rated poor and 1 group rated “fair”). On the basis of treatment and developmental stage, only the fertilized embryos at the one-cell stage showed a consistently “good” mitochondrial morphology, regardless of the culture medium. Cloned embryos, however, displayed the most pronounced difference in mitochondrial morphology between WM+ and WM− (2 of 2 good and 2 of 2 poor, respectively). Clones cultured in WM− displayed greater variability in mitochondrial size, fewer mitochondria with cristae, and degenerated mitochondria compared with clones cultured in WM+ (Fig. 4).
Fig. 3.
Mitochondrial ultrastructure in fertilized (A–C), cloned (D and E), and parthenogenetically activated (F and G) embryos. A: round mitochondria with circular cristae. B: mitochondrion with a large, regularly shaped central vacuole. C: disintegrating mitochondrion displaying loss of mitochondrial cristae and matrix. D: lamellar organization of membranes. E and G: central vacuole debris. F: loss of outer mitochondrial membrane.
Fig. 4.
Mitochondrial ultrastructure in WM+ (A and c) and WM− (B and D) cloned 2-cell embryos. WM+ embryos had mostly even-sized mitochondria with circular or transvere cristae (arrowheads in A) and distinct perinuclear distribution (C). WM− embryos showed greater variability in mitochondrial size (note very large mitochondrion, arrow in B), fewer mitochondria with cristae (arrowheads in B), degenerated mitochondria (* in B), and less pronounced perinuclear accumulation of mitochondria (D).
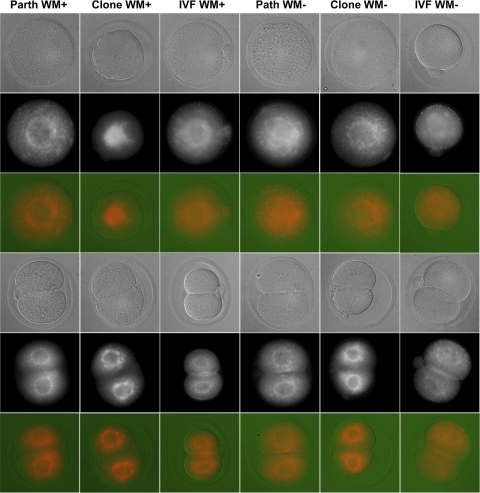
To explore mitochondrial properties further, we labeled embryos with MitoTracker dye to visualize the overall distribution of active mitochondria (Table 8). For the one-cell stage in WM+, fertilized embryos displayed predominantly cortical mitochondrial localization, parthenotes displayed a combination of diffuse and perinuclear localization, and clones displayed predominantly perinuclear labeling. In WM−, the parthenogenetic and fertilized embryos tended to display enhanced perinuclear localization, whereas cloned embryos tended to display more diffuse localization across the cytoplasm. At the two-cell stage, clones and parthenotes displayed predominantly perinuclear localization, whereas the fertilized embryos displayed a combination of perinuclear and cortical localization (Fig. 5). There was little difference attributable to glucose content, although perinuclear localization appeared more intense in all three types of WM+ embryos (Fig. 5). Because we observed a difference between clones and parthenotes in ATP content at the two-cell stage, we also measured overall staining intensity at the two-cell stage (Table 9). We observed no statistically significant difference in overall MitoTracker staining intensities in any of the groups, although fertilized embryos tended to have the highest relative intensity averages.
Table 8.
Mitochondrial distribution visualized by MitoTracker labeling
| Embryo 1 | Embryo 2 | Embryo 3 | Embryo 4 | Embryo 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1-Cell stage | |||||||||
| WM+ | |||||||||
| Parthenogenetic | Perinuclear, punctuate across ooplasm | Perinuclear, punctuate across ooplasm | Diffuse | Diffuse | Diffuse | ||||
| Cloned | Perinuclear | Perinuclear | Perinuclear, diffuse ooplasmic | Perinuclear | Perinuclear | ||||
| IVF | Cortical | Cortical | Cortical | Cortical | Cortical | ||||
| WM− | |||||||||
| Parthenogenetic | Perinuclear | Diffuse | Perinuclear/start to diffuse | Perinuclear | Perinuclear | ||||
| Cloned | Perinuclear, strong across cytoplasm | Perinuclear, strong across cytoplasm | Strong across cytoplasm | Strong across cytoplasm | Perinuclear, across cytoplasm | ||||
| IVF | Cortical | Cortical | Cortical, perinuclear | Cortical, perinuclear | Cortical, perinuclear | ||||
| 2-Cell stage | |||||||||
| WM+ | |||||||||
| Parthenogenetic | Perinuclear | Perinuclear | Perinuclear | Perinuclear | Perinuclear | ||||
| Cloned | Perinuclear | Perinuclear | Perinuclear | Perinuclear | Perinuclear | ||||
| IVF | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | ||||
| WM− | |||||||||
| Parthenogenetic | Perinuclear | Perinuclear | Perinuclear, cortical | Perinuclear | Perinuclear | ||||
| Cloned | Perinuclear | Perinuclear | Perinuclear | Perinuclear | Perinuclear | ||||
| IVF | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | Perinuclear, cortical | ||||
IVF, in vitro fertilized.
Fig. 5.
Embryo uptake of the vital mitochondrial dye chloromethyltetramethylrosamine (MitoTracker), reflective of mitochondrial activity and distribution. All groups displayed perinuclear localization of mitochondria, to various degrees, whereas cortical localization was observed uniquely in in vitro fertilized (IVF) embryos. IVF embryos also showed highest relative intensity of MitoTracker labeling, which was further enhanced by glucose in the culture medium (see Table 8). Perfocal images of MitoTracker-labeled embryos were obtained with differential interference contrast optics. All MitoTracker images are shown as acquired, i.e., as raw data without rebalancing of contrast and brightness. Mitotracker images are shown in both grayscale and in false color.
Table 9.
MitoTracker staining intensity measurements at the 2-cell stage
|
Cloned Embryos |
Parthenogenetic Embryos
|
Fertilized Embryos
|
||||
|---|---|---|---|---|---|---|
| WM− | WM+ | WM− | WM+ | WM− | WM+ | |
| Expt 1 | 1,211±56 | 1,320±74 | 1,289±65 | 1,348±59 | 1,309±36 | 1,396±85 |
| Expt 2 | 1,366±70 | 1,343±45 | 1,332±69 | 1,354±66 | 1,501±66 | 1,484±52 |
Values are means ± SE, expressed in arbitrary units, for 4–5 embryos. The same microscope settings were used for all measurements.
DISCUSSION
The results of the present study constitute the first detailed examination of carbohydrate metabolism combined with analysis of mitochondrial ultrastructure, distribution, and number in early cloned embryos of any mammalian species. Previous studies revealed that cloned embryos exhibit a unique preference for glucose-containing medium as early as the one-cell stage, resulting in enhanced development to the blastocyst stage when glucose is provided (11). In the present study, we show increased rates of glucose metabolism via the pentose phosphate pathway and glycolysis in cloned embryos at the two-cell stage prepared with cumulus cell nuclei relative to parthenogenetic controls; however, clones and parthenotes displayed lower overall rates of metabolism than fertilized two-cell embryos. Clones displayed an ATP deficit at the two-cell stage compared with parthenotes and fertilized controls. This suggests that cloned embryos are likely handicapped in their ability to execute ATP-dependent cellular processes, thus restricting developmental progress. The ATP deficit also suggests that cloned embryos may have higher energy demands than control embryos, a characteristic that may arise from aberrant expression of ATP-requiring processes expressed in the somatic donor cell type, from increased ATP demands associated with the need to reprogram chromatin structure, or from increased ATP demands related to other aberrantly expressed gene activities. This result also indicates that the developmental potential of cloned embryos might be improved if the increased ATP demand could be satisfied by alteration of the culture environment or manipulation of the mitochondrial composition of clones.
The ATP deficiency might conceivably be related to deficiencies in mitochondrial function. Mitochondrial copy number appeared unaltered in clones, but the ultrastructural examination revealed that mitochondrial morphology and ultrastructure were not always favorable in cloned embryos, particularly in the WM− group. We have noted a greater incidence of aberrant mitochondrial morphologies in embryos cultured in WM−, regardless of stage. Cloned bovine embryos also display deficiencies in mitochondrial ultrastructure (19). We observed little difference in MitoTracker staining of mitochondria at the two-cell stage. This indicates that clones are likely not experiencing an accelerated or deficient mitochondrial replication, but they may be affected by inherent problems with structure and mitochondrial function not revealed by these assays. These problems, to some extent, might be resolved by adjustment of the glucose content of the culture medium.
We also found that cloned two-cell embryos contain more glycogen than the parthenogenetic embryos but less than fertilized embryos at a comparable stage of development. Glycogen content in all three types of embryos was sensitive to the presence/absence of glucose in the medium, and the increase in glycogen content in response to glucose was greater in cloned than in parthenogenetic embryos. This may reflect the glycogen synthesis activity of the cumulus cell nuclear donors, with a donor cell gene expression pattern being imposed on the cloned embryos. There was no difference in glycogen synthase activity, however, and the much higher glycogen content in fertilized two-cell embryos indicates that glycogen synthesis and accumulation in clones are within the range of normal embryogenesis. Thus clones may simply be undergoing a slower rate of glycogen degradation than parthenotes and fertilized embryos. Also, a higher oxygen concentration can promote glycogen accumulation in fertilized embryos (23). In this study, neither clones nor parthenotes displayed the same high concentration of glycogen found in the cultured fertilized embryos.
We previously observed that cloned embryos prepared with cumulus cell nuclei prefer glucose-containing medium for progression through the first cell cycle (11), a result that contrasts greatly with normal embryos and has not been explained. Glucose uptake and expression of glucose transporters are also altered in clones (15). These unusual characteristics of cloned embryos indicate that the cumulus cell donor nucleus alters embryo phenotype even before the first cleavage division. The metabolic data obtained here for the one-cell stage fail to identify a metabolic basis for this early glucose requirement, inasmuch as none of the metabolic parameters display expected differences to account for this effect. This suggests that glucose may be serving some other function in the one-cell cloned embryo. Studies of normal one-cell embryos revealed the presence of glucose transporters in the nuclear compartment, suggesting possible signaling functions (4). Additionally, glucose may be required for protein glycosylation. A more likely explanation, however, is that glucose affects mitochondrial structure or function, even at this early stage. Indeed, at the one-cell stage, we observe a beneficial effect of glucose availability on mitochondrial morphology, ultrastructure, and perinuclear distribution. The perinuclear clustering of mitochondria in hamster embryos at the one-cell stage is associated with enhanced developmental potential (3), suggesting that the availability of glucose could influence clone developmental progression by affecting mitochondrial distribution. Interestingly, the differences between normal and abnormal mitochondrial morphology appeared to be more pronounced between clones grown in the two media than between parthenotes grown in the two media. This suggests that clones may respond more favorably than parthenotes to the presence of glucose, at least within the context of the WM formulation. We reported previously increased expression of a number of mRNAs encoding mitochondrial proteins, including the key regulator Tfam, in cumulus cell cloned embryos cultured in MEMα, which also contains glucose (43). Thus the preference of clones for glucose may be related to intracellular signaling, which ultimately controls mitochondrial function via a transcription-dependent mechanism. The difference between cloned and control embryos with respect to the beneficial effects of glucose on mitochondrial ultrastructure and distribution indicates that some form of downstream signaling in response to glucose is lacking in normal embryos but is present in clones.
The significant increases in the carbon substrate metabolism of cloned embryos prepared with cumulus cell nuclei vs. parthenogenetic and fertilized embryos, the beneficial effects of glucose on development and mitochondrial structure and localization, and the previously reported increase in expression of genes related to oxidative phosphorylation (43) indicate a failure to complete early reprogramming of gene expression. As a result, clones display characteristics of their somatic donor cells (cumulus cells), which are differentiated to metabolize glucose at increased rates in support of oogenesis (13). In this sense, the enhancement of glucose metabolism through the pentose phosphate pathway is reminiscent of the hormone-responsive increase in the pentose phosphate pathway in cumulus cells (13). We propose that the slow pace of nuclear reprogramming constitutes a fundamental restriction that must be overcome if cloning efficiency is to be improved. The results presented here offer clues into how this may be accomplished by addressing the increased metabolic demands and deficiencies in the ATP production manifested in cloned embryos.
GRANTS
This work was supported in part by National Institute of Child Health and Human Development Grant HD-43092 to K. E. Latham. P. Sutovsky was supported by the Food for the 21st Century Program of the University of Missouri-Columbia and by National Research Initiative Competitive Grants 2002-35203-12237 and 2007-35203-18274 from the US Department of Agriculture Cooperative State Research, Education, and Extension Service. K. H. Moley was supported by National Institute of Child Health and Human Development Grant U01-HD-044691.
Supplementary Material
Acknowledgments
We thank Cheryl Jensen (Electron Microscopy Core of the University of Missouri-Columbia) for transmission electron microscopy sample processing.
Present address of R. Vassena: Stem Cell Bank CMR[B] Av., Dr. Aiguader 88, Barcelona, Spain.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aghayan M, Rao LV, Smith RM, Jarett L, Charron MJ, Thorens B, Heyner S. Developmental expression and cellular localization of glucose transporter molecules during mouse preimplantation development. Development 115: 305–312, 1992. [DOI] [PubMed] [Google Scholar]
- 2.Barbehenn EK, Wales RG, Lowry OH. Measurement of metabolites in single preimplantation embryos: a new means to study metabolic control in early embryos. J Embryol Exp Meophol 43: 29–46, 1978. [PubMed] [Google Scholar]
- 3.Bavister BD, Squirrell JM. Mitochondrial distribution and function in oocytes and early embryos. Hum Reprod 15, Suppl 2: 189–198, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod 16: 153–163, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Brackett BG In vitro culture of the zygote and embryo. In: Fertilization and Embryonic Development In Vitro, edited by Mastroanni L and Biggers JD. New York: Plenum, 1981, p. 63–79.
- 6.Brown JJ, Whittingham DG. The roles of pyruvate, lactate and glucose during preimplantation development of embryos from F1 hybrid mice in vitro. Development 112: 99–105, 1991. [DOI] [PubMed] [Google Scholar]
- 7.Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA 97: 7313–7318, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology 145: 1435–1443, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Chi MM, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. Am J Physiol Endocrinol Metab 283: E226–E232, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem 275: 40252–40257, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Chung YG, Mann MR, Bartolomei MS, Latham KE. Nuclear-cytoplasmic “tug-of-war” during cloning: effects of somatic cell nuclei on culture medium preferences in the preimplantation cloned mouse embryo. Biol Reprod 66: 1178–1184, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Chung YG, Ratnam S, Chaillet JR, Latham KE. Abnormal regulation of DNA methyltransferase expression in cloned mouse embryos. Biol Reprod 69: 146–153, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte-cumulus cell complexes. Biol Reprod 60: 1446–1452, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Gao S, Chung YG, Parseghian MH, King GJ, Adashi E, Latham KE. Rapid H1 linker histone transitions following fertilization or somatic cell nuclear transfer: evidence for a uniform developmental program in mice. Dev Biol 266: 62–75, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Chung YG, Williams JW, Riley J, Moley K, Latham KE. Somatic cell-like features of cloned mouse embryos prepared with cultured myoblast nuclei. Biol Reprod 69: 48–56, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Czirr E, Chung YG, Han Z, Latham KE. Genetic variation in oocyte phenotype revealed through parthenogenesis and cloning: correlation with differences in pronuclear epigenetic modification. Biol Reprod 70: 1162–1170, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gao S, McGarry M, Latham KE, Wilmut I. Cloning of mice by nuclear transfer. Cloning Stem Cells 5: 287–294, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Hardy K, Hooper MA, Handyside AH, Rutherford AJ, Winston RM, Leese HJ. Non-invasive measurement of glucose and pyruvate uptake by individual human oocytes and preimplantation embryos. Hum Reprod 4: 188–191, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Heyman Y, Degrolard J, Adenot P, Chesne P, Flechon B, Renard JP, Flechon JE. Cellular evaluation of bovine nuclear transfer embryos developed in vitro. Reprod Nutr Dev 35: 713–723, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Hillman N, Tasca RJ. Ultrastructural and autoradiographic studies of mouse cleavage stages. Am J Anat 126: 151–173, 1969. [DOI] [PubMed] [Google Scholar]
- 21.Hogan A, Heyner S, Charron MJ, Copeland NG, Gilbert DJ, Jenkins NA, Thorens B, Schultz GA. Glucose transporter gene expression in early mouse embryos. Development 113: 363–372, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Houghton FD, Thompson JG, Kennedy CJ, Leese HJ. Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 44: 476–485, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Khurana NK, Wales RG. Effects of oxygen concentration on the metabolism of [U-14C]glucose by mouse morulae and early blastocysts in vitro. Reprod Fertil Dev 1: 99–106, 1989. [DOI] [PubMed] [Google Scholar]
- 24.Krisher RL, Bavister BD. Enhanced glycolysis after maturation of bovine oocytes in vitro is associated with increased developmental competence. Mol Reprod Dev 53: 19–26, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Leese HJ, Barton AM. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 72: 9–13, 1984. [DOI] [PubMed] [Google Scholar]
- 26.Leppens-Luisier G, Urner F, Sakkas D. Facilitated glucose transporters play a crucial role throughout mouse preimplantation embryo development. Hum Reprod 16: 1229–1236, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Lequarre AS, Grisart B, Moreau B, Schuurbiers N, Massip A, Dessy F. Glucose metabolism during bovine preimplantation development: analysis of gene expression in single oocytes and embryos. Mol Reprod Dev 48: 216–226, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Martin KL, Leese HJ. Role of glucose in mouse preimplantation embryo development. Mol Reprod Dev 40: 436–443, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto H, Shoji N, Sugawara S, Umezu M, Sato E. Microscopic analysis of enzyme activity, mitochondrial distribution and hydrogen peroxide in two-cell rat embryos. J Reprod Fertil 113: 231–238, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Moley KH, Chi MM, Mueckler MM. Maternal hyperglycemia alters glucose transport and utilization in mouse preimplantation embryos. Am J Physiol Endocrinol Metab 275: E38–E47, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Moley KH, Chi MMY, Manchester JK, McDougal DB, Lowry OH. Alterations of intraembryonic metabolites in preimplantation mouse embryos exposed to elevated concentrations of glucose: a metabolic explanation for the development retardation seen in preimplantation embryos from diabetic animals. Biol Reprod 54: 1209–1216, 1996. [DOI] [PubMed] [Google Scholar]
- 32.Morita Y, Tsutsumi O, Oka Y, Taketani Y. Glucose transporter GLUT1 mRNA expression in the ontogeny of glucose incorporation in mouse preimplantation embryos. Biochem Biophys Res Commun 199: 1525–1531, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Nagai S, Mabuchi T, Hirata S, Shoda T, Kasai T, Yokota S, Shitara H, Yonekawa H, Hoshi K. Correlation of abnormal mitochondrial distribution in mouse oocytes with reduced developmental competence. Tohoku J Exp Med 210: 137–144, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Pantaleon M, Kaye PL. Glucose transporters in preimplantation development. Rev Reprod 3: 77–81, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleon M, Ryan JP, Gil M, Kaye PL. An unusual subcellular localization of GLUT1 and link with metabolism in oocytes and preimplantation mouse embryos. Biol Reprod 64: 1247–1254, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Clifton, NJ: Humuna, 1993.
- 37.Sathananthan AH, Trounson AO. Mitochondrial morphology during preimplantational human embryogenesis. Hum Reprod 15, Suppl 2: 148–159, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Shepard TH, Muffley LA, Smith LT. Mitochondrial ultrastructure in embryos after implantation. Hum Reprod 15, Suppl 2: 218–228, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Shepard TH, Muffley LA, Smith LT. Ultrastructural study of mitochondria and their cristae in embryonic rats and primate (N. nemistrina). Anat Rec 252: 383–392, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Taylor KD, Piko L. Mitochondrial biogenesis in early mouse embryos: expression of the mRNAs for subunits IV, Vb, and VIIc of cytochrome c oxidase and subunit 9 (P1) of H+-ATP synthase. Mol Reprod Dev 40: 29–35, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Valojerdi MR, Salehnia MJ. Developmental potential and ultrastructural injuries of metaphase II (MII) mouse oocytes after slow freezing or vitrification. Assist Reprod Genet 22: 119–127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Bosch BJ, van den Burg CM, Schoonderwoerd K, Lindsey PJ, Scholte HR, de Coo RF, van Rooij E, Rockman HA, Doevendans PA, Smeets HJ. Regional absence of mitochondria causing energy depletion in the myocardium of muscle LIM protein knockout mice. Cardiovasc Res 65: 411–418, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Vassena R, Han Z, Gao S, Baldwin DA, Schultz RM, Latham KE. Tough beginnings: alterations in the transcriptome of cloned embryos during the first two cell cycles. Dev Biol 304: 75–89, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394: 369–374, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Wu G, Hao L, Han Z, Gao S, Latham KE, de Villena FP, Sapienza C. Maternal transmission ratio distortion at the mouse Om locus results from meiotic drive at the second meiotic division. Genetics 170: 327–334, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.