Abstract
Although elevated plasma prorenin levels are commonly found in diabetic patients and correlate with microvascular complications, the pathological role of these increases, if any, remains unclear. Prorenin/renin binding to the prorenin/renin receptor [(p)RR] enhances the efficiency of angiotensinogen cleavage by renin and unmasks prorenin catalytic activity. We asked whether plasma prorenin could be activated in local vascular tissue through receptor binding. Immunohistochemical staining showing localization of the (p)RR in the aorta to vascular smooth muscle cells (VSMCs). After cultured rat VSMCs were incubated with 10−7 M inactive prorenin, cultured supernatant acquired the ability to generate ANG I from angiotensinogen, indicating that prorenin had been activated. Activated prorenin facilitated angiotensin generation in cultured VSMCs when exogenous angiotensinogen was added. Small interfering RNA (siRNA) against the (p)RR blocked this activation and subsequent angiotensin generation. Prorenin alone induced dose- and time-dependent increases in mRNA and protein for the profibrotic molecule plasminogen activator inhibitor (PAI)-1, effects that were blocked by siRNA, but not by the ANG II receptor antagonist saralasin. When inactive prorenin and angiotensinogen were incubated with cells, PAI-1 mRNA increased a striking 54-fold, 8-fold higher than the increase seen with prorenin alone. PAI-1 protein increased 2.75-fold. These effects were blocked by treatment with siRNA + saralasin. We conclude that prorenin at high concentration binds the (p)RR on VSMCs and is activated. This activation leads to increased expression of PAI-1 via ANG II-independent and -dependent mechanisms. These data provide a mechanism by which elevated prorenin levels in diabetes may contribute to the progression of fibrotic disease.
Keywords: diabetes, fibrosis, renin-angiotensin system
although type 1 diabetes is associated with a greatly increased risk of vascular complications, the basic mechanisms underlying this association are unclear. Activation of the renin-angiotensin system (RAS) contributes to diabetic vascular complications, and RAS blockade has become a crucial therapy that slows progression of disease but does not halt it (27). These results suggest that additional pathways lead to vascular complications. Among the consequences of ANG II blockade is an increase in levels of circulating prorenin/renin through compensatory feedback mechanisms. Of great interest are studies showing that the onset of micro- and macrovascular disease in patients with type 1 diabetes coincides with elevated plasma prorenin, suggesting that prorenin may contribute to vascular complications (21).
Prorenin is the inactive precursor of renin. A 43-amino acid NH2-terminal prosegment of prorenin usually covers the enzymatic cleft and obstructs access of angiotensinogen (AGT). Classically, only the kidneys process prorenin to renin proteolytically and secrete renin into the circulation, and kidneys secrete much more prorenin than renin (10). Once prorenin enters the circulation, it has been thought to have no enzymatic activity. Recently, a specific prorenin/renin receptor [(p)RR] that binds prorenin as effectively as it binds renin was identified (26). Prorenin bound to this receptor cleaves AGT with kinetics similar to kinetics of fully active renin in solution without proteolytic removal of its prosegment (26). (p)RR is found in vascular smooth muscle cells (VSMCs) in human heart and kidney and is widely distributed in most other organs (26).
Ichihara et al. (16) developed a decoy-related epitope of the prorenin prosegment termed the handle region peptide (HRP), which is thought to block binding of prorenin to the (p)RR by competing for the same binding site. Infusion of HRP reduced local ANG II generation and fibrosis in an impressive set of experimental rat models, including diabetic nephropathy in streptozotocin-induced diabetic rats, suggesting an ANG II-dependent mechanism (reviewed in Ref. 32). ANG II-independent effects are suggested by the finding that HRP administration largely abolished the increased ERK1/2 activation and diabetic nephropathy in angiotensin type 1a receptor-deficient mice, which no longer display the normal response to ANG II (17). Also, human prorenin activates p38 MAPK and phosphorylates heat shock protein-27 in neonatal rat cardiomyocytes, an effect not altered in the presence of the renin inhibitor aliskiren or the ANG II type 1 receptor antagonist eprosartan (30).
Taken together, these data suggest that the elevated prorenin levels in patients with type 1 diabetes mellitus may correlate with microvascular complications, because they directly contribute to these complications by ANG II-dependent and -independent mechanisms.
However, the clarified receptor-mediated action of prorenin is still lacking. The decoy peptide HRP is thought to compete with prorenin for receptor binding that is not found on mature renin. The model of HRP is not able to explain the findings that the receptor responds to renin and prorenin and that renin induces synthesis of a number of molecules involved in renal fibrosis via the same receptor (14, 15). Therefore, the prorenin receptor-dependent mechanism in diabetic vascular diseases remains obscure.
Since all components of the RAS except renin have been demonstrated to be present in the vasculature, local generation of ANG II in the vasculature is probably dependent on circulating renin or prorenin (31). In diabetes, levels of active renin in plasma are decreased or not changed (4, 28). Thus the elevated prorenin is a more likely source of increased local ANG II generation. Very recently, it was shown that inactive recombinant human prorenin binds rat VSMC with overexpression of transfected human (p)RR cDNA and that bound prorenin induces ANG I generation in vitro (1). Furthermore, bound prorenin activates cellular ERK phosphorylation in these cells, independent of ANG II generation or action (6, 29). Thus the (p)RR-mediated prorenin activation and action may occur locally in diabetic vasculature and, thereby, contribute to the development of vascular complications. We further asked whether, at high concentration, rat prorenin could be activated through binding of its receptor on rat VSMCs in the vasculature without heterogenic human (p)RR gene expression, whether this activation leads to increases in the profibrotic molecule PAI-1, and whether these effects were ANG II dependent, ANG II independent, or both.
METHODS
Reagents
Serum from binephrectomized normal male Sprague-Dawley (SD) rats (∼250 g body wt) was used as a source of rat AGT, as previously described (19). Unless otherwise indicated, other reagents were purchased from Sigma (St. Louis, MO).
Expression and Purification of Recombinant Rat Prorenin
A new method was used to produce rat prorenin. The cDNA encoding prorenin was amplified by PCR as described elsewhere (15). The resulting cDNA was ligated into a modified version of the pCEP4 expression plasmid containing an NH2-terminal polyhistidine encoding sequence (9). A prorenin-expressing cell line was created by transfection of the resulting plasmid into 293-EBNA cells. Stable expressing cells were selected by culture of the transfected cells in the presence of 200 μg/ml hygromycin B. Cells were then amplified and grown to confluence in T175 flasks. On confluence, the culture medium was replaced with serum-free DMEM. Medium was then collected every 48 h, concentrated, and subjected to immobilized metal affinity chromatography using a chromatography system (ÄKTA Explorer, Amersham Biosciences). Fractions containing purified prorenin were collected, concentrated, and further processed through a desalting column (model HR26/10, Amersham Biosciences).
Verification of (p)RR Expression in Normal Rat Aorta
Immunofluorescent staining for (p)RR was performed on sections of frozen normal rat aorta. A phage-display technique (8) was used to generate the monoclonal human anti-rat (p)RR against the extracellular domain containing the first 1–156 amino acids of the receptor. A specific renin receptor-binding ELISA was established in our laboratory using this newly developed monoclonal anti-rat (p)RR antibody. Our data demonstrate that recombinant rat renin and prorenin have a similar binding capacity for the rat (p)RR (data not shown). The colocalization of (p)RR and α-smooth muscle actin (α-SMA), a marker of VSMCs, was investigated. Frozen sections of aorta were stained with the monoclonal receptor antibody and then incubated in tetramethylrhodamine isothiocyanate-conjugated goat anti-human F(ab')2 secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). α-SMA was stained with FITC-conjugated monoclonal mouse anti-human α-SMA IgG. A recombinant monoclonal antibody fragment [F(ab)-Dhlx-MH] served as negative control antibody.
VSMC Culture
Rat VSMCs were obtained from aorta of 100- to 150-g male SD rats that were housed under conditions consistent with National Institutes of Health guidelines; the animal protocols were approved by the University of Utah Animal Care Committee. VSMCs were verified by staining for α-SMA and myosin according to published methods [the detailed protocol was kindly provided by Dr. E. Wilson's group (3)]. Cells from passages 4–7 were maintained in DMEM supplemented with 20% FBS (Hyclone Laboratories), 100 U/ml penicillin, 100 μg/ml streptomycin, and 25 mM HEPES buffer at 37°C in a 5% CO2 incubator. Cells were seeded in six-well plates, grown to subconfluency, and made quiescent in serum-free medium for 48 h before experimental studies.
Expression of RAS Components and (p)RR in Rat VSMCs
Total RNA was isolated from cultured VSMCs or liver tissue of normal SD male rats for the determination of rat AGT, angiotensin-converting enzyme (ACE), renin, and (p)RR gene expression by RT-PCR or real-time RT-PCR (see below). The receptor protein expressed by rat VSMCs and mesangial cells was observed by Western blot using the monoclonal anti-rat (p)RR. Mesangial cells obtained and cultured as described previously (15) served as controls.
VSMC-Mediated Activation of Prorenin and Its (p)RR Dependence
(p)RR was depleted by Stealth RNA interference as described previously (15). Depleted and control cells were treated with prorenin in the presence or absence of AGT with or without the ANG II receptor antagonist saralasin. The culture supernatant was harvested for determination of prorenin activation and ANG I and ANG II levels. Prorenin activation was measured as the ability of a sample to generate ANG I from AGT in nephrectomized rat serum as described elsewhere (19). Generated ANG I was quantitated using a commercially available RIA kit (Phoenix Pharmaceuticals, Belmont, CA). ANG II levels were determined using a commercially available ELISA kit (Division of BACHEM, Peninsula Laboratories, San Carlos, CA). Cellular RNA was used for (p)RR and PAI-1 mRNA determination by real-time RT-PCR. PAI-1 protein levels in the supernatant were determined by Western blot as described elsewhere (15).
RNA Preparation and Real-Time RT-PCR Assay
Total RNA was extracted immediately from cells with use of TRIzol reagent (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instructions. Total RNA (2 μg) was reverse transcribed using the SuperScript III first-stand synthesis system for RT-PCR kit (Invitrogen). Real-time RT-PCR was performed using SYBR Green Dye I (Applied Biosystems, Foster City, CA) with the ABI 7900 Sequence Detection System (PE Applied Biosystems) as described previously (12). Samples were run as triplicates in separate tubes to permit quantification of the target gene normalized to GAPDH used for equal loading. Primer sequences are shown in Table 1. The specificity of the PCR products was confirmed on a 1.5% agarose gel by a specific single band with the expected size.
Table 1.
Primers used for real-time RT-PCR
| Gene | Location (complementary to nucleotides) | Sequence (5′–3′) |
|---|---|---|
| Rattus PAI-1 (M24067) | ||
| Forward | 681–700 | TGG TGA ACG CCC TCT ATT TC |
| Reverse | 909–928 | GAG GGG CAC ATC TTT TTC AA |
| Rattus renin receptor (AB188298) | ||
| Forward | 642–661 | CTG AAC TGC AAG TGC TGC AT |
| Reverse | 773–792 | TCC CGG AAT TGT TCA GAG TC |
| Rattus renin (NM_012642) | ||
| Forward | 811–834 | GAT CAC CAT GAA GGG GGT CTC TGT |
| Reverse | 1,061–1,084 | GTT CCT GAA GGG ATT CTT TTG CAC |
| Rattus ACE (NM_012544) | ||
| Forward | 2,447–2,447 | GAG CCA TCC TTC CCT TTT TC |
| Reverse | 2,674–2,693 | CCA CAT GTT CCC TAG CAG GT |
| Rattus GAPDH (NM_017008) | ||
| Forward | 29–48 | AGA CAG CCG CAT CTT CTT GT |
| Reverse | 248–267 | TTC CCA TTC TCA GCC TTG AC |
| Rattus AGT (NM_134432) | ||
| Forward | 1,452–1,471 | AGC ATC CTC CTT GAA CTC CA |
| Reverse | 1,676–1,695 | TGA TTT TTG CCC AGG ATA GC |
PAI-1, plasminogen activator inhibitor-1; ACE, angiotensin-converting enzyme; AGT, angiotensinogen.
Statistical Analysis
Values are means ± SD. Groups were analyzed by one-way ANOVA, and the Student-Newman-Keuls multiple comparisons test was used to compare differences among the groups. P < 0.05 was considered statistically significant (t-test and ANOVA). Duplicate wells were analyzed for each experiment, and each experiment was performed independently at least three times.
RESULTS
(p)RR Expression in Normal Aorta
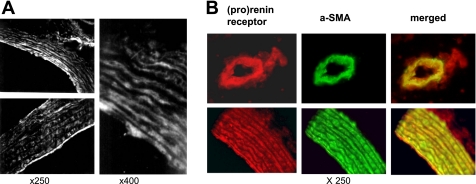
The (p)RR was present in all aortic structures, including intima, cell layer, and outer membrane (Fig. 1A). Red immunostaining for (p)RR and green for α-SMA produced strong yellow staining when viewed with a double filter, indicating colocalization of (p)RR with rat VSMCs in the aorta (Fig. 1B). These results are similar to those in human kidney cortex artery and coronary artery (26).
Fig. 1.
Prorenin/renin receptor [(p)RR] production in normal rat aorta tissue. A: immunofluorescent staining for (p)RR in normal rat aorta tissue. Original magnification ×250 (left) and ×400 (right). B: double immunofluorescent staining for (p)RR and α-smooth muscle actin (α-SMA) in normal rat aorta tissue. Red-stained (p)RR and green-stained α-SMA produced strong yellow staining when viewed with a double filter. Original magnification ×250.
Catalytic Activation of Prorenin by Incubation With VSMCs In Vitro
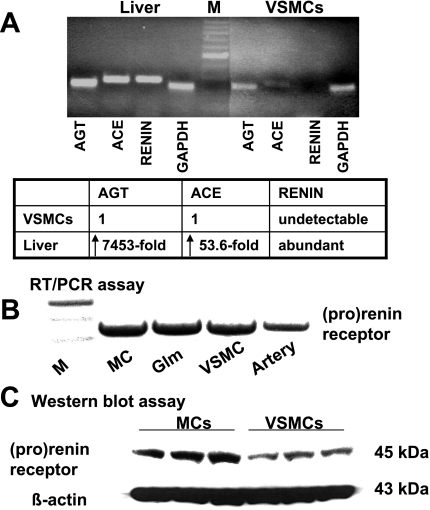
As expected, primary cultures of rat VSMCs expressed much less AGT and ACE mRNA than did rat liver tissue, and prorenin/renin gene expression was not visible (Fig. 2A). (p)RR was abundant not only in rat renal mesangial cells but also in rat VSMCs (Fig. 2, B and C), consistent with observations in aorta in vivo (Fig. 1). Because VSMCs contain no endogenous prorenin/renin but abundant (p)RR, they are ideal for investigation of the receptor-mediated effects of exogenous (circulating) prorenin.
Fig. 2.
Expression of renin-angiotensin system (RAS) components and (p)RR in rat vascular smooth muscle cells (VSMCs). A: mRNA for RAS components in rat liver and VSMCs by real-time RT-PCR. Top: PCR products (30 μl) were electrophoresed on 1.5% agarose gel, and 245-bp angiotensinogen (AGT), 266-bp angiotensin-converting enzyme (ACE), 274-bp renin, and 239-bp GAPDH PCR products were visualized by ethidium bromide staining under UV light. Bottom: changes in mRNA levels were determined by normalization of amplification of each sample to that of GAPDH. For comparison, this ratio was set at unity for rat VSMC sample, and level for liver tissue was expressed as fold increase over this value. B: (p)RR mRNA expression by 1-step RT-PCR described previously (15) in rat renal mesangial cells (MC), normal glomeruli (Glm), VSMCs, and artery. M, DNA marker. C: Western blot analysis of (p)RR in cell membrane extracts from rat MCs and VSMCs. Total protein was separated by SDS-PAGE and analyzed by immunoblotting with anti-rat prorenin receptor F(ab')2. β-Actin staining was used as a control for sample loading of gel wells.
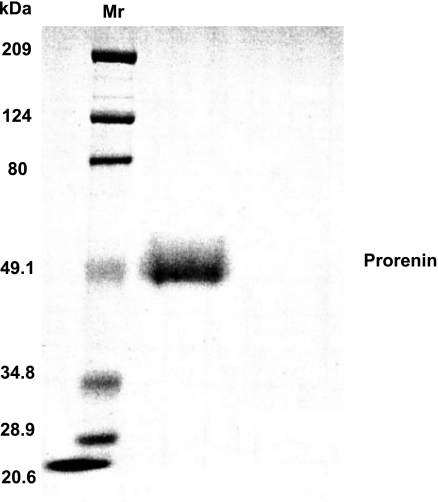
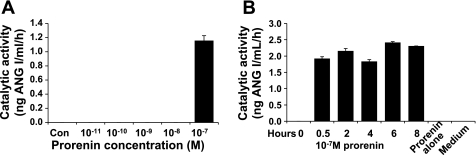
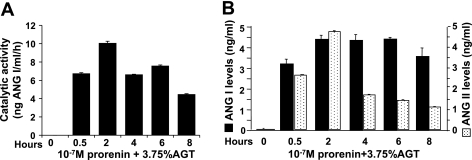
The recombinant rat prorenin that was produced was stored in PBS (pH 7.4), and its size, as visualized by SDS-PAGE, was as predicted (Fig. 3). When culture supernatant was removed from VSMCs incubated for 6 h with 10−11–10−7 M prorenin and its catalytic ability to generate ANG I with exogenous AGT was determined, only culture supernatant with the highest prorenin concentration (10−7 M) showed catalytic activity (Fig. 4A). When the effect of 10−7 M prorenin was investigated over time, catalytic activity was present as early as 0.5 h and lasted ≥8 h (Fig. 4B). Without cells, neither prorenin nor medium alone produced culture supernatant that was able to generate ANG I. These surprising results suggest that, at high concentrations, inactive prorenin binds to (p)RR and is activated. This activated prorenin remains active after it is released from the receptor, as indicated by the ability of cell-free culture supernatants to generate ANG I when a source of AGT was added. However, neither ANG I nor ANG II was detectable in these cultured supernatants in the absence of an exogenous source of AGT. This finding, along with the very limited [7,453-fold lower than in rat liver tissues (Fig. 2A)] cellular AGT mRNA expression in VSMCs, indicates that a reservoir of ANG I in cultured VSMCs is not sufficient for conversion to ANG II. An exogenous source of rat AGT is required for further observation of activated prorenin facilitation of angiotensin generation. When VSMCs were incubated with 10−7 M prorenin and serum as a source of AGT, the culture supernatant was able to generate ANG I (Fig. 5B), and ANG I and ANG II were present in the cultured supernatant after incubation (Fig. 5B). ANG II levels peaked at 2 h. No catalytic cleavage of AGT to ANG I was seen without cells in the presence or absence of 3.75% rat AGT serum or 10−7 M prorenin. Neither ANG I nor ANG II was measurable in the supernatant of cells treated with AGT alone or prorenin alone. Neither ANG I nor ANG II was measurable during incubation of prorenin with AGT without VSMCs (data not shown). These results indicate that there is no detectable active renin/prorenin, ANG I, or ANG II in the prepared rat AGT serum and that the amount of soluble prorenin supposed to be activated under physiological conditions (37°C, pH 7.4) is not sufficient to induce angiotensin generation in this system. The data presented in Fig. 5 suggest that prorenin binds to VSMCs and is activated. When exogenous AGT is added, ANG I and ANG II are produced.
Fig. 3.
SDS-PAGE analysis of pure rat recombinant prorenin. After purification, recombinant prorenin was subjected to SDS-PAGE. Bands were visualized by Coomassie blue staining. Mr, molecular weight marker.
Fig. 4.
Prorenin activation after incubation with rat VSMCs. A: catalytic activity of 0–10−7 M recombinant inactive prorenin incubated with VSMCs for 6 h. B: catalytic activity of culture supernatant from VSMCs incubated with 10−7 M recombinant inactive prorenin for 0–8 h.
Fig. 5.
Activated prorenin facilitates angiotensin generation when exogenous AGT is present. Catalytic activity (A) and ANG I and ANG II generation (B) of 10−7 M recombinant prorenin incubated with VSMCs for 0–8 h in the presence of 3.75% rat AGT serum were detected by RIA and ELISA, respectively.
Receptor-Mediated Catalytic Activation of Prorenin in VSMCs
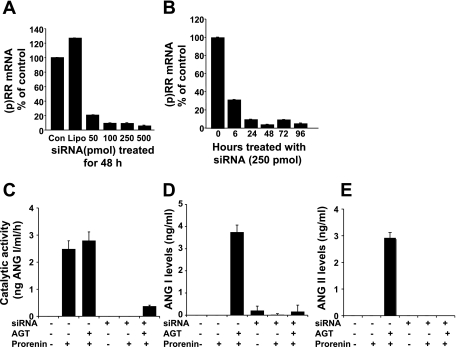
To determine whether the activation of prorenin by VSMCs was mediated by the (p)RR that was shown to be abundant on VSMCs in Fig. 2B, we inhibited receptor expression with siRNA. Cells were pretransfected with (p)RR Stealth siRNA to silence receptor mRNA expression and then treated with 10−7 M prorenin for 6 h. As previously shown in mesangial cells (15) and as shown in Fig. 6 for VSMCs, transfection of a Stealth siRNA molecule that targeted the (p)RR induced a dose-dependent decrease in receptor mRNA after 48 h (Fig. 6A). As shown in Fig. 6B, 250 pmol of Stealth siRNA induced a time-dependent, 80% reduction of receptor mRNA expression lasting 24 to ≥72 h. Cell transfection with Lipofectamine 2000 and 250 pmol of Stealth siRNA for 48 h significantly reduced increases in catalytic activity and ANG I and ANG II generation (Fig. 6, C–E). Treatment of cells with Lipofectamine 2000 alone, Opti-MEM alone, or control siRNA had no effect on (p)RR mRNA expression, prorenin activation, or angiotensin generation. These results indicate that prorenin activation and ANG I and ANG II generation are mediated by (p)RR.
Fig. 6.
Effect of (p)RR on activation of prorenin and subsequent angiotensin generation in cultured VSMCs. Dose (A) and time (B) responses of rat (p)RR mRNA expression are plotted against Stealth small interfering RNA (siRNA) targeting (p)RR. (p)RR mRNA was measured by real-time RT-PCR and standardized to GAPDH mRNA levels. Levels of mRNA and protein are expressed relative to nontransfected normal control (Con) and Lipofectamine 2000 (Lipo)-transfected values in A and to Stealth siRNA-transfected value at time 0 in B, 2 of which were set at 100%. Catalytic activity (C) and ANG I (D) and ANG II (E) generation in the culture supernatant when cellular (p)RR was deleted by Stealth siRNA followed by treatment of 10−7 M inactive prorenin in the presence of 3.75% rat AGT serum for 6 h were detected by RIA and ELISA, respectively.
Effect of Activated Prorenin on PAI-1 mRNA and Protein Production by VSMCs
ANG II-independent effects.
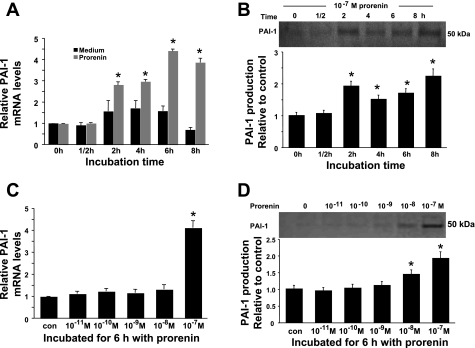
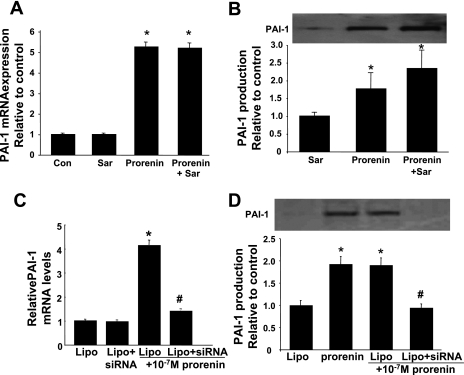
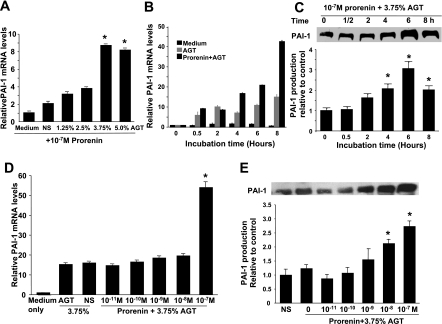
Prorenin at 10−7 M increased PAI-1 mRNA expression in a time-dependent manner by almost threefold (P < 0.05) at 2 h, with a peak (a 4.4-fold increase) at 6 h (P < 0.05; Fig. 7A). PAI-1 protein showed a similar pattern, with a peak (a 223% increase) at 8 h (P < 0.05; Fig. 7B). This increase in PAI-1 mRNA was seen with prorenin at 10−7 M, but not at lower concentrations (Fig. 7C). Interestingly, 10−8 M prorenin induced a small, but significant, increase in PAI-1 protein. At 10−7 M prorenin, PAI-1 protein was increased 191% after 6 h (P < 0.05; Fig. 7D). The nonselective ANG II receptor blocker saralasin had no effect on PAI-1 mRNA and protein levels in the absence or presence of prorenin (Fig. 8, A and B), indicating that blocking the action of ANG II does not alter prorenin's effect. In contrast, targeting rat (p)RR mRNA with siRNA blocked the prorenin-induced increase in PAI-1 mRNA and protein (Fig. 8, C and D). These results indicate that 10−7 M prorenin induces PAI-1 mRNA and protein expression by VSMCs through a receptor-mediated, ANG II-independent mechanism.
Fig. 7.
Effect of prorenin on plasminogen activator inhibitor (PAI)-1 mRNA and protein expression. Time course of PAI-1 mRNA (A) and protein (B) induction by 10−7 M prorenin was detected by real-time RT-PCR and Western blot, respectively. Relative values are expressed relative to time 0 control, which was set at unity. *P < 0.05 vs. time 0 medium-only control (A) or time 0 control (B). C and D: effect of prorenin dose on PAI-1 mRNA (C) and protein (D) production. PAI-1 mRNA and protein values are expressed relative to no-additive control (con), which was set at unity. *P < 0.05 vs. control.
Fig. 8.
Effects of saralasin and (p)RR Stealth siRNA on prorenin-induced PAI-1 mRNA and protein synthesis by VSMCs. Saralasin (Sar, 10−5 M) had no effect on 10−7 M prorenin-induced PAI-1 mRNA (A) and protein (B) production determined by real-time RT-PCR and Western blot. PAI-1 mRNA and protein values are expressed relative to no-additive control (Con) in A or saralasin-alone control in B, which was set at unity. *P < 0.05 vs. control. C and D: effect of (p)RR Stealth siRNA on prorenin-induced PAI-1 mRNA expression and protein production. Measurement of PAI-1 mRNA (C) and protein (D) production in prorenin-treated VSMCs was measured when cellular (p)RR mRNA expression was suppressed by siRNA. PAI-1 mRNA and protein values are expressed relative to Lipofectamine 2000-transfected, no-prorenin control, which was set at unity. *P < 0.05 vs. Lipo control. #P < 0.05 vs. 10−7 M prorenin-treated cells without siRNA transfection.
ANG II-dependent effects.
As shown in Fig. 5, AGT is required for generation of ANG II by activated prorenin. Prorenin at 10−7 M dramatically stimulated PAI-1 mRNA expression in a manner dependent on the concentration of AGT (Fig. 9A). PAI-1 mRNA expression peaked with 10−7 M prorenin + 3.75% AGT serum: it was increased more than eightfold compared with cells treated with prorenin alone (P < 0.05; Fig. 9A). Thus 3.75% AGT was used to evaluate the ANG II-dependent effect of activated prorenin in culture.
Fig. 9.
Effect of prorenin on PAI-1 mRNA and protein expression by VSMCs in the presence of exogenous AGT. A: dose effect of rat AGT serum on prorenin-induced PAI-1 mRNA expression. Serum-free medium (medium) and 3.75% normal rat serum (NS) served as controls. PAI-1 mRNA levels are expressed relative to 10−7 M prorenin in serum-free medium-treated control, which was set at unity. *P < 0.05 vs. control. B and C: time course of PAI-1 mRNA and protein induction by 10−7 M prorenin in the presence of 3.75% AGT detected by real-time RT-PCR and Western blot. Values are expressed relative to time 0 control, which was set at unity. *P < 0.05 vs. time 0 control. D and E: effect of prorenin in the presence of 3.75% AGT on PAI-1 mRNA and protein production. Serum-free medium and 3.75% normal rat serum served as controls. PAI-1 mRNA and protein values are expressed relative to no-additive serum-free medium control in D and 3.75% normal rat serum control in E, which were set at unity. *P < 0.05 vs. control.
Addition of 3.75% AGT + 10−7 M prorenin increased PAI-1 mRNA expression 9-fold at 0.5 h (P < 0.05) and >40-fold at 8 h compared with time 0 (P < 0.05; Fig. 9B). Addition of 3.75% AGT + 10−7 M prorenin maximally increased PAI-1 protein production by 306% at 6 h (P < 0.05; Fig. 9C). Interestingly, the induction of PAI-1 mRNA expression by prorenin could not be detected until the concentration of prorenin reached 10−7 M (Fig. 9D) in the presence of 3.75% AGT. At 10−7 M, prorenin was activated and converted AGT to ANG I. As seen in Fig. 5B, 4.75 ng/ml or 0.45 × 10−8 M ANG II was then produced. ANG II at 10−8 M effectively stimulates PAI-1 activity and expression in cell culture (33). Prorenin + AGT produced increases in PAI-1 mRNA expression that were much greater than those produced by 10−8 M ANG II (18) or 10−7 M prorenin alone (Fig. 7A), indicating an additive or synergistic effect of prorenin and ANG II on PAI-1 expression in VSMCs. Addition of 3.75% AGT + prorenin also increased PAI-1 protein production by 212% at 10−8 M and 273% at 10−7 M (P < 0.05; Fig. 9E). However, 3.75% AGT serum alone had no effect on PAI-1 mRNA and protein expression compared with 3.75% normal rat serum (Fig. 9, D and E), which was consistent with the failure to detect active renin/prorenin, ANG I, or ANG II in AGT serum (Fig. 5).
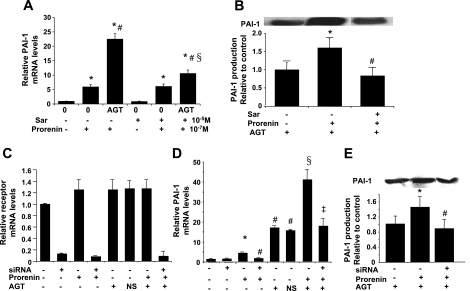
Saralasin did not affect prorenin-induced PAI-1 mRNA and protein expression in VSMCs when AGT was absent (Fig. 8, A and B). In contrast, prorenin-induced increases in PAI-1 were significantly reduced by saralasin when AGT was present (Fig. 10, A and B). When siRNA was used to silence (p)RR (Fig. 10C), prorenin-induced PAI-1 mRNA and protein were blocked in the absence and presence of AGT (Fig. 10, D and E), indicating that prorenin also induces ANG II-dependent PAI-1 expression when a source of AGT is available. Prorenin-induced ANG II-dependent and -independent effects on PAI-1 mRNA and protein production are mediated by the (p)RR.
Fig. 10.
Effects of saralasin and (p)RR Stealth siRNA on prorenin-induced PAI-1 mRNA and protein synthesis by VSMCs in the presence of exogenous AGT. A: PAI-1 mRNA expressed relative to no-additive control, which was set at unity. *P < 0.05 vs. control. #P < 0.05 vs. prorenin. §P < 0.05 vs. prorenin + AGT. B: PAI-1 protein expressed relative to 10−5 M saralasin + 3.75% AGT control, which was set at unity. *P < 0.05 vs. control. #P < 0.05 vs. prorenin + AGT. C–E: effect of (p)RR Stealth siRNA on prorenin-induced PAI-1 mRNA expression and protein production in the presence of exogenous AGT. C: measurement of (p)RR mRNA expression by real-time RT-PCR after transfection with 250 pmol of (p)RR Stealth siRNA for 48 h followed by 6 h of prorenin and/or AGT treatment. (p)RR mRNA expression was suppressed >95% only by siRNA treatment. D: measurement of PAI-1 mRNA expression by real-time RT-PCR in prorenin-treated VSMCs with or without 3.75% AGT after siRNA suppression of cellular (p)RR mRNA expression. PAI-1 mRNA values are expressed relative to no-additive control, which was set at unity. *P < 0.05 vs. no-additive control. #P < 0.05 vs. prorenin alone. §P < 0.05 vs. AGT alone. ‡P < 0.05 vs. prorenin + AGT. E: measurement of PAI-1 protein production by Western blot in VSMCs treated with prorenin + AGT after siRNA suppression of cellular (p)RR mRNA expression. PAI-1 protein values are expressed relative to Lipofectamine 2000-transfected, non-prorenin-treated, but AGT-treated, control, which was set at unity. *P < 0.05 vs. control. #P < 0.05 vs. non-Stealth siRNA-transfected cells treated with 10−7 M prorenin + 3.75% AGT.
DISCUSSION
Our data indicate that a solution containing 10−7 M inactive prorenin cannot convert AGT to ANG I until it is incubated with VSMCs. Catalytic activity is detectable at 30 min (Fig. 4B). Acquisition of this catalytic ability was mediated through binding to the (p)RR, since suppression of the receptor by RNA interference diminished it. These data suggest that inactive prorenin binds to (p)RR, is activated, and then remains in this activated form in the culture supernatant. Since <2% of prorenin usually displays catalytic activity under physiological conditions through temperature- and pH-mediated prosegment unfolding from the enzymatic cleft (5), it is important to define the expected activation of soluble prorenin in this cell culture system. First, it has been determined that neither ANG I nor ANG II was measurable during the incubation of prorenin with AGT without VSMCs. Second, the catalytic activity of active rat renin at 10−8 M prepared as described previously (15) was used as control after incubation with VSMCs for 2 h in the presence of 3.75% rat AGT. Renin at 10 nM produced ∼2.1 nM ANG I and ANG II in the cultured VSMCs (see supplemental data in the online version of this article). Neither ANG I nor ANG II was measurable again in the supernatant of cells treated with active renin alone. When prorenin was incubated with VSMCs and AGT for 2 h (Fig. 5B), the catalytic activity of prorenin was calculated as 100 nM prorenin to produce ∼7.9 nM ANG I and ANG II in cultured supernatant. The catalytic activity of the cell-activated prorenin is ∼37.6% of that of renin on the basis of angiotensin generation, which is much higher than the expected 2% activity of soluble prorenin. It thus appears that prorenin is activated by binding its receptor on the surface of VSMCs. Whether inactive prorenin is activated proteolytically or nonproteolytically is unknown. The presence of the intact prorenin in the cultured supernatant by end of the experiment, determined by Western blot (data not shown), suggests that the nonproteolytic mechanism is likely involved in the activation of prorenin. These results further confirmed the (p)RR-mediated prorenin activation not only in VSMCs with overexpressed heterogeneous human (p)RR (1), but also in normal VSMCs. Consistent with the in vitro work are data from a double-transgenic mouse expressing human native prorenin and human AGT (22). When transgene expression was targeted specifically to the somatotrophs of the pituitary gland by placement of the human renin and AGT cDNAs under the transcriptional control of the rat growth hormone gene promoter, human prorenin and AGT mRNA were detectable only within the pituitary gland of the transgenic mouse. These double-transgenic mice demonstrated an elevation of pituitary ANG I content, which suggests that human prorenin is enzymatically active against its substrate within the mouse pituitary gland. Interestingly, the relative amounts of ANG I generated in the pituitary glands of the various crosses correlated well with the levels of expression of prorenin mRNA in those lines (22). When mice expressing human AGT were crossed with a line expressing a human mutant prorenin that was noncleavable, the elevation of ANG I content in the pituitary gland continued, suggesting that prorenin activation within the pituitary gland is not due to proteolytic cleavage of prorenin (22). Consistent with the data presented here using VSMCs, the nonproteolytic activation of prorenin in vivo in the double-transgenic mouse is likely mediated by the (p)RR, since (p)RR is abundant in the pituitary gland (11).
Data shown in Fig. 2 indicate that (p)RR expression is abundant in normal aorta but prorenin message is absent in VSMCs. Since plasma levels of prorenin are known to be elevated in diabetic subjects (20), it is likely that circulating prorenin may bind (p)RR and be activated at tissue sites. Active rat prorenin, as expected, facilitates angiotensin generation in cell culture when rat AGT is present (Fig. 5). These findings support the idea that the high levels of prorenin often seen in diabetes actually contribute to tissue RAS activation, particularly in the vascular wall. When human recombinant prorenin was infused at 150 ng/ml (∼10−9 M) into rats transgenic for human AGT, prorenin was not activated in tissue or in the circulation, and mean arterial pressure was neither increased nor decreased (23). This may be due to infusion of an insufficient amount of prorenin. However, it is unclear whether tissue sites, other than glomeruli, that are involved in diabetic complications are ever exposed to 10−7 M prorenin. There are no data to answer this question. Plasma prorenin has been reported to be as high as 400 mU/l in diabetic patients with proliferative retinopathy (7). No molar concentration of plasma prorenin was ever given. When prorenin levels were increased 400-fold, such as in transgenic rats engineered to secrete prorenin from liver, pathological changes, including glomerulosclerosis and cardiomyocyte hypertrophy, occurred in the absence of elevated renin (34). No molar concentration of plasma prorenin in those mice was given, since the restandardization of direct prorenin/renin assays by use of a pure standard of prorenin or renin is not available. However, this work and the in vitro data presented here indicate that high levels of prorenin are likely to contribute to disease. When Nguyen et al. (26) incubated human (p)RR-transfected mesangial cells with 10−9 M human prorenin, the prorenin was activated and amounts of ANG I almost comparable to those seen with fully active renin were generated. That 10−9 M prorenin was not activated when infused but was activated when applied to (p)RR-transfected mesangial cells could be due to different receptor densities in different cells. Rat mesangial cells express more (p)RRs than rat VSMCs (Fig. 2C), and (p)RR-transfected mesangial cells should express even more (p)RR. Thus the level of prorenin activation may depend on prorenin concentration and the cellular (p)RR density. Our dose-response data indicate that, at the low level of receptor expression seen in VSMCs in culture, high concentrations of prorenin are required for prorenin activation. Consistently, transgenic rats overexpressing the human (p)RR gene in smooth muscle cells developed a cardiovascular phenotype with elevated systolic blood pressure and increased heart rate (2). Since cell culture data showed increased binding of renin and prorenin at the cell surface after transfection of human or rat (p)RR cDNA (16, 26), the human receptor transgenic animals would be expected to bind rat prorenin/renin in vivo, enhancing prorenin/renin activity. Although our data and those of others suggest a role for (p)RR density and prorenin concentration in prorenin activation and increased tissue fibrosis, definitive experiments have not been done.
The present study showed that, when combined with rat AGT, inactive prorenin was activated on VSMCs, PAI-1 mRNA was increased, and PAI-1 was released from VSMCs. Since saralasin significantly decreased this effect and (p)RR Stealth siRNA blocked this effect, PAI-1 production was dependent on receptor-mediated prorenin activation and subsequent ANG II generation and action. Our data are consistent with the previously reported finding of ANG II-induced upregulation of PAI-1 expression upon addition of human AGT and prorenin to neonatal rat cardiomyocytes (30), although it was not known whether the receptor-prorenin binding was species specific. Although Saris et al. (30) reported a significant PAI-1 increase at 10−9 M prorenin, we observed an upward trend at 10−9 M prorenin and a significant increase at 10−8 M prorenin (Fig. 9D). They observed no PAI-1 increase when rat cardiomyocytes were treated with 10−9 M human prorenin without AGT (30), consistent with our data for 10−9 M rat prorenin. However, we saw ANG II-independent prorenin-induced increases in PAI-1 with 10−7 M rat prorenin (Figs. 7 and 8).
The presence of ANG II-dependent and -independent effects and the magnitude of PAI-1 increases upon addition of AGT + prorenin suggest the possibility of an interesting synergistic effect between ANG II and prorenin on PAI-1 expression. Such interconnections between members of the RAS and profibrotic molecules have been reported: Kagami et al. (18) reported that ANG II upregulates PAI-1 directly and indirectly by upregulating expression of transforming growth factor-β, which then also increases PAI-1.
PAI-1 has emerged as a powerful fibrogenic molecule in cardiovascular, lung, and kidney diseases, and its overexpression has effects beyond its role in regulating the fibrinolytic system (for review see Ref. 13). Plasma PAI-1 levels are increased in patients with diabetes (13). The db/db mouse develops obesity and diabetes resembling type 2 diabetes in humans and a fivefold increase in plasma PAI-1 levels (13). It is therefore quite likely that the elevated prorenin levels in diabetes are involved in generating the increased PAI-1 levels, which then contribute to diabetes-associated vascular disease.
Perspectives
As we and others have shown, prorenin and renin bind to the same receptor and induce similar cellular signaling and gene expression (15, 24, 25). This raises the following interesting question: What is the relationship between renin and prorenin: a couple of competitors, or partners in disease? The answer will remain obscure until we have greater understanding of the (p)RR binding sites on prorenin and renin and the conformational changes during binding. The data presented here strongly suggest that high levels of prorenin may bind to (p)RR, be activated, and contribute to disease by angiotensin-dependent and -independent mechanisms and that the proteolytic activation of prorenin seems unnecessary for activation of the (p)RR-mediated signaling. Thus the rise in prorenin in diabetes may be pathogenic. Blocking (p)RR binding and enzymatic activity may represent a future therapeutic approach in patients with diabetic complications.
GRANTS
This work was supported by the Kidney Foundation of Utah and Idaho (Y. Huang) and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-43609 (W. A. Border) and DK-60508 (N. A. Noble).
Supplementary Material
Acknowledgments
We thank Linda Hoge for excellent technical assistance with animal studies and immunofluorescent staining.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burckle CA, Muller DN, Bader M, Nguyen G, Danser AH. Prorenin is the endogenous agonist of the (pro)renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro)renin receptor. J Hypertens 25: 2441–2453, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Burckle CA, Jan Danser AH, Muller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Chao JT, Meininger GA, Patterson JL, Jones SA, Partridge CR, Neiger JD, Williams ES, Kaufman SJ, Ramos KS, Wilson E. Regulation of α7-integrin expression in vascular smooth muscle by injury-induced atherosclerosis. Am J Physiol Heart Circ Physiol 287: H381–H389, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Christlieb AR, Kaldany A, D'Elia JA. Plasma renin activity and hypertension in diabetes mellitus. Diabetes 25: 969–974, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Danser AH, Deinum J. Renin, prorenin and the putative (pro)renin receptor. Hypertension 46: 1069–1076, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Feldt S, Maschke U, Dechend R, Luft FC, Muller DN. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol 19: 743–748, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franken AA, Derkx FH, Man in't Veld AJ, Hop WC, van Rens GH, Peperkamp E, de Jong PT, Schalekamp MA. High plasma prorenin in diabetes mellitus and its correlation with some complications. J Clin Endocrinol Metab 71: 1008–1015, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Frisch C, Brocks B, Ostendorp R, Hoess A, von Ruden T, Kretzschmar T. From EST to IHC: human antibody pipeline for target research. J Immunol Methods 275: 203–212, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Goldoni S, Owens RT, McQuillan DJ, Shriver Z, Sasisekharan R, Birk DE, Campbell S, Iozzo RV. Biologically active decorin is a monomer in solution. J Biol Chem 279: 6606–6612, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Hsueh WA, Baxter JD. Human prorenin. Hypertension 17: 469–477, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Border WA, Noble NA. Functional renin receptors in renal mesangial cells. Curr Hypertens Rep 9: 133–139, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Border WA, Yu L, Zhang J, Lawrence DA, Noble NA. A PAI-1 mutant, PAI-1R, slows progression of diabetic nephropathy. J Am Soc Nephrol 19: 329–338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Noble NA. PAI-1 as a target in kidney disease. Curr Drug Targets 8: 1007–1015, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-β1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int 72: 45–52, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border WA. Renin increases mesangial cell transforming growth factor-β1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int 69: 105–113, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y, Koura Y, Nishiyama A, Okada H, Uddin MN, Nabi AH, Ishida Y, Inagami T, Saruta T. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J Clin Invest 114: 1128–1135, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M, Inagami T. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol 17: 1950–1961, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Kagami S, Kuhara T, Okada K, Kuroda Y, Border WA, Noble NA. Dual effects of angiotensin II on the plasminogen/plasmin system in rat mesangial cells. Kidney Int 51: 664–671, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Katz SA, Opsahl JA, Lunzer MM, Forbis LM, Hirsch AT. Effect of bilateral nephrectomy on active renin, angiotensinogen, and renin glycoforms in plasma and myocardium. Hypertension 30: 259–266, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med 312: 1412–1417, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Makimattila S, Summanen P, Matinlauri I, Mantysaari M, Schlenzka A, Aalto M, Irjala K, Yki-Jarvinen H. Serum total renin, an independent marker of the activity and severity of retinopathy in patients with IDDM. Br J Ophthalmol 82: 939–944, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Methot D, Silversides DW, Reudelhuber TL. In vivo enzymatic assay reveals catalytic activity of the human renin precursor in tissues. Circ Res 84: 1067–1072, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Muller DN, Hilgers KF, Mathews S, Breu V, Fischli W, Uhlmann R, Luft FC. Effects of human prorenin in rats transgenic for human angiotensinogen. Hypertension 33: 312–317, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Nabi AH, Kageshima A, Uddin MN, Nakagawa T, Park EY, Suzuki F. Binding properties of rat prorenin and renin to the recombinant rat renin/prorenin receptor prepared by a baculovirus expression system. Int J Mol Med 18: 483–488, 2006. [PubMed] [Google Scholar]
- 25.Nguyen G, Burckle CA, Sraer JD. Renin/prorenin-receptor biochemistry and functional significance. Curr Hypertens Rep 6: 129–132, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa S, Takeuchi K, Mori T, Nako K, Tsubono Y, Ito S. Effects of monotherapy of temocapril or candesartan with dose increments or combination therapy with both drugs on the suppression of diabetic nephropathy. Hypertens Res 30: 325–334, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Price DA, Porter LE, Gordon M, Fisher ND, De'Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK. The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10: 2382–2391, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res 30: 1139–1146, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Saris JJ, 't Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AJ. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension 48: 564–571, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 52: 639–672, 2000. [PubMed] [Google Scholar]
- 32.Uddin MN, Nabi AH, Nakagawa T, Ichihara A, Inagami T, Suzuki F. Non-proteolytic activation of prorenin: activation by (pro)renin receptor and its inhibition by a prorenin prosegment, “decoy peptide.” Front Biosci 13: 745–753, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Van Leeuwen RT, Kol A, Andreotti F, Kluft C, Maseri A, Sperti G. Angiotensin II increases plasminogen activator inhibitor type 1 and tissue-type plasminogen activator messenger RNA in cultured rat aortic smooth muscle cells. Circulation 90: 362–368, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Veniant M, Menard J, Bruneval P, Morley S, Gonzales MF, Mullins J. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J Clin Invest 98: 1996–1970, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.