Abstract
Insulin has an exercise-like action to increase microvascular perfusion of skeletal muscle and thereby enhance delivery of hormone and nutrient to the myocytes. With insulin resistance, insulin's action to increase microvascular perfusion is markedly impaired. This review examines the present status of these observations and techniques available to measure such changes as well as the possible underpinning mechanisms. Low physiological doses of insulin and light exercise have been shown to increase microvascular perfusion without increasing bulk blood flow. In these circumstances, blood flow is proposed to be redirected from the nonnutritive route to the nutritive route with flow becoming dominant in the nonnutritive route when insulin resistance has developed. Increased vasomotion controlled by vascular smooth muscle may be part of the explanation by which insulin mediates an increase in microvascular perfusion, as seen from the effects of insulin on both muscle and skin microvascular blood flow. In addition, vascular dysfunction appears to be an early development in the onset of insulin resistance, with the consequence that impaired glucose delivery, more so than insulin delivery, accounts for the diminished glucose uptake by insulin-resistant muscle. Regular exercise may prevent and ameliorate insulin resistance by increasing “vascular fitness” and thereby recovering insulin-mediated capillary recruitment.
Insulin-Mediated Increases in Microvascular Perfusion
Present status.
The role of blood flow in insulin action has had a checkered history, and as pointed out in our previous review on this topic five years ago (28), the association of increased limb blood flow due to insulin and the increase in “downstream” glucose uptake by muscle remains controversial. What appears to be accepted by many (9, 21, 45, 76, 94, 107, 108, 151, 180, 185, 194) is that physiological insulin acts at key sites in the vasculature of muscle to increase available capillary surface area, with the potential for increased delivery of both insulin and glucose (and perhaps other nutrients) to the myocytes. This is termed “capillary recruitment” or “increased microvascular perfusion,” and it clearly involves what we have interpreted as an increase in nutritive blood flow for muscle. A key component of, and contributing factor for, insulin-mediated increase in microvascular perfusion is the vasodilatory action of this hormone on terminal arterioles that control access to nutritive capillary beds of muscle that are receiving little, intermittent, or no blood flow in the basal state. There is now strong evidence for insulin's vasodilatory action with microvessels exposed in vivo such as that of the spinotrapezius muscle (132) as well as from isolated microvessels and endothelial cells (46, 196).
A second component of insulin's action that we have proposed previously is a vasoconstrictor activity (28). This may result from insulin-mediated endothelin-1 (ET-1) release (46) or other as-yet-unidentified vasoconstrictors, which in turn gives rise to vasoconstriction of arterioles controlling entry to nonnutritive vessels of connective tissue (28) to further increase nutritive flow. However, although the release of ET-1 from endothelial cells by insulin is generally accepted (e.g., see Ref. 156), its place in the control of nutritive flow in healthy muscle is presently unknown. Recent application of a microdialysis method (discussed in Developments in techniques for determining capillary recruitment) gave no evidence for an insulin-mediated decease in nonnutritive flow (113), and so this weakens the notion of an accompanying vasoconstriction with normal insulin action. Thus a role for ET-1-mediated vasoconstriction in insulin action in healthy tissue remains to be resolved. Eringa et al. (46), using isolated first-order microvessels, reported that insulin (applied abluminally) had no vasodilatory action unless an ET-1 receptor antagonist was present, suggesting that the first-order vessels are controlled by both ET-1 (vasoconstriction) and nitric oxide (NO; vasodilation). If indeed ET-1 is released by insulin action and its proposed role is to constrict flow in the nonnutritive route, as indicated above, then a population of arterioles that vasoconstrict with ET-1 with minimal NO-mediated vasodilation should be present. So on balance, the data of Eringa et al. (46) are more consistent with the view that ET-1 plays a role only in insulin resistance when insulin-mediated NO-dependent vasodilation is impaired and ET-1-mediated vasoconstriction dominates to contribute to both hypertension and diminished nutritive blood flow in muscle (e.g., see Ref. 151).
Not all workers in this field have embraced the notion that delivery is a vital part of insulin's action (e.g., see Refs. 82, 117, and 143). Indeed, most, if not all, of these groups have avoided the issue and instead focused entirely on events occurring after the insulin and glucose have crossed the capillary walls and insulin has commenced the insulin-signaling cascade by interacting with myocyte insulin receptors (e.g., see Refs. 82 and 120). To date, there has been only one study where the notion that insulin increases delivery to muscle has been challenged (183), but that study specifically examined inulin distribution volume and not microvascular perfusion. This reviewer is unaware of findings that directly dispute that insulin-mediated capillary recruitment occurs or that capillary recruitment is impaired in insulin-resistant states.
History of insulin-mediated capillary recruitment in muscle.
Defronzo et al. (39) studied the relationship between exercise and insulin on muscle glucose uptake and suggested that the synergism was the result of increased blood flow and increased capillary surface area for enhanced insulin delivery to exercising muscle. However, Baron et al. (see Ref. 4 and references therein) must be given credit for being the first to show an independent action of insulin to increase limb blood flow that had the potential to increase capillary recruitment. Even so, a number of groups subsequently showed that an increase in limb blood flow did not always associate with increased myocyte glucose uptake (e.g., see Refs. 111, 116, 144, and 162), and a lively controversy ensued (167, 191). Because of the absence of techniques to detect actual changes in microvascular perfusion, direct evidence for insulin-mediated capillary recruitment in vivo did not appear until 1997, when Steve Rattigan from our group, working with Gene Barrett, perfected the 1-methylxanthine (1-MX) method in rats (127). Subsequently, we have extended the rat studies by showing that capillary recruitment is impaired under a number of circumstances relating to insulin resistance, whether this was induced acutely [α-methyl serotonin (128), TNFα (192), intralipid/heparin (29), glucosamine (181)] or genetically inherited in rats [Zucker obese rats (182)]. Augmentation of the process was also possible, and exercise training was shown to enhance insulin-mediated capillary recruitment in association with enhanced glucose uptake (129). In addition, insulin's action to increase microvascular perfusion along with the action to increase limb blood flow and muscle glucose uptake were shown to be partly NO dependent (176, 177). Taken together, and as shown elsewhere, the correlation between insulin-mediated capillary recruitment as measured by 1-MX metabolism and glucose uptake is remarkable (see review in Ref. 24 and Fig. 1 therein).
By 2002, it was shown that insulin-mediated increase in microvascular perfusion preceded any detectable change in muscle glucose uptake (175), and a detailed time course study was published in 2004 (177). This advance was possible largely as the result of being able to continuously measure changes in microvascular perfusion using contrast-enhanced ultrasound (CEU; cf. 1-MX method, which is an end-point determination made at steady state) (33). In addition, and by using both CEU and 1-MX metabolism, capillary recruitment was shown to be more sensitive to insulin than glucose uptake or the increase in limb blood flow (197). It was also shown that, although insulin-mediated capillary recruitment was sensitive to inhibitors of NO synthase (NOS), exercise-mediated capillary recruitment was not NO-dependent (136).
It is important to note that measurement of capillary recruitment in muscle of human subjects cannot be conducted using 1-MX metabolism (Rattigan S and Richter EA, unpublished observations) probably because human muscle capillary endothelium contains much less xanthine oxidase than rats, and thus the arterio-venous difference across the leg at the high blood flow rates encountered in humans is too small to allow an accurate assessment of 1-MX metabolism. Thus CEU has been the method of choice in humans, and using this technique, insulin-mediated capillary recruitment was first reported by Coggins et al. (31) in 2001. Subsequently, capillary recruitment was reported in the forearm of healthy humans following a mixed meal and was found to follow closely the time-dependent rise in plasma insulin (178). In light exercise, the increase in microvascular volume preceded the increase in limb blood flow (178). In addition, insulin-mediated capillary recruitment in the forearm was shown to be impaired in obese women when they were exposed to a physiological insulin clamp (30).
Our findings and those by our colleagues at the University of Virginia have resulted in the CEU method being used by others. For example, Mulder et al. (105) have recently shown positive stimulatory effects of both exercise and insulin on microvascular blood volume in healthy nonobese humans, using real-time contrast imaging of forearm muscle. This is a departure from the original CEU method and involves a bolus injection of contrast medium with image measurement over 90 s rather than continuous infusion and intermittent harmonic imaging. In addition, Muniyappa et al. (106) have published a study where forearm capillary recruitment was determined following oral glucosamine in lean and obese subjects.
In heart, Scognamiglio et al. (149) have used CEU to describe an increase in postprandial myocardial perfusion in healthy subjects, which was markedly diminished in type 2 diabetic patients; this defect was partly corrected by acutely administered fast-acting insulin analog prior to the meal (150).
Other methods have been used to determine changes in capillary perfusion in muscle. For example, de Jongh et al. (35) used an impaled laser Doppler flowmetry (LDF) probe to assess the change in muscle microvascular perfusion in normal healthy subjects when they were subjected to a hyperinsulinemic euglycemic clamp. Although the increase in microvascular perfusion itself was not significant, a decrease occurred in the time needed to reach peak intramuscular perfusion during reactive hyperemia, and there was a dramatic increase in vasomotion due to the insulin. The increase in vasomotion mediated by insulin has been confirmed in rats in vivo in this laboratory (114) and may play a crucial role in facilitating increased microvascular perfusion for insulin and glucose delivery (this is discussed in more detail below) (35).
LDF has also been used to assess insulin responsiveness of muscle in individuals with features of the metabolic syndrome (92). Measurements were made under basal conditions and during reactive hyperemia to arterial occlusion before and during the clamp. Functional vasodilatory reserve was reduced in individuals with features of the metabolic syndrome. Similarly, in rats in vivo, acute insulin resistance resulting from α-methyl serotonin infusion blocks the stimulatory effect of insulin on vasomotion at 0.06–0.3 Hz (114), attributed to smooth muscle response as deduced from wavelet analysis of the LDF signal (165).
Indirect evidence for insulin-mediated capillary recruitment.
A particularly novel approach to assess change in microvascular perfusion of muscle was used by Ellmerer and colleagues (43, 44). During hyperinsulinemic euglycemic clamps in dogs, transport parameters and distribution volumes of [14C]inulin (a polymer of d-fructose of similar molecular size to insulin) were determined. Insulin was found to stimulate the extravascular distribution volume of inulin (44), and in a later study (43), direct cannulation of the hindlimb lymphatics was made and the appearance of intravenously injected [14C]inulin determined in lymph during physiological hyperinsulinemia. The effects of insulin in control and insulin-resistant animals from a high-fat diet were compared. The high-fat diet resulted in a significant reduction of the peripheral distribution volume of [14C]inulin and a marked decrease in inulin appearance in the lymph. The authors in the later study proposed that the observed diet-induced defect in stimulation of tissue perfusion contributes to the development of peripheral insulin resistance (43). However, in a recent report, physiological hyperinsulinemia was found to have no detectable effect on access of inulin to insulin-sensitive tissues in healthy humans (183). The significance of this difference between dogs and humans remains to be explained.
Yet another indirect approach for assessing capillary recruitment has been to use microdialysis to determine interstitial delivery of glucose and from this, together with extraction fraction, and blood flow across the region of interest calculate permeability surface (PS) area changes. In one of the first studies by this group (61), an oral glucose load by healthy males was shown to increase PS for glucose by almost 10-fold. Again using this approach, a second study involving eight men with type 2 diabetes and eight age-, sex-, and weight-matched controls was conducted during the last 30 min of a 330-min hyperinsulinemic euglycemic clamp at 120 mU·m2·min−1. PS areas for glucose of the diabetics were ∼45% of the controls (60). Most recently, local vasodilation with methacholine was shown to attenuate the impairment of insulin actions on muscle capillary recruitment and glucose disposal in obese insulin-resistant subjects (109); capillary recruitment was measured by determining the capillary PS area product for glucose using intramuscular microdialysis (61).
Bertoldo et al. (11) has used positron emission tomography (PET) to study the interactions between delivery, transport, and phosphorylation of glucose in regulating glucose uptake by muscle. PET imaging was conducted on human muscle using a mix of various tracers that enabled quantitative determinations of glucose delivery, transport, and its phosphorylation. During fasting, with no added insulin, regulation was found to be exerted predominantly at glucose transport, but under insulin-stimulated conditions control was distributed equally between delivery and transport. However, it must not be forgotten that insulin-mediated increase in glucose transport can be activated only if insulin delivery occurs, and as yet there are only sketchy details on where control points are exerted for this process. Attempts to determine interstitial insulin concentrations by microdialysis following insulin infusions have been hampered by low recovery due to relatively low permeability of the microdialysis probes to insulin (77, 163). However, there is considerable evidence that the delay in insulin reaching the interstitium reflects a delay in delivery involving the time required for insulin-mediated increase in microvascular perfusion and/or transendothelial transport (see below). A recent study shows that hyperinsulinemia increases human forearm capillary recruitment and insulin uptake; capillary recruitment was significant at 15–20 min and insulin uptake at 40 min after commencement of insulin infusion (42).
Capillary recruitment in skin: inferences for muscle.
A number of studies have reported a stimulatory effect of insulin on microvascular perfusion of skin (e.g., see Refs. 35, 71, 138, and 154) and a defect in this process in association with hypertension (152, 153, 155), obesity (36–38), and insulin resistance (37). Two types of approaches have been used to assess changes in microvascular perfusion. Most commonly, human subjects undergo a hyperinsulinemic euglycemic clamp, and skin capillary recruitment is assessed before, and immediately after, a short period of reactive hyperemia. For example, nailfold capillaries in the dorsal skin of one of the fingers are visualized by a capillary microscope. A visual field of ∼1 mm2 is recorded before and after 4 min of arterial occlusion with a finger cuff. Erythrocyte-perfused capillaries are counted at baseline and directly after release of the cuff by analysis of the recordings. The responses vary, but in general, physiological insulin (∼400 pM) results in a 35% increase relative to basal following cuff removal (37). The second approach has been to apply insulin directly on the skin on the inside of the wrist in a specially designed cell to which a laser Doppler flow probe is fitted. The cell also can act as a cathode, to which a small current is periodically applied (e.g., 200 microamps) every 90 s with an anode placed ∼6 cm away. Vehicle controls are used for comparison, but using iontophoresed insulin in this manner, the laser Doppler signal can increase between two- and sixfold in healthy human subjects (e.g., see Ref. 154). However, there are a number of issues that need to be resolved before insulin-mediated capillary recruitment can be concluded to have occurred from each of these approaches. First, it is not clear why the hyperinsulinemic clamp, which readily reveals a direct increase in muscle microvascular perfusion (31), does not increase nailfold capillary perfusion (35) or skin blood flow (71), nor is it clear why insulin-mediated increase can be deduced only by measuring the reactive hyperemic response before and after insulin (35) or by systemic administration of a rapidly absorbed form of insulin (glulisine) (71). Second, the increase in laser Doppler signal from iontophoresed insulin on the skin of the wrist may not necessarily reflect capillary recruitment. This is because LDF appears to be more sensitive to changes in nonvectorial speed resulting from increased blood flow rate than an increase in cell number from an increase in the number of perfused capillaries (25). Thus an increase in LDF signal during iontophoresis could arise from a vasodilatory action of insulin to increase local blood flow rate in vessels already perfused rather than a vasodilatory action of insulin at branch points of the terminal arterioles to increase the number of capillaries perfused. This latter issue is particularly important when the responses of skin determined by LDF and muscle determined by 1-MX or CEU are compared. For muscle, it has been shown that a number of agents can markedly increase flow without an increase in capillary recruitment (e.g., see Ref. 28). With the exception of methacholine (99, 109), it is likely that a number of other vasodilators that have no effect on muscle glucose uptake, such as nitroprusside, adenosine, and IGF-I, would simply increase skin blood flow in nonnutritive vessels (shunts) without recruiting nutritive capillaries.
Developments in techniques for determining capillary recruitment.
There are now a number of techniques that have been claimed to measure changes in microvascular perfusion in muscle, and these are shown in Table 1. Some are direct, others are indirect, and several depend on a number of assumptions. In this section, each of these techniques is examined to highlight the most recent developments. The methodological aspects of the techniques of 1-MX metabolism and CEU have been dealt with in detail in previous reviews (28, 130). Each of these two methods essentially measures a change in capillary surface area available for substrate and hormone exchange. The 1-MX metabolism method is predicated on the assumption that the target enzyme xanthine oxidase is located predominantly in the capillary endothelium, and thus any change in the available capillary surface area resulting from capillary recruitment is reflected by an increase in the metabolism of entering 1-MX. For changes in capillary recruitment that might be mediated by insulin or exercise, limb blood flow is multiplied by the arterio-venous difference determined across the limb. In general, the rates of metabolism are determined under experimental (e.g., insulin infusion) and control conditions (e.g., saline infusion) to establish that a change has taken place. However, it is important to note that this technique does not allow determination of the proportion of total flow that is passing through the nutritive route before or after intervention (e.g., insulin infusion). Similarly, the 1-MX method does not allow detection of a flow pattern change between the nutritive and nonnutritive routes. For example, capillary recruitment occurs at physiological insulin when there is no accompanying change in limb blood flow (177), and low levels of muscle contraction also induce capillary recruitment in the absence of flow increase (178). In both of these circumstances, flow redistribution from the nonnutritive to the nutritive route could very likely account for the increase in 1-MX metabolism because the nonnutritive route comprises short capillaries (of low resistance and therefore high capacitance) and thus has a low opportunity for metabolism of 1-MX to occur.
Table 1.
Techniques purported to measure capillary recruitment (microvascular perfusion in muscle)
| Technique | Assumptions | Limitations | Particular Advantages | References for Details |
|---|---|---|---|---|
| 1-MX metabolism | 1) XO is predominantly in capillaries. | 1) Not suitable for human use. | Total tissue is sampled from which venous sample is taken. | 127 |
| 2) XO activity does not change as a result of changing the experimental paradigm. | 2) Cannot distinguish proportion of total flow that is nutritive. | |||
| 3) Reflects metabolism in capillaries of muscle | ||||
| CEU | Nutritive capillaries fill more slowly than either nonnutritive route or feed vessels. | 1) Subjects must remain motionless; animals need to be anesthetized. | Essentially noninvasive. | Rats (33, 136) Humans (31, 178) |
| 2) Yet to be adapted to mice. | ||||
| 3) Cannot distinguish proportion of total flow that is nutritive. | ||||
| LDF | Signal for capillary recruitment not confounded by signal from cell motion. | 1) Requires impaling into muscle. | 1) Allows Fourier or wavelet analysis to assess change in vasomotion. | 35, 75, 137, 138, 154 |
| 2) Sampling volume relatively small. | 2) Particularly useful for skin measurements in conjunction with iontophoresis. | |||
| 3) Skin studies are noninvasive. | ||||
| Microdialysis | 1) Local tissue damage by impaled probe is negligible. 2) Sampling region is homogeneous | 1) Sampling volume relatively small. 2) Sampling during exercise affected by pressure of contracting fibers. | 1) Can determine capillary permeability-surface area product for glucose. | 61, 113, 134 |
| 2) Using out/in ratio of L-[14C]glucose allows calculation of fraction of total flow that is nutritive. | ||||
| Washout kinetics From intramuscular site (e.g., 133Xe) | Direct relationship between total flow and microvascular perfusion. Injection is into interstitial fluid. | Label cleared only by nutritive flow. Thus increase in total flow may not necessarily remove label. | 12, 16 | |
| 133Xe diffusion limitations may exist. | ||||
| From intravenous bolus injection (e.g., labeled inulin) | Three-compartment model, where main central compartment = blood volume; 2 peripheral compartments represent rapid and slow equilibrating interstitial fluid compartments with rapid = splanchic and slow = muscle. | 44, 69, 166 | ||
| Muscle lymph sampling | Lymph draining from muscle derives exclusively from nutritive flow. | 1) Suitable only for larger animals where lymphatics can be sampled. 2) Lymph is difficult to collect and may require massaging of muscle. 3) Ideally, rate of lymph production should be measured. | Direct measurement of change in delivery to interstitium. | 44, 166 |
| Venous occlusion Plesymethography | Sometimes claimed to be measuring microvascular perfusion. | Actually measures total flow in forearm. | Noninvasive. | 186 |
| Near-infrared method with ICG | ICG does not extravasate. | Measures entitre vascular volume and thus cannot determine microvascular perfusion. | Allows blood flow distribution measurements, e.g., in tendons. | 16, 32 |
1-MX, 1-methylxanthine; XO, xanthine oxidase; CEU, contrast-enhanced ultrasound; LDF, laser Doppler flowmetry; ICG, indocyanine green.
When using the 1-MX method, it is also important to determine that the level of expression of capillary xanthine oxidase does not change as a result of the experimental intervention or different experimental paradigm. For example, when 1-MX metabolism was used to assess insulin-mediated capillary recruitment in Zucker obese vs. lean rats, it was necessary to establish that xanthine oxidase levels did not differ between the two phenotypes. In acute interventions (e.g., 2-h exposure to TNFα), this is of less concern. Calculation of 1-MX metabolism requires accurate measurement of limb blood flow; so far this has been conducted only in anesthetized animals but is theoretically possible for conscious unrestrained animals, provided that movement is minimal.
CEU (Table 1) measures the volume occupied by the contrast agent (gas-filled microbubbles), and by constructing pulse interval curves and subtracting the volume that can be attributed to rapidly filling larger feed vessels (31), it is possible to deduce the perfused microvascular (capillary) volume. Throughout the studies published thus far by us and our colleagues (30, 31, 33, 42, 74, 136, 175–179, 197), this substraction has been made and the group of rapid-fill vessels may include those of the nonnutritive route, but this requires confirmation by conducting experiments where a high proportion of nonnutritive flow is created so that characterization is possible. Often the field of interest from which determination of microvascular volume is made represents a wedge of muscle of ∼1 g in the rat and 15 g in the human. Regardless of the mass of muscle studied, only 8% of the capillaries need to be short, of low resistance (therefore slightly larger in diameter; e.g., see Ref. 66), and traveling through connective tissue to meet the requirement for a nonnutritive route of muscle with the capacity to carry ∼50% of the blood flow at rest. CEU only allows determination of a change in microvascular volume and is used in essentially the same way as 1-MX metabolism comparing an experimental intervention with a control that is conducted separately. Thus, at this stage, using either 1-MX or CEU, the proportion of total blood flow that is nutritive cannot be determined. Since muscle contraction readily leads to increases in microvascular perfusion, subjects must remain motionless when infused agents are being tested. Obviously, experimental animals must be anesthetized to avoid the confounding effects of muscle contraction. Because of the plethora of genetically modified mice important to cardiovascular research, it is an imperative to adapt this technique for muscle studies in these small animals.
As discussed earlier in this review, LDF (Table 1) has been used more commonly to measure changes in capillary flow in skin than in muscle. This is simply because only low-intensity laser light is used and tissue penetration is limited. To date, the only full publication describing an insulin-mediated increase in relative perfusion and a decrease in time to peak perfusion from LDF signal in skeletal muscle is by de Jongh et al. (35). In that study, plasma insulin levels increased more than sixfold and leg blood flow was increased by 36% (35).
Interpretation of an increase in primary LDF signal as representing an increase in microvascular perfusion may be misleading. Using various constructed models of fine tubing, we showed that the reflected LDF signal arose predominantly from red blood cell movement (nonvectorial speed) and less so from cell number (25), emphasizing that any change in LDF signal, obtained from tissue measurements, should be treated with caution. One way to avoid this potentially confounding issue is to use experimental conditions where bulk flow does not change (e.g., low concentrations of insulin or low levels of exercise). Alternatively, attention can simply be focused on analyzing the signal frequencies (reflecting vasomotion), changes of which may not necessarily be linked to change in limb blood flow, although this requires future investigation.
Microdialysis has been applied largely to sample interstitial fluid and thereby determine concentrations of various metabolites that readily cross the membrane of the microdialysis probe threaded through the muscle. Gudbjörnsdóttir et al. (61) have successfully adapted the method to measure capillary recruitment by determining the capillary PS area product for glucose. In contrast, use of this method to determine limb blood flow from the out/in ratio of either 3H2O or ethanol (164) can lead to serious errors. Recognition that muscle blood flow has two components (nutritive and nonnutritive) needs to be taken into account, because any agent that changes the proportion of total flow that is nutritive changes the out/in ratio independently of a change in total flow (112). It would thus seem reasonable to conclude that microdialysis out/in ratios largely reflect nutritive flow. In fact, Newman et al. (113) and Roberts et al. (134) have specifically adapted the microdialysis technique to determine the proportion of total blood flow that is nutritive (Table 1). A mathematical model using the microdialysis outflow/inflow (O/I) ratio of l-[14C]glucose was developed. This steady-state model is based on a cylindrical probe geometry and assumes that diffusion from the probe is only in the radial (r) direction, with none in the axial direction (z), and that the tracer is not metabolized (134). This means that there is an implied assumption that the interstitial space is an unstirred compartment. Considering a differential length of probe (dz), a mass flux (M) leaves the probe volume to the outside surface of the probe membrane. At steady state, this mass flux must be matched by a mass flux from the interstitial fluid into surrounding capillaries (which must be nutritive by definition). From derived equations (134), knowledge of the diffusion coefficient, and total blood flow around the microdialysis probe, determination of the nutritive fraction of blood flow in the tissue can be made (113). This is now the second method available for determining nutritive flow as a proportion of total flow (e.g., limb blood flow). An earlier method based on the indicator-dilution pattern of radioiodoalbumin and of 86Rb was reported in 1966 (55).
Application of the microdialysis method (113) has been conducted on anesthetized rats, although anesthesia may not be essential, provided that the microdialysis probe does not move or dislodge. The microdialysis probe carrying l-[14C]glucose was inserted through the calf muscle group (tibialis/plantaris/gastrocnemius), and the nutritive fraction of total blood flow was determined under basal conditions and in response to contraction (electrical field stimulation), insulin (hyperinsulinemic euglycemic clamp with 10 mU·min−1·kg−1 insulin), or saline control. Limb blood flow and the microdialysis O/I ratio of l-[14C]glucose were measured. It was found that both contraction and insulin infusion decreased the O/I ratio of l-[14C]glucose and increased total limb blood flow. Calculations using l-[14C]glucose O/I ratio revealed that, during basal conditions, the nutritive fraction of total flow was ∼38%, indicating that under these conditions before the infusion of insulin ∼62% of the total flow was passing through a vascular route that had little or no exchange with the interstitial fluid surrounding the muscle fibers (i.e., the “nonnutritive” route), or indeed the probe. Insulin at a high physiological dose increased limb blood flow from 1.2 to 1.8 ml/min and, based on calculations using l-[14C]glucose O/I ratio, resulted in an increase in the nutritive fraction to ∼52%. Muscle contraction also increased limb blood flow (to 2.0 ml/min) and the nutritive fraction to ∼82%. Calculations of the absolute nutritive flow rates revealed that insulin and contraction had increased nutritive flow by 113 and 250%, respectively. Insulin did not affect the absolute nonnutritive flow rate, but contraction significantly reduced it. These data indicate that there was a preferential increase of flow to the nutritive route as a result of insulin action, thus consistent with the known vasodilatory action of this hormone (127). In addition to a preferential increase of flow to the nutritive route, contraction seems to have actively reduced flow to the nonnutritive route, suggestive of an accompanying vasoconstriction. The source of the vasoconstriction has yet to be identified but may be due to the accompanying increase in sympathetic tone that occurs with muscle contraction in vivo. A drawback of the microdialysis technique is that the sampling volume is relatively small. To increase the sampling accuracy, a number of probes simultaneously located in slightly different positions in the limb can be used. Since there is always potential for damage to the tissue surrounding the probe due to its insertion and physical presence, some caution should be exercised in the interpretation of the data.
Washout kinetics (Table 1) following the injection or infusion of a labeled marker substance has been applied in various forms to measure total blood flow and, by default, microvascular perfusion. The classical approach has been to use an intramuscular injection of 133Xe and follow the decay of radioactivity from the injection site with a surface probe placed over the site. Blood flow is calculated (16) as blood flow = −100 × λ × K (ml × 100 ml tissue/min), where λ is the partition coefficient of tissue/blood (in units of radioactivity per unit mass of tissue per units of radioactivity per unit volume of blood) and K is the elimination rate constant for the monoexponential washout of 133Xe. It is assumed that the injection is into the interstitial fluid, and thus any change in flow calculated by this procedure reflects a change in nutritive flow; this will not necessarily correlate with a change in total flow, as argued previously. To illustrate this point, vasodilators that increase total flow (limb blood flow), such as sodium nitroprusside (SNP) or bradykinin, but do not increase nutritive flow would not be expected to have an effect on the rate of 133Xe clearance. Similarly, a systematic error attributed to this method at exercise work rates of >60% maximal oxygen consumption (V̇o2max) may not be entirely due to diffusion limitations of 133Xe (12). It may be that, at high work rates and high total flow rates, a greater proportion of nonnutritive flow occurs commensurate with a need for redirecting some blood flow to skin for cooling.
Another approach using washout kinetics has been to estimate distribution volumes following a bolus intravenous injection of a labeled marker. Recently, Ellmerer et al. (44) used the Henthorn et al. (69) three-compartment model to assess the effect of hyperinsulinemia on transport parameters and distribution volumes of labeled inulin (a marker that has access to the vascular and interstitial spaces) in the dog. The original model described by Henthorn et al. delineated the mean central compartment, as the blood volume and the two peripheral compartments of the model were said to represent rapid and slow equilibrating interstitial fluid compartments of the whole animal. Indirect evidence suggested that the fast equilibrating compartment represented splanchnic blood flow, as this could be accounted for by the presence of porous capillaries and thus minimal resistance to movement of inulin (44). Subsequently, Steil et al. (166) claimed the slow equilibrating compartment to be attributable to muscle interstitial fluid, and Ellmerer et al. (44) showed that there was a strong and highly significant correlation between the labeled inulin response of the slowly equilibrating compartment from the three-compartment model using plasma data only and the labeled inulin response directly measured in the hindlimb lymph compartment. These later data were possible only by directly sampling the hindlimb lymph (44) at two levels (low and high) of insulin. However, it is important to note that, because the vascular (or blood) compartment did not simultaneously increase during hyperinsulinemia, the increase in the slowly exchanging compartment was concluded to be due to increased access (44), possibly attributable to an insulin-mediated increase in vasomotion. It is also interesting to note that, in a similar study, l-[14C]glucose disappearance from plasma and appearance in lymph were not altered by maximally effective insulin infusion (166). One potential difficulty that is not addressed in these studies where lymph is sampled is the rate of lymph formation. Measured levels of labeled inulin and l-glucose in lymph are each expressed as disintegrations per minute per milliliter and would clearly be influenced by the rate of lymph formation if the rate of transendothelial inulin and l-glucose differed. For example, it is established that lymph albumin concentration decreases as the rate of lymph formation increases (58), and it is not known whether insulin has any effect on the rate of lymph formation. Overall, this method is at best an indirect approach to assessing changes in capillary recruitment because it has yet to show an increase in microvascular perfusion volume when other more direct methods have done so under similar conditions (e.g., see Ref. 33). There is also the added complication that hindlimb lymph was collected by continuously massaging the hindlimb (124), and this may have affected microvascular perfusion.
Venous occlusion plethysmography (Table 1) involves placing a strain gauge around the midforearm with a rapid occlusion cuff sited on the upper arm. Rapid inflation of the cuff to 40 mmHg is initiated within 1.5 s by means of gas from a compressed air cylinder. With the blood pressure cuff around the bicep, the subject's arm is supported so that the midforearm is slightly above heart level. During the time of elevated pressure, venous outflow is prevented, but arterial inflow is unimpeded. Consequently, the limb swells as the arterial inflow accumulates. The rate of inflow is determined from the slope of the accompanying record of forearm circumference, obtained from a gauge around the midforearm. Venous occlusion plethysmography is usually used to measure total forearm blood flow (e.g., see Ref. 186), but occasionally researchers have used this technique to study impairment of microcirculation, as assessed by the time required for reactive hyperemia to reach peak value (e.g., see Ref. 1) or microcirculatory endothelial function (188); hence, this technique is listed in Table 1. Whether or not responses determined in this manner partly or fully reflect microcirculatory function remains to be resolved. However, venous occlusion plethysmography would almost certainly perturb microvascular flow and cannot be used to determine capillary recruitment.
Indocyanine green (ICG; also known as Cardio-Green), unlike Evans Blue, is bound to plasma proteins with no retention in extravascular tissue spaces (16). Because of this and the ability to detect ICG by near-infrared spectroscopy (NIRS), it is possible to determine blood flow by applying the Fick principle, where the rate of accumulation of ICG in a given tissue is equal to its rate of inflow minus its rate of outflow (Table 1). Thus, if ICG is introduced rapidly and its rate of accumulation measured over time, blood flow can be measured as a ratio of ICG accumulated to the quantity of tracer introduced over a given time. Provided that it remains only in the vascular space, the total tissue signal if the ICG were to be infused at a constant rate to achieve a steady-state concentration should be indicative of the total perfused volume. This would of course include large vessels, macrocirculation, and microcirculation, including nutritive and nonnutritive routes. Unless corrections can be made for the volumes other than nutritive route, microvascular volume cannot be determined. NIRS-ICG was first used to measure cerebral blood flow in the duck (32), and although changes in flow estimated by ICG paralleled those of 133Xe washout, the clearance curve for ICG was very short (<30 s) compared with 133Xe of 10–30 min (32). This discrepancy between the two methods may reflect a low fraction of total flow that is nutritive. In the study by Boushel et al. (16), regional blood flow in calf muscle and around the Achilles tendon was quantified during plantar flexion using NIRS-ICG; blood flow was found to be proportional to workload.
Capillary recruitment from redistribution of microvascular flow or flow sharing: indications from isolated vessel studies and models.
It has been reported that capillary recruitment can occur under circumstances when total flow does not increase (31, 177–179); this can be with low physiological doses of local insulin (31) or at low levels of exercise in human forearm (178) or low levels of systemic insulin (197) in rats. Capillary recruitment has been detected either with an imaging approach (CEU) or from metabolism of 1-MX, both of which imply an increase in capillary surface area for exchange. For this to have occurred, blood flow has been either redistributed from previously perfused vessels (these might be the low-resistance, high-capacitance, and shorter nonnutritive capillaries) into previously unperfused longer, more tortuous, nutritive capillaries or shared from a few into additional similar capillary units. In either case, insulin-mediated vasodilation could be responsible for lowering the resistance at terminal arterioles leading into the recruited route. With these notions in mind, the challenge has been to devise ways of assessing the flow patterns discussed above, which might explain the capillary recruitment that occurs with physiological insulin when total blood flow does not increase. Two of the methods, CEU and LDF, have been tried with the two most likely models, each of which was constructed and tested in vitro. In the first model, recruitment of capillaries by flow sharing (i.e., recruiting further arms of a manifold) would predict that red cell velocity would decrease. In contrast, in the second model, if flow is redistributed from a shorter into a longer, more tortuous route where capillary surface area increases, red cell velocity would not change. However, recent studies using CEU (135), which provides a measurement of both microvascular volume and the rate of fill of that volume, proved inconclusive. CEU estimates of capillary volume increased and measurements of capillary filling rate decreased, regardless of whether flow was switched from shorter to longer capillaries, or flow was recruited from one to many capillaries within a manifold. From similar model studies using LDF (25), an increase in signal in the absence of an increase in bulk flow would favor the model where flow was switched from shorter to longer capillaries. However, these are only model studies in vitro, and the issues remains to be resolved in vivo.
Parallels and differences between insulin action and exercise.
Both insulin action and exercise result in capillary recruitment in muscle. This has been evident from recent use of either the 1-MX metabolism method or CEU (31, 33, 177–179). Capillary recruitment due to muscle contraction has been known for some time (72, 90) and can be visualized directly by microscopic examination of muscle sections for capillary content of red blood cells following movement or stimulus. Surprisingly little exercise (effort and/or frequency of electrical stimuli) is required to initiate capillary recruitment, but in general, the increase with maximum exercise exceeds that with maximum insulin, suggesting that there are regions of the nutritive route that are recruited by exercise but which cannot be recruited by insulin. In addition, insulin-mediated capillary recruitment is very sensitive and occurs at lower doses of insulin than those required to increase leg glucose uptake and femoral blood flow in the rat (197). Time course studies indicate that muscle contraction-mediated capillary recruitment in rats in vivo begins to occur as soon as contraction is started (Ross RM, Rattigan S, and Clark MG, unpublished observations), and precise detection of when this actually takes place is limited by the resolving power of the CEU technique. In comparison, insulin-mediated capillary recruitment from systemic insulin infusion is slower in onset than that due to exercise, but capillary recruitment due to insulin precedes signaling and glucose uptake in the myocytes (177). If insulin is injected directly in the interstitium, the metabolic response occurs at least 5 min sooner than when insulin is infused systemically (23). Mechanistic differences are also apparent when insulin and exercise-mediated capillary recruitment are compared; only insulin is blocked by inhibitors of NOS (136), and in the obese insulin-resistant Zucker rat, capillary recruitment due to insulin is absent but the response to exercise is normal (184). Similarly, exercise-mediated rates of glucose utilization, carbohydrate and fat oxidation in patients with non-insulin-dependent diabetes mellitus do not differ from healthy controls (81), possibly suggesting that exercise-mediated capillary recruitment may also be normal.
Insulin-mediated increases in vasomotion: possible implications.
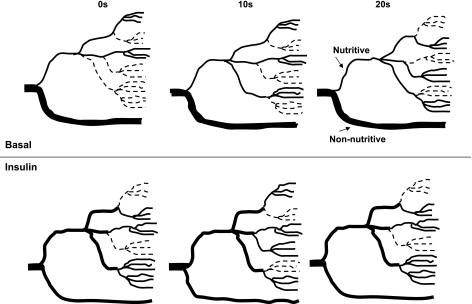
Vasomotion is a spontaneous rhythmic change of arteriolar diameter that almost certainly plays an important role in ensuring that tissue such as muscle is perfused sufficiently to sustain the prevailing metabolic demand (141) by periodically redistributing blood from one region of the muscle to another (Fig. 1). It is most active under conditions of low bulk flow rates or reduced overall perfusion (115) and is readily detectable in skeletal muscle vasculature at rest. Thus a snapshot of resting muscle at any one time might suggest that a certain region is “underperfused.” However, a snapshot at a later time point will show that the previously underperfused region is now perfused and another region is underperfused. The consequence is that, over an extended period, all of the muscle receives sufficient oxygen and nutrients to meet energy demands required for the basal state. Vasomotion is an oscillation of vascular tone that is thought to be generated in the vascular wall and is not a consequence of heartbeat, respiration, or neuronal input (115). A noninvasive technique (for skin) that allows detection of changes in vasomotion in vivo is LDF. A number of very useful papers from Stefanovska et al. (165) and Rossi and colleagues (137–139) have analyzed the reflected LDF signal from skin to provide indirect assessment of vasomotion (10). In humans they have interpreted the spectrum as follows: the highest frequency peak that manifests in the arterial tree and in the microvascular blood flow signal reflects the heart beat that ranges from 0.6 Hz in sportsmen to 1.6 Hz in subjects with impaired cardiovascular function. Simultaneous monitoring of heart rate confirms the origin of this peak in the LDF signal from the microvasculature. There is very little evidence of the respiratory activity peak, which is nominally indicated to be 0.15 to 0.4 Hz and can be readily confirmed by monitoring lung excursions. The third highest frequency peak is ∼0.1 Hz and is thought to correspond to blood pressure regulation, where the smooth muscle cells in the vessel's walls respond to changes in intravascular pressure, and this is known as the myogenic response (78). Although attributed to spontaneous intrinsic stretch-dependent ion channels, it may also be controlled by the sympathetic nervous system (122). Since this peak stands out as the one that changes most with insulin-mediated changes in LDF signal from skin (138) and muscle (114), it may indicate an important vasomotor input mediated by insulin. Another peak occurs at the interval from 0.02 to 0.06 Hz, and this is thought to represent neurogenic activity and is eliminated following denervation (84). The lowest frequency peak occurs at ∼0.01 Hz and is thought to derive from endothelial activity (e.g., see Ref. 138). There are two reports of an increase in vasomotion in muscle as a result of insulin action, where an LDF probe was impaled in the leg of either human subjects (35) or rats (114) undergoing a hyperinsulinemic clamp. In the human study, insulin increased the contribution of frequencies between 0.01 and 0.04 Hz (determined by Fourier analysis), which the authors attributed to increased endothelial and neurogenic activity (35). It remains to be explained why this result differs from results from iontophoresed insulin in human skin, where there has been a consistently marked increase in amplitude of the frequency at ∼0.1 Hz (138). Similarly, rat muscle studies showed the main increase due to insulin, deduced by wavelet analysis, to be myogenic at 0.1 Hz (114). The increase in vasomotion at around this frequency is considered to be due to spontaneous rhythymic oscillations in intracellular Ca2+ concentration of the vascular smooth muscle (115), and the increase in amplitude of the myogenic response may imply that insulin activates some aspect of the process, leading to an increase in intracellular Ca2+. An increase in vasomotion due to insulin would enhance microvascular perfusion (Fig. 1) without necessarily requiring an accompanying increase in bulk flow, as is commonly the case with physiological insulin (31, 177–179). It is important to note that some workers have shown that the peak at 0.133–0.03 Hz is related to rhythymic contractions of the terminal arterioles, which can be induced during hemorrhagic hypotension and visualized using intravital microscopy (14). These are not always detectable because the terminal arterioles or precapillary sphincters are not synchronized over significant portions of the microvasculature, and so the signal is averaged out when sampled by LDF (145). It follows that synchronization of the oscillations may explain their detection. A slower wave vasomotion at 0.02–0.05 Hz is not evident until mean arterial pressure falls to ∼35 mmHg (145), and this is thought to originate in larger-sized vessels, possibly the transverse arterioles.
Fig. 1.
Proposed schematic blood flow patterns in muscle in vivo under basal conditions and following a physiological rise in plasma insulin. Insulin increases microvascular perfusion as a result of lowering the resistance in terminal arterioles and gives rise to increased perfusion of nutritive capillary beds. This is accompanied by a passive decrease in nonnutritive flow when bulk flow does not increase. Overall, there is an increase in perfused microvascular volume, as detected by contrast-enhanced ultrasound and laser Doppler flowmetry (LDF) and an average increase in available capillary surface area for 1-methylxanthine metabolism and glucose uptake. Insulin's increase in microvascular perfusion is also associated with an increase in vasomotion that is responsible for the rhythmic redistribution of blood flow throughout the muscle and which very likely contributes to the increase in microvascular perfusion. Wavelet analysis of the LDF signal reveals that insulin predominantly augments the myogenic component of vasomotion occurring at 0.1 Hz; hence, 3 different (random) patterns are shown to occur at intervals of 10 s. Perfused and unperfused capillaries are shown as solid and broken lines, respectively. During the increased rhythmic dilatation resulting from insulin, the arterioles allow greater penetration of blood flow into the microvasculature, analogous to “waves breaking further up the beach,” and more capillaries are perfused. In insulin resistance, insulin-mediated increase in microvascular perfusion and vasomotion are impaired, resulting in predominantly nonnutritive flow. With limited capillary recruitment and limited vasomotion, periodic blood flow to a capillary unit impacts more on glucose uptake than insulin signaling. Because the extraction fraction of glucose is high, interruption of blood flow leads to a decline in capillary blood glucose level sufficient to limit uptake. Periodic delivery of insulin may be sufficient to allow insulin signaling, which, once activated, continues unabated, increasing the demand for glucose that cannot be met.
On balance, it would now seem likely that insulin-mediated increase in intensity of vasomotion at the terminal arterioles involving primarily smooth muscle could play a major part in the increased microvascular perfusion by contributing to increased penetration and distribution of blood flow (Fig. 1). If this is initiated by insulin acting on smooth muscle insulin receptors, then it might explain why vascular endothelial insulin receptor knockout (VENIRKO) mice are not insulin resistant (174). However, it cannot readily account for eNOS knockout mice being insulin resistant (157) unless eNOS is involved in nonvascular mediation of glucose uptake (136).
Capillary recruitment and the concept of partly perfused muscle at rest.
Insulin- and exercise-mediated capillary recruitment is predicated on the assumption that skeletal muscle at rest and before a meal is only partly perfused (i.e., there exists a reserve of unperfused capillaries that are recruited to carry flow not only by muscle contraction but also by insulin). A corollary to this concept is the possibility that rhythmic redistribution of blood flow by vasomotion operates so that on average about one-half to one-third of the muscle is perfused at any one time. Even so, there is some evidence that, when muscle is exposed and examined at rest by intravital microscopy, all capillaries are perfused (e.g., see Ref. 123). Clearly, if all capillaries are fully perfused with red blood cells at rest, then the observed increases in microvascular perfusion mediated by insulin or exercise create a dilemma. The evidence in favor of capillary recruitment and, by default, a capillary reserve at rest derives largely from findings obtained using five different approaches discussed in detail elsewhere (27) and relates to observations of red cell distribution in cryosections of muscle before and after contraction (72, 90), intravital microscopy of surgically exposed transparent muscle of anesthetized animals (e.g., see Ref. 97), 1-MX metabolism (127), CEU (178), and LDF (35). The reader is directed to a point-counterpoint discussion (27, 123) on this controversy where the above evidence for, as well as the evidence against, a capillary reserve in muscle at rest is presented in more detail.
Obesity, Insulin Resistance, and Impaired Insulin-Mediated Capillary Recruitment
Identifying the rate-limiting step of impaired muscle glucose uptake.
In animal models of muscle insulin resistance and in human subjects who show decreased ability of insulin to increase muscle glucose uptake, there has been considerable debate as to where the rate-limiting step(s) occurs as the insulin resistance becomes entrenched. A number of approaches have been used to assess the situation. Halseth et al. (63, 64) used radioactive-labeled markers to monitor the muscle [glucose]/plasma [glucose] ratio in rats following insulin administration and concluded that glucose delivery, reflected by an increased ratio, was a rate-limiting step in one of the muscles examined (gastrocnemius) when animals were on a normal balanced diet. However, following a period on a high-fat diet that led to insulin resistance, the ratio of muscle [glucose]/plasma [glucose] became elevated in all three of the muscles examined (soleus, gastrocnemius, and superficial vastus lateralis) following insulin administration (63). Overall, these data suggest that glucose delivery is a rate-limiting step in normal insulin action and that this step becomes further limiting as muscle insulin resistance develops.
Bertoldo et al. (11) have used dynamic PET imaging of human muscle in vivo to assess interactions between delivery, transport, and phosphorylation of glucose in governing uptake into muscle of lean healthy subjects under normal conditions. Three tracers used were [15O]H2O, [11C]3-O-methyl-d-glucose, and [18F]fluoro-deoxy-d-glucose to quantify determinations of glucose delivery, transport, and its phosphorylation, respectively. Control during insulin-stimulated conditions indicated delivery and transport of glucose to be the key steps. In essence, insulin-mediated stimulation of glucose transport unmasked a greater dependence on glucose delivery and phosphorylation than was previously evident.
It is important to note that there is no consensus in this debate over where the rate-limiting step for glucose uptake occurs in insulin-resistant muscle. A number of studies by Perseghin et al. (118), Petersen et al. (119), and Rothman et al. (140) examining early stages of insulin resistance including healthy, young, lean, insulin-resistant individuals have shown that insulin resistance in these subjects can be attributed mostly to defects in insulin-stimulated glycogen synthesis. In these studies, magnetic resonance spectroscopy and a combination of 13C- and 31P-enriched substrates were used to noninvasively measure changes in glycogen synthesis, intracellular glucose, and intracellular glucose 6-phosphate. In essence, it was found that the reduction in insulin-stimulated muscle glycogen synthesis was associated with a blunted increment of insulin-stimulated intramuscular glucose 6-phosphate concentrations and a markedly decreased concentration of intracellular glucose, particularly evident in muscle from patients with type 2 diabetes (see review in Ref. 120). Overall, these data were interpreted by those authors to reflect that defective insulin-stimulated glucose transport activity was the major factor responsible for insulin resistance in patients with type 2 diabetes. However, no mention was made of defective glucose delivery resulting from impaired vascular function, although the data would not exclude this as the defective step.
Another approach to assess the rate-limiting step that is responsible for impaired muscle glucose uptake in insulin resistance has been to use microdialysis. But in these studies (161), the focus has been on insulin delivery. To do this, microdialysis probes were inserted in muscle of obese and nonobese subjects and the time-rate of rise of interstitial insulin concentration was determined. Inulin, a polymer of d-fructose and of similar molecular mass to insulin, was used as a comparison. It was found that the time-dependent rise in interstitial insulin and inulin were similar in nonobese subjects but considerably slower in obese subjects. These data imply that, in the obese insulin-resistant subjects, insulin delivery from plasma to interstitium is rate limiting.
As indicated in an earlier section of this review, monitoring the delivery of radioactive inulin to the interstitium and lymph has been used by Ellmerer and colleagues (43, 44). During hyperinsulinemic euglycemic clamps in dogs, insulin was found to stimulate the extravascular distribution volume of inulin (44), and in a later study (43), insulin was shown to increase movement of inulin to the lymph that drained from hindlimb muscles. A high-fat diet resulted in a significant reduction of the peripheral distribution volume of [14C]inulin and a marked decrease in inulin appearance in the hindlimb lymph. Again, consistent with the findings of Sjöstrand et al. (161), insulin-mediated delivery of inulin would appear to be impaired with insulin resistance.
Transendothelial transport of insulin as a rate-limiting step in insulin action and insulin resistance.
From studies discussed earlier in this review, it would seem highly probable that insulin's transport from the central circulation into muscle is rate limiting for the stimulation of glucose metabolism under normal conditions. Consequently, by recruiting muscle microvasculature, insulin may promote its own movement into muscle interstitium. There are a number of studies indicating insulin's transendothelial transport to be rate limiting for its action in stimulating muscle glucose uptake (103, 124, 159, 189). However, separating insulin-mediated capillary recruitment from insulin-mediated transendothelial insulin transport has proven to be complicated. In one recent study (42), microvascular blood volume, a reflection of the extent of capillary recruitment (measured by CEU), total blood flow and muscle insulin, and glucose uptake (by forearm balance technique), was measured in lean healthy subjects before and during an insulin clamp. It was found that capillary recruitment increased due to insulin. Forearm insulin uptake abruptly increased with the increase in capillary recruitment, but further uptake did not occur and indeed clearance gradually declined. These data suggest that insulin clearance is not passive and that a process exists at the capillary that can become saturated. However, it does not tell us whether insulin mediates its own entry into the interstitium by a process additional to the effect of increased capillary surface area, the result of capillary recruitment. In a separate study, the same group showed that low frequency muscle contraction increased transendothelial movement of both albumin and insulin (at basal insulin levels) in association with capillary recruitment (74). The question of whether insulin mediates insulin transendothelial transport would still seem to be unresolved, including its role, if any, in insulin resistance, where general transendothelial movement of macromolecules may increase (193).
Implications of insulin resistance on muscle fatty acid uptake.
The relationship between fatty acid uptake, fatty acid oxidation, and insulin resistance has come under close scrutiny in recent times. This is largely because elevated plasma fatty acids have invariably been linked to insulin resistance (120, 143) and infusion of intralipid with heparin induces an acute state of insulin resistance (29). Moreover, established insulin resistance corresponds with fewer subsarcolemmal mitochondria of diminished size, potentially reflecting impaired fatty acid oxidation, thus causing intermediates to accumulate intracellularly to impair insulin-mediated glucose uptake and metabolism in muscle (120). Given that insulin normally increases capillary recruitment as part of its action in healthy individuals and thereby potentially increases fatty acid delivery to muscle, it is of interest to examine the effect of insulin clamp on fatty acid uptake. Fatty acid uptake has been difficult to measure, but a labeled (essentially nonmetabolized) fatty acid has been used. This is 18F-FTHA (fluoro-6-thia-heptadecanoic acid), and its clearance from plasma and appearance in muscle was determined by measurement of label in muscle biopsies (animals; see Ref. 62) and by PET (humans; see Ref. 100). In healthy pigs (that have similar lipoprotein profiles to humans), using physiological insulin there was a threefold increase in the tissue-to-plasma input radioactivity ratio of skeletal muscle (when fasted and hyperinsulinemic conditions were compared). But because the arterial concentration of free fatty acids (FFAs) decreases proportionately more due to insulin-mediated inhibition of lipolysis, a net increased uptake into muscle is generally masked. The masking effect of decreased lipolysis was less in rats where, using 3H-R-bromopalmitate [another fatty acid tracer (68)], FFA clearance increased into red gastrocnemius muscle when insulin clamp and basal conditions were compared. However, it remains to be seen whether increased capillary area due to insulin has contributed to the apparent increased muscle fatty acid uptake in normal healthy animals and humans.
Other data in the literature using a more reductionist approach are not helpful in assessing the role of blood flow in muscle fatty acid uptake. For example, in isolated perfused rat hindlimbs, insulin increased fatty acid uptake by 51% (98); this was deduced to be due to the insulin-mediated translocation of the fatty acid transporter protein FAT/CD30 to the sarcolemma. Keeping in mind that the perfused rat hindlimb is fully dilated and not subject to insulin-mediated capillary recruitment, then increased uptake has occurred independent of change in capillary surface area. As another example, in insulin-resistant type 2 diabetic and obese subjects, free fatty uptake was reported to be upregulated (13). Giant sarcolemmal vesicles prepared from the muscle of these patients and incubated with labeled palmitate showed that fatty acid uptake was increased by approximately fourfold compared with similar vesicles prepared from lean subjects. Furthermore, fatty acid uptake by isolated perfused hindlimbs (no insulin) from obese Zucker rats showed higher rates of FFA uptake than their lean counterparts (170). Again, because the isolated perfused rat hindlimb preparation is fully dilated, a potential role for changes in microvascular perfusion cannot be made. Taken together, the effects of increased capillary recruitment and, hence, delivery on fatty acid uptake by muscle in vivo are yet to be resolved. Similarly, impaired delivery of fatty acids that is likely to be occurring in insulin resistance also needs to be addressed. In addition, the increased metabolic uptake of fatty acids by skeletal muscle sometimes ascribed to insulin resistance (e.g., see Ref. 158) needs to be rigorously examined given that interfibrillar adipocytes, located on the nonnutritive route, may be receiving preferential blood flow in insulin resistance.
Vascular Dysfunction
Definition.
The microvasculature of muscle has the major function of ensuring optimal delivery of hormones and nutrients to the myocytes. There are now several studies where loss of function reflected by impaired capillary recruitment in response to insulin occurs in association with impaired insulin-mediated glucose uptake by the myocytes (see review in Ref. 28 and the references therein). However, more research is required before it can be concluded that vascular dysfunction per se translates to impaired delivery in muscle following insulin action. For example, microvascular dysfunction is ascribed when any one of the following can be demonstrated to be different from normal: 1) decreased vasodilation in response to classic endothelium-dependent vasodilators, such as acetylcholine; 2) decreased vasodilator function of resistance vessels and capillary recruitment in response to reactive hyperemia; 3) increased vasoconstrictor responsiveness; and 4) decreased insulin-mediated capillary recruitment. Change of either decreased vasodilation in response to classic endothelium-dependent vasodilators, decreased vasodilator function of resistance vessels and capillary recruitment in response to reactive hyperemia, or increased vasoconstrictor responsiveness has not yet been shown to correlate with loss of insulin-mediated capillary recruitment. Measurements are often made of microvascular responses in skin, where it is relatively simple to apply acetylcholine, SNP, or insulin by iontophoresis and measure change in LDF signal. However, the vast majority of studies have been to measure flow in a major blood vessel such as the carotid artery (20) or brachial artery (6). Typically, vascular endothelial function is assessed by flow-induced vasodilation determined by acquiring ultrasound images of the artery 1 min after cuff deflation; at this time point, vasodilation is considered to be NO dependent (6). Yet others have examined responses in isolated vessels from experimental animals. One such recent study using isolated aortas from 3- and 24-mo-old rats showed that NO-dependent relaxation to insulin was markedly impaired in the older rats but that endothelial NO-dependent (acetylcholine) and endothelial-independent (SNP) vasorelaxation were similar in the young and older rats (148). In general, obese subjects show impaired responses in skin and resistance arteries to classic endothelium-dependent vasodilators (34, 38, 168), and this may extend to impaired capillary recruitment in response to reactive hyperemia (2, 34, 38, 56) as well as shear stress (2). Unfortunately, very few in vivo studies have attempted to assess whether the loss of response to insulin occurs alongside the loss of response to acetylcholine. However, a number of studies that have involved therapeutic intervention have reported recovery of response to acetylcholine without amelioration of the impaired insulin-mediated glucose disposal (e.g., see Ref. 110). If impaired insulin-mediated capillary recruitment is indeed responsible for part of the impaired insulin-mediated glucose uptake by muscle in insulin resistance, then an improvement in microvascular function should also lead to an improvement in glucose uptake. Thus the relationship between acetylcholine-mediated vasodilation and insulin-mediated capillary recruitment in skin and insulin-mediated glucose uptake in muscle needs to be further explored. One elegant study by de Jongh et al. (37) measured capillary recruitment in skin with capillaroscopy (after 4 min of arterial occlusion with a digital cuff) and vasodilation by iontophoresis of acetylcholine before and after hyperinsulinemia in lean women following FFA elevation. They found that FFA elevation impaired capillary recruitment and acetylcholine-mediated vasodilation before and during hyperinsulinemia (SNP effects were unchanged). Provided that regulation of skin microvascular perfusion represents what is occurring in muscle, these results certainly suggest that insulin-mediated capillary recruitment in muscle may parallel acetylcholine vasodilation. Similarly, it might be expected that the general loss of response to endothelial-dependent vasodilators and not just muscarinic receptor agonists as determined in human forearm blood flow (by strain-gauge plethysmography) in overweight/obese individuals (171) accompanies the loss of insulin-mediated capillary recruitment, as reported by others on similar subjects (30).
Because of the dissociation between limb blood flow and capillary recruitment, it might not be meaningful to attempt to relate macrovascular responses to endothelium-dependent vasodilators to insulin-mediated muscle glucose uptake. As indicated elsewhere in this article, insulin-mediated capillary recruitment may be regulated predominantly by insulin receptors on the vascular smooth muscle cells of the terminal arterioles and only partly dependent on endothelial mechanisms. For example, there is a report of insulin acting to dilate coronary epicardial vessels in vitro in an endothelial-independent manner involving activation of potassium channels and independently of cyclic guanosine monophosphate (cGMP) production (67). Also, the microvascular action of insulin when iontophoresed into skin causes a marked increase in the amplitude of the vasomotion frequency attributed to a vascular smooth muscle response when LDF signals are subjected to wavelet analysis (138). Thus vascular function tests on larger vessels based on the response to endothelium-dependent vasodilators might not address the key issue on two counts.
Vascular shunts.
The presence in muscle of a nonnutritive route, or functional shunt, conveys a character on this tissue similar to many others that have shunts and raises the question of the role that these might play in physiological and pathophysiological states. As early as 1952, it was pointed out that a paradox exists in type 1 diabetics where skin blood flow can be normal or even increased, yet tissue hypoxia is clearly evident (15, 18, 41, 50). One explanation for this has been that a malfunction of microvascular regulation (79, 88, 133) occurs, leading to a maldistribution of blood flow between nutritional and nonnutritional routes (with the latter sometimes referred to as “thoroughfare channels”). Thus, in the skin of a type 1 diabetic, blood flow is carried predominantly by the nonnutritive route. However, it is important to point out that the nonnutritional route may not necessarily be a discrete vascular entity. For example, and as delineated by Fagrell et al. (51) in a thoughtful editorial comment, the nonnutritive route may simply occur as a preferential pathway in the microvascular network. For example, when skin vessels in vivo are visualized under microscopy, it is clear that red blood cells distribute at capillary bifurcations and concentrate in the branch with greater velocity. Therefore, in successive bifurcations of nonidentical vessels, there are natural and passive mechanisms that cause increasing hematocrit in the preferential channels relative to the surrounding capillaries. These preferential channels are necessarily of lower resistance than the surrounding capillary beds, and a change (i.e., increase) in distribution of blood flow is achieved only by vasodilation of the higher resistance branch point and/or vasoconstriction of the lower resistance branch point. Indeed, in a number of early studies, we have shown that vasoconstrictors act to increase nutritive flow or nonnutritive flow in the constant-flow, pump-perfused rat hindlimb by vasoconstricting to decrease entry to the alternate route (e.g., see Ref. 26).
Preferential capillaries, or channels, have been observed in a number of tissues, including muscle (87). A deleterious contribution to pathology results from the bypass function of the preferential pathway. Examples include hemorrhagic shock and ischemia reperfusion injury, where the normal capillary endothelium swells proportionately more than those of the preferential channels, leading to increased resistance and thus diminished nutritive flow (101). It seems entirely plausible that the inflammation associated with obesity may be a primary cause of swelling of the nutritive capillary endothelium, leading to impaired delivery of glucose and insulin and, hence, to muscle insulin resistance with regard to capillary recruitment and myocyte metabolism. Flow is then locked into a predominantly nonnutritive state, favoring attendant adipocyte growth as interfibrillar fat. There is one recent report where insulin resistance from intralipid infusion resulted in the diversion of intramuscular insulin into the exiting capillary network, concluded to be vascular shunts (22). Thus lipid directly or indirectly, through oxidative stress, may influence insulin-signaling pathways in either, or both, the endothelium or vascular smooth muscle so that insulin-mediated capillary recruitment becomes impaired and blood flow is carried predominantly via the nonnutritive (or shunt) route (Fig. 1).
Myocyte vs. vascular dysfunction as the cause of insulin resistance in muscle.
The cause of insulin resistance in muscle is still not resolved, and, as mentioned above, there are those who firmly believe that the key impairment lies within the myocytes (which henceforth should be called the “myocyte hypothesis”; e.g., see Refs. 82 and 143). Of course, this may well be the case when insulin resistance becomes fully established. However, there is also a very real possibility that changes in the microvasculature precede impairments in the myocytes. Thus an early microvascular dysfunction leads to extensive periods of poor delivery of insulin, glucose, and other nutrients, and the muscle cells may respond by adapting to this scenario. There is at least one study where vascular function has been assessed in relation to the onset of diabetes and hypertension in a well-proven animal model, the Zucker diabetic fatty rat (96). These workers found that increases in vasoconstrictor responsiveness resulting from diminished NO signaling in skeletal muscle arterioles preceded the development of diabetes and hypertension in these animals. There is also evidence that vascular insulin resistance in aging may contribute to the development of hypertension and precede the development of metabolic insulin resistance (148). However, in general, the widely held notion at present is that endothelial dysfunction results from the hyperglycemia and other metabolic consequences of insulin resistance (e.g., see Ref. 126). For example, there is evidence that optimizing glycemic control reduces the development and progression of microvascular dysfunction (53).
Proponents of the myocyte hypothesis have applied a number of in vivo and in vitro approaches to try and identify the insulin-signaling defects associated with type 2 diabetes (95). Even so, it is not clear whether insulin-signaling defects are a cause or a consequence of skeletal muscle insulin resistance (82). Thus the notion that impaired perfusion has caused myocyte adaptations to occur merits careful consideration. This becomes even more important when one observes that published results of insulin-signaling defects in skeletal muscle are claimed to be inconsistent (82), having been drawn largely from subjects with long-standing type 2 diabetes (82). Take, for example, the question of whether or not insulin receptors (IR) in skeletal muscle from type 2 diabetics are altered. Protein expression studies indicate that expression of IR is normal (3, 91), and other signaling proteins are also expressed at normal or near-normal levels (82). This could imply that impaired perfusion, were it to have been occurring for some time, has had no effect on the expression level of IR or other insulin-signaling proteins. However, when assessing an acute insulin response in vivo, insulin-mediated glucose uptake by muscle is clearly impaired. Measurements of insulin binding to IR or indeed signaling further along the pathway conducted in vitro, where delivery of insulin and glucose is simply by diffusion, cannot address this issue. Recent studies that have examined this question in human subjects in vivo (83) suggest that all steps of insulin signaling are normal until the step immediately preceding glucose transporter-4 translocation involving AS160. Thus the increment of insulin-mediated phosphorylation over basal state for AS160 was markedly reduced in skeletal muscle from type 2 diabetic patients compared with healthy controls (83). This may be due to an impairment in activation of the kinase (Akt) that phosphorylates AS160, where phosphorylation of Akt on Thr308 over basal was also reduced in type 2 diabetic patients (83), a problem that may result from Akt isoform-specific differences or indeed the altered metabolic milieu, particularly hyperglycemia (82). In this respect, insulin-mediated Thr308 phosphorylation of Akt was similar between normal and type 2 diabetic patients after glycemia was normalized (102). Findings of this kind clearly suggest that, once type 2 diabetes has become entrenched, major defects develop in the insulin-signaling pathway of the myocytes. However, this does not necessarily identify the intial cause of the insulin resistance, where loss of myocyte insulin signaling may have been the outcome from an intial loss of vascular function affecting insulin and/or glucose delivery. Another concept is that insulin resistance is in essence an adaptive phenomenon and that impairments in insulin signaling in vessels and muscle develop in parallel, without one causing the other (after having taken into account that impaired insulin delivery will cause metabolic insulin resistance). It is pertinent to note that insulin signaling in muscle and endothelium is very similar.
Taking all possibilities into account, it is this reviewer's opinion that impaired delivery of glucose due to vascular dysfunction may be the key issue. For this argument, much depends on the role and extent of vasomotion (Fig. 1), which periodically interchanges regions of underperfusion of the skeletal muscle (see Insulin-mediated increases in vasomotion: possible implications) and which is very likely stimulated by insulin in normal muscle (35). In this scenario, insulin delivery, although impaired, may be sufficient for signaling to become activated throughout the entire muscle tissue. A brief exposure allowing insulin to bind to its receptor occurs when flow is occurring, and although further delivery of insulin to that region may halt when vasomotion redirects the blood flow to other regions of the muscle, the signaling cascade is activated. In contrast, periodic glucose delivery may mean that the concentration falls in those regions that are periodically without delivery so that the average interstitial concentration is lower than arterial, resulting in a lower glucose uptake over a finite period (e.g., as in a clamp or glucose tolerance test) despite full insulin signaling. Clearly, these issues must be addressed in the future.
How does impaired delivery occur as a result of vascular dysfunction, and can it be the cause of muscle insulin resistance?
There seems to be no question that obesity is associated not only with insulin resistance, reflected by impaired insulin-mediated glucose disposal (80), but also with hypertension (131). Importantly, these associations can be explained by vascular dysfunction, where an imbalance between vasodilatory and vasoconstictor processes occurs such that delivery becomes compromised. Thus impaired insulin-mediated endothelium-dependent vasodilation in the resistance arteries, together with impaired endothelium- or smooth muscle-dependent vasodilation of terminal arterioles, increases vascular resistance, which in muscle contributes to decreased insulin-mediated glucose disposal (28) and elevated vascular resistance. Both vasodilatory and vasoconstrictor effects of insulin have been described, and the normal response to insulin for isolated resistance arteries can be vasodilator or neutral (46, 173). This can be accounted for by NO-dependent vasodilator effects of insulin being antagonized by endothelin-mediated vasoconstrictor effects. The signaling mechanisms for insulin's vasodilatory response in muscle resistance arteries involve phosphatidylinositol (PI) 3-kinase, Akt, and eNOS (46, 48), whereas insulin's vasoconstriction are mediated by endothelin and are independent of PI 3-kinase. Blocking of NO production in these normal vessels unmasks the insulin-mediated vasoconstriction (46, 147). In obese patients the vasodilatory response of insulin is greatly impaired and subsumed by a vasoconstrictor response. Thus, although insulin-stimulated endothelin is normal in diabetics (52), it is increased in obesity (19), and the relative lack of NO-dependent vasodilation gives rise to increased resistance. Recently, Eringa et al. (47) explored these relationships using isolated resistance arteries from the obese insulin-resistant and hypertensive Zucker rats compared with their lean counterparts. In vivo the obese Zucker is hyperinsulinemic, hyperglycemic, and highly insulin resistant at maximal insulin doses; more importantly, insulin-mediated capillary recruitment in muscle is essentially absent (182). Eringa et al. (47) showed that, in arteries of lean rats, insulin induced activity of both NO and endothelin, resulting in a neutral effect under basal conditions; blockade of NO synthesis revealed strong endothelin-mediated vasoconstrictor activity. This contrasted with arteries from obese rats, where insulin induced only vasoconstrictor activity that was not enhanced by NO synthesis blockade. In addition, protein levels of eNOS were markedly decreased in arteries of obese animals, leading to a situation where insulin-mediated vasoconstriction predominated and insulin-mediated vasodilatory function was grossly impaired. Thus a basis is provided whereby impaired insulin-mediated capillary recruitment in the presence of hypertension in these same obese rats (182) can be explained.
The situation may not be restricted to endothelial processes. Vascular dysfunction may also derive from impairments in the NO-cGMP-cGMP-dependent protein kinase (PKG) pathway in vascular smooth muscle. In a recent study, again using tissue (vascular smooth muscle cells) from the obese Zucker rat, Russo et al. (142) showed a defect in NO-mediated soluble guanylyl cyclase activation and a reduction in NO and cGMP's ability to activate PKG. Most important, and as a clue to the cause of these defects, was the detected presence of higher levels of superoxide anions. Thus the addition of antioxidants partially prevented the defects of the NO-cGMP-PKG pathway observed in the vascular smooth muscle cells from the obese Zucker, which were reproduced by hydrogen peroxide in vascular smooth muscle cells from lean counterparts. These findings underscore the probable role played by oxidative stress in causing vascular dysfunction, which in turn impairs nutrient and hormone delivery.
Can the loss of an insulin-mediated vasodilatory function explain the impairment in insulin-mediated glucose uptake? It would seem so. For example, insulin-mediated capillary recruitment and glucose uptake by the muscle tissue in which capillary recruitment is measured are closely linked (24). Thus deliberate prevention of insulin-mediated capillary recruitment in rats in vivo by a pharmacological agent (α-methyl serotonin) that vasoconstricts to prevent access to nutritive route results in decreased insulin-mediated glucose uptake by muscle (128). This is accompanied by a total blockade of insulin's action to decrease hindlimb vascular resistance in the same hindlimb in which capillary recuitment is blocked, thus reflecting loss of insulin-mediated vasodilatory function. Similarly, acute insulin resistance can be introduced by infusion of TNFα (192), FFAs (29), or glucosamine (181). There is reasonable evidence that each of these nonvasoactive agents prevents insulin's action to increase capillary surface area by discrete molecular events to intercede in insulin signaling within the vascular tissue to cause vasodilation. TNFα and FFAs each activate kinases that phosphorylate and inactivate elements of the insulin-signaling cascade (86), leading to NO production (85).
The exact source of agents that increase with obesity and probably cause insulin resistance has yet to be determined. Inhibitory cytokines and FFA produced locally by intermuscular (interfibrillar) adipose tissue may play a key role (57, 190). Just how this might occur is embodied by the hypothesis referred to as “vasocrine” signaling by Yudkin et al. (195). In any event, although insulin's vascular action may be markedly compromised, exercise-mediated vascular and metabolic actions may remain essentially intact. Thus exercise-mediated capillary recruitment and muscle glucose uptake were normal in hindlimb muscles of rats that show grossly impaired responses to insulin (184). However, this may not be the case in other animal models of insulin resistance where vascular remodelling/rarefaction has taken place (e.g., see Ref. 56) or in humans with well-established type 2 diabetes. Indeed, in diabetic subjects, peak exercise intensity and V̇o2max (49) can be reduced along with slowed microvascular blood flow kinetics (5) and accompanying mitochondrial dysfunction (146), although this is not fully resolved (125). The clue to understanding the basis by which insulin-mediated capillary recruitment has become impaired in insulin resistance, and to developing possible novel therapeutic approaches, may come from an understanding of how exercise mediates its effects. Even so, the mechanism whereby exercise leads to capillary recruitment is unclear. Possibilities include mechanical coupling (70) or a transient decrease in high-energy intermediates with the activation of AMPK, leading to the phosphorylation and activation of eNOS (104), or AMPK activation may lead to vasorelaxation (and capillary recruitment) in an endothelium- and eNOS-independent manner (59). In this regard, 5-aminoimidazole-4-carboxamide, an activator of AMPK, has recently been shown (in preliminary studies) to mediate capillary recruitment in vivo (17).
The loss of insulin-mediated capillary recruitment with insulin resistance: can this result from muscle insulin resistance?
This question is certainly valid, and an answer should be possible. For example, insulin-mediated capillary recruitment needs to be assessed in conditional knockout mice where myocyte insulin resistance has been created. Do mice with downregulated glucose transporter-4 in muscle show a normal response to insulin's action to increase capillary recruitment in this tissue? Clearly, experiments of this kind need to be addressed.
Documented chronological development of vascular dysfunction and muscle insulin resistance from longitudinal studies suggest that the former occurs first (96) and thus makes it unlikely that the loss of insulin-mediated capillary recruitment is the result of muscle insulin resistance. However, much depends on whether the indexes of vascular dysfunction (acetylcholine and SNP responses, plasma E-selectin, enhanced vasoconstrictor responsiveness, or others) are indicative of relative insulin response in terms of capillary recruitment in muscle. On balance, the available data would favor the view that vascular dysfunction precedes, and is a cause of, insulin resistance in muscle (e.g., see reviews in Refs. 76 and 151). One property of the vasculature that is common in many of the models of muscle insulin resistance is the loss of NO production (47). In the animal model where eNOS is deleted (eNOS−/−), there is insulin resistance, hypertension, elevated triglycerides, and FFAs (40) as well as fewer skeletal muscle mitochondria that have less β-oxidation capacity (93). This adds support to the notion that loss of vascular function through loss of NO leads to impaired perfusion, to which the myocytes are forced to adapt. As a result, fewer mitochondria of lower oxidative capacity (7) ensue, leading to elevated FFAs and triglycerides. Long-term loss of the capacity to recruit nutritive blood flow by insulin, and perhaps also by exercise, may mean that flow is predominantly nonnutritive, favoring connective tissue vessels that are interspersed between muscle fibers and bundles. The long-term consequence of this is the proliferation and growth of interfibrillar fat cells that constitute the marbling of meat. Indeed, recent studies examining the extent of intermuscular adipose tissue using whole body magnetic resonance imaging have found significant independent associations for intermuscular adipose tissue with fasting glucose, protein-bound glucose, and total cholesterol (190).
“Vascular Fitness”
Beneficial effects of regular exercise.
The immediate or acute effects of a single bout of exercise are directed primarily at supporting the working fibers with adequate nutrients and, by the same token, adequate removal of metabolic end products. Repeated bouts of exercise result in adaptation (73), and a number of conditioning effects take place that enhance the response to insulin (187). In addition, changes also occur where fuel preference may change (172) so that, during subsequent bouts of exercise by the trained individual, glucose flux for a given power output decreases (54). There are also data to indicate that regular exercise (aerobic and/or resistance) delays the onset of age-related insulin resistance in muscle (121) and can ameliorate insulin resistance in obese insulin-resistant subjects (89, 160) as well as in obese children without a change in body weight (8). In addition, exercise training was shown to improve vasodilatory function, which was measured as the response to infused acetylcholine in the brachial artery in conjunction with improved insulin-stimulated whole body glucose disposal (34). Accordingly, it is possible that regular exercise leads to a generalized “vascular fitness,” along with the other now obvious changes that occur throughout the body (e.g., muscle fitness, bone strength, cardiac conditioning, etc.), as well as having a number of beneficial effects in improving endothelial vasodilator function and left ventricular diastolic function and decreasing total and abdominal fat (169). We already know that exercise training of rats enhances insulin-mediated capillary recruitment and glucose uptake in muscle (129) as well as skin blood flow responses to insulin in elderly humans (139). Could regular exercise have a general effect to enhance vascular fitness? One way in which this might be assessed is to monitor the morphological appearance of retinal vessels. Insofar as this does not appear to have been directly assessed (169), a significant independent association was demonstrated between diabetic retinopathy and exercise capacity in 419 non-insulin-dependent diabetes mellitus patients who underwent graded exercise testing (49).
From the opposite viewpoint, physical inactivity can rapidly induce insulin resistance and microvascular dysfunction (65), so maintained physical activity has to be at least prophylactic in preventing both.
Conclusion
Insulin's action to increase microvascular perfusion (capillary recruitment) of muscle is impaired in insulin resistance and reflects early-onset vascular dysfunction and results in diminished delivery of glucose. In addition, insulin resistance is characterized by blood flow being carried predominantly by nonnutritive vessels in connective tissue where intrafibriller adipocyes can grow. Regular exercise, through its actions to substantially increase limb blood flow and fully recruit capillary flow, clearly has positive effects on the vasculature that leads to vascular fitness and enhancement of insulin-mediated capillary recruitment.
GRANTS
The work both from our laboratory and cited in this review has been supported by grants from the National Health and Medical Research Council, Heart Foundation, and Australian Research Council. Collaborative research with Gene Barrett at the University of Virginia has been supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK-057878).
Acknowledgments
Over the last 25 years, I have had the privilege of working with some outstanding scientists. In particular, I acknowledge my colleague Stephen Rattigan, whose work underpins most of what I have cited from our laboratory. I also thank the numerous students and associates for their contributions. I thank John Newman for allowing me to cite his unpublished findings (114).
REFERENCES
- 1.Alexandraki K, Protogerou AD, Papaioannou TG, Piperi C, Mastorakos G, Lekakis J, Panidis D, Diamanti-Kandarakis E. Early microvascular and macrovascular dysfunction is not accompanied by structural arterial injury in polycystic ovary syndrome. Hormones (Athens) 5: 126–136, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord 23: 936–942, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Arner P, Pollare T, Lithell H, Livingston JN. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 30: 437–440, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Baron AD, Tarshoby M, Hook G, Lazaridis EN, Cronin J, Johnson A, Steinberg HO. Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes 49: 768–774, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 30: 2880–2885, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Beckman JA, Goldfine AB, Dunaif A, Gerhard-Herman M, Creager MA. Endothelial function varies according to insulin resistance disease type. Diabetes Care 30: 1226–1232, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, Shulman GI. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes 56: 1376–1381, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell LM, Watts K, Siafarikas A, Thompson A, Ratnam N, Bulsara M, Finn J, O'Driscoll G, Green DJ, Jones TW, Davis EA. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab 92: 4230–4235, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bergman RN Insulin action and distribution of tissue blood flow. J Clin Endocrinol Metab 88: 4556–4558, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Bernjak A, Stefanovska A. Importance of wavelet analysis in laser Doppler flowmetry time series. Conf Proc IEEE Eng Med Biol Soc 1: 4064–4067, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Bertoldo A, Pencek RR, Azuma K, Price JC, Kelley C, Cobelli C, Kelley DE. Interactions between delivery, transport, and phosphorylation of glucose in governing uptake into human skeletal muscle. Diabetes 55: 3028–3037, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Bonde-Petersen F, Henriksson J, Lundin B. Blood flow in thigh muscle during bicycling exercise at varying work rates. Eur J Appl Physiol Occup Physiol 34: 191–197, 1975. [DOI] [PubMed] [Google Scholar]
- 13.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Borgstrom P, Lindbom L, Meyer JU, Sjoquist M, Arfors KE, Intaglietta M. Hemodynamic responses in rabbit tenuissimus muscle arterioles during local reduction in perfusion pressure. Int J Microcirc Clin Exp 9: 175–186, 1990. [PubMed] [Google Scholar]
- 15.Boulton AJ, Scarpello JH, Ward JD. Venous oxygenation in the diabetic neuropathic foot: evidence of arteriovenous shunting? Diabetologia 22: 6–8, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Boushel R, Langberg H, Olesen J, Nowak M, Simonsen L, Bulow J, Kjaer M. Regional blood flow during exercise in humans measured by near-infrared spectroscopy and indocyanine green. J Appl Physiol 89: 1868–1878, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Bradley EA, Clark MG, Rattigan S. Acute AICAR infusion stimulates capillary recruitment in skeletal muscle (Abstract). Diabetes 55, Suppl 1: A336, 2006. [Google Scholar]
- 18.Cameron NE, Cotter MA. The relationship of vascular changes to metabolic factors in diabetes mellitus and their role in the development of peripheral nerve complications. Diabetes Metab Rev 10: 189–224, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Cardillo C, Campia U, Iantorno M, Panza JA. Enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension 43: 36–40, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet 340: 1111–1115, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev 22: 423–436, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Chiu JD, Kolka C, Richey JM, Harrison LN, Zuniga E, Kirkman E, Ellmerer M, Bergman RN. Intralipid dramatically reduces access of insulin to its target tissue (Abstract). Diabetologia 50, Suppl 1: S253, 2007. [Google Scholar]
- 23.Chiu JD, Richey JM, Harrison LN, Zuniga E, Kolka CM, Kirkman E, Ellmerer M, Bergman RN. Direct administration of insulin into skeletal muscle reveals that the transport of insulin across the capillary endothelium limits the time course of insulin to activate glucose disposal. Diabetes 57: 828–835, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Clark MG, Barrett EJ, Richards SM, Clark ADH, Rattigan S. Microvascular involvement in insulin resistance of skeletal muscle. Proceedings of the 7th World Congress for Microcirculation 1: 31–37, 2001. [Google Scholar]
- 25.Clark MG, Clark AD, Rattigan S. Failure of laser Doppler signal to correlate with total flow in muscle: is this a question of vessel architecture? Microvasc Res 60: 294–301, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Clark MG, Colquhoun EQ, Rattigan S, Dora KA, Eldershaw TP, Hall JL, Ye J. Vascular and endocrine control of muscle metabolism. Am J Physiol Endocrinol Metab 268: E797–E812, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Clark MG, Rattigan S, Barrett EJ, Vincent MA. Point:Counterpoint: There is/is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol 104: 889–891, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284: E241–E258, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Clerk LH, Rattigan S, Clark MG. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51: 1138–1145, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Coggins M, Lindner J, Rattigan S, Jahn L, Fasy E, Kaul S, Barrett E. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 50: 2682–2690, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Colacino JM, Grubb B, Jobsis FF. Infra-red technique for cerebral blood flow: comparison with 133Xenon clearance. Neurol Res 3: 17–31, 1981. [DOI] [PubMed] [Google Scholar]
- 33.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab 282: E714–E720, 2002. [DOI] [PubMed] [Google Scholar]
- 34.De Filippis E, Cusi K, Ocampo G, Berria R, Buck S, Consoli A, Mandarino LJ. Exercise-induced improvement in vasodilatory function accompanies increased insulin sensitivity in obesity and type 2 diabetes mellitus. J Clin Endocrinol Metab 91: 4903–4910, 2006. [DOI] [PubMed] [Google Scholar]
- 35.de Jongh RT, Clark AD, IJzerman RG, Serné EH, de Vries G, Stehouwer CD. Physiological hyperinsulinaemia increases intramuscular microvascular reactive hyperaemia and vasomotion in healthy volunteers. Diabetologia 47: 978–986, 2004. [DOI] [PubMed] [Google Scholar]
- 36.de Jongh RT, Ijzerman RG, Serné EH, Voordouw JJ, Yudkin JS, de Waal HA, Stehouwer CD, van Weissenbruch MM. Visceral and truncal subcutaneous adipose tissue are associated with impaired capillary recruitment in healthy individuals. J Clin Endocrinol Metab 91: 5100–5106, 2006. [DOI] [PubMed] [Google Scholar]
- 37.de Jongh RT, Serné EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 53: 2873–2882, 2004. [DOI] [PubMed] [Google Scholar]
- 38.de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation 109: 2529–2535, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Defronzo RA, Ferrannini E, Sato Y, Felig P, Wahren J. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest 68: 1468–1474, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Edmonds ME, Roberts VC, Watkins PJ. Blood flow in the diabetic neuropathic foot. Diabetologia 22: 9–15, 1982. [DOI] [PubMed] [Google Scholar]
- 42.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56: 2958–2963, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Ellmerer M, Hamilton-Wessler M, Kim SP, Huecking K, Kirkman E, Chiu J, Richey J, Bergman RN. Reduced access to insulin-sensitive tissues in dogs with obesity secondary to increased fat intake. Diabetes 55: 1769–1775, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Ellmerer M, Kim SP, Hamilton-Wessler M, Hücking K, Kirkman E, Bergman RN. Physiological hyperinsulinemia in dogs augments access of macromolecules to insulin-sensitive tissues. Diabetes 53: 2741–2747, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Eringa EC, Bakker W, Smulders YM, Serné EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation 14: 389–402, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56: 464–471, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Eringa EC, Stehouwer CD, Walburg K, Clark AD, van Nieuw Amerongen GP, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-alpha dependence on c-Jun N-terminal kinase. Arterioscler Thromb Vasc Biol 26: 274–280, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Estacio RO, Regensteiner JG, Wolfel EE, Jeffers B, Dickenson M, Schrier RW. The association between diabetic complications and exercise capacity in NIDDM patients. Diabetes Care 21: 291–295, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Fagerberg SE Diabetic neuropathy: a clinical and histological study on the significance of vascular affections. Acta Med Scand Suppl 345: 1–97, 1959. [PubMed] [Google Scholar]
- 51.Fagrell B, Jörneskog G, Intaglietta M. Disturbed microvascular reactivity and shunting - a major cause for diabetic complications. Vasc Med 4: 125–127, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Ferri C, Carlomagno A, Coassin S, Baldoncini R, Faldetta MR, Laurenti O, Properzi G, Santucci A, De Mattia G. Circulating endothelin-1 levels increase during euglycemic hyperinsulinemic clamp in lean NIDDM men. Diabetes Care 18: 226–233, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Franklin VL, Khan F, Kennedy G, Belch JJ, Greene SA. Intensive insulin therapy improves endothelial function and microvascular reactivity in young people with type 1 diabetes. Diabetologia 51: 353–360, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Friedlander AL, Casazza GA, Horning MA, Huie MJ, Brooks GA. Training-induced alterations of glucose flux in men. J Appl Physiol 82: 1360–1369, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Friedman JJ Total, non-nutritional, and nutritional blood volume in isolated dog hindlimb. Am J Physiol 210: 151–156, 1966. [DOI] [PubMed] [Google Scholar]
- 56.Frisbee JC Impaired skeletal muscle perfusion in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 285: R1124–R1134, 2003. [DOI] [PubMed] [Google Scholar]
- 57.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr 81: 903–910, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garlick DG, Renkin EM. Transport of large molecules from plasma to interstitial fluid and lymph in dogs. Am J Physiol 219: 1595–1605, 1970. [DOI] [PubMed] [Google Scholar]
- 59.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of AMP kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol 581: 1163–1171, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gudbjornsdottir S, Sjostrand M, Strindberg L, Lonnroth P. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J Clin Endocrinol Metab 90: 1078–1082, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P. Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab 88: 4559–4564, 2003. [DOI] [PubMed] [Google Scholar]
- 62.Guiducci L, Gronroos T, Jarvisalo MJ, Kiss J, Viljanen A, Naum AG, Viljanen T, Savunen T, Knuuti J, Ferrannini E, Salvadori PA, Nuutila P, Iozzo P. Biodistribution of the fatty acid analogue 18F-FTHA: plasma and tissue partitioning between lipid pools during fasting and hyperinsulinemia. J Nucl Med 48: 455–462, 2007. [PubMed] [Google Scholar]
- 63.Halseth AE, Bracy DP, Wasserman DH. Limitations to basal and insulin-stimulated skeletal muscle glucose uptake in the high-fat-fed rat. Am J Physiol Endocrinol Metab 279: E1064–E1071, 2000. [DOI] [PubMed] [Google Scholar]
- 64.Halseth AE, Bracy DP, Wasserman DH. Functional limitations to glucose uptake in muscles comprised of different fiber types. Am J Physiol Endocrinol Metab 280: E994–E999, 2001. [DOI] [PubMed] [Google Scholar]
- 65.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol 27: 2650–2656, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrison DK, Birkenhake S, Knauf SK, Kessler M. Local oxygen supply and blood flow regulation in contracting muscle in dogs and rabbits. J Physiol 422: 227–243, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hasdai D, Rizza RA, Holmes DR Jr, Richardson DM, Cohen P, Lerman A. Insulin and insulin-like growth factor-I cause coronary vasorelaxation in vitro. Hypertension 32: 228–234, 1998. [DOI] [PubMed] [Google Scholar]
- 68.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51: 1477–1484, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Henthorn TK, Avram MJ, Frederiksen MC, Atkinson AJ Jr. Heterogeneity of interstitial fluid space demonstrated by simultaneous kinetic analysis of the distribution and elimination of inulin and gallamine. J Pharmacol Exp Ther 222: 389–394, 1982. [PubMed] [Google Scholar]
- 70.Hocking DC, Titus PA, Sumagin R, Sarelius IH. Extracellular matrix fibronectin mechanically couples skeletal muscle contraction with local vasodilation. Circ Res 102: 372–379, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Hohberg C, Forst T, Larbig M, Safinowski M, Diessel S, Hehenwarter S, Weber MM, Schöndorf T, Pfützner A. Effect of insulin glulisine on microvascular blood flow and endothelial function in the postprandial state. Diabetes Care 31: 1021–1025, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Honig CR, Odoroff CL, Frierson JL. Active and passive capillary control in red muscle at rest and in exercise. Am J Physiol Heart Circ Physiol 243: H196–H206, 1982. [DOI] [PubMed] [Google Scholar]
- 73.Hoppeler H, Klossner S, Fluck M. Gene expression in working skeletal muscle. Adv Exp Med Biol 618: 245–254, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2200, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Jaap AJ, Shore AC, Stockman AJ, Tooke JE. Skin capillary density in subjects with impaired glucose tolerance and patients with type 2 diabetes. Diabet Med 13: 160–164, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Jansson PA Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med 262: 173–183, 2007. [DOI] [PubMed] [Google Scholar]
- 77.Jansson PA, Fowelin JP, von Schenck HP, Smith UP, Lonnroth PN. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Importance of the endothelial barrier for insulin. Diabetes 42: 1469–1473, 1993. [DOI] [PubMed] [Google Scholar]
- 78.Johnson PC The myogenic response. News Physiol Sci 6: 41–42, 1991. [Google Scholar]
- 79.Jörneskog G, Brismar K, Fagrell B. Skin capillary circulation severely impaired in toes of patients with IDDM, with and without late diabetic complications. Diabetologia 38: 474–480, 1995. [DOI] [PubMed] [Google Scholar]
- 80.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 106: 473–481, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang J, Kelley DE, Robertson RJ, Goss FL, Suminski RR, Utter AC, Dasilva SG. Substrate utilization and glucose turnover during exercise of varying intensities in individuals with NIDDM. Med Sci Sports Exerc 31: 82–89, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Karlsson HK, Zierath JR. Insulin signaling and glucose transport in insulin resistant human skeletal muscle. Cell Biochem Biophys 48: 103–113, 2007. [DOI] [PubMed] [Google Scholar]
- 83.Karlsson HK, Zierath JR, Kane S, Krook A, Lienhard GE, Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes 54: 1692–1697, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Kastrup J, Bulow J, Lassen NA. Vasomotion in human skin before and after local heating recorded with laser Doppler flowmetry. A method for induction of vasomotion. Int J Microcirc Clin Exp 8: 205–215, 1989. [PubMed] [Google Scholar]
- 85.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol 25: 989–994, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Kim JA, Koh KK, Quon MJ. The union of vascular and metabolic actions of insulin in sickness and in health. Arterioscler Thromb Vasc Biol 25: 889–891, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Klitzman B, Duling BR. Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol Heart Circ Physiol 237: H481–H490, 1979. [DOI] [PubMed] [Google Scholar]
- 88.Kohner EM The retinal blood flow in diabetes. Diabete Metab 19: 401–404, 1993. [PubMed] [Google Scholar]
- 89.Kraus WE, Levine BD. Exercise training for diabetes: the “strength” of the evidence. Ann Intern Med 147: 423–424, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Krogh A August Krogh - Nobel Lecture: a contribution to the physiology of the capillaries. In: Nobel Lectures, Physiology or Medicine, 1901–1921. Amsterdam, The Netherlands: Elsevier Publishing, 1967, p. 1–8.
- 91.Krook A, Bjornholm M, Galuska D, Jiang XJ, Fahlman R, Myers MG Jr, Wallberg-Henriksson H, Zierath JR. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49: 284–292, 2000. [DOI] [PubMed] [Google Scholar]
- 92.L'Esperance VS, Turzyniecka M, Krentz AJ, Byrne CD, Clough GF. Decreased microvascular functional vasodilatory reserve and features of the metabolic syndrome (Abstract). Diabet Med 25, Suppl 1: 42, 2008. [Google Scholar]
- 93.Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, Scherrer U, Vollenweider P. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes 56: 2690–2696, 2007. [DOI] [PubMed] [Google Scholar]
- 94.LeBrasseur NK, Ruderman NB. Why might thiazolidinediones increase exercise capacity in patients with type 2 diabetes? Diabetes Care 28: 2975–2977, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Leng Y, Karlsson HK, Zierath JR. Insulin signaling defects in type 2 diabetes. Rev Endocr Metab Disord 5: 111–117, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 294: H1840–H1850, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lindbom L Microvascular blood flow distribution in skeletal muscle. Acta Physiol Scand Suppl 525: 1–40, 1983. [PubMed] [Google Scholar]
- 98.Luiken JJ, Arumugam Y, Bell RC, Calles-Escandon J, Tandon NN, Glatz JF, Bonen A. Changes in fatty acid transport and transporters are related to the severity of insulin deficiency. Am J Physiol Endocrinol Metab 283: E612–E621, 2002. [DOI] [PubMed] [Google Scholar]
- 99.Mahajan H, Richards SM, Rattigan S, Clark MG. Local methacholine but not bradykinin potentiates insulin-mediated glucose uptake in muscle in vivo by augmenting capillary recruitment. Diabetologia 47: 2226–2234, 2004. [DOI] [PubMed] [Google Scholar]
- 100.Maki MT, Haaparanta M, Nuutila P, Oikonen V, Luotolahti M, Eskola O, Knuuti JM. Free fatty acid uptake in the myocardium and skeletal muscle using fluorine-18-fluoro-6-thia-heptadecanoic acid. J Nucl Med 39: 1320–1327, 1998. [PubMed] [Google Scholar]
- 101.Mazzoni MC, Borgstrom P, Arfors KE, Intaglietta M. The efficacy of iso- and hyperosmotic fluids as volume expanders in fixed-volume and uncontrolled hemorrhage. Ann Emerg Med 19: 350–358, 1990. [DOI] [PubMed] [Google Scholar]
- 102.Meyer MM, Levin K, Grimmsmann T, Beck-Nielsen H, Klein HH. Insulin signalling in skeletal muscle of subjects with or without Type II-diabetes and first degree relatives of patients with the disease. Diabetologia 45: 813–822, 2002. [DOI] [PubMed] [Google Scholar]
- 103.Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 44: 947–953, 1995. [DOI] [PubMed] [Google Scholar]
- 104.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem 278: 31629–31639, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Mulder AH, van Dijk AP, Smits P, Tack CJ. Real-time contrast imaging: a new method to monitor capillary recruitment in human forearm skeletal muscle. Microcirculation 15: 203–213, 2008. [DOI] [PubMed] [Google Scholar]
- 106.Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, Hortin GL, Quon MJ. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes 55: 3142–3150, 2006. [DOI] [PubMed] [Google Scholar]
- 107.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. [DOI] [PubMed] [Google Scholar]
- 108.Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Murdolo G, Sjöstrand M, Strindberg L, Gudbjörnsdóttir S, Lind L, Lönnroth P, Jansson PA. Effects of intrabrachial methacholine infusion on muscle capillary recruitment and forearm glucose uptake during physiologic hyperinsulinaemia in obese, insulin-resistant individuals. J Clin Endocrinol Metab 93: 2764–2773, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Natali A, Baldeweg S, Toschi E, Capaldo B, Barbaro D, Gastaldelli A, Yudkin JS, Ferrannini E. Vascular effects of improving metabolic control with metformin or rosiglitazone in type 2 diabetes. Diabetes Care 27: 1349–1357, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Natali A, Bonadonna R, Santoro D, Galvan AQ, Baldi S, Frascerra S, Palombo C, Ghione S, Ferrannini E. Insulin resistance and vasodilation in essential hypertension. Studies with adenosine. J Clin Invest 94: 1570–1576, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Newman JMB, Di Maria CA, Rattigan S, Clark MG. Nutritive blood flow affects microdialysis O/I ratio for [14C]ethanol and 3H2O in perfused rat hindlimb. Am J Physiol Heart Circ Physiol 281: H2731–H2737, 2001. [DOI] [PubMed] [Google Scholar]
- 113.Newman JM, Ross RM, Richards SM, Clark MG, Rattigan S. Insulin and contraction increase nutritive blood flow in rat muscle in vivo determined by microdialysis of l-[14C]glucose. J Physiol 585: 217–229, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Newman JMB, Ross RM, Richards SM, Clark MG, Rattigan S. Acute insulin resistance results in altered vasomotion in rat muscle (Abstract). Proc Eur Soc Microcirc. In press.
- 115.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv 3: 79–89, 51, 2003. [DOI] [PubMed] [Google Scholar]
- 116.Nuutila P, Raitakari M, Laine H, Kirvelä O, Takala T, Utriainen T, Mäkimattila S, Pitkänen OP, Ruotsalainen U, Iida H, Knuuti J, Yki-Järvinen H. Role of blood flow in regulating insulin-stimulated glucose uptake in humans. Studies using bradykinin, [15O]water, and [18F]fluoro-deoxy-glucose and positron emission tomography. J Clin Invest 97: 1741–1747, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Patel N, Huang C, Klip A. Cellular location of insulin-triggered signals and implications for glucose uptake. Pflugers Arch 451: 499–510, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med 335: 1357–1362, 1996. [DOI] [PubMed] [Google Scholar]
- 119.Petersen KF, Dufour S, Shulman GI. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med 2: e233, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Petersen KF, Shulman GI. New insights into the pathogenesis of insulin resistance in humans using magnetic resonance spectroscopy. Obesity (Silver Spring) 14, Suppl 1: 34S–40S, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Phillips SM Resistance exercise: good for more than just Grandma and Grandpa's muscles. Appl Physiol Nutr Metab 32: 1198–1205, 2007. [DOI] [PubMed] [Google Scholar]
- 122.Ping P, Johnson PC. Autoregulatory responses in cat skeletal muscle during enhanced vascular tone (Abstract). FASEB J 3: A270, 1989. [Google Scholar]
- 123.Poole DC, Brown MD, Hudlicka O. Counterpoint: There is not capillary recruitment in active skeletal muscle during exercise. J Appl Physiol 104: 891–893, 2008. [DOI] [PubMed] [Google Scholar]
- 124.Poulin RA, Steil GM, Moore DM, Ader M, Bergman RN. Dynamics of glucose production and uptake are more closely related to insulin in hindlimb lymph than in thoracic duct lymph. Diabetes 43: 180–190, 1994. [DOI] [PubMed] [Google Scholar]
- 125.Rabol R, Boushel R, Dela F. Mitochondrial oxidative function and type 2 diabetes. Appl Physiol Nutr Metab 31: 675–683, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56, 2007. [DOI] [PubMed] [Google Scholar]
- 127.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes 46: 1381–1388, 1997. [DOI] [PubMed] [Google Scholar]
- 128.Rattigan S, Clark MG, Barrett EJ. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes 48: 564–569, 1999. [DOI] [PubMed] [Google Scholar]
- 129.Rattigan S, Wallis MG, Youd JM, Clark MG. Exercise training improves insulin-mediated capillary recruitment in association with glucose uptake in rat hind limb. Diabetes 50: 2659–2665, 2001. [DOI] [PubMed] [Google Scholar]
- 130.Rattigan S, Zhang L, Mahajan H, Kolka CM, Richards SM, Clark MG. Factors influencing the hemodynamic and metabolic effects of insulin in muscle. Curr Diabetes Rev 2: 61–70, 2006. [DOI] [PubMed] [Google Scholar]
- 131.Reaven GM Role of insulin resistance in human disease. Diabetes 37: 1595–1607, 1988. [DOI] [PubMed] [Google Scholar]
- 132.Renaudin C, Michoud E, Rapin JR, Lagarde M, Wiernsperger N. Hyperglycaemia modifies the reaction of microvessels to insulin in rat skeletal muscle. Diabetologia 41: 26–33, 1998. [DOI] [PubMed] [Google Scholar]
- 133.Riva CE, Petrig B. Blue field entoptic phenomenon and blood velocity in the retinal capillaries. J Opt Soc Am 70: 1234–1238, 1980. [DOI] [PubMed] [Google Scholar]
- 134.Roberts JL, Newman JM, Warner R, Rattigan S, Clark MG. Axially symmetric semi-infinite domain models of microdialysis and their application to the determination of nutritive flow in rat muscle. J Physiol 563: 213–228, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ross RM, Downey K, Newman JM, Richards SM, Clark MG, Rattigan S. Contrast-enhanced ultrasound measurement of microvascular perfusion relevant to nutrient and hormone delivery in skeletal muscle: a model study in vitro. Microvasc Res 75: 323–329, 2008. [DOI] [PubMed] [Google Scholar]
- 136.Ross RM, Wadley GD, Clark MG, Rattigan S, McConell GK. Local nitric oxide synthase inhibition reduces skeletal muscle glucose uptake but not capillary blood flow during in situ muscle contraction in rats. Diabetes 56: 2885–2892, 2007. [DOI] [PubMed] [Google Scholar]
- 137.Rossi M, Bertuglia S, Varanini M, Giusti A, Santoro G, Carpi A. Generalised wavelet analysis of cutaneous flowmotion during post-occlusive reactive hyperaemia in patients with peripheral arterial obstructive disease. Biomed Pharmacother 59: 233–239, 2005. [DOI] [PubMed] [Google Scholar]
- 138.Rossi M, Maurizio S, Carpi A. Skin blood flowmotion response to insulin iontophoresis in normal subjects. Microvasc Res 70: 17–22, 2005. [DOI] [PubMed] [Google Scholar]
- 139.Rossi M, Santoro G, Ricco R, Pentimone F, Carpi A. Effect of chronic aerobic exercise on cutaneous microcirculatory flow response to insulin iontophoresis and to ischemia in elderly males. Int J Sports Med 26: 558–562, 2005. [DOI] [PubMed] [Google Scholar]
- 140.Rothman DL, Magnusson I, Cline G, Gerard D, Kahn CR, Shulman RG, Shulman GI. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA 92: 983–987, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rucker M, Strobel O, Vollmar B, Roesken F, Menger MD. Vasomotion in critically perfused muscle protects adjacent tissues from capillary perfusion failure. Am J Physiol Heart Circ Physiol 279: H550–H558, 2000. [DOI] [PubMed] [Google Scholar]
- 142.Russo I, Del Mese P, Doronzo G, Mattiello L, Viretto M, Bosia A, Anfossi G, Trovati M. Resistance to the nitric oxide/cyclic guanosine 5′-monophosphate/protein kinase G pathway in vascular smooth muscle cells from the obese Zucker rat, a classical animal model of insulin resistance: role of oxidative stress. Endocrinology 149: 1480–1489, 2008. [DOI] [PubMed] [Google Scholar]
- 143.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87: 507–520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest 94: 2511–2515, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schmidt JA, Intaglietta M, Borgstrom P. Periodic hemodynamics in skeletal muscle during local arterial pressure reduction. J Appl Physiol 73: 1077–1083, 1992. [DOI] [PubMed] [Google Scholar]
- 146.Schrauwen-Hinderling VB, Roden M, Kooi ME, Hesselink MK, Schrauwen P. Muscular mitochondrial dysfunction and type 2 diabetes mellitus. Curr Opin Clin Nutr Metab Care 10: 698–703, 2007. [DOI] [PubMed] [Google Scholar]
- 147.Schroeder CAJ, Chen YL, Messina EJ. Inhibition of NO synthesis or endothelium removal reveals a vasoconstrictor effect of insulin on isolated arterioles. Am J Physiol Heart Circ Physiol 276: H815–H820, 1999. [DOI] [PubMed] [Google Scholar]
- 148.Schulman IH, Zhou MS, Jaimes EA, Raij L. Dissociation between metabolic and vascular insulin resistance in aging. Am J Physiol Heart Circ Physiol 293: H853–H859, 2007. [DOI] [PubMed] [Google Scholar]
- 149.Scognamiglio R, Negut C, de Kreutzenberg SV, Tiengo A, Avogaro A. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation 112: 179–184, 2005. [DOI] [PubMed] [Google Scholar]
- 150.Scognamiglio R, Negut C, de Kreutzenberg SV, Tiengo A, Avogaro A. Effects of different insulin regimes on postprandial myocardial perfusion defects in type 2 diabetic patients. Diabetes Care 29: 95–100, 2006. [PubMed] [Google Scholar]
- 151.Serné EH, de Jongh RT, Eringa EC, RGIJzerman, Stehouwer CD. Microvascular dysfunction: a potential pathophysiological role in the metabolic syndrome. Hypertension 50: 204–211, 2007. [DOI] [PubMed] [Google Scholar]
- 152.Serné EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38: 238–242, 2001. [DOI] [PubMed] [Google Scholar]
- 153.Serné EH, Gans RO, ter Maaten JC, ter Wee PM, Donker AJ, Stehouwer CD. Capillary recruitment is impaired in essential hypertension and relates to insulin's metabolic and vascular actions. Cardiovasc Res 49: 161–168, 2001. [DOI] [PubMed] [Google Scholar]
- 154.Serné EH, IJzerman RG, Gans RO, Nijveldt R, De Vries G, Evertz R, Donker AJ, Stehouwer CD. Direct evidence for insulin-induced capillary recruitment in skin of healthy subjects during physiological hyperinsulinemia. Diabetes 51: 1515–1522, 2002. [DOI] [PubMed] [Google Scholar]
- 155.Serné EH, Stehouwer CD, ter Maaten JC, ter Wee PM, Rauwerda JA, Donker AJ, Gans RO. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation 99: 896–902, 1999. [DOI] [PubMed] [Google Scholar]
- 156.Setola E, Losa M, Lanzi R, Lucotti P, Monti LD, Castrignano T, Galluccio E, Giovanelli M, Piatti P. Increased insulin-stimulated endothelin-1 release is a distinct vascular phenotype distinguishing Cushing's disease from metabolic syndrome. Clin Endocrinol (Oxf) 66: 586–592, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 49: 684–687, 2000. [DOI] [PubMed] [Google Scholar]
- 158.Shearer J, Coenen KR, Pencek RR, Swift LL, Wasserman DH, Rottman JN. Long Chain Fatty Acid Uptake In Vivo: Comparison of [(125)I]-BMIPP and [(3)H]-Bromopalmitate. Lipids. In press. [DOI] [PMC free article] [PubMed]
- 159.Sherwin RS, Kramer KJ, Tobin JD, Insel PA, Liljenquist JE, Berman M, Andres R. A model of the kinetics of insulin in man. J Clin Invest 53: 1481–1492, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud'homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P, Jennings A, Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med 147: 357–369, 2007. [DOI] [PubMed] [Google Scholar]
- 161.Sjöstrand M, Gudbjörnsdottir S, Holmäng A, Lönn L, Strindberg L, Lönnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 51: 2742–2748, 2002. [DOI] [PubMed] [Google Scholar]
- 162.Sjöstrand M, Gudbjörnsdottir S, Lind L, Strindberg L, Lönnroth P. Vasodilatation does not increase capillary permeability for glucose or transport of insulin to muscle tissue in obese subjects. Diabetologia 48: A230–A231, 2005. [Google Scholar]
- 163.Sjöstrand M, Holmäng A, Lönnroth P. Measurement of interstitial insulin in human muscle. Am J Physiol Endocrinol Metab 276: E151–E154, 1999. [DOI] [PubMed] [Google Scholar]
- 164.Stallknecht B, Donsmark M, Enevoldsen LH, Fluckey JD, Galbo H. Estimation of rat muscle blood flow by microdialysis probes perfused with ethanol, [14C]ethanol, and 3H2O. J Appl Physiol 86: 1054–1061, 1999. [DOI] [PubMed] [Google Scholar]
- 165.Stefanovska A, Bracic M, Kvernmo HD. Wavelet analysis of oscillations in the peripheral blood circulation measured by laser Doppler technique. IEEE Trans Biomed Eng 46: 1230–1239, 1999. [DOI] [PubMed] [Google Scholar]
- 166.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 97: 1497–1503, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Steinberg HO, Baron AD. Insulin-mediated vasodilation: why one's physiology could be the other's pharmacology. Diabetologia 42: 493–495, 1999. [DOI] [PubMed] [Google Scholar]
- 168.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest 97: 2601–2610, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stewart KJ Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA 288: 1622–1631, 2002. [DOI] [PubMed] [Google Scholar]
- 170.Turcotte LP, Swenberger JR, Zavitz TM, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes 50: 1389–1396, 2001. [DOI] [PubMed] [Google Scholar]
- 171.Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 294: H1685–H1692, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van Loon LJ, Jeukendrup AE, Saris WH, Wagenmakers AJ. Effect of training status on fuel selection during submaximal exercise with glucose ingestion. J Appl Physiol 87: 1413–1420, 1999. [DOI] [PubMed] [Google Scholar]
- 173.Verma S, Yao L, Stewart DJ, Dumont AS, Anderson TJ, McNeill JH. Endothelin antagonism uncovers insulin-mediated vasorelaxation in vitro and in vivo. Hypertension 37: 328–333, 2001. [DOI] [PubMed] [Google Scholar]
- 174.Vicent D, Ilany J, Kondo T, Naruse K, Fisher SJ, Kisanuki YY, Bursell S, Yanagisawa M, King GL, Kahn CR. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J Clin Invest 111: 1373–1380, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vincent MA, Barrett EJ. Insulin-induced capillary recruitment precedes changes in skeletal muscle glucose uptake (Abstract). Diabetes 51, Suppl 2: A31, 2002. [Google Scholar]
- 176.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285: E123–E129, 2003. [DOI] [PubMed] [Google Scholar]
- 177.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53: 1418–1423, 2004. [DOI] [PubMed] [Google Scholar]
- 178.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290: E1191–E1197, 2006. [DOI] [PubMed] [Google Scholar]
- 179.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 180.Wagenmakers AJ Insulin resistance in the offspring of parents with type 2 diabetes. PLoS Med 2: e289, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wallis MG, Smith ME, Kolka CM, Zhang L, Richards SM, Rattigan S, Clark MG. Acute glucosamine-induced insulin resistance in muscle in vivo associated with impaired capillary recruitment. Diabetologia 48: 2131–2139, 2005. [DOI] [PubMed] [Google Scholar]
- 182.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51: 3492–3498, 2002. [DOI] [PubMed] [Google Scholar]
- 183.Weinhandl H, Pachler C, Mader JK, Ikeoka D, Mautner A, Falk A, Suppan M, Pieber TR, Ellmerer M. Physiological hyperinsulinemia has no detectable effect on access of macromolecules to insulin-sensitive tissues in healthy humans. Diabetes 56: 2213–2217, 2007. [DOI] [PubMed] [Google Scholar]
- 184.Wheatley CM, Rattigan S, Richards SM, Barrett EJ, Clark MG. Skeletal muscle contraction stimulates capillary recruitment and glucose uptake in insulin-resistant obese Zucker rats. Am J Physiol Endocrinol Metab 287: E804–E809, 2004. [DOI] [PubMed] [Google Scholar]
- 185.Wiernsperger N, Nivoit P, De Aguiar LG, Bouskela E. Microcirculation and the metabolic syndrome. Microcirculation 14: 403–438, 2007. [DOI] [PubMed] [Google Scholar]
- 186.Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol 52: 631–646, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wojtaszewski JF, Richter EA. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem 42: 31–46, 2006. [DOI] [PubMed] [Google Scholar]
- 188.Woodman RJ, Watts GF, Playford DA, Best JD, Chan DC. Oxidized LDL and small LDL particle size are independently predictive of a selective defect in microcirculatory endothelial function in type 2 diabetes. Diabetes Obes Metab 7: 612–617, 2005. [DOI] [PubMed] [Google Scholar]
- 189.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84: 1620–1628, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, Gallagher D. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 31: 1400–1405, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yki-Jarvinen H, Utriainen T. Insulin-induced vasodilatation: physiology or pharmacology? Diabetologia 41: 369–379, 1998. [DOI] [PubMed] [Google Scholar]
- 192.Youd JM, Rattigan S, Clark MG. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNFa. Diabetes 49: 1904–1909, 2000. [DOI] [PubMed] [Google Scholar]
- 193.Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, Wu MH. Microvascular permeability in diabetes and insulin resistance. Microcirculation 14: 363–373, 2007. [DOI] [PubMed] [Google Scholar]
- 194.Yudkin JS Insulin resistance and the metabolic syndrome—or the pitfalls of epidemiology. Diabetologia 50: 1576–1586, 2007. [DOI] [PubMed] [Google Scholar]
- 195.Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 365: 1817–1820, 2005. [DOI] [PubMed] [Google Scholar]
- 196.Zeng GY, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes 53: 447–453, 2004. [DOI] [PubMed] [Google Scholar]