Abstract
In health insulin is secreted in discrete insulin secretory bursts from pancreatic β-cells, collectively referred to as β-cell mass. We sought to establish the relationship between β-cell mass, insulin secretory-burst mass, and hepatic insulin clearance over a range of age-related insulin sensitivity in adult rats. To address this, we used a novel rat model with chronically implanted portal vein catheters in which we recently established the parameters to permit deconvolution of portal vein insulin concentration profiles to measure insulin secretion and resolve its pulsatile components. In the present study, we examined total and pulsatile insulin secretion, insulin sensitivity, hepatic insulin clearance, and β-cell mass in 35 rats aged 2–12 mo. With aging, insulin sensitivity declined, but euglycemia was sustained by an adaptive increase in fasting and glucose-stimulated insulin secretion through the mechanism of a selective augmentation of insulin pulse mass. The latter was attributable to a closely related increase in β-cell mass (r = 0.8, P < 0.001). Hepatic insulin clearance increased with increasing portal vein insulin pulse amplitude, damping the delivery of insulin in the systemic circulation. In consequence, the curvilinear relationship previously reported between insulin secretion and insulin sensitivity was extended to both insulin pulse mass and β-cell mass vs. insulin sensitivity. These data support a central role of adaptive changes in β-cell mass to permit appropriate insulin secretion in the setting of decreasing insulin sensitivity in the aging animal. They emphasize the cooperative role of pancreatic β-cells and the liver in regulating the secretion and delivery of insulin to the systemic circulation.
Keywords: pulse mass, deconvolution analysis, insulin sensitivity, aging, rat
in health, the blood glucose concentration is closely regulated. Pancreatic β-cells play a central role by secreting insulin in a glucose concentration-dependent manner. Both regulation of insulin secretion and the delivery of insulin to target tissues are complex. Insulin is secreted in discrete secretory bursts at ∼4-min intervals with regulation accomplished through modulation of burst size (56). As a result, hepatocytes (directly exposed to portal venous blood in hepatic sinusoids) are exposed to an insulin concentration wavefront with oscillations of ∼200–500 pmol/l in the fasting state and increasing to 1,000–5,000 pmol/l after meal ingestion (50, 56). Regulation of insulin delivery to extrahepatic insulin tissues depends on both the rate of insulin secretion and the extent of hepatic insulin clearance (40). In addition to minute-by-minute regulation of insulin secretion, pancreatic β-cells also adapt over a longer period to overall demand. For example, in response to an increased insulin demand in pregnancy, there is an adaptive increase in β-cell number per given pancreatic weight (often collectively termed β-cell mass) (28).
In type 2 diabetes, there is an ∼65% deficit in β-cell mass (8) and impaired insulin secretion (49, 55, 58) in the setting of insulin resistance (15). The mechanism subserving impaired insulin secretion is reduced amplitude of insulin secretory bursts, particularly in response to hyperglycemia (32). However, impaired insulin secretion is partially offset by decreased hepatic clearance (7, 38, 54). These findings highlight the important relationships between insulin secretion, insulin clearance, and β-cell mass. While elegant studies have examined the relationship between insulin secretion and insulin sensitivity (6, 27), none has established a relationship between β-cell mass and insulin sensitivity. Moreover, most clinical analyses have utilized systemic measurements of inadequate sampling intensity to permit evaluation of pulsatile insulin (27). Evaluation of insulin secretion by use of insulin concentrations measured in the systemic circulation, while technically feasible in humans, presents significant limitations. Because hepatic insulin clearance is related to the magnitude of insulin pulses in the portal vein (40), and approximately eighty percent of insulin is cleared in the first pass through the liver (40), systemic insulin measurements predictably underestimate decreased insulin secretion. Estimates of β-cell mass in humans have been confined to autopsy studies, precluding analysis of the relationships among β-cell mass, insulin secretion, and insulin action (8, 30).
Our goal was to examine the relationship among β-cell mass, insulin secretion (to include direct sampling of the portal vein for pulsatile insulin secretion), hepatic insulin clearance, and insulin sensitivity in the same animal. Moreover, we sought to establish how these relationships adapt to increasing insulin demand (decreasing insulin sensitivity) during normal aging. We hypothesized that the primary adaptive response to an age-related reduction in insulin sensitivity is an adaptive increase in β-cell mass. We further postulated that an increase in β-cell mass could permit appropriate glucose-stimulated insulin secretion in response to hyperglycemia through the mechanism of increased insulin burst mass presented to the liver. Finally, we hypothesized that the extent of increased insulin secretion in the portal vein is attenuated by a related increase in hepatic insulin clearance.
RESEARCH DESIGN AND METHODS
Study Design
We applied newly developed methods of direct measurement of pulsatile insulin secretion in the rat portal vein (37) along with those previously available to quantify the fasting and glucose-stimulated insulin secretion, hepatic insulin clearance, and insulin sensitivity as well as β-cell mass in Sprague-Dawley rats at age 2, 7, and 12 mo. With this ensemble of data, we were thus able to examine the adaptive changes of β-cell mass, insulin secretion, and hepatic insulin clearance in health to age-related declining insulin sensitivity.
Animal studies were approved by The University of California Los Angeles Institutional Animal Care and Use Committee. Rats were bred and housed individually at the University of California Los Angeles animal housing facility. Rats were maintained in a standard 12:12-h light-dark cycle and fed ad libitum regular rodent chow.
Surgical Implantation of Catheters
The surgical implantation of the portal vein and arterial sampling catheters as well as jugular vein infusion catheters has been described in detail (37). In brief, rats were anesthetized by isoflourane (2.5%) inhalation until effect. Under aseptic conditions, the portal vein was dissected free of surrounding connective tissue, and the portal vein sampling catheter was placed in the hepatic portal vein at the bifurcation of the portal vein in the liver. Additionally, indwelling catheters were inserted in the right internal jugular vein and left carotid artery. All catheters were exteriorized to the back of the neck and encased in the infusion harness (Instech). Immediately postoperatively, and for the next 3 days, all animals received preventative antibiotic treatment (200 mg sulfamethoxazole in drinking water; Hi-Tech Pharmaceutical, Amityville, NY). The rats maintained their preoperative body weight and had normal food intake and a normal hematocrit (42 ± 2%) at the time of subsequent studies.
Fasting Insulin Secretion and Clearance
To assess the impact of aging on fasting insulin secretion, male Sprague-Dawley rats ages 2 (n = 7), 7 (n = 5), and 12 (n = 5) mo old were studied in the conscious unrestrained state 12 h after food withdrawal the preceding day and 5–7 days after surgery as described in detail (37). In short, rats were placed in a cage outfitted with a counter-weighted swivel mount (Instech), and, following a 30-min equilibration period (−30 to 0 min), portal vein blood (∼80 μl) was sampled every minute for 35 min, and arterial samples (∼100 μl) were sampled every 10 min. Blood samples were collected in prechilled microcentrifuge tubes containing protease inhibitor cocktail solution and EDTA and immediately centrifuged. At the end of the sampling period, animals were euthanized and the pancreas was removed for subsequent morphological analysis.
Glucose-stimulated insulin secretion and clearance.
To assess the impact of aging on glucose-stimulated insulin secretion, Sprague Dawley rats age 2 (n = 7), 7 (n = 5), and 12 (n = 6) mo underwent a hyperglycemic clamp. On the morning of the study, animals were weighed, and the jugular vein venous line was connected to an adjustable infusion pump (Harvard Apparatus, Holliston, MA) for glucose infusion. The portal vein and carotid artery line were used for simultaneous blood sampling. Following an equilibration period, animals received an intravenous glucose bolus (375 mg/kg) followed by a variable 50% (wt/vol) glucose infusion to clamp arterial glucose at ∼250 mg/dl (Fig. 1). Arterial blood samples (50 μl) were taken at baseline and every 15 min during the clamp for immediate determination of plasma glucose and arterial insulin concentrations. Portal vein blood (∼80 μl) was sampled every minute for 35 min (5–40 min) during the steady-state period of the hyperglycemic clamp. At the end of the sampling period, animals were euthanized and the pancreas was removed for subsequent morphological analysis.
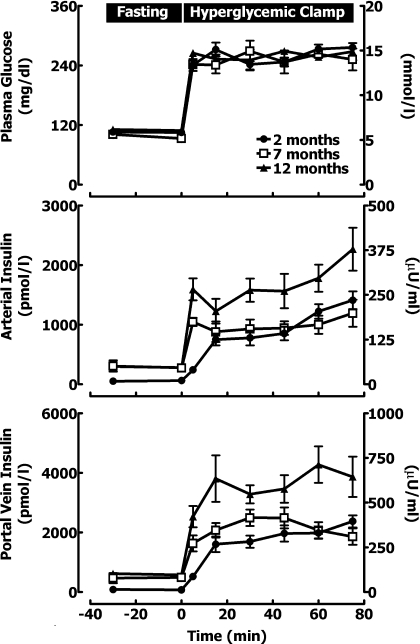
Fig. 1.
The mean arterial glucose (top), arterial insulin (middle), and portal vein insulin concentrations (bottom) at fasting (−30 to 0 min) and during a hyperglycemic clamp (0–75 min) in rats at 2, 7, and 12 mo of age.
Islet morphology and immunohistochemistry.
Pancreas was rapidly removed from rats, and all nonpancreatic tissue was removed; the pancreas was weighed and immediately fixed in 4% paraformaldehyde overnight at 4°C. The pancreas was embedded in paraffin, and, subsequently, complete longitudinal sections (4-μm) of pancreas (head, body, and tail) were obtained through its maximal width. These sections were stained for hematoxylin/eosin and insulin (guinea pig anti-insulin, 1:100; Zymed, Carlsbad, CA). The β-cell mass was measured by first quantifying pancreatic cross-sectional area positive for insulin and multiplying this by pancreatic weight.
Analytical procedures.
Insulin concentrations were measured in duplicate by an insulin ELISA (ALPCO Diagnostics, Salem, NH). C-peptide levels were measured using a competitive colorimetric enzyme-linked immunosorbent assay (ALPCO Diagnostics). Plasma free fatty acid levels were measured using the in vitro standard enzymatic colorimetric method (WAKO Chemicals, Richmond, VA). Glucose concentrations were measured by the glucose oxidase method (Glucose Analyzer 2; Beckman, Fullerton, CA).
Calculations.
The portal vein plasma insulin concentrations were subjected to multiparameter deconvolution analysis as previously described in detail and recently validated for use in rats (37). Briefly, multiparameter deconvolution technique used in this study assumes that time-varying insulin concentrations can be decomposed mathematically into 1) a finite number of discrete insulin secretory bursts occurring at specific times and having 2) individual amplitudes and 3) a common half-duration, wherein bursts are superimposed upon 4) a basal time-invariant insulin secretory rate, and 5) insulin disappearance in the portal vein is modeled via rapid and slow half-lives of 0.18 and 1.67 min and fractional slow-component amplitude of 0.065. Mean rates of insulin clearance were calculated during the fasting sampling period and during the hyperglycemic clamp studies in each rat using the following equation: C (l/min) = S (pmol/min)/I (pmol/l) where C is insulin clearance rate, S is insulin secretion rate (by deconvolution), and I is the corresponding arterial plasma insulin concentration. Insulin sensitivity index was calculated as a ratio of a mean glucose infusion rate during the hyperglycemic clamp (in mg·kg−1·min−1) to prevailing insulin concentrations (in pmol/l) and has been previously validated for the use in rats (45).
Statistical Analysis
Statistical analysis was performed using standard one-way ANOVA and regression analysis as stated (Statsoft, Tulsa, OK). Data in graphs and tables are presented as means ± SE. Findings were taken to be statistically significant at the P < 0.05.
RESULTS
Fasting Metabolic Characteristics
As expected, aging resulted in a progressive increase in body weight from 2 to 12 mo of age (P < 0.05 between the groups, Table 1). Fasting plasma glucose levels remained within the nondiabetic range in all groups. However, the mean in 12-mo-old rats was slightly but significantly elevated compared with that in 2- and 7-mo-old rats (108 ± 1 vs. 100 ± 3 and 93 ± 3 mg/dl, P < 0.05; Table 1). Aging led to a progressive increase in arterial insulin and C-peptide concentrations from 2 to 12 mo age (P < 0.05, Table 1), which was associated with a progressive decrement in insulin sensitivity (P < 0.05 between all groups; Table 1).
Table 1.
Metabolic and demographic characteristics
|
Age, mo |
|||
|---|---|---|---|
| 2 | 7 | 12 | |
| n | 14 | 10 | 11 |
| Weight, g | 374±13 | 604±17* | 668±26†‡ |
| Fasting glucose, mg/dl | 100±3 | 93±3 | 108±1†‡ |
| Fasting insulin, pmol/l | 66±8 | 256±47* | 313±39† |
| Fasting C-peptide, ng/ml | 0.9±0.1 | 2.5±0.6* | 3.1±0.6† |
| Fasting FFA, mmol/l | 0.7±0.2 | 0.8±0.2 | 0.7±0.2 |
| Insulin sensitivity index | 0.064±0.003 | 0.032±0.006* | 0.017±0.001†‡ |
Data are expressed as means ± SE; n, no. of animals. FFA, free fatty acid. Insulin sensitivity index was calculated as a ratio of the mean glucose infusion rate during the hyperglycemic clamp (in mg·kg−1·min−1) to prevailing insulin concentrations (in pmol/l).
P < 0.05 for 7 vs. 2 mo,
12 vs. 2 mo,
and 12 vs. 7 mo.
Fasting Pulsatile Insulin Secretion
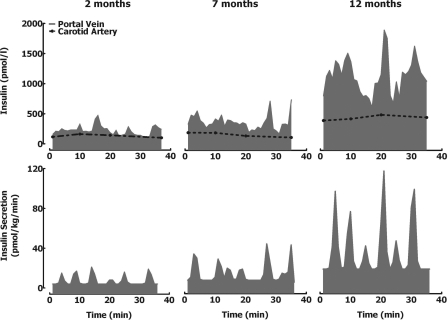
Visual examination of portal vein insulin concentration profiles in the fasting state (representative examples shown in Fig. 2) revealed distinct insulin oscillations with a periodicity of ∼5 min and an amplitude of ∼400 pmol/l at 2 mo of age increasing to ∼2,000 pmol/l by 12 mo of age. Deconvolution of fasting insulin concentration time series revealed that the fasting insulin secretion rate increased by 12-fold between ages 2 and 12 mo (4 ± 1 vs. 21 ± 5 vs. 49 ± 7 pmol·kg−1·min−1 for 2, 7, and 12 mo-old rats, respectively, P < 0.05 between all groups; Table 2 and Fig. 2). Increased insulin secretion was achieved through the mechanism of increased insulin secretory burst mass (5 ± 1 vs. 34 ± 5 vs. 77 ± 22 pmol/pulse for 2, 7, and 12 mo-old rats, respectively, P < 0.05 between all groups; Table 2 and Fig. 2). There was no change in the frequency of insulin pulses (P > 0.05). Deconvolution analysis indicated that the majority of insulin secreted (62%) was pulsatile.
Fig. 2.
Fasting state. Insulin concentration (top) and deconvolved corresponding insulin secretion rates (bottom) (measured at 1-min intervals) at the portal vein in representative rats at 2, 7, and 12 mo of age in the fasting state.
Table 2.
Pulsatile insulin secretion
| Pulse Mass, pmol/l | Pulse Amplitude, pmol | Pulse Interval, min | Insulin Secretion, pmol·kg−1·min−1 | Insulin Clearance, ml/min | Pulsatile Secretion, % | |
|---|---|---|---|---|---|---|
| 2 Months | ||||||
| Fasting | 5±1 | 666±108 | 4.7±0.3 | 4±1 | 19±3 | 72±6 |
| Glucose stimulated | 59±7 | 7,066±594 | 4.7±0.3 | 56±4 | 35±5 | 61±6 |
| 7 Months | ||||||
| Fasting | 34±9* | 2,407±723* | 4.7±0.4 | 21±5* | 54±9* | 59±8 |
| Glucose stimulated | 117±19* | 9,437±1,427* | 4.6±0.4 | 79±6 | 48±4 | 58±9 |
| 12 Months | ||||||
| Fasting | 77±22† | 5,206±1,627† | 4.5±0.7 | 49±7†‡ | 82±8†‡ | 57±5 |
| Glucose stimulated | 182±25†‡ | 13,356±1,882†‡ | 4.6±0.3 | 134±12†‡ | 62±7† | 46±3 |
Data are expressed as means ± SE, and statistical analysis was restricted to effects of aging.
P < 0.05 for 7 vs. 2 mo,
12 vs. 2 mo,
and 12 vs. 7 mo.
Glucose-Stimulated Pulsatile Insulin Secretion
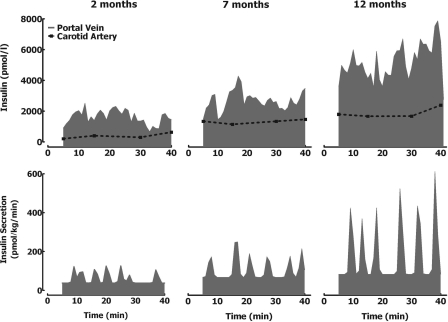
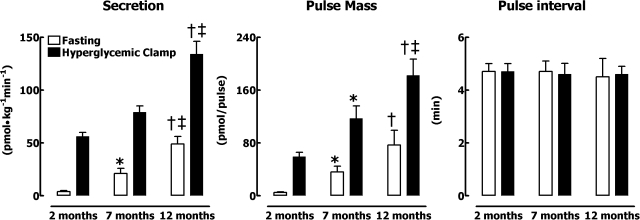
Inspection of individual 1-min sampled portal vein insulin concentration profiles during the hyperglycemic clamp (Fig. 3) revealed that the amplitude of insulin oscillations increased in response to hyperglycemia at all ages. The glucose-stimulated increase in insulin secretion rate compared with baseline was achieved by a selective increase in insulin pulse mass (P < 0.05 for all age groups) with no change in pulse interval (P > 0.05). Despite comparable arterial glucose concentrations during the hyperglycemic clamps (Fig. 1), glucose-stimulated insulin secretion was approximately threefold greater in 12-mo-old than 2-mo-old rats (P < 0.01). The age-related increase in glucose-stimulated insulin secretion was also attributed to a selective threefold increase in insulin pulse mass (59 ± 7 vs. 117 ± 19 vs. 182 ± 25 pmol/pulse for 2-, 7-, and 12-mo-old rats, respectively, P < 0.05 between all groups; Table 2 and Fig. 4) with no change in the frequency of insulin release (P > 0.05).
Fig. 3.
Glucose-stimulated state. Insulin concentration (top) and deconvolved corresponding insulin secretion rates (bottom) (measured at 1-min intervals) at the portal vein in representative rats at 2, 7, and 12 mo of age during the hyperglycemic clamp.
Fig. 4.
Mean insulin secretion rate (left), insulin pulse mass (middle), and interpulse interval (right) at fasting and during the hyperglycemic clamp in rats at 2, 7, and 12 mo of age. Data are expressed as means ± SE. Statistical analysis was restricted to effects of aging. P < 0.05 for 7 vs. 2 mo (*), for 12 vs. 2 mo (†), and for 12 vs. 7 mo (‡).
Insulin Clearance
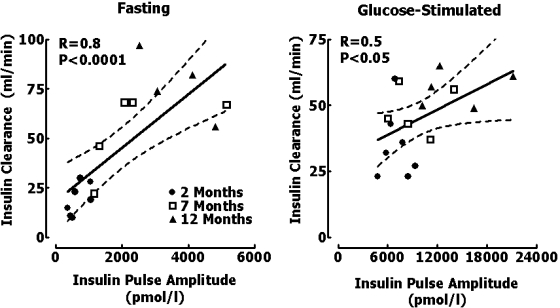
The calculated clearance rate of endogenously secreted insulin increased with age both at fasting (19 ± 3 vs. 54 ± 9 vs. 82 ± 8 ml/min for 2-, 7-, and 12-mo-old rats, respectively, P < 0.05 between all groups; Table 2) and glucose-stimulated (35 ± 5 vs. 48 ± 4 vs. 62 ± 7 ml/min for 2-, 7-, and 12-mo-old rats, respectively, P < 0.05; Table 2) states. Thus the systemic accessibility of increased insulin secretion in response to the age-related decline in insulin sensitivity is attenuated by greater hepatic insulin clearance. Consequently, exposure of the liver to age-related elevation of insulin concentrations was greater than that of extrahepatic tissues. Moreover, had we relied on sampling insulin at an extrahepatic sampling site, we would have markedly underestimated the extent of increased insulin secretion with aging. In extension of prior studies in which we have shown that hepatic clearance of endogenously secreted insulin is proportionate to the amplitude of portal vein insulin concentrations (29, 38, 40, 52), insulin clearance in this study was correlated with the magnitude of insulin pulses under fasting (r = 0.8, P < 0.001; Fig. 5) and hyperglycemic clamp conditions (r = 0.5, P < 0.05; Fig. 5).
Fig. 5.
Relationship between the clearance of endogenously secreted insulin and the mean insulin pulse amplitude in each rat at fasting (left) and during the hyperglycemic clamp at 2, 7, and 12 mo of age.
Relationship of Insulin Pulse Mass and Total Insulin Secretion to β-Cell Mass
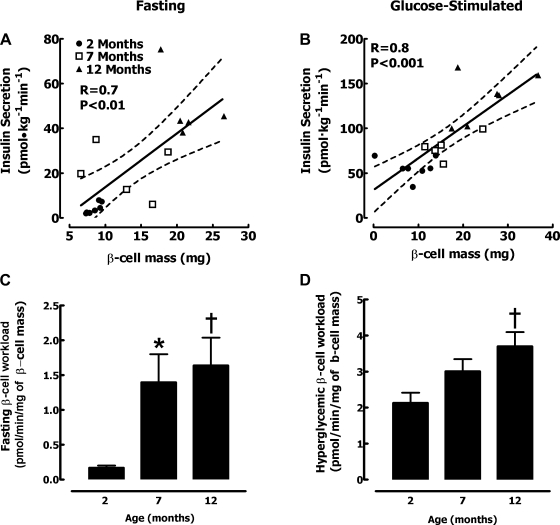
To putatively compensate for increased insulin demand in response to the age-associated decline in insulin sensitivity, β-cell mass increased almost threefold from 2 to 12 mo of age (8.6 ± 0.8 vs. 14.4 ± 1.6 vs. 23.4 ± 1.8 mg for 2-, 7-, and 12-mo-old rats, respectively, P < 0.05 between all groups). To test the postulate that β-cell mass adaptively increases to permit the appropriate rate of insulin secretion, we examined the relationship between insulin secretion and β-cell mass in the same animal. There was a strong linear relationship between the rate of glucose-stimulated insulin secretion and β-cell mass when all age groups were included (r = 0.8, P < 0.001, Fig. 6B). Because insulin secretion is derived from insulin secretory bursts, not surprisingly there was a comparable close relationship between insulin pulse mass and β-cell mass (r = 0.8, P < 0.001). These data imply that, in the aging rat, β-cell mass adaptively increases in response to the demand for insulin secretion under glucose drive. The relationship between β-cell mass and insulin secretion in the fasting state was also linear (r = 0.7, P < 0.01, Fig. 6A); however, the rate of insulin secretion initially increased more rapidly than β-cell mass. As a consequence of these relationships, the β-cell workload (insulin secretion per mg of β-cell mass) increased ∼10-fold in the fasting state (Fig. 6C) and ∼2-fold in the glucose-stimulated state from age 2 to 12 mo (Fig. 6D).
Fig. 6.
Relationship between the individual β-cell mass (mg) and deconvolved total insulin secretion rate in each rat at 2, 7, and 12 mo of age at fasting (A) and during the hyperglycemic clamp (B). The mean fasting (C) and glucose-stimulated (D) β-cell workload calculated as the ratio of insulin secretion to mg of β-cell mass. Data are expressed as means ± SE. P < 0.05 for 7 vs. 2 mo (*) and for 12 vs. 2 mo (†).
Relationship Between β-Cell Mass, Insulin Secretion, Hepatic Insulin Clearance, and Insulin Sensitivity
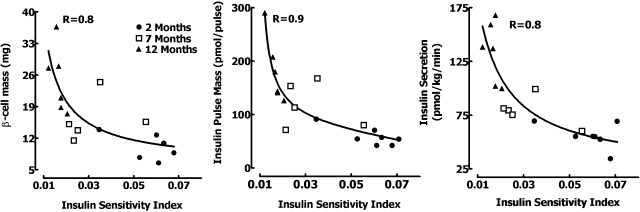
Uniquely, in this study, we were able to examine the interrelationship among adaptive changes in β-cell mass, insulin secretion (including direct measurement of prehepatic insulin pulses), hepatic insulin clearance, and age-associated increased insulin demand (decline in insulin sensitivity). We reaffirm the hyperbolic relationship between insulin sensitivity and insulin secretion (r = 0.8; Fig. 7) previously noted in humans (6, 23). We demonstrate for the first time a similar curvilinear relationship between both β-cell mass (r = 0.8; Fig. 7) and pulse mass (r = 0.9; Fig. 7) vs. insulin sensitivity.
Fig. 7.
Hyperbolic relationship between insulin sensitivity vs. the β-cell mass (left), pulse mass (middle), and deconvolved total insulin secretion (right) in each rat at 2, 7, and 12 mo of age.
DISCUSSION
In the present study, we report that, consistent with canine and human studies (51, 56), 1) most insulin in rats is secreted in a high-frequency pulsatile manner (pulse interval ∼4–5 min); 2) glucose concentrations determine insulin secretion by amplification of insulin burst mass with no change in pulse frequency; 3) as a consequence of this mode of insulin secretion, the liver is exposed to insulin oscillations of ∼1,000 pmol/l in the fasting state, increasing to 6–10,000 pmol/l in the glucose-stimulated state; and 4) hepatic clearance of endogenous insulin secretion is proportionate to the amplitude of portal vein insulin pulses.
Having recently established this methodology in rats, we were able to examine the adaptive changes in pulsatile insulin secretion and β-cell mass over a range of insulin sensitivity and age. As previously reported, β-cell mass in rats increases from 2 to 12 mo of age (36, 44). Although there is a rapid period of postnatal expansion of β-cell mass in the first 2 mo after birth in rodents, growth of β-cell mass thereafter appears to be determined by insulin sensitivity (18, 44). Consistent with this impression, β-cell mass increased as insulin sensitivity declined in the present studies (Fig. 7). As insulin sensitivity declined, normal blood glucose values were maintained by a progressive increase in insulin secretion accomplished by an increased pulse mass, exposing the liver to markedly greater oscillations in insulin concentrations (Figs. 2 and 3).
The deconvolution approach used here assumes that insulin clearance is constant at the sampling site. To the extent that the insulin clearance varies from minute to minute at the sampling site, the deconvolved insulin secretory rate may be in error. We previously reported that first-pass hepatic insulin extraction of endogenous insulin secretion increases in phase with each secretory burst (40) and is proportionate to amplitude of the insulin pulse in the portal vein (38, 40, 50). As a result, the deconvolved insulin secretion rate during insulin pulses may be underestimated when sampling from the systemic circulation. Fortunately, this error appears to have little impact on measured insulin secretion when sampling at the portal vein sampling site, since the major determinants of insulin concentration at this sampling site are insulin secretion and blood flow through the portal vein, as previously discussed (37, 51). Of note, the deconvolution approach used here was directly validated in the portal vein previously in the dog (51) over a threefold range of insulin secretion, implying that variance in first-pass hepatic insulin extraction (and therefore insulin clearance) at the portal vein sampling site has a negligible effect on the dynamics of insulin concentration compared with the rate of insulin secretion.
Hepatic clearance of endogenously secreted insulin was directly related to the amplitude of portal vein insulin oscillations (Fig. 5), consistent with prior studies in the dog and human (38, 40, 52). We have previously reported that hepatic insulin clearance is pulsatile, the pulses being synchronous with pulsatile insulin secretion (40). In a variety of other studies in which blood was sampled directly from the portal vein, we were able to report that hepatic insulin clearance of endogenously secreted insulin increases with the amplitude of portal vein insulin pulses (38, 50). Collectively these observations imply that hepatocytes appear to respond minute-by-minute to changes in the delivered insulin concentration by adaptively changing insulin clearance. Hepatic insulin clearance and subsequent degradation is a receptor-mediated endocytosis process (16). Receptor-bound insulin is internalized for degradation within endosomes, and this process requires insulin receptor (2), but not necessarily insulin receptor substrate (IRS)-1 or IRS-2 tyrosine phosphorylation (11). More recently, evidence has emerged to reveal an important role of the transmembrane glycoprotein CEACAM1 in regulation of hepatic insulin clearance (53). CEACAM1 is phosphorylated by the insulin receptor kinase after insulin binding and can subsequently bind to insulin receptor and is thus internalized (46). Because CAECAM1-null mice or mice that express a dominant-negative CAECAM1 mutant have impaired hepatic insulin clearance, this close relationship between CAECAM1 and the insulin receptor clearly has mechanistic importance (14, 53). Based on these data, it is plausible that the explanation for the relationship between hepatic insulin clearance and the amplitude of insulin presented to the liver in a pulse is mediated at least in part through the property of insulin-stimulated CAECAM1 facilitation of hepatic insulin clearance. Whether this mechanism is responsible for the increase in insulin clearance observed in aging-associated insulin resistance in the present study remains to be elucidated.
Our data again emphasize that, under conditions of daily living, the liver is intermittently exposed to much higher insulin concentrations than extrahepatic tissues. Moreover, the insulin concentration profile to which the liver is exposed in health far exceeds the range of insulin concentrations represented during hyperinsulinemic-euglycemic clamp studies in which suppression of hepatic glucose production is conventionally measured (31). It remains to be established what role the large insulin concentration oscillations in the portal vein have, if any, on hepatic insulin signaling. They do not appear to be important in insulin-mediated hepatic glucose uptake (21), but their role in suppression of hepatic glucose release remains unresolved.
Another consequence of increased hepatic insulin clearance of increasing portal vein insulin concentration oscillations is that changes in insulin secretion across the range present in healthy individuals will be markedly damped when evaluated by measurement of insulin concentrations in the systemic circulation. Defective insulin secretion in type 2 and early type 1 diabetes is characterized by loss of insulin pulse mass (32, 47). It is therefore not surprising that the systemic insulin concentrations are often comparable in individuals with type 2 diabetes mellitus and nondiabetic controls in the basal state despite decreased C-peptide concentrations, contributing to an underappreciation of the importance of reduced insulin secretion in the pathophysiology of type 2 diabetes (7, 54).
When evaluating the relationship between the rate of insulin secretion and β-cell mass (Fig. 6), we noted distinct relationships in the fasting and glucose-stimulated states. There was a close linear relationship between the rate of glucose-stimulated insulin secretion and the β-cell mass (r = 0.8). Not surprisingly, because most insulin is derived in secretory bursts, the relationships were comparable between total rate or insulin pulse mass and β-cell mass during glucose stimulation (r = 0.8). The data serve to underscore the importance of β-cell mass in defining glucose-stimulated insulin secretion. The relationship between β-cell mass and insulin secretion in the fasting state was also linear (r = 0.7); however, the rate of insulin secretion initially increased more rapidly than β-cell mass. Although it remains controversial precisely how β-cell mass adapts to increased insulin demand (insulin resistance) (24), one hypothesis is that β-cell formation is prompted by the unfolded protein response of the endoplasmic reticulum under an increased load of protein folding and processing. The present data would suggest that it is the glucose-stimulated rate of insulin secretion that drives expansion of β-cell mass rather than the rate of insulin secretion in the fasting state. The data also serve to emphasize the importance of β-cell mass in the adaptive response to increased demand for insulin secretion.
However, it also important to point out that the close relationship between the adaptive change in β-cell mass and pulsatile insulin secretion in response insulin resistance observed in the current study doesn't necessarily imply that the same relationship is true in humans. Although the β-cell mass can adaptively increase in humans in response to obesity (8, 30), the extent of this increase is less than that reported in rodent studies (9). It is also important to emphasize that the current study was designed to establish the adaptive changes in β-cell mass and insulin secretion (specifically the pulsatile component) to age-related increasing insulin demand (decreasing insulin sensitivity) with normal aging in rats. The same relationships may not necessarily occur in response to insulin resistance induced through other mechanisms (for example, obesity). Also, adaptive changes in β-cell mass and insulin secretion to insulin resistance may also be different with onset of insulin resistance at different ages, since the ability to expand β-cell mass and function may be altered in animals and humans with aging (39, 59).
The relationship between insulin secretion and insulin sensitivity in vivo is often portrayed as a hyperbolic relationship as first described by Bergman and colleagues (6). It has been postulated that a feedback mechanism operates between insulin sensitivity and β-cell function such that as insulin sensitivity declines (e.g., in response to obesity), insulin secretion increases, thereby maintaining a constant product of insulin secretion and insulin sensitivity (27). Here we report that there is a comparable curvilinear relationship between the adaptive increase in insulin secretion and β-cell mass to declining insulin sensitivity. As expected, since most insulin secretion is derived from insulin secretory bursts, a relationship can be delineated between insulin pulse mass and insulin sensitivity (Fig. 7).
In humans, evaluation of the effects of aging on β-cell function has yielded conflicting results, with some investigators reporting increases in both fasting (1, 5, 12, 19, 22) and glucose-stimulated (13, 19, 22, 26) insulin and/or C-peptide levels and others a decrease or no change in insulin secretion (1, 12, 20, 41, 48). These differences are likely attributed to many factors such as differences in methods used to measure insulin secretion (12, 22), varying degrees of insulin sensitivity (27), differences in body composition and physical fitness (13), and varying levels of impaired fasting glucose and glucose tolerance often associated with aging (1, 12, 20). Most of these studies used systemic insulin concentrations to assess insulin secretion (12, 13, 20, 41). Studies that assessed the rates of prehepatic insulin secretion via deconvolution analysis of C-peptide concentrations reported both increased fasting and glucose-stimulated insulin secretion with aging in humans (22) and increased hepatic insulin clearance (5).
In the present study, the aging-associated increase in insulin pulse mass correlated with increased hepatic clearance of endogenously secreted insulin. We have previously reported that the amplitude of insulin pulses directed to the liver is related to hepatic insulin clearance (40), implying that the islet dictates the rate of systemic insulin delivery through the dual mechanisms of pulsatile insulin secretion and the influence of the latter on hepatic insulin clearance. The present data support this hypothesis. Additionally, consistent with the present model, others have shown an increase in hepatic insulin extraction with aging in humans (1, 5). Thus, as pulse amplitude presented to the liver increases in response to insulin resistance, the liver preferentially extracts large insulin pulses, thus regulating systemic insulin delivery. Subsequently, the marked increase in fasting prehepatic pulsatile insulin secretion (∼12-fold) is largely offset by a compensatory increase in hepatic insulin clearance, resulting in a lesser (∼4-fold) increase in fasting systemic insulin concentrations. This observation suggests that an adaptive increase in pulsatile insulin secretion in response to aging-associated insulin resistance primarily modulates hepatic glucose production and not necessarily extrahepatic glucose disposal, an observation supported by others (3, 4, 23).
Our observation that the clearance of endogenously secreted insulin increased in response to age-related insulin resistance in the rat contradicts previous data that reports a decline in insulin clearance in insulin-resistant states such as obesity (17) and high-fat feeding (43). These differences are likely attributed to many factors, one of which is the methods used to measure insulin clearance. It is important to emphasize that the insulin clearance reported here is that of endogenously secreted insulin that is delivered in the portal vein and thus directly to the liver in large discrete pulses. Insulin clearance of endogenously secreted insulin was obtained by dividing the insulin secretion rate (obtained by direct sampling from the portal vein) by the insulin concentration in the systemic circulation.
In contrast, most prior studies either measure the clearance of exogenously delivered insulin by a constant systemic or intraportal insulin infusion (43, 60) or estimate the clearance of endogenously secreted insulin by comparing systemic insulin and C-peptide concentrations (17). Moreover, differences in plasma free fatty acid levels in the current study (no change between 2- and 12-mo-old rats) and others (elevated free fatty acid levels observed in obesity) may account for the discrepancy in the results. Elevation in systemic free fatty acid concentrations has been shown in vivo and in vitro to impair hepatic insulin clearance (57, 60), presumably by direct inhibition of insulin binding and subsequent degradation (60).
In summary, we report that the β-cell adaptation to age-associated insulin resistance is accomplished via a selective increase in both fasting and glucose-stimulated insulin secretory burst mass. Moreover, the increase in insulin secretory burst mass in the rat can be attributed to a parallel increase in β-cell mass. Concurrently, hepatic insulin clearance increases due to higher amplitude of portal vein insulin oscillations, so that the age-related increase in systemic insulin concentrations is substantially damped. These data serve to emphasize the complex but highly coordinated relationship between pancreatic islets (through pulsatile insulin secretion) and the liver (through both metabolic responses and regulation of systemic insulin delivery). The data also permit an appreciation of the primacy of β-cell mass in determining insulin secretion over a range of insulin sensitivity.
GRANTS
Thease studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-59579 and the Larry Hillblom Foundation to P. C. Butler. A. V. Matveyenko is supported by the National Institutes of Health Ruth L. Kirschstein National Research Service Award.
Acknowledgments
We are grateful to Heather Gerber and Ryan Galasso for excellent technical support and acknowledge the support and excellent suggestions of our colleagues at the Larry Hillblom Islet Research Center, Dr. Anil Bhushan, Dr. Alexandra Butler, Dr. Tatyana Gurlo, and Dr. Leena Haataja.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahren B, Pacini G. Age-related reduction in glucose elimination is accompanied by reduced glucose effectiveness and increased hepatic insulin extraction in man. J Clin Endocrinol Metab 83: 3350–3356, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Backer JM, Kahn CR, Cahill DA, Ullrich A, White MF. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor β-subunit. J Biol Chem 265: 16450–16454, 1990. [PubMed] [Google Scholar]
- 3.Barzilai N, Banerjee S, Hawkins M, Chen W, Rossetti L. Caloric restriction reverses hepatic insulin resistance in aging rats by decreasing visceral fat. J Clin Invest 101: 1353–1361, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barzilai N, She L, Liu BQ, Vuguin P, Cohen P, Wang J, Rossetti L. Surgical removal of visceral fat reverses hepatic insulin resistance. Diabetes 48: 94–98, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52: 1738–1748, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 32: 438–446, 1983. [DOI] [PubMed] [Google Scholar]
- 8.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 52: 102–110, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Janson J, Soeller WC, Butler PC. Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 52: 2304–2314, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Butler PC, Chou J, Carter WB, Wang YN, Bu BH, Chang D, Chang JK, Rizza RA. Effects of meal ingestion on plasma amylin concentration in NIDDM and nondiabetic humans. Diabetes 39: 752–756, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Carpentier JL, Paccaud JP, Backer J, Gilbert A, Orci L, Kahn CR, Baecker J. Two steps of insulin receptor internalization depend on different domains of the β-subunit. J Cell Biol 122: 1243–1252, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AM, Smith MJ, Bloem CJ, Galecki AT, Halter JB, Supiano MA. Limitation of the homeostasis model assessment to predict insulin resistance and β-cell dysfunction in older people. J Clin Endocrinol Metab 91: 629–634, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Coon PJ, Rogus EM, Drinkwater D, Muller DC, Goldberg AP. Role of body fat distribution in the decline in insulin sensitivity and glucose tolerance with age. J Clin Endocrinol Metab 75: 1125–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Deangelis AM, Heinrich G, Dai T, Bowman TA, Patel PR, Jun Lee S, Hong EG, Young Jung D, Assmann A, Kulkarni RN, Kim JK, Najjar SM. CEACAM1: a link between insulin and lipid metabolism. Diabetes. In press. [DOI] [PMC free article] [PubMed]
- 15.DeFronzo RA, Simonson D, Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23: 313–319, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev 19: 608–624, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Faber OK, Christensen K, Kehlet H, Madsbad S, Binder C. Decreased insulin removal contributes to hyperinsulinemia in obesity. J Clin Endocrinol Metab 53: 618–621, 1981. [DOI] [PubMed] [Google Scholar]
- 18.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of β-cell mass in the growing rat pancreas Estimation with a simple mathematical model. Diabetes 44: 249–256, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Fink RI, Revers RR, Kolterman OG, Olefsky JM. The metabolic clearance of insulin and the feedback inhibition of insulin secretion are altered with aging. Diabetes 34: 275–280, 1985. [DOI] [PubMed] [Google Scholar]
- 20.Fritsche A, Madaus A, Stefan N, Tschritter O, Maerker E, Teigeler A, Haring H, Stumvoll M. Relationships among age, proinsulin conversion, and β-cell function in nondiabetic humans. Diabetes 51, Suppl 1: S234–S239, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Grubert JM, Lautz M, Lacy DB, Moore MC, Farmer B, Penaloza A, Cherrington AD, McGuinness OP. Impact of continuous and pulsatile insulin delivery on net hepatic glucose uptake. Am J Physiol Endocrinol Metab 289: E232–E240, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Gumbiner B, Polonsky KS, Beltz WF, Wallace P, Brechtel G, Fink RI. Effects of aging on insulin secretion. Diabetes 38: 1549–1556, 1989. [DOI] [PubMed] [Google Scholar]
- 23.Gupta G, Cases JA, She L, Ma XH, Yang XM, Hu M, Wu J, Rossetti L, Barzilai N. Ability of insulin to modulate hepatic glucose production in aging rats is impaired by fat accumulation. Am J Physiol Endocrinol Metab 278: E985–E991, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic β-cell proliferation. Annu Rev Cell Dev Biol 22: 311–338, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56: 2016–2027, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Kahn SE, Larson VG, Beard JC, Cain KC, Fellingham GW, Schwartz RS, Veith RC, Stratton JR, Cerqueira MD, Abrass IB. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol Endocrinol Metab 258: E937–E943, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, Porte D. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 42: 1663–1672, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK. Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318: 806–809, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, Yang D, Lefebvre P, Butler PC. Decrease in β-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes 50: 2001–2012, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 4: 110–125, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Kraegen EW, James DE, Jenkins AB, Chisholm DJ. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am J Physiol Endocrinol Metab 248: E353–E362, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Laedtke T, Kjems L, Porksen N, Schmitz O, Veldhuis J, Kao PC, Butler PC. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab 279: E520–E528, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ. Endoplasmic reticulum stress contributes to β-cell apoptosis in type 2 diabetes. Diabetologia 50: 752–763, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Leahy JL, Bumbalo LM, Chen C. Diazoxide causes recovery of β-cell glucose responsiveness in 90% pancreatectomized diabetic rats. Diabetes 43: 173–179, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ 13: 385–392, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Matveyenko AV, Butler PC. β-Cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes 55: 2106–2114, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Matveyenko AV, Veldhuis JD, Butler PC. Measurement of pulsatile insulin secretion in the rat, direct sampling from the hepatic portal vein. Am J Physiol Endocrinol Metab doi: 10.1152/ajpendo.90335.2008. [DOI] [PMC free article] [PubMed]
- 38.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by ∼50% pancreatectomy. Diabetes: 2347–2356, 2006. [DOI] [PubMed]
- 39.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57: 1584–1594, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 54: 1649–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Meneilly GS, Veldhuis JD, Elahi D. Disruption of the pulsatile and entropic modes of insulin release during an unvarying glucose stimulus in elderly individuals. J Clin Endocrinol Metab 84: 1938–1943, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke β-cell regeneration. Diabetes 57: 142–149, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Mittelman SD, Van Citters GW, Kim SP, Davis DA, Dea MK, Hamilton-Wessler M, Bergman RN. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced β-cell response. Diabetes 49: 2116–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Montanya E, Nacher V, Biarnes M, Soler J. Linear correlation between β-cell mass and body weight throughout the lifespan in Lewis rats: role of β-cell hyperplasia and hypertrophy. Diabetes 49: 1341–1346, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes 53: 441–446, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Najjar SM Regulation of insulin action by CEACAM1. Trends Endocrinol Metab 13: 240–245, 2002. [DOI] [PubMed] [Google Scholar]
- 47.O'Meara NM, Sturis J, Herold KC, Ostrega DM, Polonsky KS. Alterations in the patterns of insulin secretion before and after diagnosis of IDDM. Diabetes Care 18: 568–571, 1995. [DOI] [PubMed] [Google Scholar]
- 48.Pacini G, Beccaro F, Valerio A, Nosadini R, Crepaldi G. Reduced β-cell secretion and insulin hepatic extraction in healthy elderly subjects. J Am Geriatr Soc 38: 1283–1289, 1990. [DOI] [PubMed] [Google Scholar]
- 49.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest 46: 1954–1962, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes 45: 1317–1323, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Porksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol Endocrinol Metab 269: E478–E488, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Porksen N, Munn SR, Steers JL, Veldhuis JD, Butler PC. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am J Physiol Endocrinol Metab 270: E1043–E1049, 1996. [DOI] [PubMed] [Google Scholar]
- 53.Poy MN, Yang Y, Rezaei K, Fernstrom MA, Lee AD, Kido Y, Erickson SK, Najjar SM. CEACAM1 regulates insulin clearance in liver. Nat Genet 30: 270–276, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Sando H, Lee YS, Iwamoto Y, Ikeuchi M, Kosaka K. Isoproterenol-stimulated C-peptide and insulin secretion in diabetic and nonobese normal subjects: decreased hepatic extraction of endogenous insulin in diabetes. J Clin Endocrinol Metab 51: 1143–1149, 1980. [DOI] [PubMed] [Google Scholar]
- 55.Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46: 323–335, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 85: 4491–4499, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Svedberg J, Bjorntorp P, Smith U, Lonnroth P. Free-fatty acid inhibition of insulin binding, degradation, and action in isolated rat hepatocytes. Diabetes 39: 570–574, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Temple RC, Carrington CA, Luzio SD, Owens DR, Schneider AE, Sobey WJ, Hales CN. Insulin deficiency in non-insulin-dependent diabetes. Lancet 1: 293–295, 1989. [DOI] [PubMed] [Google Scholar]
- 59.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of β-cells in aged adult mice. Diabetes 54: 2557–2567, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Wiesenthal SR, Sandhu H, McCall RH, Tchipashvili V, Yoshii H, Polonsky K, Shi ZQ, Lewis GF, Mari A, Giacca A. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 48: 766–774, 1999. [DOI] [PubMed] [Google Scholar]