Abstract
The present studies examined the relationship between fasting blood glucose and Hb A1c in C57BL/6J, DBA/2J, and KK/HlJ mice with and without diabetes mellitus. Daily averaged blood glucose levels based on continuous glucose monitoring and effects of 6-h vs. overnight fasting on blood glucose were determined. Daily averaged blood glucose levels were highly correlated with Hb A1c, as determined with a hand-held automated device using an immunodetection method. R2 values were 0.90, 0.95, and 0.99 in KK/HIJ, C57BL/6J, and DBA/2J, respectively. Six-hour fasting blood glucose correlated more closely with the level of daily averaged blood glucose and with Hb A1c than did blood glucose following an overnight fast. To validate the immunoassay-determined Hb A1c, we also measured total glycosylated hemoglobin using boronate HPLC. Hb A1c values correlated well with total glycosylated hemoglobin in all three strains but were relatively lower than total glycosylated hemoglobin in diabetic DBA/2J mice. These results show that 6-h fasting glucose provides a superior index of glycemic control and correlates more closely with Hb A1c than overnight-fasted blood glucose in these strains of mice.
Keywords: glycosylated hemoglobin, methodology, mice
blood glucose varies widely together with food intake during a typical day; thus fasting blood glucose has been used to assess daily averaged level of blood glucose (1, 1a, 10, 16, 17, 31). In humans, overnight-fasted blood glucose is routinely used to diagnose diabetes mellitus and evaluate glycemic control because it correlates with glycosylated hemoglobin (GlyHb) (1a, 1b, 5). Overnight fasting has also been adopted for determination of blood glucose in mice (4, 35). Recent studies have raised concerns regarding the potential physiological stress imposed by long-term fasting in mice (3, 6). Thus, the Animal Models of Diabetes Complications Consortium (www.amdcc.org) recommends a short-term (i.e., 6-h) fasted protocol for blood glucose determination in mice (6). However, the effectiveness with which short-term fasting glucose reflects glycemic control over the course of a day remains to be validated.
Measurement of GlyHb provides a critical parameter for assessing long-term glycemic control and predicting the incidence of diabetic complications in diabetic patients (1, 1a). In humans, glycosylation of NH2-terminal valine of the hemoglobin β-chain is known as hemoglobin A1c (Hb A1c) and represents the major form of GlyHb (7). Several methods for determining GlyHb exist, including ion-exchange high-performance liquid chromatography (HPLC), boronate affinity HPLC (23), and an antibody-based approach (2). Ion- exchange HPLC determines GlyHb on the basis of altered Hb charge caused by linkage with glucose molecules, whereas the boronate affinity HPLC measures GlyHb by the presence of coplanar vicinal hydroxyl and/or carbonyl groups of hexoses (23). It has been demonstrated in humans that the boronate affinity HPLC approach determines total GlyHb with less interference from nonglycosylated hemoglobin compounds (23, 24).
Clinically, an immunochemistry-based approach that detects Hb A1c using a monoclonal antibody against human Hb A1c has been widely used (e.g., DCA 2000 analyzer) (2, 13). Advantages of this technique include the requirement for only a few microliters of blood, making it suitable for determining Hb A1c in mice and the rapid simplicity of measurement (18, 36). Although it has been demonstrated in humans that Hb A1c determined using DCA 2000 analyzer correlates with GlyHb measured using ion-exchange HPLC (2), a similar relationship in mice remains to be established. Validation of the relationship between Hb A1c, GlyHb, and glucose control in mice remains to be established but is critical for studies of diabetic complications in this species. Therefore, we undertook the present studies to characterize the relationship between fasting blood glucose and Hb A1c in mice.
MATERIALS AND METHODS
Materials.
Inbred C57BL/6J, DBA/2J, and KK/HlJ male mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animal protocols were approved by the Institutional Animal Care and Use Committee of Vanderbilt University, and the animal studies were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Generating diabetic mice.
Diabetes was induced using a low-dose streptozotocin protocol (40 mg·kg−1·day−1 for DBA/2J and 50 mg·kg−1·day−1 for C57BL/6J and KK/HlJ mice, made freshly in 0.1 mol/l citrate buffer, pH 4.0, ip injection daily for 5 consecutive days), as described previously (25). The induction of diabetes mellitus was defined as a blood glucose level measured using a B-Glucose Analyzer (HemoCue, Lake Forest, CA) >250 mg/dl in mice following a 6-h fast starting at 6:00 AM (25). More than 90% of mice developed hyperglycemia within 2 wk of the streptozotocin injection, and hyperglycemia persisted for the duration of the experimental period. All diabetic mice included in the present studies were diabetic for ≥8 wk.
Continuous monitoring of blood glucose in conscious mice.
To definitely determine daily average blood glucose level in mice, we used the Continuous Glucose Monitoring System (CGMS; Meditronic MiniMed, Northridge, CA) to continuously measure blood glucose for 24 h. This system utilizes a subcutaneous glucose sensor such that interstitial fluid glucose reacts with the sensor's glucose oxidase layer, producing an electrical current that is proportional to glucose concentrations (21, 28). The electrical signal was collected every 10 s. These signals were averaged and saved to memory of the cable-tethered glucose monitor every 5 min. The data stored in the monitor were periodically downloaded into a computer for analysis. A correlation between interstitial glucose and blood glucose levels has previously been demonstrated in several species, including mice and humans (19, 27, 28). In the present studies, we adapted this system to mice.
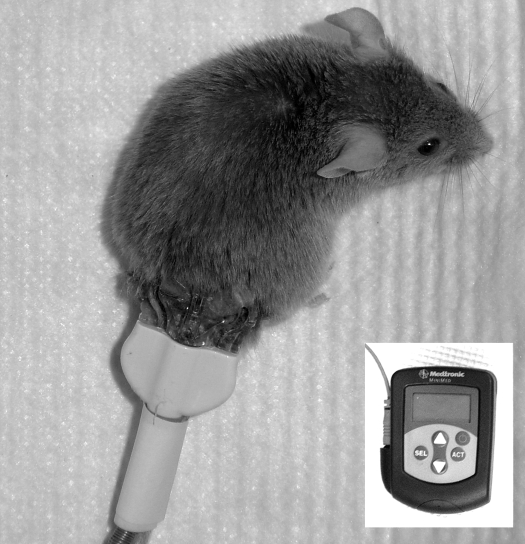
A method for implantation of the subcutaneous glucose sensor in mice was established as described below. Briefly, a diabetic or control inbred mouse was restrained in a 50-ml centrifuge tube with air holes drilled in its tip. The fur on the lower back was shaved and the skin sterilized with an iodine pad, followed by washing with a 70% ethanol pad. The glucose sensor was inserted subcutaneously and fixed to skin using tissue glue (3M Vetbond Tissue Adhesive 1469SB). The mouse was then housed individually in a cage and allowed free access to water and food. The glucose signal was recorded by the glucose monitor placed outside the cage via a cable tethered to the mouse. Three to four measurements of blood glucose were performed during the daytime by puncturing saphenous vein as described previously (26), and the blood glucose values were input into the monitor to calibrate the glucose level. To minimize the effect of sensor implantation on blood glucose, the data recorded by the glucose sensor in the first 5 h were discarded.
Correlation between glucose levels in subcutaneous interstitium and blood.
The validity of the CGMS has been established by studies in humans indicating that the interstitial glucose is proportional to blood glucose (27, 28). In the present studies, we examined the relationship between glucose levels in these two compartments in mice. To do this, we injected a 20% of glucose solution (1.5 g/kg body wt) intraperitoneally in mice implanted with the glucose sensor. Blood glucose concentration determined via a saphenous vein bleed and the electric current recorded using the glucose sensor were examined at several time points following glucose injection. A significant correlation between blood glucose level and the sensor current (n = 10, R2 = 0.762, P < 0.05) was observed, indicating the interstitial glucose sensitively reflects blood glucose in inbred mice.
Determination of Hb A1c.
Mouse immunoreactive Hb A1c was determined using DCA 2000 analyzer (Bayer, Elkhart, IN). This system automatically measures both Hb A1c and total hemoglobin in an ∼5-μl blood sample (2, 13). Total Hb is determined using potassium ferricyanide to oxidize Hb in the sample to methemoglobin. The methemoglobin then complexes with thiocyanate to form thiocyan-methemoglobin, and the colored species is measured. The extent of color development at 531 nm is proportional to the concentration of total hemoglobin in the sample. For the specific measurement of Hb A1c, the assay relies on inhibition of latex agglutination. An agglutinator causes agglutination of latex coated with an Hb A1c-specific monoclonal antibody. Increased agglutination causes increased light scattering, which is measured as an increase in absorbance at 531 nm. Hb A1c in whole blood specimens competes for the limited number of antibody-latex binding sites, reducing agglutination and scattering of light. Decreased light scattering is measured as decreased absorbance at 531 nm. The Hb A1c concentration is then quantified using a calibration curve of absorbance vs. Hb A1c concentration.
Approximately 30 μl of blood was collected via a saphenous vein in conscious mouse. Ten microliters of blood was used to measure Hb A1c. The remaining 20 μl of the blood was sent to Case Western Reserve University via an overnight service to determine total GlyHb (see below). Preliminary studies showed that there was no difference in Hb A1c levels determined in freshly collected blood and that which had been stored for 1 day (data not shown).
Determination of total GlyHb.
Total GlyHb in mouse blood was determined using boronate affinity HPLC (Bio-Rad Variant Hemoglobin Testing Systems; Bio-Rad Laboratories, Hercules, CA). Sample hemolysate is passed over the boronate column, and glycated hemoglobin is preferentially bound due to the formation of a stable complex between the coplanar cis-diol group of glycated hemoglobin and the immobilized 3-aminophenylboronic acid in the affinity cartridge. The nonglycated hemoglobin is eluted from the column with subsequent displacement of the glycated hemoglobins using a separate buffer. The separated hemoglobins pass through the flow cell of the filter photometer, where changes in the absorbance are measured at 415 nm. A secondary filter at 690 nm corrects for matrix effects caused by mixing buffers of different ionic strength.
Statistical analysis.
All data are expressed as means ± SE. One-way ANOVA followed by a Bonferroni post hoc test was used to compare the difference among three groups, and two-tailed t-test was used for comparison of data between two groups. The level of statistical significance was assigned at P < 0.05 by convention.
RESULTS
Continuous blood glucose profile in inbred mice.
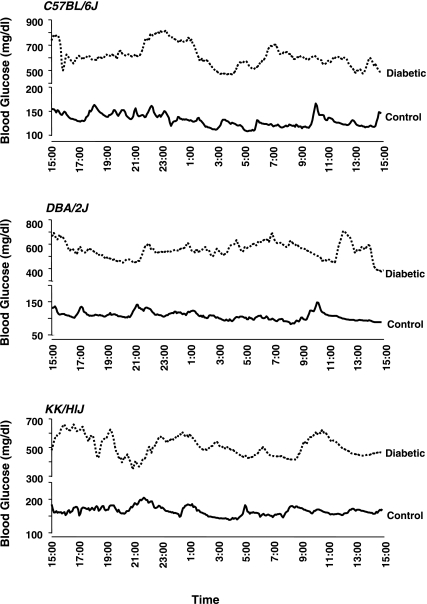
Continuous 24-h monitoring of interstitial fluid glucose levels using the MiniMed CGMS in diabetic and control C57BL/6J, DBA/2J, and KK/HlJ mice (Fig. 1) provides an estimate of daily blood glucose profile in each strain of mouse. Figure 2 shows representative blood glucose profiles in control and diabetic inbred mice. Diabetic mice exhibited significantly higher levels of blood glucose and greater glucose excursions than nondiabetic controls (mean standard deviations of daily blood glucose in diabetic vs. control KK/HlJ, DBA/2J, and C57BL/6J mice were 63.7 vs. 32.0, 67.4 vs. 17.4, and 79.9 vs. 16.4 mg/dl, respectively; P < 0.005 in all 3 strains). Among the controls, KK/HlJ mice, an inbred strain developing spontaneous glucose intolerance (33), exhibited higher daily averaged blood glucose levels than nondiabetic DBA/2J mice (Table 1).
Fig. 1.
A mouse with an implanted glucose sensor. Inset: the glucose monitor used for storing glucose data collected by glucose sensor. The monitor was connected to glucose sensor through a cable.
Fig. 2.
Representative daily blood glucose profiles determined using MiniMed glucose sensor in control and streptozotocin-induced diabetic C57BL/6J, DBA/2J, and KK/HlJ mice.
Table 1.
Daily averaged blood glucose levels in control and diabetic inbred mice
| C57BL/6J | DBA/2J | KK/HlJ | |
|---|---|---|---|
| Control | 158.3±2.49 (5) | 131.7±6.12 (8) | 171.2±14.0 (9)* |
| Diabetes | 626.8±16.2 (5)† | 498.4±60.0 (5)† | 463.1±30.7 (4)† |
Values are means ± SE. The mean was calculated using averaged daily glucose level of each mouse, and the numbers inside parentheses represent the mouse numbers.
P < 0.05 vs. value in control DBA/2J;
P < 0.0001 vs. values in respective control mice.
Correlation between daily averaged blood glucose and Hb A1c.
To study whether the daily averaged blood glucose correlates with glycosylated hemoglobin in mice, as previously shown in humans (1), we measured Hb A1c using the DCA 2000 analyzer in mice undergoing continuous glucose monitoring. Daily averaged blood glucose was tightly correlated with Hb A1c in all three strains of mice with R2 values of 0.90, 0.95, and 0.99 in KK/HlJ (n = 10), C57BL/6J (n = 10), and DBA/2J mice (n = 11), respectively (P < 0.05 in all 3 strains).
Blood glucose level following 6 h vs. overnight fasting.
To define the optimal fasting time for determining blood glucose in mice, we measured saphenous venous glucose following a 6-h fast in diabetic and control C57BL/6J, DBA/2J, and KK/HlJ mice. One week later, the blood glucose was measured again in the same mice following an overnight fast. The fasting blood glucose was then compared with the daily averaged blood glucose determined with the use of an implanted interstitial glucose sensor in strain- and age-matched mice. Overnight fasting resulted in significantly lower blood glucose in all groups of mice except diabetic C57BL/6J mice (overnight fasting vs. daily averaged blood glucose in control and diabetic C57BL/6J: 109.5 ± 3.2 vs. 158.3 ± 2.5 and 445.8 ± 60.5 vs. 626.8 ± 16.5 mg/dl, respectively; in control and diabetic KK/HlJ mice: 91.5 ± 2.5 vs. 171.2 ± 14.0 and 243.2 ± 16.3 vs. 463.1 ± 30.7 mg/dl, respectively; in control and diabetic DBA/2J mice: 73.5 ± 2.9 vs. 131.7 ± 6.1 and 191.9 ± 57.1 vs. 498.4 ± 60.0 mg/dl, respectively). In contrast, a 6-h fast yielded blood glucose values that were no different from the daily averaged MiniMed determined glucose values.
Six-hour fasting slightly decreased body weight compared with the same mice without a fast (<7%). Overnight fasting resulted in a more marked decrease in body weight (8.0 ± 0.8, 11.0 ± 1.0, and 11.5 ± 1.9% less than 6-h-fasted body weight in control KK/HlJ, DBA/2J, and C57BL/6J mice, respectively, and 6.2 ± 0.7, 5.1 ± 2.1, and 8.7 ± 1.1% in diabetic KK/HlJ, DBA/2J, and C57BL/6J mice, respectively; P < 0.05 in all groups compared with body weight in 6-h-fasted mice). These data indicate that a 6-h fast imposes less metabolic stress on mice than an overnight fast.
Correlation between fasting blood glucose and Hb A1c.
The relationship between Hb A1c and 6-h or overnight-fasted blood glucose was also analyzed in C57BL/6J, KK/HlJ, and DBA/2J mice. Six-hour-fasted blood glucose correlated more closely with Hb A1c than overnight-fasted blood glucose, although overnight-fasted blood glucose also correlated with Hb A1c (Fig. 3).
Fig. 3.
Correlation of 6-h-fasted vs. overnight-fasted blood glucose with Hb A1c in C57BL/6J, KK/HlJ, and DBA/2J mice.
Hb A1c determination in mice.
To validate Hb A1c determined using DCA 2000 as an index of total GlyHb, we measured total GlyHb using a boronate affinity HPLC method on two blood samples collected from the same mouse at the same time. The Hb A1c is comparable with GlyHb in C57BL/6J mice (n = 45) but slightly higher than GlyHb in KK/HlJ mice (with a difference of 0.46 ± 0.12%; n = 32, P < 0.05). In contrast, the Hb A1c in DBA/2J mice is significantly lower than total GlyHb (with a mean difference of 2.34 ± 0.30% between these 2 measurements; n = 49, P < 0.0001). Despite this difference, Hb A1c correlates closely with total GlyHb in all strains studied (R2 = 0.8823, 0.8765, and 0.8445 in C57BL/6J, KK/HlJ, and DBA/2J strains, respectively; P < 0.0001 in all strains). These results indicate that the Hb A1c determined using the DCA 2000 analyzer reflects GlyHb.
The predictive value of total GlyHb for diabetic nephropathy in inbred mice.
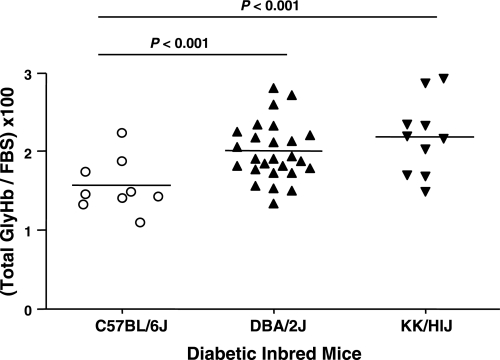
We have demonstrated previously that diabetic KK/HlJ and DBA/2J mice develop severe nephropathy, whereas C57BL/6J mice appear to resist diabetic nephropathy (25). In the present study, we examined whether the total GlyHb was different in these three strains when correlated with 6-h fasting blood glucose (total GlyHb/fasting blood glucose). The present studies revealed that KK/HlJ and DBA/2J mice, which are susceptible to diabetic nephropathy, exhibit significantly higher GlyHb for any given fasting blood glucose than C57BL/6J mice (Fig. 4).
Fig. 4.
Ratio of total glycosylated Hb (GlyHb) to 6-h fasting blood glucose in diabetic C57BL/6J, DBA/2J, and KK/HlJ mice.
DISCUSSION
Daily averaged blood glucose more closely correlates with Hb A1c than fasted blood glucose in humans (1, 5, 29). Determining the effect of fast duration on blood glucose and the relationship between blood glucose levels and Hb A1c in mice requires the determination of daily averaged blood glucose levels as a reference in this species.
Therefore, we established a method to continuously measure blood glucose level over 24 h in mice using the CGMS. This system determines glucose level in interstitial fluid. A correlation between interstitial fluid and blood glucose levels has been demonstrated in humans and several other species (19, 21, 28). The present studies further demonstrate that this correlation also exists in inbred mice.
Glucose excursions were observed in both control and diabetic mice, but the extent of glucose excursions was significantly greater in diabetic mice than their controls. The glucose excursion appears to be more pronounced during the night, consistent with the nocturnal nature of mice. Glucose excursions were also seen in some mice during the daytime following saphenous vein bleeding for blood glucose determination. Thus, interruption of daytime sleep by blood sampling may lead mice to consume food, contributing to daytime glucose excursions.
Daily averaged glucose levels are strain dependent. Of the three strains of mice studied, KK/HlJ mice exhibited the highest level of daily blood glucose, whereas the DBA/2J mice had the lowest averaged daily blood glucose. This observation is consistent with previous studies indicating that both KK/HlJ and C57BL/6J mice develop glucose intolerance (8, 33) and that glucose intolerance in KK/HlJ mice is more severe than in C57BL/6J mice (33).
Using daily averaged blood glucose as a reference value, we compared the effect of a 6-h vs. overnight fast on mouse blood glucose. The results indicate that 6-h-fasted blood glucose was closer to the level of daily averaged blood glucose than blood glucose following an overnight fast. In addition, body weight was greater in mice following a 6-h fast than following an overnight fast, consistent with the notion that a 6-h fast imposes less stress than an overnight fast. More importantly, blood glucose following a 6-h fast correlates with Hb A1c better than blood glucose following an overnight fast. This may be explained by the fact that mice are nocturnal feeders, so that initiation of a fast in the evening could effectively result in a 24-h fast, causing inordinately low blood glucose.
In humans, Hb A1c represents the major form of GlyHb and correlates with the averaged blood glucose levels over the preceding 3 mo and the development of diabetic complications (1, 7). The DCA 2000 system is designed to determine Hb A1c on the basis of an immunochemical technique that has been validated against human Hb A1c (2, 13) but has been widely used in mice (18, 36).
To validate Hb A1c values determined using the DCA 2000 analyzer as accurately reflecting GlyHb in mice, we compared these values with a boronate affinity HPLC method that measures total GlyHb on the basis of the presence of coplanar vicinal hydroxyl and/or carbonyl groups of hexoses (23, 24). Comparable values for Hb A1c and total GlyHb in C57BL/6J mice indicate that the Hb A1c determined using DCA 2000 analyzer represents a valid determination of GlyHb in this strain. Considering the small volume of blood required and the convenience and simplicity of operation, this method has considerable utility.
Interestingly, the Hb A1c values were consistently lower than total GlyHb in DBA/2J mice. A difference in Hb A1c determined using DCA 2000 has been observed previously (34). This may be explained by the variation in Hb β-chain alleles in inbred mice (9, 30). For example, DBA/2J appears to be homozygous for the Hbbd β-chain allele, whereas C57Bl/6J is homozygous for the Hbbs allele (30). The allele variation may result in changes in antigenicity and/or glycosylation of hemoglobin. Nevertheless, the close correlations between Hb A1c and total glycosylated Hb, as well as 6-h-fasted blood glucose, indicate that the DCA 2000 is a reliable approach for determination of Hb glycosylation and blood glucose levels in the strains studied. However, the correlations in other strains need to be validated prior to using DCA 2000 approach.
Although the boronate affinity HPLC method has been used as the gold-standard method for GlyHb in humans (23, 24), direct evidence indicating that this is also true in mice is lacking. However, agreement of strain susceptibility to diabetic nephropathy (14, 25) with the ratio of total GlyHb to fasting blood glucose among the strains of mice studied is consistent with boronate HPLC-determined total GlyHb providing a meaningful measurement of GlyHb in mice. The ratio of GlyHb to blood glucose has been suggested to predict diabetic complications in humans (15, 22, 32).
Both blood glucose level and glycosylated Hb are important and widely used parameters in diabetic research. For a short-term study or in settings involved in altered life span of erythrocytes or hemoglobinopathy (20), daily glucose profile and/or fasted blood glucose are more valuable. For long-term studies, especially pertaining to diabetic complications, glycosylated Hb is advantageous in that it is not subject to momentary changes due to stress or some other acute factor. Thus establishing the relationship of glycosylated Hb to fasting glucose will facilitate studies requiring assessment of glucose control.
In summary, we adapted a method to continuously monitor blood glucose in humans to use in conscious mice. The daily averaged blood glucose level determined using the glucose sensor data correlates closely with Hb A1c. Compared with an overnight fast, 6-h fasting blood glucose levels correlate more closely with daily averaged blood glucose levels and Hb A1c. The present studies also demonstrated that Hb A1c determined using the DCA 2000 analyzer correlates with total glycosylated Hb that was determined using a boronate HPLC approach in the three strains of mice studied. Nevertheless, this relationship is strain dependent, and Hb A1c values in DBA/2J mice are relatively lower than total glycosylated hemoglobin compared with C57BL/6J and KK/HlJ mice. Differences in other strains may also exist and should be explored on a strain-by-strain basis. The results of these studies show that Hb A1c measured using a low-cost, hand-held, automated analyzer (DCA 2000) provides an accurate means of assessing glycemic control in the mouse. Use of Hb A1c measurements should permit a more reliable determination of the contribution of blood glucose control to diabetic complications in mouse models.
GRANTS
This study was supported by a Pilot & Feasibility Grant (to Z. Qi) from the Vanderbilt Mouse Metabolic Phenotyping Center (National Institute of Diabetes and Digestive and Kidney Diseases Grant No. DK-59637 to D. H. Wasserman) and by the Animal Models of Diabetic Complication Consortium (to M. D. Breyer).
Acknowledgments
We thank Dr. Phillip E. Williams for technical support in the use of the MiniMed glucose sensor.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.No authors listed. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986, 1993. [DOI] [PubMed] [Google Scholar]
- 1a.No authors listed. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853, 1998. [PubMed] [Google Scholar]
- 1b.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 28, Suppl 1: S37–S42, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Arsie MP, Marchioro L, Lapolla A, Giacchetto GF, Bordin MR, Rizzotti P, Fedele D. Evaluation of diagnostic reliability of DCA 2000 for rapid and simple monitoring of HbA1c. Acta Diabetol 37: 1–7, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, Steppan CM, Ahima RS, Obici S, Rossetti L, Lazar MA. Regulation of fasted blood glucose by resistin. Science 303: 1195–1198, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bouma M, Dekker JH, de Sonnaville JJ, van der Does FE, de Vries H, Kriegsman DM, Kostense PJ, Heine RJ, van Eijk JT. How valid is fasting plasma glucose as a parameter of glycemic control in non-insulin-using patients with type 2 diabetes? Diabetes Care 22: 904–907, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Breyer MD, Böttinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K; AMDCC. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bunn HF, Gabbay KH, Gallop PM. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science 200: 21–27, 1978. [DOI] [PubMed] [Google Scholar]
- 8.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 55: 2153–2156, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gilman JG Hemoglobin Beta chain structural variation in mice: evolutionary and functional implications. Science 178: 873–874, 1972. [DOI] [PubMed] [Google Scholar]
- 10.Graf RJ, Halter JB, Porte D Jr. Glycosylated hemoglobin in normal subjects and subjects with maturity-onset diabetes. Evidence for a saturable system in man. Diabetes 27: 834–839, 1978. [DOI] [PubMed] [Google Scholar]
- 13.Guerci B, Durain D, Leblanc H, Rouland JC, Passa P, Godeau T, Charbonnel B, Mathieu Daude JC, Boniface H, Monnier L, Dauchy F, Slama G, Drouin P. Multicentre evaluation of the DCA 2000 system for measuring glycated haemoglobin. DCA 2000 Study Group. Diabetes Metab 23: 195–201, 1997. [PubMed] [Google Scholar]
- 14.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications 16: 313–320, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Holman RR, Turner RC. The basal plasma glucose: a simple relevant index of maturity-onset diabetes. Clin Endocrinol (Oxf) 14: 279–286, 1981. [DOI] [PubMed] [Google Scholar]
- 17.Holman RR, Turner RC. Optimizing blood glucose control in type 2 diabetes: an approach based on fasting blood glucose measurements. Diabet Med 5: 582–588, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Inada A, Nagai K, Arai H, Miyazaki J, Nomura K, Kanamori H, Toyokuni S, Yamada Y, Bonner-Weir S, Weir GC, Fukatsu A, Seino Y. Establishment of a diabetic mouse model with progressive diabetic nephropathy. Am J Pathol 167: 327–336, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klueh U, Liu Z, Cho B, Ouyang T, Feldman B, Henning TP, Kaur M, Kreutzer D. Continuous glucose monitoring in normal mice and mice with prediabetes and diabetes. Diabetes Technol Ther 8: 402–412, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Ly J, Marticorena R, Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis 44: 715–719, 2004. [PubMed] [Google Scholar]
- 21.Mastrototaro JJ The MiniMed continuous glucose monitoring system. Diabetes Technol Ther 2, Suppl 1: S13–S18, 2000. [DOI] [PubMed] [Google Scholar]
- 22.McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 27: 1259–1264, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Nuttall FQ Comparison of percent total GHb with percent HbA1c in people with and without known diabetes. Diabetes Care 21: 1475–1480, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Peterson KP, Pavlovich JG, Goldstein D, Little R, England J, Peterson CM. What is hemoglobin A1c? An analysis of glycated hemoglobins by electrospray ionization mass spectrometry. Clin Chem 44: 1951–1958, 1998. [PubMed] [Google Scholar]
- 25.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Rebrin K, Steil GM. Can interstitial glucose assessment replace blood glucose measurements? Diabetes Technol Ther 2: 461–472, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Rebrin K, Steil GM, van Antwerp WP, Mastrototaro JJ. Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol Endocrinol Metab 277: E561–E571, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 25: 275–278, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Russell ES, Gerald PS. Inherited electrophoretic hemoglobin patterns among 20 inbred strains of mice. Science 128: 1569–1570, 1958. [DOI] [PubMed] [Google Scholar]
- 31.Singer DE, Coley CM, Samet JH, Nathan DM. Tests of glycemia in diabetes mellitus. Their use in establishing a diagnosis and in treatment. Ann Intern Med 110: 125–137, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Snieder H, Sawtell PA, Ross L, Walker J, Spector TD, Leslie RD. HbA(1c) levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins. Diabetes 50: 2858–2863, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Suto J, Matsuura S, Imamura K, Yamanaka H, Sekikawa K. Genetic analysis of non-insulin-dependent diabetes mellitus in KK and KK-Ay mice. Eur J Endocrinol 139: 654–661, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Tabata H, Kubo M, Suzuki H, Matsuzawa T. Rapid determination of haemoglobin A1c and glucose in mice: strain difference, glucose tolerance tests and the neonatal streptozotocin-induced diabetic model. Comparative Haematology International 8: 53–57, 1998. [Google Scholar]
- 35.Wang Z, Moro E, Kovacs K, Yu R, Melmed S. Pituitary tumor transforming gene-null male mice exhibit impaired pancreatic beta cell proliferation and diabetes. Proc Natl Acad Sci USA 100: 3428–3432, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest 108: 261–268, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]