Abstract
The mitochondrial permeability transition (MPT) plays an important role in hepatocyte death caused by ischemia-reperfusion (IR). This study investigated whether activation of the cellular oxygen-sensing signal cascade by prolyl hydroxylase inhibitors (PHI) protects against the MPT after hepatic IR. Ethyl 3,4-dihyroxybenzoate (EDHB, 100 mg/kg ip), a PHI, increased mouse hepatic hypoxia-inducible factor-1α and heme oxygenase-1 (HO-1). EDHB-treated and untreated mice were subjected to 1 h of warm ischemia to ∼70% of the liver followed by reperfusion. Mitochondrial polarization, cell death, and the MPT were assessed by intravital confocal/multiphoton microscopy of rhodamine 123, propidium iodide, and calcein. EDHB largely blunted alanine aminotransferase (ALT) release and necrosis after reperfusion. In vehicle-treated mice at 2 h after reperfusion, viable cells with depolarized mitochondria were 72%, and dead cells were 2%, indicating that depolarization preceded necrosis. Mitochondrial voids excluding calcein disappeared, indicating MPT onset in vivo. NIM811, a specific inhibitor of the MPT, blocked mitochondrial depolarization after IR, further confirming that mitochondrial depolarization was due to MPT onset. EDHB decreased mitochondrial depolarization to 16% and prevented the MPT. Tin protoporphyrin (10 μmol/kg sc), an HO-1 inhibitor, partially abrogated protection by EDHB against ALT release, necrosis, and mitochondrial depolarization. In conclusion, IR causes the MPT and mitochondrial dysfunction, leading to hepatocellular death. PHI prevents MPT onset and liver damage through an effect mediated partially by HO-1.
Keywords: ethyl 3,4-dihyroxybenzoate; heme oxygenase; hepatic ischemia-reperfusion; mitochondrial permeability transition; prolyl hydroxylase inhibitor
ischemia-reperfusion (IR) injury to the liver occurs in trauma, hemorrhagic and cardiac shock, vascular diseases, and hepatic surgery, including tumor resection and liver transplantation. A variety of pathophysiological processes likely contribute to development of IR injury. Reactive oxygen species (ROS) play a critical role in the injury caused by IR (18, 36, 57). ROS not only directly damage cell membranes, DNA, and protein; they also trigger formation of toxic cytokines and increase adhesion molecules leading to inflammatory responses, tissue damage, and multiple organ failure (1, 10, 17, 41). Recently, growing evidence supports an important role of the mitochondrial permeability transition (MPT) in cell injury after IR (24, 25, 45, 58). The mitochondrial membrane potential collapses when the MPT occurs, leading to failure of ATP synthesis, release of cytochrome c, and cell death (24, 25, 55). ROS cause opening of MPT pores (22, 31, 43, 59).
Low oxygen tension elicits a variety of complex cellular responses by altering the activity of many signaling pathways, such as AMP kinase, mammalian target of rapamycin, and oxygen-dependent proline hydroxylase-domain containing enzymes (PHD, prolyl hydroxylases). Reduction of PHD activity during hypoxia leads to stabilization and accumulation of hypoxia-inducible factor-1α (HIF-1α), a transcription factor that regulates the expression of target genes in response to hypoxia. Several studies have found that PHD inhibitors (PHI) recapitulate various cellular responses to hypoxia and preconditioning stimuli. These include HIF-1α stabilization, induction of hypoxia-inducible genes [e.g., heme oxygenase 1 (HO-1) and GLUT-1], stimulation of angiogenesis, and protection against metabolic stress (3, 42, 50, 52). Although upregulation of the HIF-1α target gene HO-1 was reported to protect liver from IR injury (2, 40), it is unknown, however, whether increased HIF-1α or HO-1 can prevent onset of the MPT after IR in vivo.
Study of mitochondrial dysfunction and the MPT in vivo has been difficult. Developments in intravital confocal/multiphoton microscopy in recent years provide novel approaches to visualize mitochondrial depolarization and onset of the MPT in live animals (45, 58). Therefore, this study investigated the potential of pharmacological activation of the PHD/HIF-1α pathway by a cell-permeable PHI, ethyl-3,4 dihydroxybenzoate (EDHB), to prevent mitochondrial dysfunction after warm IR in vivo using novel intravital confocal/multiphoton microscopic techniques.
MATERIALS AND METHODS
Animals.
Male C57Bl/6 (8–9 wk) mice were pretreated with EDHB (100 mg/kg ip; Sigma, St. Louis, MO) or an equivalent volume of DMSO daily for 3 days prior to surgery. Under ether anesthesia, body temperature was maintained at ∼37°C with warming lamps. Hepatic ischemia was induced by clamping the artery and portal vein to the top three lobes of the liver (i.e., ∼70% of total liver). Thirty minutes to 1 h later, the ischemic liver was reperfused by opening the vascular clamp. In some mice, Sn(IV) protoporphyrin IX dichloride (SnPP, 10 μmol/kg sc, Frontier Scientific, Logan, UT) was prepared as described elsewhere (51) and injected 1 h before ischemia to inhibit HO-1 (Fig. 1A). SnPP at doses of 3 μmol/kg or higher completely inhibits HO-1 activity (51). A timeline for the EDHB and SnPP treatments, the ischemia-reperfusion procedure and sample collection is shown in Fig. 1A. N-methyl-4-isoleucine cyclosporin (NIM811), a nonimmunosuppressive derivative of cyclosporin A, was given to some mice (20 mg/kg ig) 1 h before surgery to inhibit onset of the MPT (46). All animals were given humane care in compliance with institutional guidelines using protocols approved by the Institutional Animal Care and Use Committee.
Fig. 1.
Ethyl 3,4-hydroxybenzoate (EDHB) activates hypoxia inducible factor (HIF)-1α, upregulates heme oxygenase-1 (HO-1), and protects against hepatic necrosis after hypoxia-reperfusion. A: timeline shows the sequence of EDHB and Sn(IV) protoporphyrin IX dichloride (SnPP) treatments, the ischemia-reperfusion (IR) procedure, and sample collection. MPT, mitochondrial permeability transition; ROS, reactive oxygen species; ALT, alanine aminotransferase. B: mice were pretreated with EDHB (100 mg/kg ip) or an equivalent volume of DMSO. Livers were harvested 4 h after EDHB treatment without hypoxia-reperfusion, and HIF-1α (120 kDa) was detected by Western blotting in nuclear extracts. C: mice were pretreated with EDHB daily for up to 3 days. Livers were harvested 16 h after 1 dose of EDHB or 24 h after 1 day and 3 days of EDHB treatment without hypoxia-reperfusion. HO-1 (32 kDa) was detected by Western blotting. D: mice were pretreated with EDHB daily for 3 days. Livers were harvested 2 h after reperfusion following 1 h of ischemia. HO-1 was detected by immunoblotting. Representative Western blot images are shown in B, C, and D (n = 3–4 in each group). E: mice were pretreated with EDHB or DMSO daily for 3 days prior to liver ischemia for 0.5 or 1 h followed by reperfusion, as described in materials and methods. At 6 h after reperfusion, livers were fixed, sectioned, and stained with hematoxylin and eosin. Representative images are shown (n = 4 in each group). Left, 0.5 h of ischemia; right, 1 h of ischemia. Top, sham operation; middle, ischemia-reperfusion; bottom, ischemia-reperfusion + EDHB treatment. Bar is 100 μm.
Serum transaminase and total bilirubin.
Before ischemia and at 2 and 6 h after reperfusion, mice were anesthetized with pentobarbital (80 mg/kg ip), and blood samples were collected from the vena cava. Serum alanine aminotransferase (ALT) activity and bilirubin were determined using analytical kits from Pointe Scientific (Uncoln Park, MI).
Histology and immunohistochemical staining for hypoxia inducible factor-1α and 4-hydroxynonenal.
Livers were harvested under pentobarbital anesthesia (80 mg/kg ip), and slides were prepared as described elsewhere (58). Liver sections stained with hematoxylin-eosin were imaged using a Universal Imaging Image-1/AT image acquisition and analysis system (West Chester, PA) incorporating an Axioskop 50 microscope (Carl Zeiss, Thornwood, NY) and a ×10 objective lens, and necrotic areas were quantified by image analysis in a blinded manner (56).
Hypoxia inducible factor-1α (HIF-1α) expression in liver sections was determined by immunohistochemical staining. Briefly, sections were deparaffinized with xylene (Mallinckrodt Baker, Paris, KY) and taken through a graded series of alcohol/water mixtures to rehydrate the tissue (58). Sections were incubated with 10 mM citric acid buffer (pH 6.0) at 95–100°C for 20 min to retrieve antigen. Sections were then exposed to anti-HIF-1α monoclonal antibody (1:1,000 dilution) in 0.1 M phosphate buffer (pH 7.1) containing 0.1% Tween-20 and 1% bovine albumin (Sigma, St. Louis, MO) overnight at 4°C. Peroxidase-conjugated anti-mouse IgG1 antibody (DAKO, Carpinteria, CA) was applied, and then 3,3′-diaminobenzidine chromogen was added as the peroxidase substrate. 4-Hydroxynonenal (4-HNE) adducts, a marker of lipid peroxidation (11), were detected immunohistochemically (56).
Western blotting.
HIF-1α was not detectable by Western blotting in whole liver extracts, probably because the transcription factor is present in very low levels even when induced by hypoxia. Accordingly, we used nuclear extracts that were prepared from livers as previously described (14). Nuclear extracts were subjected to electrophoresis, as described below, and HIF-1α protein was assessed using standard immunoblotting procedures (Santa Cruz, Lexington, KY).
To detect HO-1, liver tissue was homogenized in 0.1% Triton X-100 buffer containing protease and phosphatase inhibitors, and the extract was centrifuged at 14,000 g for 15 min at 4°C. Aliquots of supernatant (40 μg of protein) were separated on 4–12% SDS-PAGE gels, transferred onto nitrocellulose membranes, and immunoblotted with primary antibodies (Affinity Bioreagents, Golden, CO) specific for HO-1 (1:1,000 overnight at 4°C). Horseradish peroxidase-conjugated secondary antibodies were applied, and detection was by chemiluminescence (ECL, Amersham).
Detection of thiobarbituric acid reactive substances.
Liver tissue was homogenized in 50 mM phosphate buffer (pH 7.4). Formation of malondialdehyde, a product of lipid peroxidation, was determined as thiobarbituric acid reactive substances (TBARS) using 1,1,3,3,-tetraethoxypropane (Science Lab, Houston, TX) as a standard (21). Protein in the liver homogenates was determined by using a Bio-Rad Protein Assay reagent (Bio-Rad Laboratories, Hercules, CA).
Intravital confocal and multiphoton microscopy.
Rhodamine 123 (Rh123, Sigma, St. Louis, MO), a cationic fluorophore that is taken up by polarized mitochondria in response to their negative membrane potential, was used to monitor mitochondrial polarization after hepatic IR. At 2 h after reperfusion or sham operation, mice were anesthetized with pentobarbital (80 mg/kg ip) and connected to a small animal ventilator via tracheotomy and a respiratory tube (20-gauge catheter). Rh123 (2 μmol/mouse) and propidium iodide (PI, 0.04 μmol/mouse), which labels nuclei of nonviable cells, were infused via polyethylene (PE10) tubing inserted into the carotid artery over 10 min.
MPT pore opening was assessed using calcein acetoxymethyl ester (calcein-AM) (5). When calcein-AM enters cells, esterases cleave it to form calcein-free acid. Because mitochondria are normally impermeant to calcein, calcein fluoresces in the cytosol but not in mitochondria, which appear as dark voids. These voids disappear after onset of the MPT as calcein, a 623-Da solute, gains entrance to the mitochondrial matrix space through MPT pores (35). At 2 h after reperfusion or sham operation, calcein-AM (1 mg/mouse) was injected slowly into the rectal vein. To prevent biliary excretion of calcein, an anion channel inhibitor, bromosulfophthalein (6.6 μmol/mouse), was injected into the rectal vein 5 min before calcein-AM.
For intravital confocal microscopy, laparotomized mice were put in a prone position over a coverslip mounted on the stage of CARV spinning disk confocal microscopic system (ATTO Bioscience, Rockville, MD) with a ×40 water immersion objective lens (Zeiss C-Apochromat, 1.2 numerical aperture) using excitation wavelengths of 488 and 555 nm, respectively, and a multiwavelength emission filter. During image acquisition, the respirator was turned off for ∼5 s to eliminate breathing movement artifacts. In 10 fields per mouse, parenchymal cells were scored in a blinded fashion for bright punctate Rh123 fluorescence representing cells with polarized mitochondria or a dimmer diffuse cytosolic fluorescence representing cells with depolarized mitochondria. Nonviable PI-positive cells, indicated by red nuclear fluorescence, were also counted.
Mitochondrial depolarization and onset of the MPT were also detected in some mice by intravital multiphoton microscopy which can achieve higher resolution of images and deeper penetration into the tissue (30). Intravital multiphoton microscopy was performed using a laser scanning confocal/multiphoton microscopic system (Zeiss LSM510; Zeiss, Thornwood, NY) with a ×63 water-immersion objective lens. Fluorescence of Rh123 and PI was imaged with excitation wavelengths of 820 and 500–550 nm and 650- to 710-nm band pass emission filters, respectively. For imaging of calcein, an excitation wavelength of 720 nm and a 500- to 550-nm band pass emission filter were used. Approximately 10 randomized images throughout the liver were collected from each mouse.
Statistical analysis.
All groups were compared by ANOVA plus Student-Newman-Keuls post hoc test, Student's t-test, or Kaplan-Meier test, as appropriate. Values are means ± SE. Differences were considered significant at P < 0.05.
RESULTS
EDHB induces HIF-1α and HO-1 protein levels after hepatic IR.
Broad spectrum PHIs, including EDHB, stabilize and activate HIF-1α in vitro (39, 54). However, the pharmacokinetics of EDHB in vivo have not been reported, so it was important to show that the EDHB dosing protocol used in these studies was sufficient to activate the PHD pathway in liver. Previously, we found that HIF-1α protein accumulation is maximal after ∼4 h of EDHB treatment in cultured myocytes (42), so this time point was selected to establish HIF-1α accumulation in liver after EDHB treatment (Fig. 1A). Nuclear extracts from the livers of EDHB-treated mice had elevated HIF-1α compared with vehicle-treated animals which showed no detectable HIF-1α (Fig. 1B). Similarly, HIF-1α was undetectable in the nuclei of hepatocytes after immunohistochemical staining of liver sections from vehicle-treated mice. By contrast, 4 h after EDHB treatment, nuclear HIF-1α staining increased markedly and was maintained at higher levels after 1 and 2 days of EDHB treatment (data not shown).
To ensure that the EDHB-induced HIF-1α accumulation we observed was sufficient to drive the expression of target genes in the liver, the levels of HIF-1α-inducible HO-1 were examined. A basal level of HO-1 protein was detected in livers of mice not exposed to EDHB (Fig. 1C). HO-1 increased at 24 h after the first dose of EDHB and remained elevated after three daily doses of EDHB (Fig. 1, A and C). HO-1 levels after sham operation were similar to untreated mice but increased after 1-h ischemia plus 2-h reperfusion (Fig. 1, A and D). HO-1 increased to a higher level in the EDHB-treated group compared with the vehicle-treated group at 2 h after reperfusion (Fig. 1D).
HO-1 catalyzes the breakdown of heme into biliverdin, which is further converted to bilirubin (32). Serum bilirubin concentration was 0.19 ± 0.028 mg/dl in mice receiving vehicle but increased to 0.52 ± 0.011 mg/dl after 3 days treatment with EDHB (means ± SE, P < 0.01, n = 4 per group), consistent with upregulation of HO-1. These data indicate that in vivo EDHB treatment activates the PHD pathway and increases expression of HIF-1α target genes in liver.
EDHB treatment prevents liver injury and improves survival after hepatic IR.
No pathological changes were observed in liver tissue after sham operation (Fig. 1E, top). Focal necrosis occurred after 0.5-h ischemia plus 6 h of reperfusion (Fig. 1E, middle left). Necrotic areas became panlobular after 1-h ischemia plus 6 h of reperfusion (Fig. 1E, middle right), but necrosis was barely discernable at 2 h after reperfusion (data not shown). After EDHB pretreatment, necrosis was rarely detectable after 0.5-h ischemia plus 6-h reperfusion (Fig. 1E, bottom left) and decreased markedly in livers after 1-h ischemia plus 6 h of reperfusion compared with the vehicle-treated livers (Fig. 1E, bottom right).
Before ischemia, serum ALT was ∼35 U/l. At 6 h after reperfusion, ALT increased to ∼7,000 and ∼25,000 U/l in livers exposed to 0.5-h and 1-h ischemia, respectively (Fig. 2A). EDHB decreased ALT to ∼600 and 3,000 U/l in livers exposed to 0.5-h ischemia and 1-h ischemia, respectively, which reflects a ∼90% decrease compared with livers without EDHB treatment (Fig. 2A). These results indicate that EDHB markedly decreases hepatic IR injury.
Fig. 2.
EDHB protects against alanine aminotransferase release and improves survival after hepatic ischemia-reperfusion. Mice were pretreated with EDHB or DMSO daily for 3 days prior to liver IR, as described in Fig. 1. Blood samples were collected at 6 h after reperfusion for ALT (A). Nonischemic liver tissue was removed immediately after ischemia for observation of survival (B). Group sizes were 4–5 livers per group. aP < 0.05 vs. sham operation; bP < 0.05 vs. corresponding ischemia group (isch).
All mice survived after sham operation (data not shown) and 0.5-h ischemia plus reperfusion (Fig. 2B). Survival decreased to 10% after 1-h ischemia plus reperfusion (Fig. 2B). Death occurred mainly in the first 18 h after reperfusion (Fig. 2B). By contrast, in EDHB-pretreated mice, survival was 70% after 1-h ischemia plus reperfusion (Fig. 2B).
Mitochondrial depolarization occurs after hepatic IR: prevention by EDHB.
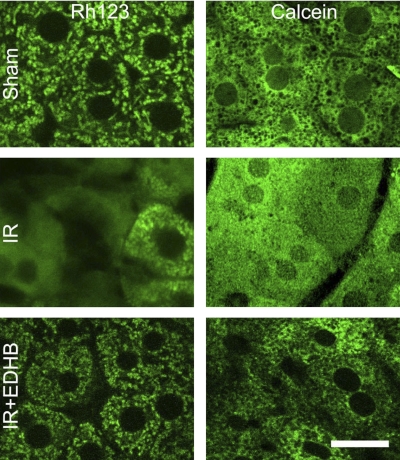
If the MPT occurs, mitochondria will depolarize. To detect whether mitochondrial depolarization occurs after hepatic IR in vivo, we performed intravital multiphoton fluorescent microscopy to image liver cell mitochondria using Rh123, a fluorophore that is taken up by polarized mitochondria. In sham-operated mice, green Rh123 fluorescence was punctate in virtually all hepatocytes, indicating mitochondrial polarization (Fig. 3, top left). By contrast, mitochondria in many hepatocytes did not take up Rh123 at 2 h of reperfusion after 1-h ischemia (Fig. 3, middle left). Importantly, mitochondrial depolarization was rare in the livers of EDHB-treated animals (Fig. 3, bottom left).
Fig. 3.
EDHB prevents mitochondrial depolarization and onset of the mitochondrial permeability transition after hepatic ischemia-reperfusion. Mice were pretreated with EDHB or DMSO daily for 3 days prior to 1 h of liver ischemia. At 2 h after reperfusion, rhodamine 123 (Rh123) and propidium iodide (PI) were infused, and intravital multiphoton microscopy was performed as described in materials and methods. Representative images are shown. Left, Rh123; right, PI. Top, sham operation; middle, ischemia-reperfusion; bottom, ischemia-reperfusion plus EDHB treatment. Bar is 20 μm.
Calcein, a fluorophore that loads into the cytosol, outlined mitochondria as dark voids in the hepatocytes from sham-operated mice (Fig. 3, top right). These voids disappeared at 2 h after reperfusion (Fig. 3, middle right). Since calcein gains entrance to the mitochondrial matrix space only when MPT pores open, disappearance of voids indicated onset of the MPT. This new finding shows directly that the MPT occurs in vivo and is a sequel of IR insult to the liver. Furthermore, EDHB effectively prevented onset of the MPT in vivo (Fig. 3, bottom right).
HO-1 partly mediates protective effects of EDHB on hepatic IR injury.
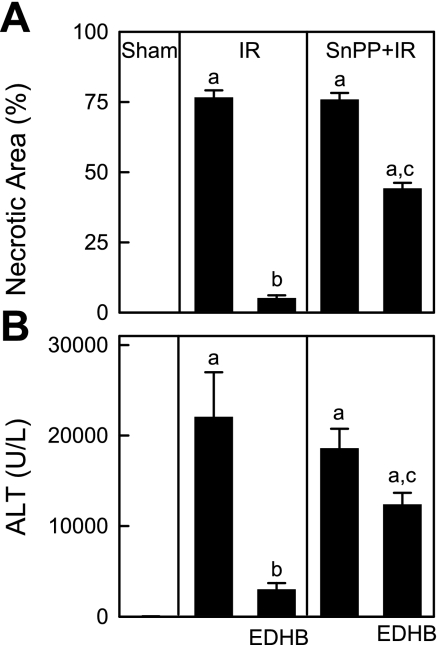
Expression of HO-1, a HIF-1 responsive gene, was induced in liver by EDHB treatment (Fig. 1, C and D). Accordingly, we investigated whether HO-1 mediates the protection conferred by EDHB on hepatic injury after 1 h of ischemia followed by reperfusion. Massive hepatic necrosis of 75% occurred 6 h after IR (Fig. 4A), which was markedly attenuated by EDHB to 5% (Fig. 4A). By contrast, in mice given SnPP, a specific inhibitor of HO-1, protection of EDHB on necrosis was partly abrogated, and necrosis increased from 5 to 44% (Fig. 4A).
Fig. 4.
HO-1 mediates protection of EDHB against hepatic ischemia-reperfusion injury. Mice were pretreated with EDHB daily for 3 days. Some mice were pretreated with SnPP, a specific inhibitor of HO-1, 1 h before surgery. After 1 h of ischemia and 6 h of reperfusion, livers were harvested for histology, as described in materials and methods. Five random fields per slide were captured in a blinded manner, and necrotic areas were quantified by image analysis using the IPlab 3.7v software (BD Biosciences, Rockville, MD) by dividing the necrotic areas by the total cellular area of the images (A). Blood samples were collected at 6 h after reperfusion for ALT measurement (B). Group sizes were 4–5 livers per group. aP < 0.05 vs. sham operation; bP < 0.05 vs. corresponding ischemia-reperfusion group; cP < 0.05 vs. corresponding ischemia-reperfusion group pretreated with EDHB.
At 6 h after reperfusion, ALT increased to 22,000 U/l in livers exposed previously to 1-h ischemia (Fig. 4B). EDHB decreased ALT by 86% (Fig. 4B). By contrast, in mice pretreated with SnPP, EDHB decreased ALT only by 43% (Fig. 4B). These results indicate that HO-1 participates in, but does not fully account for, the protective effect of EDHB on hepatic IR injury.
SnPP decreases protection by EDHB against mitochondrial depolarization.
ROS trigger onset of the MPT (22, 31, 43, 59), and the protective effects of HO-1 have been suggested to be due to antioxidant effects (32). Therefore, the question of the involvement of HO-1 in protection against mitochondrial depolarization was investigated. Mitochondrial depolarization and cell death were detected in living mice by use of a CARV spinning disc confocal microscopic system. The CARV microscope, unlike the laser scanning confocal/multiphoton microscope, permits video frame rate imaging, making movement artifacts less of a problem although resolution is less than with multiphoton microscopy. We used green-fluorescing Rh123 to monitor mitochondrial polarization and red-fluorescing PI to detect the nuclei of nonviable cells.
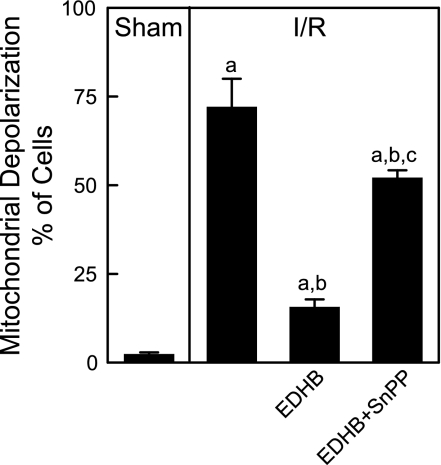
In sham-operated mice, green Rh123 fluorescence was punctate in virtually all hepatocytes, indicating mitochondrial polarization (Fig. 5, top left), and red PI staining in nuclei was negligible (Fig. 5, top middle). At 2 h after IR, mitochondria of 72% of hepatocytes did not take up Rh123 (Fig. 5, 2nd row left and Fig. 6). Despite the absence of mitochondrial polarization, the majority of hepatocytes maintained membrane integrity as indicated by lack of nuclear PI staining, and only 2% of parenchymal and nonparenchymal cells took up PI (Fig. 5, 2nd row middle). In some cells, Rh123 fluorescence was relatively bright but diffuse, possibly indicating the recent depolarization of mitochondria and release of mitochondrial Rh123 into the cytosol. These results indicated that at 2 h after hepatic IR, mitochondrial depolarization had occurred in most hepatocytes and that this event preceded hepatocyte death. NIM811 largely prevented mitochondrial depolarization, further confirming that mitochondrial depolarization after IR was caused by onset of the MPT (Fig. 5, 3th row left). Importantly, mitochondrial depolarization was decreased to 16% by EDHB (Figs. 5, 4th row left, and 6). By contrast, in mice pretreated with SnPP to inhibit HO-1, EDHB protection against mitochondrial depolarization was reverted, and 51% of hepatocytes displayed depolarized mitochondria (Figs. 5, 5th row left, 6). These results indicated that activation of the oxygen-sensing signal pathway prevented mitochondrial depolarization and that this effect was mediated, in part, by upregulation of HO-1.
Fig. 5.
Heme oxygenase-1 mediates protection of EDHB against mitochondrial depolarization after hepatic hypoxia-reperfusion. After 1 h of ischemia and 2 h of reperfusion, Rh123 and PI were infused, and intravital confocal microscopy was performed as described in materials and methods. Representative images are shown. Left, Rh123; middle, PI; right, overlay. Rows: 1st, sham operation; 2nd, ischemia-reperfusion; 3rd, ischemia-reperfusion plus NIM811 treatment; 4th, ischemia-reperfusion plus EDHB treatment; 5th, ischemia-reperfusion plus EDHB and SnPP treatment. Bar is 20 μm.
Fig. 6.
SnPP partially abrogates protection of EDHB against mitochondrial depolarization after hepatic ischemia-reperfusion. Conditions were as in Fig. 5. Viable cells with polarized mitochondria, viable cells with depolarized mitochondria, and dead cells were counted in 10 random fields for each liver. Group sizes were 4–5 livers per group. aP < 0.05 vs. sham operation; bP < 0.05 vs. corresponding ischemia-reperfusion group; cP < 0.05 vs. corresponding ischemia-reperfusion group pretreated with EDHB.
SnPP diminished protective effects of EDHB on hepatic lipid peroxidation.
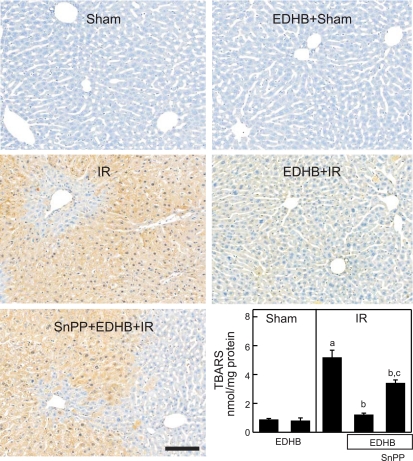
Hepatic IR increases ROS (34, 57), and HO-1 increases production of antioxidant biliverdin and bilirubin (32). Therefore, we investigated the effects of EDHB and SnPP on production of ROS. Adducts of 4-HNE, a product of lipid peroxidation (11), were barely detectable in livers from sham-operated mice with and without EDHB treatment (Fig. 7, top). 4-HNE adducts increased markedly 2 h after hepatic IR (Fig. 7, middle left), which was largely blocked by EDHB treatment (Fig. 7, middle right). SnPP alone did not significantly alter 4-HNE adduct formation after hepatic IR (data not shown). By contrast, in SnPP-treated mice prevention of 4-HNE adduct formation by EDHB was partially blunted by inhibition of HO-1 with SnPP (Fig. 7, bottom left), indicating that prevention of ROS formation after hepatic IR by EDHB is mediated, in part, by upregulation of HO-1.
Fig. 7.
Heme oxygenase-1 mediates protection of EDHB against 4-hydroxynonenal (4-HNE) adduct and thiobarbituric acid reactive substances (TBARS) formation after hepatic ischemia-reperfusion. After 1-h ischemia and 2-h reperfusion, livers were harvested and 4-HNE adducts were detected immunohistochemically. Representative images are shown. Top left, sham operation; top right, EDHB plus sham operation; middle left, IR; middle right, EDHB plus IR; bottom left, SnPP plus EDHB and IR. Bar is 100 μm. Malondialdehyde, a product of lipid peroxidation, was determined as thiobarbituric acid reactive substances (TBARS) (bottom right). Group sizes were 4 livers per group. aP < 0.05 vs. sham operation; bP < 0.05 vs. ischemia-reperfusion group; cP < 0.05 vs. ischemia-reperfusion group pretreated with EDHB.
The effects of EDHB and SnPP on production of ROS were further evaluated by detection of malondialdehyde, a product of lipid peroxidation, as TBARS (21). TBARS was ∼0.9 nmol/mg protein in livers from vehicle- or EDHB-treated mice that had undergone sham operation (Fig. 7, bottom right). TBARS increased sixfold in vehicle-treated mice at 2 h after IR but only increased 1.3-fold in EDHB-treated mice. Suppression of TBARS formation by EDHB was partially blunted by inhibition of HO-1 with SnPP (Fig. 7, bottom right).
DISCUSSION
Activation of the oxygen-sensing signal cascade increases HIF-1α in the liver and prevents liver IR injury.
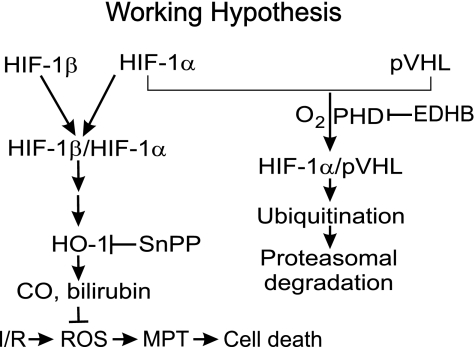
Recently, a family of oxygen-dependent PHDs has been identified as a cellular oxygen-sensing mechanism (4). During hypoxia, decreased PHD activity initiates a signaling cascade that includes the accumulation and activation of HIF-1α, a transcription factor that plays a crucial role in mediating cellular responses to low oxygen tension. The DNA-binding complex of HIF is a heterodimer of HIF-1α and HIF-1β subunits (Fig. 8) (47, 48), and the former is the target of the oxygen-sensitive signaling pathway. Under normoxic conditions, HIF-1α subunits have a short half-life and low steady-state levels due to continuous proteolytic degradation (Fig. 8) (19, 20). Oxygen availability enables PHD-dependent prolyl hydroxylation of the HIF-1α, thus allowing binding of the von Hippel Lindau tumor suppressor protein-E3 ubiquitin ligase (pVHL), leading to ubiquitination and degradation of HIF-1α subunits (Fig. 8) (4). In hypoxia, the PHD enzymes are inactive, resulting in stabilization and accumulation of HIF-1α, which forms a DNA-binding heterodimer with HIF-1β (4). Cell-permeable PHI inhibits PHDs, thus increasing HIF-1α, which in turn regulates the expression of numerous genes (Fig. 8).
Fig. 8.
Working hypothesis for EDHB protection against hepatic ischemia-reperfusion injury. Hepatic ischemia-reperfusion increases production of reactive oxygen species (ROS), which trigger onset of the MPT and cell death. The prolyl hydroxylase domain-containing enzyme inhibitor, EDHB, inhibits HIF-1α degradation by ubiquitination and proteosomal degradation. Consequently, HIF-1α increases and upregulates expressin of HO-1, which produces the antioxidants bilirubin and biliverdin, thus blocking ROS-induced MPT onset and liver injury. SnPP partially abrogates the protective effects of EDHB by inhibiting HO-1. pVHL, von Hippel Lindau tumor suppressor protein-E3 ubiquitin ligase.
In this study, we investigated whether suppression of PHDs conferred protection against hepatic IR injury. We found that EDHB, a PHI, effectively upregulated HIF-1α levels in vivo and induced expression of the HIF-1α responsive gene, HO-1 (Fig. 1). Accordingly, we used EDHB to inhibit PHD and activate the HIF-1α-dependent oxygen-sensing pathway rather than other approaches, such as siRNA, that are less amenable to clinical application. After IR, severe liver injury (marked ALT release and hepatic necrosis) occurred, and survival decreased to 10% (Fig. 1 and 2). Liver injury and increased mortality were blunted by EDHB by more than 80% (Fig. 1 and 2). These data indicate that activation of the oxygen-sensing cascade with PHI effectively prevents hepatic IR injury and suggest a pharmacological strategy to protect liver tissue in instances where IR injury is anticipated, such as prior to liver resection and transplantation.
The MPT occurs after IR in vivo: prevention by activation of the oxygen-sensing signal cascade.
Energy supply is critical for cell survival. The MPT onset is an important mechanism leading to mitochondrial dysfunction (59). MPT pores are highly conductive, nonselective “megachannels” in the mitochondrial membranes. The exact molecular composition of MPT pores remains unclear. In one model, MPT pores are composed of the adenine nucleotide translocator, the voltage-dependent anion channel, cyclophilin D, and ancillary proteins (33), whereas in an alternative model, aggregation of damaged, misfolded integral membrane proteins in association with cyclophilin D and other molecular chaperones forms MPT pores (16). Onset of the MPT leads to mitochondrial membrane potential collapse, failure of oxidative phosphorylation, and necrotic cell death (24, 25, 55). Uncoupling of oxidative phosphorylation after the MPT may also stimulate ROS production (59), thus causing a vicious cycle. In addition, the MPT causes large amplitude swelling and release of cytochrome c from the intermembrane space, which triggers apoptosis (22, 55).
Growing evidence from in vitro studies indicates that MPT onset is a common pathway of cell necrosis and apoptosis in IR injury (24, 25). For example, in cultured hepatocytes, IR induces opening of MPT pores, leading to mitochondrial inner membrane permeabilization, depolarization, and large-amplitude swelling (24, 25, 38). In isolated perfused rat heart and cultured myocytes, sanglifehrin-A and cyclosporin A, which suppress MPT pore opening, protect against IR injury (15, 26). Clearly, the MPT onset plays an important role in IR injury in vitro.
Evidence of the MPT onset in vivo is rare since detection of the MPT in live animals has been difficult. However, recent developments in fluorescent probes and confocal/multiphoton microscopy make monitoring the MPT in vivo possible (45, 58). In this study, we detected changes of mitochondrial polarization, cell death, and onset of MPT after hepatic IR in live mice using novel intravital confocal/multiphoton microscopic techniques. At 2 h after reperfusion, mitochondria depolarization, as indicated by loss of Rh123 fluorescence, occurred in many viable hepatocytes (Figs. 3 and 5). Calcein, a cytosolic flurophore, entered mitochondria after IR, indicating onset of the MPT (Fig. 3). NIM811, a specific inhibitor of the MPT, largely blocked mitochondria depolarization (Fig. 5). These data showed directly that the MPT not only occurs in vitro but also occurs in vivo after warm IR and that this event precedes cell death. Interestingly, time courses of MPT onset and cell death appear to be different for livers in vivo compared with cultured hepatocytes in vitro. In cultured hepatocytes, mitochondrial depolarization and cell death occur within about an hour of reperfusion (25, 27). By contrast in livers in vivo, mitochondrial depolarization becomes maximal in ∼2 h and remains high until 4 h after reperfusion. Onset of cell death then follows and reaches maximal after 6 h (45). These findings indicate a slower rate of MPT onset and cell death in vivo in whole liver compared with in vitro in cultured hepatocytes. The basis for this difference remains unknown. In the present study, mitochondria of ∼70% of hepatocytes depolarized at 2 h after reperfusion but cell death was still rare (Figs. 1 and 5), whereas after 6 h cell death was overt (Fig. 1). Importantly, activation of oxygen-sensing signaling cascade by EDHB effectively prevented the MPT onset and mitochondrial depolarization after hepatic IR (Figs. 3 and 5). This effect of EDHB was associated with substantially decreased cell death at later time points (Fig. 2). These findings suggest that activation of oxygen-sensing signaling cascade minimizes IR injury, most likely by preventing mitochondrial dysfunction.
Protection of PHI against the MPT and IR injury is mediated, in part, by upregulation of HO-1.
Inhibition of PHD leads to increased HIF-1α and decreased hepatic IR injury (Figs. 1 and 2). HIF-1α activates the expression of a variety of genes that mediate a myriad of compensatory responses, including upregulation of anaerobic glycolytic metabolism, increases of glucose transporter (GLUT-1) and erythropoietin expression, stimulation of angiogenesis, and induction of HO-1 (3, 50, 52). In addition to HIF-1α, PHDs also directly hydroxylate other targets to effect HIF-1α-independent cellular changes (8, 9, 28). Cell-permeable broad spectrum PHIs induce changes in gene expression and cellular responses in normoxia that closely follow those observed with authentic hypoxia. A remarkable concordance in changes of expression of hundreds of genes was noted between PHI treatment and hypoxia exposure in cultured cells (9).
HIF-1α mediates transcriptional activation of the HO-1 gene in response to hypoxia (29). HO-1 protects against IR injury in many organs. For example, inhibition of HO-1 with SnPP increases renal dysfunction after IR (13). Induction of HO-1 expression by tetramethylpyrazine attenuates rat myocardial infarction after IR (6). Induction of HO-1 protects neurons against transient forebrain ischemia (44). In the liver, upregulation of HO-1 confers protection against warm IR injury (2) and improves survival and graft function after rat liver transplantation (40). Although upregulation of HO-1 was reported to protect liver from IR injury in some studies, opposite results also exist. For example, higher level of HO-1 in human livers before transplantation correlates with graft injury and poorer liver function after transplantation (12). In this study, we observed an increase of HO-1 after PHI treatment (Fig. 1), which was associated with decreased hepatic IR injury (Figs. 1 and 2). Inhibition of HO-1 reversed the protection of EDHB on hepatic IR injury by ∼55% (Fig. 4). These data indicate that activation of oxygen-sensing signal cascade confers protection to hepatic IR injury, in part, by upregulation of HO-1. The rest of protection by EDHB might conferred by upregulation of other HIF-1α responsive genes such as erythropoietin, which increases modestly by EDHB (23), or by other direct targets of oxygen-sensing PHDs besides HIF-1α, such as activation of NF-κB (8). The specific roles of HIF-1α responsive genes other than HO-1 in protection against IR injury by EDHB will need to be evaluated in future studies.
Although upregulation of HO-1 was reported to protect liver from IR injury in some studies (2, 40); it is unknown, however, whether HO-1 works by prevention of the MPT onset after IR. In this study, we used intravital confocal/multiphoton microscopy techniques to investigate the effects of HO-1 on the MPT and mitochondrial depolarization in vivo. We found that protection of EDHB on hepatic IR injury was associated with prevention of the MPT and mitochondrial depolarization (Fig. 3 and 5), and this effect was partly reversed by inhibition of HO-1 with SnPP (Fig. 5). These data suggest that upregulation of HO-1 mediates, at least in part, the prevention of the MPT by EDHB. HO-1 protein is normally expressed in both microsomes and inner mitochondrial membranes (7). Inducers of HO-1 increase HO-1 targeting to the inner mitochondrial membrane, which is associated with a decrease of mitochondrial heme content and a reduction of NO-dependent mitochondrial oxidant production (7). Therefore, mitochondrial HO-1 may have important biological functions in regulating mitochondrial heme protein turnover and in protecting against conditions such as IR injury in which mitochondrial production of ROS substantially increases.
ROS cause opening of MPT pores (Fig. 8) (22, 31, 43, 59). Previously, we showed that free radical production increases within 1 h after reperfusion following hepatic warm ischemia (57). In the present work, we showed additionally that lipid peroxidation increased at this early stage after reperfusion (Fig. 7). This finding is consistent with the conclusion that ROS formation contributes to MPT onset rather than the MPT causing ROS formation. HO-1 catalyzes the breakdown of heme into biliverdin, carbon monoxide, and iron (Fig. 8) (32). Biliverdin is converted to bilirubin, a potent antioxidant, and carbon monoxide functions as a signaling molecule (32, 53). Consistent with HO-1 upregulation, serum bilirubin increased after EDHB pretreatment. Carbon monoxide inhibits ROS production in lung endothelial cells, thus inhibiting Bid activation and the expression and mitochondrial translocation of Bax, events that induce the MPT (37, 49). Therefore, HO-1-dependent inhibition of ROS production may be responsible for suppressing onset of the MPT (Fig. 8). Consistent with an antioxidant role for HO-1 and bilirubin, EDHB pretreatment in the present study attenuated lipid peroxidation after hepatic IR, and inhibition of HO-1 by SnPP partly reversed the protective effects by EDHB, indicating a role for HO-1 in either decreasing ROS production or enhancing ROS scavenging (Fig. 7). These data suggest that EDHB may prevent mitochondrial depolarization by HO-1-dependent inhibition of ROS formation, as illustrated schematically in Fig. 8.
Taken together, this study shows directly that the MPT occurs in vivo after warm IR to liver. Activation of the oxygen-sensing signal cascade by PHI very potently prevents onset of the MPT and IR injury. These effects are mediated, at least in part, by increased HIF-1α, upregulation of HO-1, and decreased ROS formation (Fig. 8). Thus PHIs such as EDHB might prove useful clinically to decrease or eliminate IR injury associated with liver surgery and transplantation.
GRANTS
This study was supported, in part, by Grants DK-70844, DK-37034, and DK-073336 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abello PA, Buchman TG, Bulkley GB. Shock and multiple organ failure. Adv Exp Med Biol 366: 253–268, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 104: 1631–1639, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med 38: 1002–1013, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Chauvin C, De Oliveira F, Ronot X, Mousseau M, Leverve X, Fontaine E. Rotenone inhibits the mitochondrial permeability transition-induced cell death in U937 and KB cells. J Biol Chem 276: 41394–41398, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Chen SY, Hsiao G, Hwang HR, Cheng PY, Lee YM. Tetramethylpyrazine induces heme oxygenase-1 expression and attenuates myocardial ischemia/reperfusion injury in rats. J Biomed Sci 13: 731–740, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Converso DP, Taille C, Carreras MC, Jaitovich A, Poderoso JJ, Boczkowski J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J 20: 1236–1238, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Cummins EP, Berra E, Comerford KM, Ginouves A, Fitzgerald KT, Seeballuck F, Godson C, Nielsen JE, Moynagh P, Pouyssegur J, Taylor CT. Prolyl hydroxylase-1 negatively regulates IkappaB kinase-beta, giving insight into hypoxia-induced NFkappaB activity. Proc Natl Acad Sci USA 103: 18154–18159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elvidge GP, Glenny L, Appelhoff RJ, Ratcliffe PJ, Ragoussis J, Gleadle JM. Concordant regulation of gene expression by hypoxia and 2-oxoglutarate-dependent dioxygenase inhibition: the role of HIF-1alpha, HIF-2alpha, and other pathways. J Biol Chem 281: 15215–15226, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Farber JL, Kyle ME, Coleman JB. Mechanisms of cell injury by activated oxygen species. Lab Invest 62: 670–679, 1990. [PubMed] [Google Scholar]
- 11.Ferrali M, Fulceri R, Benedetti A, Comporti M. Effects of carbonyl compounds (4-hydroxyalkenals) originating from the peroxidation of liver microsomal lipids on various microsomal enzyme activities of the liver. Res Commun Chem Pathol Pharmacol 30: 99–112, 1980. [PubMed] [Google Scholar]
- 12.Geuken E, Buis CI, Visser DS, Blokzijl H, Moshage H, Nemes B, Leuvenink HG, de Jong KP, Peeters PM, Slooff MJ, Porte RJ. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant 5: 1875–1885, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Goncalves GM, Cenedeze MA, Feitoza CQ, Wang PM, Bertocchi AP, Damiao MJ, Pinheiro HS, Antunes Teixeira VP, dos Reis MA, Pacheco-Silva A, Camara NO. The role of heme oxygenase 1 in rapamycin-induced renal dysfunction after ischemia and reperfusion injury. Kidney Int 70: 1742–1749, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Hattori M, Tugores A, Veloz L, Karin M, Brenner DA. A simplified method for the preparation of transcriptionally active liver nuclear extracts. DNA Cell Biol 9: 777–781, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia-reperfusion injury. Cardiovasc Res 60: 617–625, 2003. [DOI] [PubMed] [Google Scholar]
- 16.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512: 1–7, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med 28: 1456–1462, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg 16: 127–140, 2003. [PubMed] [Google Scholar]
- 19.Jewell UR, Gassmann M. Mammalian gene expression in hypoxic conditions. Zoology (Jena) 104: 192–197, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J 15: 1312–1314, 2001. [PubMed] [Google Scholar]
- 21.Jha HV, Recklinghausen G, Zilliken F. Inhibition of in vitro microsomal lipid peroxidation by isoflavanoids. Biochem Pharmacol 34: 1367–1369, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Kantrow SP, Tatro LG, Piantadosi CA. Oxidative stress and adenine nucleotide control of mitochondrial permeability transition. Free Radic Biol Med 28: 251–260, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Kasiganesan H, Sridharan V, Wright GL. Prolyl hydroxylase inhibitor treatment confers whole-animal hypoxia tolerance. Acta Physiol (Oxf) 190: 163–169, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun 304: 463–470, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med 3: 527–535, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol 290: H2024–H2034, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40: 1170–1179, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kuznetsova AV, Meller J, Schnell PO, Nash JA, Ignacak ML, Sanchez Y, Conaway JW, Conaway RC, Czyzyk-Krzeska MF. Von Hippel-Lindau protein binds hyperphosphorylated large subunit of RNA polymerase II through a proline hydroxylation motif and targets it for ubiquitination. Proc Natl Acad Sci USA 100: 2706–2711, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AMK. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997. [PubMed] [Google Scholar]
- 30.Lemasters JJ Fluorescence imaging microscopy. In: Encyclopedia of Analytical Chemistry, edited by Meyers RA. Chichester, UK: Wiley, 2000, p. 10351–10364.
- 31.Madesh M, Hajnoczky G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J Cell Biol 155: 1003–1015, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maines MD The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 37: 517–554, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2: 63–67, 2001. [DOI] [PubMed] [Google Scholar]
- 34.McCord JM Oxygen-derived radicals: a link between reperfusion injury and inflammation. Fed Proc 46: 2402–2406, 1987. [PubMed] [Google Scholar]
- 35.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem J 307: 99–106, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 82: 9–15, 1982. [PubMed] [Google Scholar]
- 37.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem 273: 7770–7775, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol Cell Physiol 273: C1783–C1792, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Ratcliffe PJ, O'Rourke JF, Maxwell PH, Pugh CW. Oxygen sensing, hypoxia-inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol 201: 1153–1162, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Redaelli CA, Tian YH, Schaffner T, Ledermann M, Baer HU, Dufour JF. Extended preservation of rat liver graft by induction of heme oxygenase-1. Hepatology 35: 1082–1092, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290: G583–G589, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Sridharan V, Guichard J, Bailey R, Kasiganesan H, Beeson C, Wright GL. The prolyl hydroxylase oxygen-sensing pathway is cytoprotective and allows maintenance of mitochondrial membrane potential during metabolic inhibition. Am J Physiol Cell Physiol 292: C719–C728, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Takeyama N, Matsuo N, Tanaka T. Oxidative damage to mitochondria is mediated by the Ca2+-dependent inner-membrane permeability transition. Biochem J 294: 719–725, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takizawa S, Hirabayashi H, Matsushima K, Tokuoka K, Shinohara Y. Induction of heme oxygenase protein protects neurons in cortex and striatum, but not in hippocampus, against transient forebrain ischemia. J Cereb Blood Flow Metab 18: 559–569, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Theruvath TP, Zhong Z, Pediaditakis P, Ramshesh VK, Currin RT, Tikunov A, Holmuhamedov E, Lemasters JJ. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology 47: 236–246, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldmeier PC, Feldtrauer JJ, Qian T, Lemasters JJ. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol 62: 22–29, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem 270: 1230–1237, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718–1726, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Warnecke C, Griethe W, Weidemann A, Jurgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt KU. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB J 17: 1186–1188, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 2: 87–90, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Wright G, Higgin JJ, Raines RT, Steenbergen C, Murphy E. Activation of the prolyl hydroxylase oxygen-sensor results in induction of GLUT1, heme oxygenase-1, and nitric-oxide synthase proteins and confers protection from metabolic inhibition to cardiomyocytes. J Biol Chem 278: 20235–20239, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Terakado M, Horio F, Aoki K, Tanaka M, Nakajima H. Role of bilirubin as an antioxidant in an ischemia-reperfusion of rat liver and induction of heme oxygenase. Biochem Biophys Res Commun 223: 129–135, 1996. [DOI] [PubMed] [Google Scholar]
- 54.Zagorska A, Dulak J. HIF-1: the knowns and unknowns of hypoxia sensing. Acta Biochim Pol 51: 563–585, 2004. [PubMed] [Google Scholar]
- 55.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J Exp Med 183: 1533–1544, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, Lehnert M, Mason RP, Thurman RG, Lemasters JJ. Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology 129: 652–664, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Zhong Z, Froh M, Connor HD, Li X, Conzelmann LO, Mason RP, Lemasters JJ, Thurman RG. Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am J Physiol Gastrointest Liver Physiol 283: G957–G964, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Zhong Z, Theruvath TP, Currin RT, Waldmeier PC, Lemasters JJ. NIM811, a mitochondrial permeability transition inhibitor, prevents mitochondrial depolarization in small-for-size rat liver grafts. Am J Transplant 7: 1103–1111, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim Biophys Acta 1241: 139–176, 1995. [DOI] [PubMed] [Google Scholar]