Abstract
Inorganic phosphate (Pi) plays a key role in diverse physiological functions. Recent studies have indicated that Pi affects Akt signaling through the sodium-dependent phosphate cotransporter. Akt signaling, in turn, plays an important role in liver development; however, the effects of high dietary Pi on the liver have not been investigated. Here, we examined the effects of high dietary phosphate on the liver in developing mice. We found that high dietary Pi increased liver mass through enhancing Akt-related cap-dependent protein translation, cell cycle progression, and angiogenesis. Thus careful regulation of Pi consumption may be important in maintaining normal development of the liver.
Keywords: Akt, liver development
phosphate is an essential nutrient to all living organisms because it is required for diverse cellular functions and organ development (37, 38). Since phosphate cannot be synthesized, the need for this nutrient should be met by ingestion of phosphate in the diet, both naturally and as added phosphate (39). Thus inorganic phosphate (Pi) is widely used as a food ingredient, and surveys in various countries indicate that the intake of phosphate has increased steadily as Pi-containing foods increased by ∼17% for the decade until 1993 (5) and that the use of phosphate as food additives may continue to increase (13).
Several lines of evidence indicate that elevated Pi functions as a stimulus capable of increasing or decreasing several genes related to cell cycle and angiogenesis through specific signaling pathways (12, 13). To date, many studies involving Pi have focused mainly on its effect in bone and kidneys. However, studies have not yet investigated homeostatic maintenance of normal liver and the adaptation of the liver to excess Pi. Pi enters into the cells via Na/Pi cotransporter (NPT) (27), and the NPT expression is regulated mainly by dietary and serum Pi level (38). Among three classes of NPTs (Type I, II, and III), Type II has been identified in mammalian liver, and considerable progress has been made in our understanding of their function and regulation (10).
Akt/protein kinase B is a serine/threonine kinase activated in cells exposed to diverse stimuli such as hormones, growth factors, and extracellular matrix components. Akt has also emerged as a crucial regulator of widely divergent cellular processes including apoptosis, proliferation, differentiation, and metabolism (23, 25). Our previous studies (12, 13) demonstrated that high dietary Pi perturbed normal growth of brain and lung by affecting Akt signaling in developing mice. These results suggest that high dietary Pi may affect liver development by altering Akt signaling. To investigate this possibility, we examined the effects of high dietary Pi on liver development in transgenic mice expressing CMV-LucR-cMyc-IRES-LucF dual reporter genes. CMV-LucR-cMyc-IRES-LucF reporter gene system is a convenient and powerful tool to discern cap-dependent and cap-independent protein translation since Renilla luciferase (LucR) and firefly luciferase (LucF) provide the level of cap-dependent and cap-independent internal ribosome entry site (IRES) protein translation, respectively (7). Our results clearly demonstrate that, in the livers of young mice, high dietary Pi functions as a critical signaling molecule affecting organ development via Akt-dependent cell cycle progression, angiogenesis, and cap-dependent translation.
MATERIALS AND METHODS
Cell culture and proliferation assay.
WB-F344 liver epithelial cells (American Type Culture Center, Manassas, VA) were seeded in a 96-well plate at 0.5 × 104 cells/well and grown in DMEM supplemented with 5% FBS and 1% penicillin/streptomycin at 37°C in a humidified incubator containing 5% CO2. Pi was used in the form of NaPO4, pH 7.4, and sodium sulfate was used as a control to clarify the potential salt effects. Foscarnet (Fos; Sigma-Aldrich, St. Louis, MO) was used as a phosphate transport inhibitor. After 48 h of Pi treatment with or without Fos, cell growth was evaluated by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay (MTT, Sigma-Aldrich) according to the manufacturer's instruction.
Animals and diet.
Two-week-old transgenic newly weaned male mice expressing CMV-LucR-cMyc-IRES-LucF reporter gene were used. Mice were randomly separated into two dietary groups (10 mice/group), one group on a normal diet containing 0.5% Pi (normal Pi) and another group on a high-phosphate diet containing 1.0% Pi (high Pi). All diets were prepared according to the guideline of American Institute of Nutrition and thus fulfill the requirement for normal growth, which is described precisely in Reeves et al. (29). Two weeks after of being fed Pi diet, five mice from each group were euthanized, and the remaining mice were killed at 4 wk. Serum and the liver tissues were collected and stored in −80°C for further use. Liver Pi content was measured using the methods described by Sarkar et al. (33). In this study, all animal-based procedures were in accordance with the Guidelines for the Care and Use of Experimental Animals of Seoul National University that were formulated from the Institute of Laboratory Research (ILAR) Guide for the Care and Use of Laboratory Animals. Also, all animal experiments described in this study were approved by the Institutional Animal Care and Use Committee at Seoul National University.
Liver function analysis.
Serum albumin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured with a colorimetric assay kit (Asan Pharmaceutical, Seoul, Korea).
H & E staining and determination of cell size.
For histological analysis and determination of the cell size, the paraffin sections were stained with hematoxylin and eosin (H & E) after being deparaffinized in xylene and rehydrated through a gradient series of alcohol. The cell size was analyzed using indirect measure methods as described by Baena et al. (2).
Luciferase assay.
Luciferase activities in the tissue extracts were measured with an EG&G Berthold luminometer (Bundoora, Australia). Briefly, livers were homogenized in passive lysis buffer (Promega, Madison, WI). The homogenates were centrifuged for 20 min at 4,500 revolution/min at 4°C, and the supernatant was centrifuged for an additional 15 min at 13,000 revolution/min at 4°C. LucF and LucR activities were measured using a dual luciferase assay kit (Promega).
Western blot analysis.
After measuring the protein concentration of homogenized lysates using a Bradford kit (Bio-Rad, Hercules, CA), equal amounts (30 μg) of protein were separated on SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked in Tris-buffered saline containing Tween 20 (TBST) containing 5% skim milk for 1 h; immunoblotting was performed by incubating the membranes overnight with their corresponding primary antibodies at 4°C in 5% skim milk. Anti-NPT-2b was obtained from Alpha Diagnostic International (San Antonio, TX). Monoclonal antibodies against Akt1 and phospho-Akt at Thr308 were produced using a general method described elsewhere (10a). Anti-phospho-Akt (Ser473), anti-eukaryotic initiation factor 4E (eIF-4E), anti-eIF-4E binding protein 1 (4E-BP1), anti-phospho-4E-BP1, anti-cyclin D2, anti-p21, anti-p27, anti-matrix metalloproteinase-2 (MMP-2), anti-fibroblast growth factor 2 (FGF-2), anti-vascular endothelial growth factor (VEGF), anti-cyclin-dependent kinase 4 (CDK4), CD31, CD34, and anti-proliferating cell nuclear antigen (PCNA) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies against mammalian target of rapamycin (mTOR), phospho-mTOR, and α-tubulin were obtained from Cell Signaling (Beverly, MA). After being washed in TBST, the membranes were incubated with a horseradish peroxidase (HRP)-labeled secondary antibody for 1 h at room temperature. Immunoreactive bands were detected using a luminescent image analyzer LAS-3000 (Fujifilm, Tokyo, Japan). Results were quantified using a measure program of LAS-3000.
Immunoprecipitation and protein kinase assay.
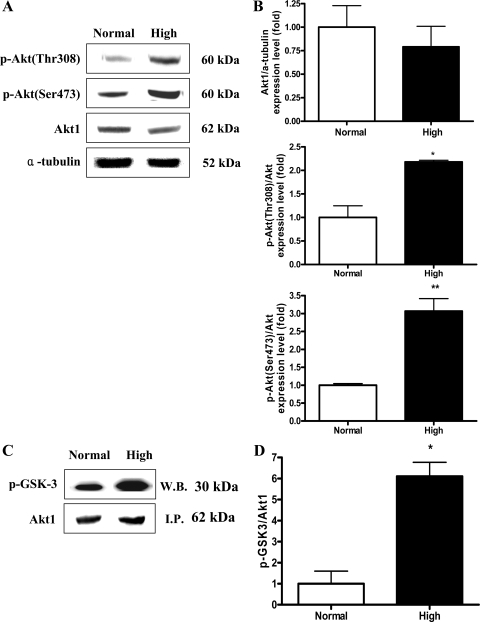
Immunoprecipitation of eIF-4E was carried out using a Seize primary mammalian immunoprecipitation kit (Pierce, Rockford, IL). The protein kinase activity of Akt was examined with an Akt kinase assay kit (Cell Signaling) according to the manufacturer's instruction. For the Akt activity assay, GSK-3 was used as a substrate of Akt because GSK-3 is one of the downstream targets of PI3K/Akt. The NH3-terminal Ser residue of GSK-3 is within the consensus sequence of the substrates for Akt (11); thus GSK-3 can be phosphorylated by activated Akt (40). Such phosphorylated GSK-3 can be detected by Western blot using specific phospho-GSK-3 antibody.
Immunohistochemistry.
The liver tissues were fixed in 10% neutral buffered formalin, paraffin processed, and sectioned at 4 μm. For immunohistochemistry (IHC), the tissue sections were deparaffinized in xylene, rehydrated through a gradient series of alcohol, incubated in 200 μl of proteinase K, and washed and incubated in 3% hydrogen peroxide (AppliChem, Darmstadt, Germany) for 30 min to quench endogenous peroxidase activity. After being washed in PBS, the tissue sections were incubated with 5% BSA in PBS for 1 h at room temperature to block nonspecific binding sites. Primary antibodies were applied on tissue sections overnight at 4°C. The following day, the tissue sections were washed and incubated with secondary HRP-conjugated antibodies for 1 h at room temperature. After being carefully washed, tissue sections were counterstained with Mayer's hematoxylin (DAKO, Carpinteria, CA) and washed with xylene. Cover slips were mounted using Permount (Fisher Scientific, Pittsburgh, PA), and the slides were viewed using a light microscope (Carl Zeiss, Thornwood, NY). Capillary vessels were counted per field of view at ×400 magnification in five consecutive fields.
Gelatin zymography assay.
Equal amounts of protein were electrophoresed in 10% SDS gel containing 1 mg/ml gelatin. The gel was then washed at room temperature for 1 h with 2.5% Triton X-100 and subsequently incubated at 37°C in a buffer containing 10 mmol/l CaCl2, 150 mmol/l NaCl, and 50 mmol/l Tris·HCl (pH 7.5) for 12 h. The gel was stained with 0.5% Coomassie brilliant blue R-250 solution and then destained with 20% acetic acid solution containing 10% methanol. Areas of gelatinase activity were detected as clear bands against the blue-stained gelatin background.
Statistical analysis.
Quantification of Western blot analysis was performed using a Multi Gauge version 2.02 program (Fujifilm). All results are given as means ± SE. Results were analyzed using an unpaired Student's t-test (GraphPad Software, San Diego, CA). *P < 0.05 was considered to indicate a significant difference, and **P < 0.01 was considered to indicate a highly significant difference compared with corresponding control.
RESULTS
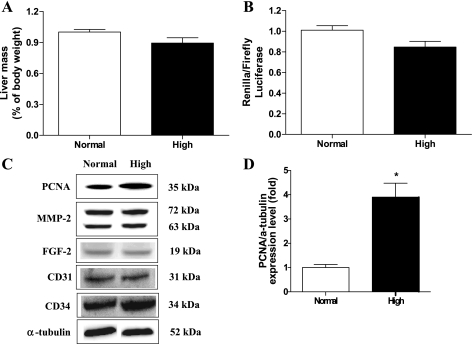
High dietary Pi increased the liver mass without affecting liver function.
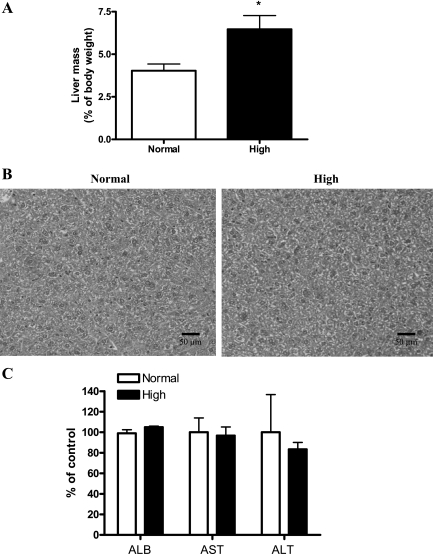
The diverse functional roles of Pi and different nutritional requirements during growth suggest that appropriate in vivo nutritional studies at specific stages of growth are needed. Therefore, we investigated the potential effects of high dietary Pi on the liver of young mice. Our results showed that high dietary Pi caused the significant increase of liver mass compared with normal Pi diet group (Fig. 1A). Such results suggest that the increased liver growth may affect the liver function. To clarify the effects of Pi in the liver function, we performed histopathological analysis and measured the levels of serum albumin, AST, and ALT; however, there was no significant change between high Pi and control-diet group (Fig. 1, B and C).
Fig. 1.
Effects of high dietary inorganic phosphate (Pi) on liver mass and function. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. A: liver mass as a percentage of body mass. B: histopathology of the livers. Original magnification was ×400. Scale bar = 50 μm. C: changes in the levels of serum albumin (ALB), aspartate aminotransferase (ATS) and alanine aminotransferase (ALT). *P < 0.05 compared with normal diet group (means ± SE, n = 5).
High dietary Pi increased the Pi level through the NPT-2b protein expression in the liver of developing mice.
Because NPT-2b is known to be a key regulator of Pi homeostasis in the liver (10), we examined the effect of high dietary Pi on NPT-2b protein expression in the livers of young mice. Western blotting (Fig. 2A) and densitometric analysis (Fig. 2B) showed that high dietary Pi significantly increased NPT-2b expression in the livers of young mice. Simultaneously, high dietary Pi increased Pi concentration in the liver as well (Fig. 2C).
Fig. 2.
Pi level and NPT-2b protein expression in the liver of young mice. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. A: expression of Na/Pi transporter (NPT)-2b protein in the liver. B: bands of NPT-2b were further analyzed by densitometer. C: Pi level in the liver tissue. *P < 0.05 compared with normal diet group (means ± SE, n = 5).
High dietary Pi regulates Akt activity in the liver of developing mice.
Our previous study demonstrated that elevated Pi affected the Akt signaling in the lung via NPT (6, 17). Therefore, we also examined the effect of high dietary Pi on Akt expression and phosphorylation status in the liver. High dietary Pi did not affect the expression of total Akt but significantly increased the Akt phosphorylation at both Ser473 and Thr308 (Fig. 3, A and B). Such increased Akt phosphorylation at both critical sites was associated with increased Akt protein kinase activity (Fig. 3, C and D).
Fig. 3.
Western blot (W.B.) analysis of Akt and phospho-Akt protein expressions and Akt kinase assay in the liver of young mice. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. A: expression of Akt and phospho-Akt proteins in the liver. B: bands of interests were further analyzed by densitometer. C: Akt kinase activity was measured in the liver homogenates. Protein was immunoprecipitated (I.P.) with Akt-specific antibody, and GSK-3 protein was used as a substrate. The immunoblotting of Akt1 was used as a control. D: bands of interests were further analyzed by densitometer. *P < 0.05, **P < 0.01 compared with normal-diet group (means ± SE, n = 5).
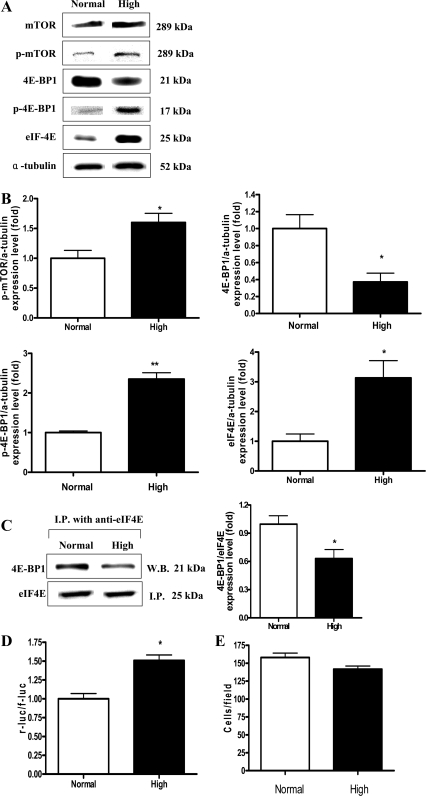
High dietary Pi enhanced cap-dependent protein translation in the liver of developing mice.
Akt-mTOR signaling regulates protein translation by enhancing phosphorylation of 4E-BP1, which causes its dissociation from eIF-4E, leading to eIF-4E activation (30). Therefore, we investigated the effects of high dietary Pi on protein translation in the liver of young dual luciferase reporter mice. Western blotting revealed that high dietary Pi significantly decreased the expression of 4E-BP1, whereas the phosphorylation of mTOR and 4E-BP1 were increased (Fig. 4, A and B). Such decreased total protein expression with increased phosphorylation of 4E-BP1 was associated with increased dissociation of eIF-4E (Fig. 4, A–C). To determine the differential levels of cap-dependent and cap-independent protein translation, we performed LucF and LucR assays. We found that high Pi enhanced cap-dependent protein translation in the liver of developing mice (Fig. 4D). In addition, the changes of liver cell size were measured by counting the cell numbers per field at ×400 magnification because change of protein translation might affect the cell growth. However, no statistical significance was observed (Fig. 4E).
Fig. 4.
Western blot analysis of Akt downstream signals, measurement of dual luciferase activity, immunoprecipitation assay, and liver cell size in the liver of young mice. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. Liver tissue homogenates were subjected to further analysis. A: expression of mammalian target of rapamycin (mTOR), phospho-mTOR (p-mTOR), eukaryotic initiation factor 4E (eIF-4E), 4 eIF binding protein (4E-BP1), and phospho-4E-BP1 (p-4E-BP1) in the liver. B: bands of interest were further analyzed by densitometer. C: immunoprecipitation analysis of eIF-4E and 4E-BP1 levels. D: luciferase activities were measured for the determination of the ratios between cap-dependent and cap-independent protein translations in the liver. r-luc, Renilla luciferase; f-luc; firefly luciferase. E: hepatocyte number per field in liver sections. The cell numbers were counted at ×400 magnification. *P < 0.05, **P < 0.01 compared with normal-diet group (means ± SE, n = 5).
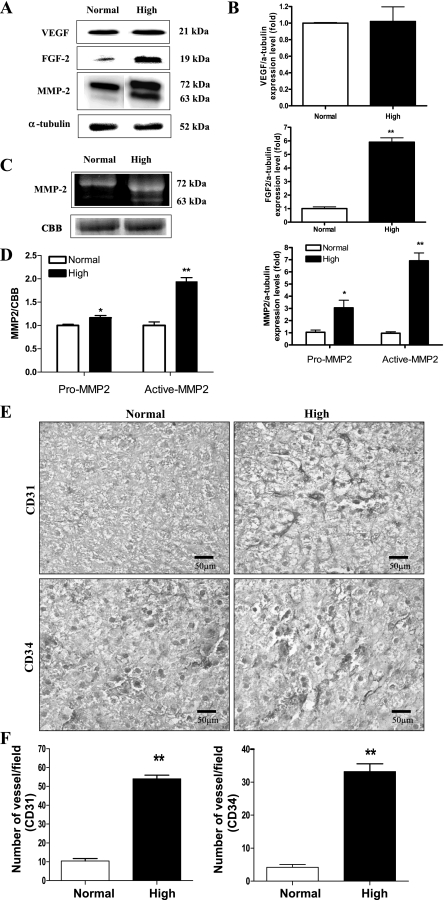
High dietary Pi increased the expression of hepatic angiogenesis factors in developing mice.
Activation of Akt/mTOR signaling (41) and increases in eIF-4E or the phosphorylation of 4E-BP1 are known to upregulate the expression of critical angiogenic factors (8). We therefore examined the effects of a high-Pi diet on the expression of angiogenesis markers. Western blotting revealed that high dietary Pi significantly increased the level of angiogenesis factors such as FGF-2 and MMP-2 in the liver of developing mice; however, the VEGF expression remained unchanged. Please note that both pro-MMP-2 as well as active-MMP2 were increased significantly (Fig. 5, A and B). Therefore, MMP-2 activity was further confirmed using the zymography assay (Fig. 5, C and D). We also measured the endothelial cell marker protein CD31 and CD34 expression by IHC because CD31 and CD34 staining are widely used to predict the tumor formation because they represent the extent of angiogenesis (28). We found that high Pi increased the expression of CD31 as well as CD34 significantly (Fig. 5, E and F).
Fig. 5.
Analysis of angiogenic factors expression in the liver of young mice. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. A: expression of matrix metalloproteinase-2 (MMP-2), fibroblast growth factor 2 (FGF-2), and vascular endothelial growth factor (VEGF) proteins in the liver. B: bands of interest were further analyzed by densitometry. C: gelatin zymography assay for the activity of MMP-2. D: zymographic band intensities were quantified by densitometry. Equivalent protein loading was confirmed by staining the gels with Coomassie brilliant blue R-250 (CBB). E: immunohistochemistry (IHC) of CD31 and CD34 in the liver. Dark brown color represents the CD31 and CD34 proteins in the liver. Original magnification was ×400. Scale bar = 50 μm. F: microvessel density quantification was determined by counting the number of CD31- or CD34-positive vessels per field of view at ×400 magnification. *P < 0.05, **P < 0.01 compared with normal-diet group (means ± SE, n = 5).
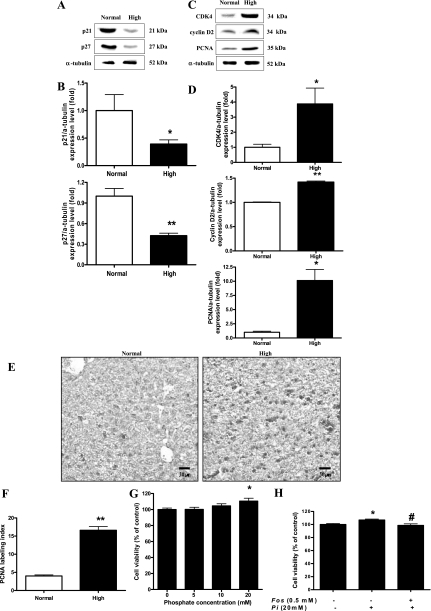
High dietary Pi facilitates cell cycle in the liver of developing mice.
Akt plays a key role in cell cycle regulation (19). Therefore, we examined the effect of high dietary Pi on the expression of cell cycle proteins. High dietary Pi significantly decreased the levels of p21 and p27 (Fig. 6, A and B), whereas the levels of cyclin D2, CDK4, and cell proliferation marker PCNA (Fig. 6, C and D) were significantly increased in the liver of developing mice. Moreover, such changes of PCNA were further confirmed by IHC (Fig. 6E) and labeling indexes (Fig. 6F). To investigate the relationship between high dietary Pi and liver cell proliferation, we performed an in vitro Pi transport inhibitor study. Our results demonstrated that the Pi treatment significantly increased liver cell proliferation at 20 mM concentration, and such increased cell proliferation was significantly inhibited by Pi transport inhibitor Fos treatment (Fig. 6, G and H).
Fig. 6.
Effects of Pi on cell cycle progression in the liver. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 4 wk. A: expression of p21 and p27 proteins in the liver. B: bands of interest were further analyzed by densitometry. C: expression of cyclin D2, cyclin-dependent kinase 4 (CDK4), and proliferating cell nuclear antigen (PCNA) proteins in the liver. D: bands of interest were further analyzed by densitometer. *P < 0.05, **P < 0.01 compared with normal-diet group (means ± SE, n = 5). E: IHC of PCNA in the liver. Dark brown color represents PCNA protein in the liver. Original magnification was ×200. Scale bar = 50 μm. F: comparison of PCNA labeling index in the liver of young mice. PCNA-positive staining was determined by counting 3 randomly chosen fields per section, determining the percentage of DAB-positive cells per 100 cells at ×400 magnification. G: WB-F344 cells were incubated for 48 h with various concentration of Pi, and then, the cell viability was measured by MTT assay. H: WB-F344 cells were pretreated with 0.5 mM foscarnet (Fos) phosphate transport inhibitor for 30 min and then treated with 20 mM of Pi. After 48-h incubation with Pi, the cell viability was measured using MTT assay. Values represent the means ± SE of 3 independent experiments. *P < 0.05 compared with control, #P < 0.05 compared with Pi treatment.
Cell proliferation stimulation is the primary event incurred by high dietary Pi.
As described above, cap-dependent protein translation, angiogenesis, and cell cycle progressions were increased by high dietary Pi for 4 wk. To demonstrate which one of these is the primary event, we performed a 2-wk feeding experiment. Results showed that high dietary Pi significantly increased the expression of cell proliferation marker protein PCNA but did not affect cap-dependent translation and the expression of angiogenesis factors in the middle of the whole period of experiment (Fig. 7).
Fig. 7.
Effects of high-Pi diet at 2 wk on the liver of developing mice. Two-week-old transgenic mice were fed a normal- or high-Pi diet for 2 wk. A: liver mass as a percentage of body mass. B: luciferase activities were measured for the determination of the ratios between cap-dependent and cap-independent protein translations in the liver. C: expression of PCNA, FGF-2, MMP-2, CD31, and CD34 proteins in the liver. D: bands of interest were further analyzed by densitometry. *P < 0.05, compared with normal diet group (means ± SE, n = 5).
DISCUSSION
Pi is an essential nutrient that can affect the development of some organs including bone, muscle, brain, and lung through controlling several pivotal genes and regulating signal transduction involved in normal growth (12, 13, 18, 37). Through this study we demonstrated the effects of high dietary Pi on the liver growth (Fig. 1).
Many studies have suggested that transport of Pi into the cell is required for molecular and cellular response. Pi transport is regulated by the family of NPT (15). NPT-2b is an isoform of the NPT family, and recent study has demonstrated that NPT-2b plays an important role in the overall Pi homeostasis of the liver (10). Our study demonstrated that high dietary Pi significantly increased the NPT-2b expression and Pi concentration in the liver of developing mice (Fig. 2). Such high Pi entrance may be responsible for regulation of signals important for maintaining cellular homeostasis. In fact, Chang et al. (6) reported that elevated Pi stimulated Akt signaling through NPT protein in lung cells and that Pi-induced Akt phosphorylation was blocked by a phosphate transport inhibitor. Similar results were also observed in an in vivo animal study by Jin et al. (12).
Akt is an important signaling protein in response to nutrient metabolism (36), and such Akt signaling strongly promotes the liver development through the regulation of diverse cellular processes (23). Protein translation is also controlled by Akt signaling, and changes in protein translation may affect organ growth and development (32). In our study, high dietary Pi significantly enhanced Akt kinase activity in the liver of developing mice by increasing the phosphorylation of Akt at both Thr308 and Ser473 (Fig. 3). The increased Akt phosphorylation also enhanced cap-dependent protein translation via the phosphorylation of mTOR and 4E-BP1 (Fig. 4, A–D). Akt directly phosphorylates and activates mTOR, a protein kinase (1). Activated mTOR kinase is known to phosphorylate 4E-BP1, causing the dissociation of 4E-BP1 from eIF-4E. The free eIF-4E then binds to mRNAs, allowing cap-dependent translation (34). Recently, Fingar et al. (9) reported that overexpressed cap-dependent protein eIF-4E increased the mammalian cell size. In addition, overexpression of eIF-4E increased the level of several proteins important for cell growth and proliferation (9). Kevil et al. (30) also reported that overexpressed eIF-4E increased FGF-2 expression. Other studies indicated that FGF-2 played an important role in the liver development (14, 35), hepatocyte migration, and proliferation as well (20). Our results showed that high dietary Pi significantly increased the cell proliferation marker protein PCNA expression in the liver through downregulating cell cycle inhibitors (p21 and p27) and upregulating cyclin D2 and CDK4 (Fig. 6, A–F). Such increased cell proliferation was dependent upon Pi treatment (Fig. 6, G and H). Together, these findings suggest that high dietary Pi may affect the normal liver development of young mice through facilitating Akt-related cap-dependent protein translation and cell cycle progression.
Angiogenesis is vitally important during organ development. Dysregulated vessel growth has a major impact on health and contributes to the pathogenesis of many disorders, some quite unexpected (26). Akt plays important roles in both physiological and pathological angiogenesis by inducing the production of angiogenic factors (22). One of the most important of these is FGF-2 (4). MMP-2 is also a major mediator of angiogenesis associated with basement membrane degradation, tumor invasion, and metastasis (17). Recent studies demonstrated that Akt signaling stimulates the expression of MMP-2 (41) and FGF-2 (24). Furthermore, eIF-4E is an oncogene that can enhance the translation of c-myc, another important angiogenic factor (32). Ruggero et al. (31) reported that the translation factor eIF-4E promoted tumor formation in lymphomagenesis. These findings agree with our detection of increased FGF-2 and MMP-2 (Fig. 5, A–D). Furthermore, immunohistochemical analysis using the angiogenesis markers CD31 and CD34 (21) revealed a significant increase in angiogenesis in the high-Pi group. Taken together, these findings strongly suggest that high dietary Pi contributes to angiogenesis in the liver of developing mice.
To obtain the information that one event was primarily induced by the high dietary Pi among the progressions of cell cycle, protein translation, and angiogenesis, we performed an additional experiment. After 2 wk of feeding with high dietary Pi, the cell cycle was significantly increased; however, protein translation and angiogenesis remained unchanged (Fig. 7). These results suggest that high dietary Pi primarily stimulates the cell cycle progression, and such stimulated cell cycle may initiate further steps for the increases of protein translation and angiogenesis.
In summary, our results suggest that high dietary Pi may disturb the normal development of liver in young mice partially through affecting Akt-related cap-dependent protein translation, cell cycle, and the expression of angiogenic factors. Also, Pi may increase the liver mass through affecting other signals because Pi is known to regulate the expression of multiple genes including transcription regulators and signal transducers (3). Thus careful regulation of Pi consumption may be important in maintaining a normal development of the liver.
GRANTS
This work was supported in part by the grant from Korea Science and Engineering Foundation (KOSEF) (M20702000006-080N0200-00601) of the Ministry of Science and Technology in Korea. M. Cho and J. Kim were supported by the Nano Systems Institute-National Core Research Center (NSI-NCRC) program of KOSEF. C. Xu, H. Jin, Y. Chung, and J. Shin are also grateful for the award of the BK21 fellowship. K. Lee was supported by grants from National Nuclear R & D Program and Human Genome Project (FG06-11-04), Korean Ministry of Science and Technology. G. Beck, Jr. was supported by National Cancer Institute Grant CA84573.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Asnaghi L, Bruno P, Priulla M, Nicolin A. mTOR: a protein kinase switching between life and death. Pharmacol Res 50: 545–549, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Baena E, Gandarillas A, Vallespinós M, Zanet J, Bachs O, Redondo C, Fabregat I, Martinez-AC, de Alborän IM. C-Myc regulates cell size and ploidy but is not essential for postnatal proliferation in liver. Proc Natl Acad Sci USA 102: 7286–7291, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck GR Jr, Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res 288: 288–300, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bremnes RM, Camps C, Sirera R. Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer 51: 143–158, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Calvo MS Dietary phosphorus, calcium metabolism, and bone. J Nutr 123: 1627–1633, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Chang SH, Yu KN, Lee YS, An GH, Beck GR Jr, Colburn NH, Lee KH, Cho MH. Elevated inorganic phosphate stimulates Akt-ERK 1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol 35: 528–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creáncier L, Mercier P, Prats A, Morello D. C-myc internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol Cell Biol 21: 1833–1840, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastasis. Oncogene 23: 3189–3199, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16: 1472–1487, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frei P, Gao B, Hagenbuch B, Mate A, Biber J, Murer H, Meier PJ, Stieger B. Identification and localization of sodium-phosphate cotransporters in hepatocytes and cholangiocytes of rat liver. Am J Physiol Gastrointest Liver Physiol 288: G771–G778, 2005. [DOI] [PubMed] [Google Scholar]
- 10a.Fuller SA, Takahashi M, Hurrell JGG. Preparation of monoclonal antibodies. In: Current Protocols in Molecular Biology, edited by Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, and Struhl K. New York: John Wiley and Sons, 2007, p.11.4.1–11.11.5.
- 11.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull 30: 1617–1623, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Jin H, Chang SH, Xu CX, Shin JY, Chung YS, Park SJ, Lee YS, An GH, Lee KH, Cho MH. High dietary inorganic phosphate affects lung through altering protein translation, cell cycle, and angiogenesis in developing mice. Toxicol Sci 100: 215–223, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Hwang SK, Yu K, Anderson HK, Lee YS, Lee KH, Prats AC, Morello D, Beck GR Jr, Cho MH. A high inorganic phosphate diet perturbs brain growth, alters Akt-ERK Signaling, and results in changes in cap-dependent translation. Toxicol Sci 90: 221–229, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science 28: 1998–2003, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Kavanaugh MP, Kabat D. Identification and characterization of a widely expressed phosphate transporter retrovirus receptor family. Kidney Int 49: 959–963, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Kevil C, Carter P, Hu B, De Benedetti A. Translational enhancement of FGF-2 by eIF-4 factors, and alternate utilization of CUG and AUG codons for translation initiation. Oncogene 7: 2339–2348, 1995. [PubMed] [Google Scholar]
- 17.Kurizaki T, Toi M, Tominaga T. Relationship between matrix metalloproteinase expression and tumor angiogenesis in human breast carcinoma. Oncol Rep 5: 673–677, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Landsman A, Lichtstein D, Bacaner M, Ilani A. Dietary phosphate-dependent growth is not mediated by changes in plasma phosphate concentration. Br J Nutr 86: 217–223, 2001. [PubMed] [Google Scholar]
- 19.Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci 114: 2903–2910, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Ma TY, Kikuchi M, Sarfeh IJ, Shimada H, Hoa NT, Tarnawski AS. Basic fibroblast growth factor stimulates repair of wounded hepatocyte monolayer: modulatory role of protein kinase A and extracellular matrix. J Lab Clin Med 134: 363–371, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Prognostic molecular markers in hepatocellular carcinoma: a systematic review. Eur J Cancer 43: 979–992, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullany LK, Nelsen CJ, Hanse EA, Goggin MM, Anttila CK, Peterson M, Bitterman PB, Raghavan A, Crary GS, Albrecht JH. Akt-mediated liver growth promotes induction of cyclin E through a novel mechanism and a p21-mediated cell cycle arrest. J Biol Chem 282: 21244–21252, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Newman DR, Li CM, Simmons R, Khosla J, Sannes PL. Heparin affects signaling pathways stimulated by fibroblast growth factor-1 and -2 in type II cells. Am J Physiol Lung Cell Mol Physiol 287: L191–L200, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal 14: 381–395, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Otrock ZK, Mahfouz RA, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells Mol Dis 39: 212–220, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Prie D, Beck L, Urena P, Friedlander G. Recent findings in phosphate homeostasis. Curr Opin Nephrol Hypertens 14: 318–324, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem 54: 385–395, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc Writing Committee on the Reformulation of the AIN-76A rodent diet. J Nutr 123: 1939–1951, 1993. [DOI] [PubMed] [Google Scholar]
- 30.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 433: 477–480, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med 10: 484–486, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Ruggero D, Sonenberg N. The Akt of translational control. Oncogene 24: 7426–7434, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar NK, Srivastava U. Biochemical changes I progressive muscular dystrophy 2. Phosphorus metabolism in normal, nutritional and hereditary dystrophic muscles, livers and brains of animals. J Nutr 83: 193–201, 1964. [DOI] [PubMed] [Google Scholar]
- 34.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell 103: 253–262, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development 132: 35–47, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9: 59–71, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki O, Kamakura S, Katagiri T, Nakamura M, Zhao B, Honda Y, Kamijo R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 27: 2671–2681, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Takeda E, Taketani Y, Sawada N, Sato T, Yamamoto H. The regulation and function of phosphate in the human body. Biofactors 21: 345–355, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Weiner ML, Salminen WF, Larson PR, Barter RA, Kranetz JL, Simon GS. Toxicological review of inorganic phosphates. Food Chem Toxicol 39: 759–786, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Woodgett JR Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol 17: 150–157, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Bar-Eli M, Meloche S, Brodt P. Dual-regulation of MMP-2 expression by the IGF-1 receptor: the PI3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem 279: 19683–19690, 2004. [DOI] [PubMed] [Google Scholar]