Abstract
The effects of trinitrobenzene sulfonic acid (TNBS)-induced inflammation on specialized, low-threshold, slowly adapting rectal mechanoreceptors were investigated in the guinea pig. Under isoflurane anesthesia, 300 μl saline or TNBS (15 mg/ml) in 30% ethanol was instilled 7 cm from the anal sphincter. Six or 30 days later, single unit extracellular recordings were made from rectal nerve trunks in flat-sheet in vitro preparations attached to a mechanical tissue stretcher. TNBS treatment caused macroscopic ulceration of the rectal mucosa at 6 days, which fully resolved by 30 days. Muscle contractility was unaffected by TNBS treatment. At 6 days posttreatment, responses of low-threshold rectal mechanoreceptors to circumferential stretch were increased, and the proportion of afferents responding with von Frey hair thresholds ≤0.1 mN and mechanoreceptor excitability in response to electrical stimulation were increased in TNBS-treated tissue, suggesting increased sensitivity of the mechanotransducer. Mechanoreceptor function at 30 days posttreatment was in most cases unchanged. The inflammatory mediator prostaglandin E2 (1 μM) activated mechanoreceptors (6 days) in conjunction with contractile activity, but capsaicin (1 μM) failed to activate mechanoreceptors. Bradykinin (1 μM) activated mechanoreceptors independently of contractile activity and responses to stretch were increased in the presence of bradykinin. Both capsaicin and bradykinin activated unidentified stretch-insensitive afferents independently of contractile activity. Mechanoreceptor function is modulated at 6 days posttreatment but not at 30 days, suggesting a moderate increase in mechanoreceptor sensitivity in inflamed tissue but not after recovery. Other unclassified stretch-insensitive afferents are responsive to inflammatory mediators and capsaicin and may be involved in aspects of visceral sensation.
Keywords: pelvic afferents, rectal nerves, hypersensitivity, trinitrobenzene sulfonic acid
it is well established that inflammation leads to visceral sensitization. Extrinsic primary afferent neurons are likely to mediate at least part of this sensitization through changes in excitability (4, 10). However, the susceptibility of specific functional classes of extrinsic visceral afferents to the processes of inflammation and to inflammatory mediators has not been systematically investigated.
Longhurst et al. (16) first described mechanically insensitive visceral afferents that were acutely sensitive to the application of algogenic substances. In addition, application of algogenic substances could recruit responses to ischemia from visceral afferents previously unresponsive to ischemia (15). Habler et al. (11) coined the phrase “silent nociceptor” for bladder afferents that had been mechanically insensitive but became active after acute inflammation. These studies contributed to the ideas that there are nociceptor-like afferents innervating the viscera and that inflammation and inflammatory mediators can increase the level of nonnoxious and noxious information reaching the spinal cord (5).
Inflammatory mediators introduced into the colon have been reported to evoke increased mechanoreceptor responses to distension (30). The function of colonic afferent nerve cell bodies isolated from dorsal root ganglia are modulated by capsaicin and some algesic substances (30, 31), and gut inflammation has profound effects on function of some colonic dorsal root ganglia cells (1), causing them to become hyperexcitable. As expected, some central neurons in spinal afferent pathways show larger responses to colorectal distension after acute inflammation (23). A number of substances also have effects on the peripheral endings of visceral spinal afferent neurons. It has been reported that ATP, released from unidentified cell types, may play a role as an important modulator during inflammation (33); however, indirect effects via changes in gut wall compliance were not taken into account in these studies. Other mediators proposed to contribute to the generation and persistence of gut hypersensitivity after inflammation include bradykinin, prostaglandins, serotonin, and nerve growth factors (4). Nerve growth factor was necessary for colonic hypersensitivity to develop in a trinitrobenzene sulfonic acid (TNBS) model of colitis (8). There is also substantial interplay between BDNF (brain-derived neurotrophic factor) and CGRP, presumably through capsaicin-sensitive afferents from the gut (7). However, there may be different patterns of responses between classes. One class of polymodal colonic mechanoreceptors, in the rat, did not show changes in mechanosensitivity during inflammation, using a well-controlled mechanical stimulus, but did show selective increased chemosensitivity to serotonin (5-HT) (6).
Most of the studies to date have examined the effects of inflammation or inflammatory mediators on nociceptors or undefined mixed populations of spinal afferents in which nociceptors are likely to be predominant (2, 19). In contrast to nociceptors, low-threshold afferents normally convey sensory information about the normal physiological state of the viscera. The extent to which they contribute to discomfort and pain, under normal or inflamed conditions, has not been resolved. Indeed, it is not clear whether low-threshold mechanoreceptors are sensitized during inflammation; to date, reports have been mixed. We have previously characterized a single functional class of low-threshold, stretch-sensitive afferents in the guinea pig rectum both electrophysiologically and morphologically (18, 20, 34). The aim of this study was to identify whether this specific class of afferents showed any changes in function as a result of TNBS-induced inflammation.
METHODS
TNBS treatment.
Adult male and female guinea pigs (180–220 g) were anesthetized with isoflurane. TNBS was administered by intracolonic injection of 300 μl of TNBS solution (15 mg/ml; 30% ethanol) 7 cm from the anal sphincter or 300 μl of saline for controls (13). The Flinders University Animal Welfare Committee approved all procedures used in this study.
Effect of TNBS treatment.
Photos of the region of inflammation 5–7 cm oral to the anus were taken and macroscopically evaluated by a previously established method (13, 20).
Tissue preparation for afferent recordings.
Six days or 21–30 days later, guinea pigs were killed by a blow to the occipital region and exsanguinated. Specimens of distal bowel, with attached rectal nerve trunks were defined as “rectum” (19, 24). Preparations from this region were excised, flushed of contents, and opened up into a flat sheet. The preparation was superfused at 3 ml/min with Krebs solution (mM: 118 NaCl, 4.75 KCl, 1.0 NaH2PO4, 25 NaHCO3, 1.2 MgSO4, 2.5 CaCl2, 11 glucose) chilled to ∼10°C, bubbled with 95% O2-5% CO2. The mucosa was removed by sharp dissection, and three to seven small extrinsic nerve trunks, 20–40 μm in diameter, were dissected free of connective tissue for a length of 2–6 mm. We defined the rectum as the region supplied by rectal nerve trunks emerging from the pelvic ganglia. Circumferentially the tissue was 8–10 mm wide under basal conditions. A 15-mm longitudinal segment was isolated and pinned, serosal side up, with 50-μm tungsten pins along one side. One rectal preparation was prepared from each animal, with each rectal preparation having three to seven nerve branches that could be individually investigated. Each nerve branch might have more than one afferent unit that could be discriminated accurately. Therefore, N was used to denote the number of rectal preparations (identical to the number of animals), and n was used to denote the number of single afferent fibers that could be recorded from the nerve branches.
Afferent dissection and recordings.
The edge of the preparation was attached to an array of hooks. This was attached to a “tissue stretcher” consisting of a microprocessor-controlled stepper motor with an in-series isometric force transducer (DSC 46-1001-01, Kistler Morse). The extrinsic nerve trunks and a strand of connective tissue were pulled under a coverslip partition into a recording chamber and sealed with silicon grease (Ajax). The recording chamber was filled with paraffin oil and platinum wire electrodes were placed in contact with both a nerve trunk and a connective tissue strand. Recorded signals were amplified and filtered through an isolated bioamplifier (ISO- 80, World Precision Instruments), digitized with a Powerlab/16SP (ADInstruments), and recorded on a Macintosh personal computer using Chart software (ADInstruments). The main chamber containing the preparation was superfused at 3 ml/min with oxygenated Krebs solution, at 34°C.
Identification of stretch-sensitive units.
After 1-h equilibration, ∼2 mN resting load was applied to the tissue. By use of this apparatus, the tissue could be stretched at rates of 20–5000 μm/s, over distances of 1–9 mm, while the tension developed by the tissue (stretching by “imposed length”) was recorded.
Hot spot identification.
Local mechanical distortion of the tissue was achieved by using von Frey hairs with tip diameters <50 μm (0.1–1.10 mN). “Hot spots,” areas of heightened responses to compression, were then identified and their positions were marked on the tissue with fine carbon particles attached to the tip of the hair. Stretch-sensitive units were investigated for their sensitivity to von Frey hairs (0.1, 0.2, 0.3, 0.5, 0.8, 1.1 mN) under isometric conditions. We assumed that the largest responses correlated with placing the von Frey hair accurately over the transduction site. Therefore, peak instantaneous frequency was determined for each von Frey hair as the mean response of the three highest values of 8–10 applications for each unit (17, 34).
Electrical activation of afferent nerve fibers.
Shielded bipolar platinum electrodes with bared tips were advanced onto a previously identified hot spot. Square pulses of 0.5- to 1-ms duration were applied to the tissue with a Grass S48 Stimulator (Grass Instruments). A range of voltages applied at 0.3 Hz was used to determine the afferent activation threshold. The test stimuli used were 130% of voltage threshold delivered over a range of frequencies (1–200 Hz). At least 10 repetitions of each frequency were applied to the hot spot. At lower frequencies each electrical pulse elicited an action potential, but at higher frequencies fewer electrical pulses elicited an action potential (27). The percentage of electrical pulses eliciting an action potential was determined and a frequency-response curve was subsequently generated.
Muscle contractility.
The number of contractions elicited in the tissue was counted by using Chart software cycle recognition with parameters of a noise threshold of 2% and 100-ms tracking. Tissue accommodation was determined by applying a 60-s stretch stimulus, then calculating the integrated mean force and afferent discharge over the last 10 s of the stretch.
Drugs.
Applications of capsaicin (1 μM) (Sigma), prostaglandin E2 (PGE2, 1–3 μM) (Sigma), and bradykinin (1 μM) (Sigma) were applied directly to the bath.
Analysis.
Single units were identified and discriminated by Chart Spike Histogram software; when single units could not be discriminated, recordings were discarded. Single unit activity was presented and analyzed by using impulses per second except where instantaneous frequency was used. For von Frey hair stimulation, both the number of spikes elicited in a 300-ms period and the peak instantaneous frequency generated during a von Frey hair application were identified for further analysis. Data is presented as means ± SE unless otherwise stated. Statistical comparison was carried out by Student's t-test, two-way analysis of variance χ2, Mann-Whitney, Wilcoxon signed-rank test, or linear regression as appropriate using GraphPad Prism software; P < 0.05 was considered significant.
RESULTS
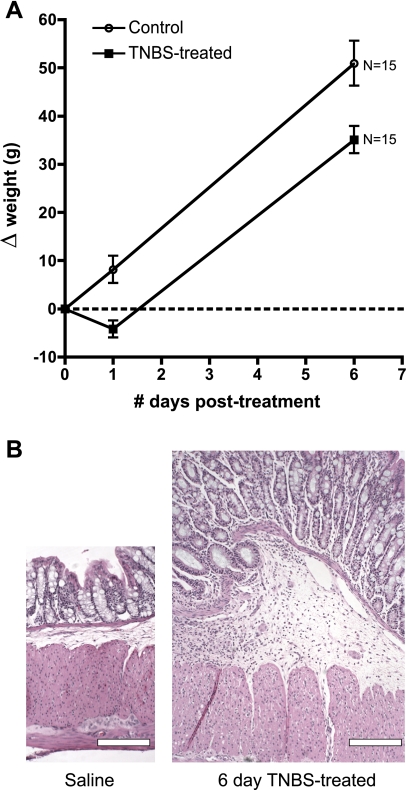
TNBS treatment in most cases evokes visible morphological changes in the rectum at 6 days posttreatment including mucosal hyperemia and edema, thickened muscle and rarely ulcerations and adhesions. Weight gain compared with saline controls was significantly decreased in the TNBS-treated animals after 24 h, and the weight deficit persisted up to 6 days posttreatment (P = 0.001, N = 14, 16, two-way ANOVA) (Fig. 1). Hematoxylin and eosin staining revealed thickened circular muscle, submucosa and mucosal layers, lesions in the muscularis mucosae, and submucosal infiltrate of leukocytes (Fig. 1B). At 30 days posttreatment, no visible morphological differences were observed in the rectum between TNBS- and saline-treated groups. At 30 days posttreatment, TNBS-treated animals had similar weights to saline-treated animals (P = 0.56, N = 5 TNBS treated and N = 4 saline treated, unpaired t-test).
Fig. 1.
Effects of trinitrobenzene sulfonic acid (TNBS) treatment. After TNBS administration, guinea pigs lost a small amount of weight compared with saline-treated animals. This weight loss was sustained until 6 days posttreatment (A) but had resolved entirely by 30 days (not shown). Sections from hematoxylin and eosin-stained sections of rectum from saline-treated and TNBS-treated animals (B) show marked hypertrophy, edema, and leukocyte infiltration in the inflamed condition. Calibration bars: 200 μm.
Spontaneous activity.
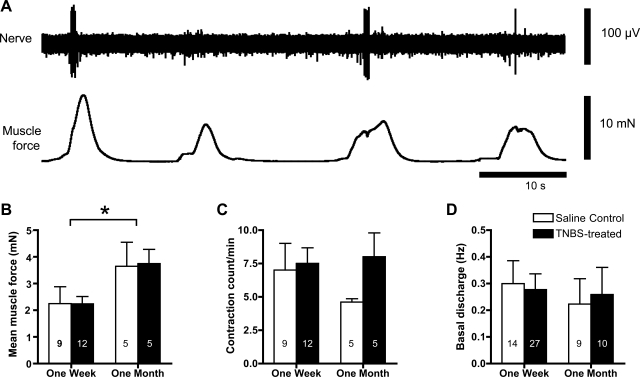
Spontaneous contractions of the circular muscle were observed in the majority of both saline-treated and TNBS-treated preparations. Stretch-sensitive mechanoreceptors fired bursts of action potentials during some but not all spontaneous contractions but were consistently silent during periods of mechanical quiescence (Fig. 2 and Refs. 17, 19). Neither muscle contractility (measured as mean force) nor mechanoreceptor firing (measured as mean frequency during a 60-s period) was affected by TNBS treatment, at either 6 days or 30 days (Fig. 2). Likewise, mean contraction amplitude and contraction frequency were not significantly affected by TNBS.
Fig. 2.
Spontaneous activity of smooth muscle and low-threshold rectal mechanoreceptors. A: preparation of rectum from a control guinea pig, pretreated with a saline enema 6 days previously, shows spontaneous contractions of circular muscle. Associated with the rising phase of most but not all of these contractions are bursts of mechanoreceptor firing. B: mean tension measured over 60 s was unaffected by TNBS treatment compared with saline. However, there was a significant increase with age (30 days after saline or TNBS enema, *P = 0.018; 2-way ANOVA). Numbers on histograms refer to the number of rectal preparations. C: frequency of contractions per minute was unaffected by TNBS treatment at both 6 days and 30 days after treatment (P = 0.26 2-way ANOVA for both). D: spontaneous firing of mechanoreceptors was not affected by either TNBS treatment or age.
Stretch sensitivity.
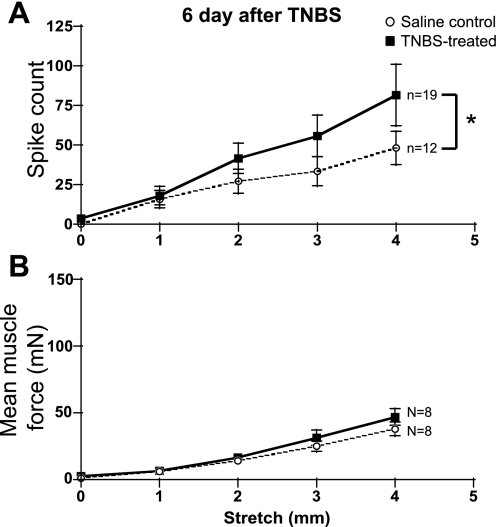
Circumferential distension of the preparation (1–4 mm stretch relative to resting length) typically evoked a single large contraction just after the onset of stretch, which was followed by an accommodation over the next 10 s. Mechanoreceptor firing, evoked by stretch, was closely associated with the active muscle contractions but could persist for up to 10 s during larger stretches (3–4 mm), despite substantial accommodation. Six days after TNBS treatment, there was a significant increase in stretch-evoked firing of rectal mechanoreceptors compared with controls (P = 0.032, linear regression) without an equivalent increase in mean muscle force (Fig. 3, A and B). Thirty days after TNBS treatment, there was no difference in either mechanoreceptor firing or mean muscle force, compared with saline-treated controls (not shown). Analysis of responses to 4-mm stretch identified age-related changes in mean muscle force (which increased significantly in both control and inflamed tissues in older animals, P = 0.0019, two-way ANOVA) and the number of spikes (which decreased significantly in both control and inflamed tissues in older animals, P = 0.046, two-way ANOVA).
Fig. 3.
Response of low-threshold mechanoreceptors and circular muscle to circumferential distension 6 days after saline or TNBS treatment. The stimulus-response curve, measured as number of action potentials during a 10-s stretch (0–4 mm), was significantly different 6 days after TNBS treatment (*P = 0.032, linear regression) compared with age-matched saline controls (A). In contrast, there was no significant difference in the mean muscle tension evoked by 0- to 4-mm stretch (10 s) at 6 days (B). At 30 days after TNBS, neither firing responses, nor mean muscle tension was significantly different between saline and TNBS-treated animals; n refers to numbers of afferent units, and N indicates the number of animals from which rectal preparations were studied.
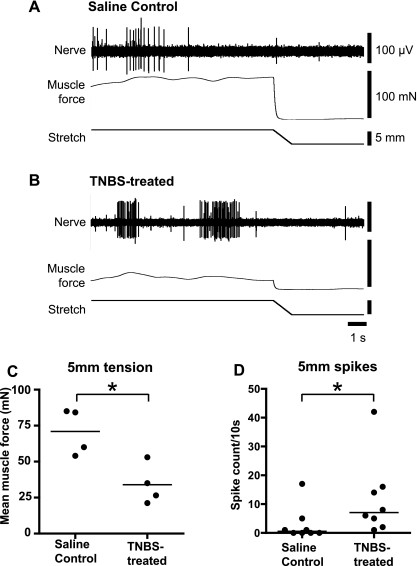
We also examined mechanoreceptor firing and muscle contractility during the fully accommodated phase of longer distensions (measuring responses during the last 10 s of a 60-s distension, Fig. 4, A and B). Thirty days after TNBS treatment, mechanoreceptor firing was significantly greater than in saline-treated controls (P = 0.020, Mann-Whitney) despite mean muscle force being slightly but significantly reduced in TNBS-treated tissue (P = 0.013, unpaired t-test; Fig. 4, C and D). This protocol was not tested 6 days after TNBS application.
Fig. 4.
Muscle activity and mechanoreceptor firing in stretch-accommodated tissue. The final 10 s of a stretch maintained for 60 s is shown in A and B. Tissue was taken from animals 30 days after either saline enema (A) or TNBS enema (B). Typically, accommodated muscle tension was reduced by TNBS treatment, but low-threshold mechanoreceptor firing was increased. Pooled data is shown in C and D. Mean muscle force, averaged over the last 10 s, was significantly lower following TNBS treatment (*P = 0.013, N = 4, N = 4, unpaired t-test; C) but the total number of mechanoreceptor action potentials during this period was increased after TNBS treatment (*P = 0.021, n = 8, n = 8, unpaired t-test; D).
Responses to focal distortion.
The transduction sites of low-threshold, rectal mechanoreceptors have previously been shown to correspond to rectal intraganglionic laminar endings (rIGLEs; Ref. 19). Direct distortion of rIGLEs using von Frey hairs activates low-threshold afferents in a manner that is relatively insensitive to changes in local muscle tone (34). Responses are graded and show slow adaptation to maintained probing (17).
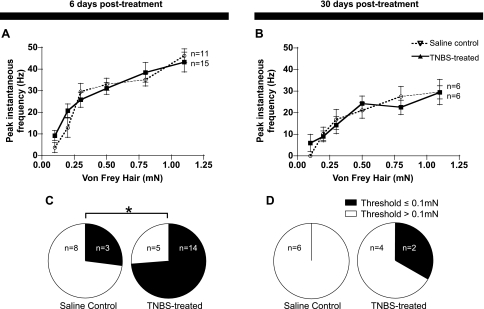
Mechanotransduction sites were activated by von Frey hairs ranging from 0.1 to 1.1 mN. Over most of this range, TNBS treatment, either 6 days or 30 days previously (Fig. 5, A and B), had no effect on firing. However, there was a significant reduction in the threshold for activation after TNBS treatment. A higher proportion of afferent units responded to the lightest von Frey hair (0.1 mN), 6 days after administration of TNBS, compared with saline-treated controls (Fig. 5C, P = 0.023, χ2). There was no significant difference at 30 days after TNBS treatment (Fig. 5D, P = 0.145, χ2), although it should be noted that fewer units (6 control, 6 TNBS) were tested at this time point.
Fig. 5.
Effects of TNBS on mechanoreceptor stimulus-response curves evoked by von Frey hairs. Stimulus-response curves to a range of von Frey hairs (0.1–1.1 mN) applied to identified mechanoreceptor transduction sites in the rectum were largely unchanged by TNBS treatment at either 6 or 30 days posttreatment (A and B). However, small but consistent differences were seen in the thresholds of units at 6 days but not 30 days. In C and D, the numbers of units (n) responding to a 0.1 mN von Frey hair is shown for TNBS-treated and saline-treated animals at 6 days and 30 days, respectively (*P < 0.05).
Excitability of afferent nerve fibers.
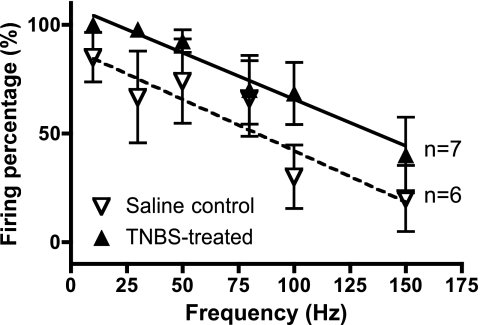
The excitability of rectal mechanoreceptors was measured by their ability to follow suprathreshold electrical stimuli, over a range of frequencies. In all afferent units, the percentage of stimuli that evoked action potentials declined, with increasing frequency. However, in preparations treated 6 days previously with TNBS, rectal mechanoreceptors followed stimuli with significantly higher fidelity across the range of frequencies tested (0–150 Hz, P = 0.0043, linear regression n = 6, n = 7, Fig. 6), indicating an increase in the electrical excitability of mechanosensitive afferents in inflamed tissue. Similar studies were attempted 30 days after treatment but reliable results were not obtained. In these slightly older animals, electrical stimulation evoked larger smooth muscle contractions which displaced the stimulating electrodes relative to afferent units, in both TNBS- and saline-treated animals.
Fig. 6.
Responses to focal electrical stimulation by low-threshold mechanoreceptors. Stimulus intensity was set to 130% of threshold and the ability to follow repetitive stimulation at frequencies from 10–150 Hz was plotted. Mechanoreceptors from TNBS-treated animals showed fewer failures over the full range of frequencies than saline controls (*P = 0.0043, linear regression).
Chemosensitivity.
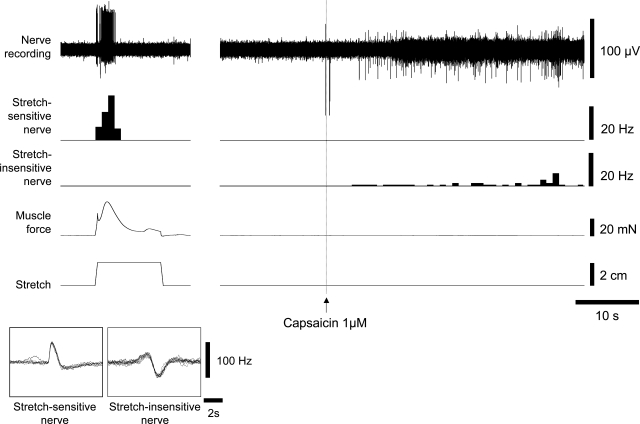
In neither control nor TNBS-treated preparations did capsaicin (1 μM) activate low-threshold stretch-sensitive afferents (n = 6, n = 5, Fig. 7). Capsaicin also did not modulate afferent responses to 50 mg von Frey hairs in saline-treated or TNBS-treated tissue (1 μM, n = 4, n = 4). We were unable to detect effects of capsaicin in naive, untreated tissue either; 1 μM capsaicin did not change basal firing or modulate the response of low-threshold stretch-sensitive afferents to a standardized stretch protocol (3 mm circumferential stretch for 10 s, n = 8). However, the capsaicin did activate some afferent fibers that were stretch insensitive. In both control and TNBS-treated tissue, afferent units that did not respond either to 3-mm stretch or to spontaneous contractile activity, frequently responded to capsaicin (1 μM), in a manner that appeared independent of contractile activity (n = 5, n = 7 units, see Fig. 7).
Fig. 7.
Responses to capsaicin of low-threshold, slowly adapting rectal mechanoreceptors. Stretch-sensitive low-threshold mechanoreceptors did not respond to 1 μM capsaicin in either TNBS-treated or saline-treated animals. However, in most preparations, other unidentified classes of afferent units (which were not stretch-sensitive) were powerfully activated. There were no detectable differences in responses between saline or TNBS-treated tissue. The example shown was from a specimen treated 6 days previously with TNBS.
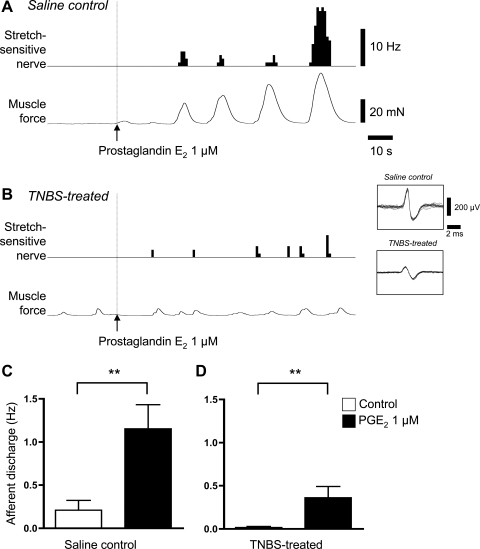
We tested the effects of potential inflammatory mediators on low-threshold rectal mechanoreceptors. The autacoid PGE2 (1–3 μM) activated low-threshold stretch-sensitive afferents in control preparations (n = 10, P = 0.0078, Wilcoxon signed-rank test) and in preparations treated 6 days previously with TNBS (n = 8, P < 0.01, Wilcoxon signed-rank test). Mean muscle force increased in the presence of PGE2 (N = 4, N = 4, P < 0.05, two-way ANOVA) in both control and TNBS-treated preparations but contraction frequency and peak force of contractions were not significantly altered. Afferent firing in the presence of PGE2 was closely associated with smooth muscle contractions (Fig. 8). Consistent with this, PGE2 (1 μM) did not modulate afferent responses to 50 mg von Frey hairs in either control (n = 4, n = 4) or TNBS-treated tissue (n = 4, n = 4). We tested this further in control preparations. In Ca2+-free Krebs containing 3.6 mM Mg2+, muscle contractility was greatly reduced and PGE2 (3 μM) did not evoke detectable contractions. Under these conditions PGE2 also did not activate rectal afferents (n = 5, N = 3). These results suggest that the effects of PGE2 on low-threshold mechanoreceptors are mediated indirectly, through changes in smooth muscle contractility, rather than by a direct effect on nerve endings.
Fig. 8.
Afferent responses to prostaglandin E2. A: a low-threshold mechanoreceptor showed conspicuous bursts of firing after application of PGE2 (1 μM) that appeared to be associated with contractile activity of the circular muscle in a saline control preparation. B: PGE2 (1 μM) also activated low-threshold, stretch-sensitive afferents in preparations treated 6 days previously with TNBS. Afferent activity in these preparations also coincided with evoked contractile activity. C and D: pooled data showed that PGE2 evoked significant increased firing in both saline control and TNBS-treated preparations (**P < 0.01). It is likely that the effects of PGE2 were mediated indirectly, through increased contractility, since the effects were abolished in Ca2+-free solution (not shown).
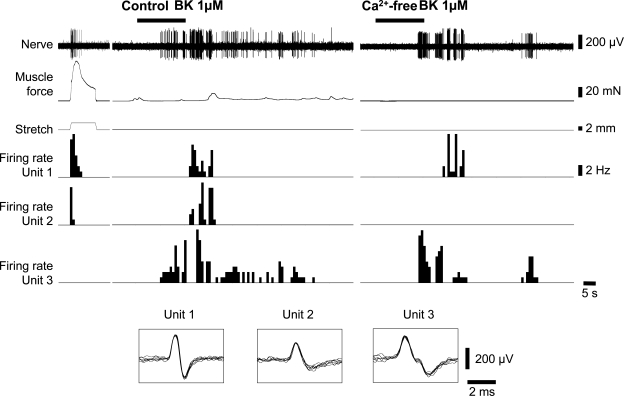
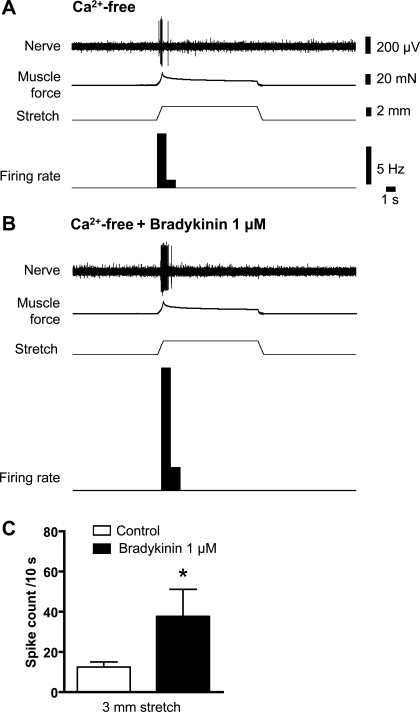
Another inflammatory mediator, bradykinin (1 μM), evoked transient contractions of smooth muscle (N = 5) and associated firing of low-threshold rectal afferents. Bradykinin-evoked firing persisted in Ca2+-free Krebs containing 3.6 mM Mg2+, when all muscle contractions were abolished (6 of 9 units, N = 5; see Fig. 9). Bradykinin (1 μM) also led to an augmentation of afferent firing evoked by standardized stretch (3 mm for 10 s) in Ca2+-free Krebs containing 3.6 mM Mg2+ (Fig. 10). These results indicate that bradykinin had direct effects on low-threshold rectal mechanoreceptors, in addition to any effects on smooth muscle contractility.
Fig. 9.
Bradykinin (BK) activated stretch-sensitive units when muscle activity was abolished in Ca2+-free Krebs solution. Effects of 1 μM bradykinin on a stretch-activated mechanoreceptors in rectum from an untreated animal. Bradykinin evoked increased circular muscle contractility and several bursts of firing in the stretch-sensitive mechanoreceptors (unit 1 and unit 2) and also activated stretch-insensitive unit 3. In Ca2+-free solution (containing 3.6 mM Mg2+), muscle activity was abolished but bradykinin still evoked several bursts of firing in the stretch-sensitive unit 1, but not 2, and activated stretch-insensitive unit 3.
Fig. 10.
Sensitization of low-threshold stretch-sensitive unit by bradykinin in Ca2+-free Krebs solution. A: mechanoreceptor responses to 3 mm stretch of 10 s duration in Ca2+-free Krebs solution (containing 3.6 mM Mg2+). B: 1 μM bradykinin potentiated response of low-threshold mechanoreceptor to 3-mm stretch. Such effects were seen in 6 of 9 units tested. C: pooled data (n = 6) show that the effect of bradykinin was statistically significant (*P = 0.0038, paired t-test).
DISCUSSION
TNBS-induced inflammation has been widely studied as a model of intestinal inflammation, sharing many similarities to Crohn's disease (29). After a short period of hyperexcitability lasting several hours, TNBS inflammation causes reduced propulsive motility in the rat colon in vivo (25) with reduced contractile responses to a range of agonists (9) and altered contractility of isolated muscle cells (32). These changes are associated with hypertrophy, hyperplasia, and edema of the gut wall, including the muscularis externa (21). At the same time, visceromotor responses to colorectal distension are significantly enhanced in the rat (26), suggesting sensitization of spinal afferent neurons. Similarly, in the guinea pig, TNBS causes a reduction in propulsive motility of the colon (13); however, its effects on the rectum have not been specifically investigated. In the present study, we were unable to detect an overall decrease in contractile activity or muscle tone of the rectum 6 days after TNBS administration, using a range of different measures. However, rectal preparations showed high variability in both spontaneous contractile activity and reflex responses to circumferential stretch (17, 19); it is possible that this variability masked a net decrease in contractility.
In the present study, recordings from extrinsic afferents were made from the most distal region of the large bowel, where a well-characterized class of low-threshold, slowly adapting mechanoreceptors is most abundant (24). These afferents make distinctive intraganglionic laminar endings in the myenteric ganglia, which function as sites for mechanotransduction (19). Detailed studies of the mechanical sensitivity of these endings has shown that they are responsive to both distension and to contraction of either longitudinal or circular smooth muscle layers within their field of innervation (17), probably by detecting distortion of the myenteric ganglia through stretch-activated ion channels in the rIGLEs (34).
In the present study, these low-threshold, slowly adapting mechanoreceptors showed a modest increase in mechanosensitivity after TNBS treatment. Mechanistically, this could be due to direct changes in the excitability of their endings or to changes in contractile activity of the smooth muscle. The latter seems improbable, since no increase in contractile force or frequency was detected. Indeed, as mentioned previously, TNBS treatment is usually associated with significant decreases in propulsive motility. This suggests that the increased mechanosensitivity was probably due to heightened excitability or sensitivity of the sensory endings themselves. Several pieces of evidence support this possibility.
The threshold for activation of mechanoreceptor transduction sites by von Frey hairs was significantly reduced after TNBS. Previous studies have shown that changes in smooth muscle compliance, or tone, can have profound effects on stretch-induced mechanoreceptor firing, confounding interpretation of the site of effects on mechanoreceptor sensitivity. However, activation of afferents using von Frey hairs is much less sensitive to the state of the smooth muscle (34). In the present study, a significantly greater proportion of mechanoreceptors were activated by a 0.1 mN von Frey hair in TNBS-treated animals, but responses to stiffer von Frey hairs were not enhanced. In contrast, stretch-evoked mechanoreceptor firing was most strongly enhanced by TNBS for the largest distensions tested (Fig. 3). There was a tendency for the peak muscle forced, evoked by stretch, to be increased slightly after TNBS, although this was not significant (Fig. 3B). Another possible mechanism by which TNBS could indirectly affect stretch-activated responses is through inflammation-induced fibrosis (29); it is possible that remodeling of connective tissue may have altered mechanical coupling between the muscle layer and mechanoreceptor transduction sites (rIGLEs).
However, another line of evidence points to a direct effect of inflammation on low-threshold, slowly adapting mechanoreceptors in the guinea pig rectum. In the present study, it was shown that at 6 days after TNBS treatment low-threshold mechanoreceptors were able to follow suprathreshold electrical stimuli at higher frequencies than after saline treatment. This indicates that TNBS caused an increase in the electrical excitability of these endings. Several mechanisms could underlie this change of excitability. TNBS inflammation causes upregulation of specific sodium channels and downregulation of several potassium channels in spinal afferent nerve cell bodies, retrogradely labeled from the gut wall (1, 28). If, as seems likely, similar changes occur in the peripheral processes of sensory neurons, this could explain the enhanced excitability of mechanoreceptors in the present study.
Ongoing release of inflammatory mediators may also have contributed to the altered excitability of sensory endings in the gut wall. It is well established that various algogenic mediators, such as bradykinin, can excite visceral afferent neurons (3, 15, 16). In the present study, bradykinin caused both an enhancement of stretch-evoked firing and direct activation of endings, which persisted in Ca2+-free solution (when changes in muscle tone were abolished). Interestingly, prostaglandin E2 also increased firing of low-threshold mechanoreceptors, but this was indirectly mediated, through increases in smooth muscle contractility, which triggered bursts of afferent firing. PGE2 did not increase responses of low-threshold mechanoreceptors to activation by von Frey hairs in the present study. PGE2 evokes increased contractile activity of guinea pig large intestine (12), which suggests that it is not a major mediator involved in TNBS-inhibition of colonic motility.
Low-threshold mechanoreceptors in the guinea pig rectum were not activated by capsaicin, an agonist at TRPv1 channels, although many non-stretch-sensitive afferents were excited. There is a close association between TRPv1 expression and nociceptive function in sensory neurons (22). It is tempting to suggest therefore that the low-threshold mechanoreceptors that were the focus of this study are probably not nociceptive. Capsaicin sensitivity has been demonstrated in subsets of two functional classes of stretch-insensitive extrinsic colonic afferents in the rat (18) in both splanchnic and pelvic pathways (2).
Guinea pig rectal muscle had highly variable responses to circumferential stretch, thus increasing individual and population variability. This has been previously reported and is believed to be a functional characteristic of guinea pig rectal tissue (17). Consequently subtle changes in motility or whole organ changes in motility are unlikely to be identified using the variables recorded in the present study. Nonetheless, afferents responded more strongly to increasing circumferential stretch in the TNBS-treated tissue independently of changes in muscle force. Inflammation has also been reported to increase afferent discharge in response to intraluminal distension in rat in vivo (23).
This study showed that the function of this low-threshold, slowly adapting class of afferents with their endings within the myenteric plexus is modestly increased after 6 days of TNBS-induced inflammation but that this resolves by 30 days. The effects of inflammation were manifested as enhanced responses to stretch of the tissue, increased electrical excitability and reduced thresholds for von Frey hair stimulation. It has been suggested that in inflammatory and some postinfective functional disorders (4, 5) patients become hypersensitive to normal motility. It is tempting to suggest that the modulation of low-threshold mechanoreceptors, as observed in this study, may contribute to the disturbed sensations from the distal bowel in a range of functional and inflammatory disorders.
GRANTS
This work was supported by Grant DK-56986 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beyak MJ, Ramji N, Krol KM, Kawaja MD, Vanner SJ. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am J Physiol Gastrointest Liver Physiol 287: G845–G855, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Brierley SM, Jones RC 3rd, Gebhart GF, Blackshaw LA. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127: 166–178, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Brunsden AM, Grundy D. Sensitization of visceral afferents to bradykinin in rat jejunum in vitro. J Physiol 521: 517–527, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut 51, Suppl 1: i19–i23, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervero F, Laird JM. Visceral pain. Lancet 353: 2145–2148, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Coldwell JR, Phillis BD, Sutherland K, Howarth GS, Blackshaw LA. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. J Physiol 579: 203–213, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delafoy L, Gelot A, Ardid D, Eschalier A, Bertrand C, Doherty AM, Diop L. Interactive involvement of brain derived neurotrophic factor, nerve growth factor, and calcitonin gene related peptide in colonic hypersensitivity in the rat. Gut 55: 940–945, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delafoy L, Raymond F, Doherty AM, Eschalier A, Diop L. Role of nerve growth factor in the trinitrobenzene sulfonic acid-induced colonic hypersensitivity. Pain 105: 489–497, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Grossi L, McHugh K, Collins SM. On the specificity of altered muscle function in experimental colitis in rats. Gastroenterology 104: 1049–1056, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Grundy D, Al-Chaer ED, Aziz Q, Collins SM, Ke M, Tache Y, Wood JD. Fundamentals of neurogastroenterology: basic science. Gastroenterology 130: 1391–1411, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425: 545–562, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizawa M Contractile effects of 16-methyl analogues of PGE2 on the circular and longitudinal muscles of the guinea-pig isolated colon. Prostaglandins 42: 579–586, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol 547: 589–601, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience 118: 69–74, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Longhurst JC, Dittman LE. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am J Physiol Heart Circ Physiol 253: H556–H567, 1987. [DOI] [PubMed] [Google Scholar]
- 16.Longhurst JC, Kaufman MP, Ordway GA, Musch TI. Effects of bradykinin and capsaicin on endings of afferent fibers from abdominal visceral organs. Am J Physiol Regul Integr Comp Physiol 247: R552–R559, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Lynn P, Zagorodnyuk V, Hennig G, Costa M, Brookes S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J Physiol 564: 589–601, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol 518: 271–282, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 125: 786–794, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol 282: G1045–G1051, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Moreels TG, De Man JG, Dick JM, Nieuwendijk RJ, De Winter BY, Lefebvre RA, Herman AG, Pelckmans PA. Effect of TNBS-induced morphological changes on pharmacological contractility of the rat ileum. Eur J Pharmacol 423: 211–222, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Nagy I, Santha P, Jancso G, Urban L. The role of the vanilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol 500: 351–369, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Ness TJ, Gebhart GF. Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett 281: 131–134, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Olsson C, Costa M, Brookes SJ. Neurochemical characterization of extrinsic innervation of the guinea pig rectum. J Comp Neurol 470: 357–371, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Pons L, Droy-Lefaix MT, Bueno L. Leukotriene D4 participates in colonic transit disturbances induced by intracolonic administration of trinitrobenzene sulfonic acid in rats. Gastroenterology 102: 149–156, 1992. [DOI] [PubMed] [Google Scholar]
- 26.Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain 79: 175–185, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Shin JB, Martinez-Salgado C, Heppenstall PA, Lewin GR. A T-type calcium channel required for normal function of a mammalian mechanoreceptor. Nat Neurosci 6: 724–730, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol 552: 797–807, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol 20: 495–549, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Su X, Gebhart GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 80: 2632–2644, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Su X, Wachtel RE, Gebhart GF. Capsaicin sensitivity and voltage-gated sodium currents in colon sensory neurons from rat dorsal root ganglia. Am J Physiol Gastrointest Liver Physiol 277: G1180–G1188, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Wells RW, Blennerhassett MG. Persistent and selective effects of inflammation on smooth muscle cell contractility in rat colitis. Pflügers Arch 448: 515–524, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol 287: G647–G657, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol 289: G397–G406, 2005. [DOI] [PubMed] [Google Scholar]