Abstract
The impact of NPC1L1 and ezetimibe on cholesterol absorption are well documented. However, their potential consequences relative to absorption and metabolism of other nutrients have been only minimally investigated. Thus studies were undertaken to investigate the possible effects of this protein and drug on fat absorption, weight gain, and glucose metabolism by using Npc1l1−/− and ezetimibe-treated mice fed control and high-fat, high-sucrose diets. Results show that lack of NPC1L1 or treatment with ezetimibe reduces weight gain when animals are fed a diabetogenic diet. This resistance to diet-induced obesity results, at least in part, from significantly reduced absorption of dietary saturated fatty acids, particularly stearate and palmitate, since food intake did not differ between groups. Expression analysis showed less fatty acid transport protein 4 (FATP4) in intestinal scrapings of Npc1l1−/− and ezetimibe-treated mice, suggesting an important role for FATP4 in intestinal absorption of long-chain fatty acids. Concomitant with resistance to weight gain, lack of NPC1L1 or treatment with ezetimibe also conferred protection against diet-induced hyperglycemia and insulin resistance. These unexpected beneficial results may be clinically important, given the focus on NPC1L1 as a target for the treatment of hypercholesterolemia.
Keywords: diabetogenic diet, glucose metabolism, insulin sensitivity, Western diet, sucrose polybehenate
the comparatively new drug ezetimibe has been shown to dramatically reduce absorption of dietary and biliary cholesterol and is now used alone or as an adjunct therapy for reducing plasma cholesterol (3, 29, 43). Results from initial studies with gene knockout mice are consistent with the drug having a direct effect on the cholesterol absorption pathway mediated by the Niemann-Pick type C-1 like protein 1 (NPC1L1) (2, 11). Both ezetimibe treatment and NPC1L1 gene inactivation effectively reduce hypercholesterolemia in humans and atherosclerosis in animal models (3, 9, 10, 42, 45, 46). Although the physiological impact of ezetimibe and NPC1L1 on cholesterol absorption is unquestioned, the mechanisms of action of both the drug and the protein are not clear. NPC1L1 has been reported to be located on the apical surface of enterocytes as expected for a transport protein (2, 24). However, other reports have localized this protein to intracellular compartments (8, 51). Similarly, ezetimibe has been reported to inhibit NPC1L1 function by direct interaction with the protein (16), but reports from several groups indicate that ezetimibe also interacts with SR-BI, the class B type I scavenger receptor (1, 13, 28, 30, 44). These reports suggest that SR-BI serves as a plasma membrane cholesterol transporter that acts in concert with NPC1L1 to facilitate cholesterol absorption (13, 30).
Although current reports indicate that ezetimibe treatment and NPC1L1 gene inactivation do not affect dietary fat (triglyceride) absorption (45–47), a different cholesterol absorption inhibitor, disodium ascorbyl phytostanyl phosphate (36), has been shown to reduce weight gain in rodent models fed diets high in fat and cholesterol (14, 48, 49). These results suggest that inhibition of cholesterol absorption may impact dietary fat absorption and/or metabolism and protect against diet-induced obesity. Interestingly, ezetimibe treatment does reduce plasma triglyceride levels in some animal models fed diets high in fat and cholesterol (45), suggesting that ezetimibe-sensitive pathways may modulate fat metabolism. The possibility that inhibiting cholesterol absorption may decrease fat absorption, or that ezetimibe directly inhibits some step in the fat absorption process has substantial clinical significance since surgical (31, 50) and pharmaceutical (6, 40) inhibition of fat absorption has become a therapeutic approach for treating obesity and its comorbidity, type II diabetes.
Hydrolysis and absorption of dietary fat (mainly triglycerides) are extremely efficient (>90%). However, because of the caloric density of lipids, relatively small decreases in absorption can significantly impact energy balance. The end products of fat digestion, i.e., monoglycerides and free fatty acids, partition into bile salt-mixed micelles and are delivered, along with dietary and biliary cholesterol, to the intestinal brush border for absorption. Whether fatty acid absorption is solely by passive diffusion or also by facilitated transport remains a matter of debate. Some studies suggest that FAT/CD36 (fatty acid transporter/cluster determinant 36) plays a role in fatty acid absorption by enterocytes (12, 35) although other mechanisms are clearly indicated (18). However, another school of thought purports that fatty acids cross the apical membrane of enterocytes by passive diffusion and that apparent transport against a concentration gradient is mediated by rapid intracellular modification of the fatty acids that prevent them from diffusing back across the cell membrane (21, 27, 34). Thus “protein-facilitated” absorption of fatty acids need not result from transporter activity. The family of fatty acid transport proteins (FATPs), which are fatty acyl:CoA synthetase enzymes, appear to be important in this regard in several cell types (22, 38), and studies indicate that FATP4, specifically, may be important for intestinal absorption of long-chain fatty acids (17, 20).
We have initiated experiments to investigate the relationship between inhibition of cholesterol absorption and efficiency of dietary fat absorption, with specific focus on susceptibility to diet-induced obesity and diabetes. Our present results show that wild-type mice treated with ezetimibe and Npc1l1−/− mice are resistant to diet-induced weight gain and hyperglycemia compared with control mice, which may result, in part, from decreased fat absorption. Interestingly, our data show that ezetimibe treatment or lack of NPC1L1 protein significantly reduces absorption of saturated fatty acids by a mechanism that may involve reduced expression of FATP4.
MATERIALS AND METHODS
Animals and diets.
Npc1l1−/− mice were generated by homologous recombination in embryonic stem cells and backcrossed 10 times into the C57BL/6J background as previously described (8). Wild-type C57BL/6J littermate mice were used in the control groups. Animals were maintained in a temperature- and humidity-controlled room with a 12-h light-dark cycle and free access to water and rodent chow (Teklad LM485). Experimental mice were fed either a basal diet (D12328, Research Diets, New Brunswick, NJ) or one of two diabetogenic diets (high in fat and sucrose). The caloric composition of diabetogenic diets were 58.5% fat (coconut oil), 25% sucrose, 16.5% protein (D12331, Research Diets) or 58.5% fat (lard), 27% sucrose, 14.5% protein (F3282, Bio-Serv, Frenchtown, NJ). The Western diet (TD88137, Harlan Teklad, Madison, WI) was 42% fat (milk fat), 43% sucrose, and 15% protein. For diets that included ezetimibe, Zetia tablets (Schering, Kenilworth, NJ) were ground and thoroughly mixed into portions of the diet at 10 mg/100 g food. Weight gain was followed by weekly measurements taken at the same time of day throughout a study. Body composition (fat and lean mass) was determined with a 1H-magnetic resonance spectroscopy (EchoMRI-100; EchoMedical Systems, Houston, TX) before animals were fed high-fat diets and periodically thereafter, usually near the end of the light cycle. All animal protocols were approved by the institutional animal care and use committee at the University of Cincinnati.
Lipid absorption.
Fat absorption in mice was determined by the sucrose polybehenate method essentially as previously described (25). Individually housed mice were fed the test diet for 3 days, after which their cages were changed and fecal samples were collected on each of 3 subsequent days. On the 4th and 5th days, fecal pellets were recovered from each cage and the fatty acid content and composition of excreted lipids were determined by gas chromatography. Since behenic acid is essentially absent from the fat sources used in the test diet and is entirely excreted when given as sucrose polybehenate, absorption is calculated from the difference between diet and feces in the ratio behenate/total fatty acid. The calorie composition of the test diet was 58% fat (1:1 mix of lard and coconut oil so that long- and medium-chain fatty acids would be similarly represented and readily detected), 27% sucrose, and 15% protein (nonfat powdered milk). Cholesterol absorption was determined by the dual-isotope method as previously described (7, 19).
Blood chemistries.
Plasma lipids were measured in tail blood collected after an overnight fast (8–10 h) by use of standard colorimetric kits (Thermo Electron, Waltham, MA). Blood glucose was measured at the same time with AccuCheck glucometers (Roche Applied Science, Penzberg, Germany). Plasma insulin was measured by radioimmunoassay (Wako Chemicals, Richmond, VA). Glucose tolerance tests were also performed after an 8- to 10-h overnight fast. Baseline glucose levels were determined and the test was initiated by oral gavage or intraperitoneal injection of 2 g glucose per kilogram body weight. Blood was obtained from the tail vein 5, 15, 30, 60, and 120 min after glucose administration.
Western blots.
Mucosal scrapings from intestines of overnight-fasted mice were homogenized in 5 volumes of 10 mM Tris·HCl, 0.25 M sucrose, 5 mM benzamidine, 1 mM EDTA, pH 7.4, plus a protease inhibitor cocktail, with 10 strokes of a Dounce homogenizer. Debris was pelleted at 1,000 g for 10 min and cell membranes were subsequently pelleted at 100,000 g for 1 h, followed by resuspension in the above buffer. All steps were carried out on ice or at 4°C and all fractions were stored at −80°C until analysis. Cytosolic and membrane proteins (50–100 μg) were resolved by SDS-PAGE, transferred to polyvinylidene difluoride or nylon membranes, and detected with antibodies against liver fatty acid binding protein (L-FABP) (SC-50380, Santa Cruz Biotechnology), FATP4 (a kind gift from Dr. Nicholas Davidson, Washington University School of Medicine), NPC1L1 (a kind gift of Dr. Helen Hobbs, University of Texas Southwestern Medical School), SR-BI (NB400-101, Novus Biologicals), CD36 (mybiosource.com), and α-tubulin (MS-581-P0, Lab Vision). Immunoreactive proteins were detected by goat anti-rabbit IgG conjugated to horseradish peroxidase (Dako) and visualized by enhanced chemiluminescence (Pierce) or by FITC-conjugated donkey anti-rabbit IgG, followed by goat anti-FITC IgG conjugated to alkaline phosphatase and visualized by enhanced chemifluorescence (Amersham). Protein concentrations were determined by a bicinchoninic acid kit (Pierce) or the Lowry procedure.
Statistical analysis.
Results are presented as means ± SD. Statistical significance (P < 0.05) between groups was assessed by one-way ANOVA with Tukey's post hoc test for all pairwise comparisons, or by Student's unpaired t-test, by use of Prism 4 (GraphPad Software) or SigmaPlot 9.01 (Systat Software).
RESULTS
Ezetimibe-treated and NPC1L1-null mice are resistant to diet-induced obesity.
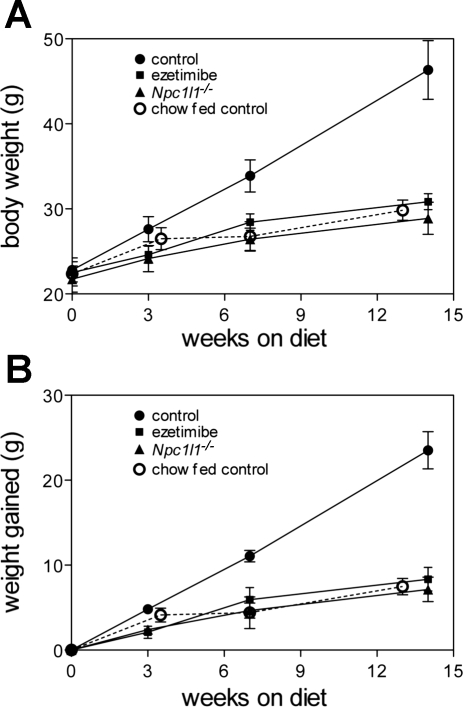
As an initial test to determine whether ezetimibe treatment or lack of NPC1L1 protein affects nutrient absorption or metabolism in a physiologically relevant way, weight gain and other metabolic parameters were monitored while mice were fed diets enriched with fat and sucrose. In the first study, weight gain was monitored for several weeks while mice were fed either a basal diet, similar in composition to standard rodent chow, or a high-fat, high-calorie, “diabetogenic” diet with 58% of calories from fat and 26% of calories from sucrose, with or without ezetimibe at 100 μg per gram of food. As shown in Fig. 1, both ezetimibe-treated and Npc1l1−/− mice were resistant to diet-induced weight gain when fed the diabetogenic diet. Control animals (C57BL/6J) gained 23.5 ± 2.2 g whereas the other groups gained an average of only 7.8 ± 1.3 g after 14 wk on the diabetogenic diet (P < 0.001). These differences were significant after only 3 wk on the diet (P < 0.015). Data shown are from male mice (4–6 per group) but female mice exhibited the same phenotype. Equivalent results were obtained when similar groups of mice were fed the standard “Western” diet, which has a less extreme fat content (42% of calories compared with 58% of calories in diabetogenic diets) and may serve as a better model for diet-induced human disease (data not shown).
Fig. 1.
Ezetimibe treatment or Niemann-Pick type C-1 like protein 1 (NPC1L1) gene inactivation prevents diet-induced weight gain. A: body weight of control mice fed diabetogenic diet with or without added ezetimibe and of Npc1l1−/− mice fed diabetogenic diet was measured for several weeks (n = 4–6 per group). B: data expressed as change from starting weight over time. Differences between control mice fed diabetogenic diet and all other groups are significant at 3 wk and all times later.
To determine whether the lack of NPC1L1 function affected diet-induced obesity or overall body growth, body composition of control and Npc1l1−/− mice was measured by NMR after 3 wk on the Western diet. The data in Table 1 show that accrual of fat mass accounted for essentially all of the difference between control and Npc1l1−/− animals in weight gained. Initially, lean mass and fat mass were the same for the two groups. Lean mass was not changed in either group after 3 wk of Western diet. However, fat mass increased more than twofold in control mice (2.15 ± 0.65 to 5.06 ± 1.22 g) but did not change significantly in Npc1l1−/− mice (1.72 ± 0.60 vs. 2.25 ± 0.96 g). The relative change in fat mass is further illustrated by the twofold change in fat-to-lean ratio for control mice (0.10 ± 0.03 to 0.22 ± 0.05). This ratio did not change for Npc1l1−/− mice (0.08 ± 0.05 vs. 0.10 ± 0.04). Similar results were obtained with the diabetogenic diets and with ezetimibe-treated mice (data not shown).
Table 1.
Western diet adds fat mass to control but not Npc1l1−/− mice
|
Start |
Western Diet, 3 wk
|
|||
|---|---|---|---|---|
| Control | Npc1l1−/− | Control | Npc1l1−/− | |
| Body weight, g | 25.77±1.16 | 26.38±1.08 | 29.67±1.16 | 27.13±1.31* |
| Fat mass, g | 2.16±0.65 | 1.72±0.60 | 5.06±1.22 | 2.25±0.96* |
| Lean mass, g | 21.68±0.78 | 22.51±0.90 | 22.67±0.46 | 22.52±0.63 |
| Fat/lean ratio | 0.10±0.03 | 0.08±0.03 | 0.22±0.05 | 0.10±0.04* |
| Weight gained, g | 3.90±1.10 | 0.75±0.52* | ||
Values are means ± SD.
P < 0.01 vs. control on diet.
Lipid absorption is decreased in ezetimibe-treated and NPCL1-null mice.
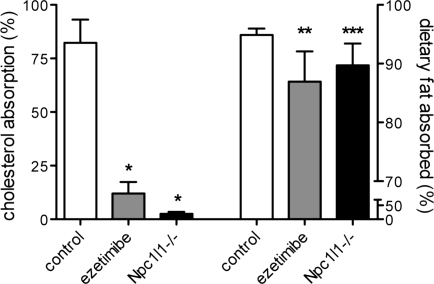
These striking differences caused us to reexamine the effect of ezetimibe and the impact of NPC1L1 on dietary triglyceride as well as cholesterol absorption. Cholesterol absorption was measured by the standard dual-isotope method (7, 19) and the results are presented in Fig. 2. Similar to previous reports (2, 8), cholesterol absorption was inhibited 90% by the absence of NPC1L1 and 80% by ezetimibe treatment. To measure dietary triglyceride absorption, a sensitive and physiologically accurate method was used. Instead of monitoring the appearance in serum of digestion products from an acutely delivered bolus of oil, fecal excretion of dietary fat was measured and normalized to the excretion of a nonabsorbable fat, sucrose polybehenate, incorporated into the diet. Briefly, a trace amount of the marker was mixed with a test diet of desired composition (see materials and methods) and fecal samples were collected after the animals had consumed this diet for 3 to 5 days. Absorption was calculated from the difference in fat content and composition between the diet and fecal samples as described (25). The data in Fig. 2 show that both ezetimibe treatment and lack of NPC1L1 inhibited fat absorption to a similar degree under normal eating conditions. Control animals absorbed 94.9% of dietary fatty acids whereas drug-treated and knockout mice absorbed only 86.9 and 89.7%, respectively, of the fat from a high-fat, high-sucrose diet (P = 0.021 for ezetimibe and P = 0.033 for Npc1l1−/− vs. controls).
Fig. 2.
Dietary fat and cholesterol absorption are decreased in ezetimibe-treated and Npc1l1−/− mice. Cholesterol absorption (left, n = 3–4) was measured by the dual-isotope method and fat absorption (right, n = 4–5) was measured by the sucrose polybehenate method as described in materials and methods. Mice were fed basal chow. *P < 0.001 compared with controls; **P = 0.033, ***P = 0.025 compared with controls.
Absorption of specific fatty acids was also determined by this method for control, ezetimibe-treated, and Npc1l1−/− mice, and the results are presented in Table 2. Only a small effect was observed for the unsaturated fatty acids oleate (18:1) and linoleate (18:2). Absorption of these fatty acids was ≥95% in controls and 2–5% less in the experimental groups. Long-chain saturated fatty acids were absorbed less efficiently than unsaturated fatty acids by all groups, as expected. However, drug treatment and lack of NPC1L1 significantly impaired absorption of saturated fatty acids in a graded manner that correlated with chain length. Thus stearate (18:0) absorption was decreased from ∼69% in controls to ∼50% in experimental mice, and palmitate (16:0) absorption was decreased from ∼91% in controls to ∼80% in experimental mice. Absorption of medium-chain saturated fatty acids was more moderately affected. Myristate (14:0) absorption was decreased 7–10% compared with controls, and laurate (12:0) absorption was reduced ∼4%. Data shown are from mice that were naive to diabetogenic diet except for the test period. Virtually identical results were obtained from a different cohort of mice that were fed diabetogenic diet for 6 wk (data not shown).
Table 2.
Reduced fatty acid absorption by Npc1l1−/− and ezetimibe-treated mice
|
% Absorbed |
|||
|---|---|---|---|
| Control | Ezetimibe | Npc1l1−/− | |
| Laurate | 97.9±1.6 | 94.4±2.8* | 94.9±2.1* |
| Myristate | 93.5±2.6 | 87.7±5.2† | 84.5±4.8* |
| Palmitate | 89.0±5.3 | 79.2±7.3* | 80.4±5.2* |
| Stearate | 68.8±10.4 | 49.4±13.7* | 49.9±10.3* |
| Oleate | 95.9±0.6 | 91.2±3.7* | 92.1±3.0* |
| Linoleate | 97.1±0.3 | 94.9±2.0† | 95.1±1.2* |
Values are means ± SD.
P < 0.045 vs. control;
P < 0.06 vs. control.
FATP4 expression is suppressed by ezetimibe and by the lack of NPC1L1.
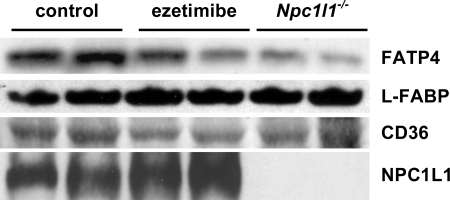
To begin to understand the mechanism(s) by which ezetimibe and NPC1L1 might affect absorption of long-chain saturated fatty acids, intestinal expression of proteins thought to contribute to fatty acid absorption was investigated. Membrane and cytosol fractions were prepared from mucosal scrapings of the duodena and jejuna from fasted ezetimibe-treated, Npc1l1−/−, and control mice that had been maintained on basal diet. Western blots were used to determine the relative amounts of FATP4, CD36, and L-FABP (liver fatty acid binding protein) protein present in these preparation. FATP4 is the only member of the fatty acyl:CoA synthetase family present at significant levels in small intestine (41). It has a distinct preference for very long-chain fatty acids and for long-chain saturated fatty acids, and some studies suggest that it plays a role in dietary fat absorption (17, 20). As shown in Fig. 3, Western blot analysis revealed that FATP4 was present at much lower levels in membrane fractions from Npc1l1−/− and ezetimibe-treated mice than from control animals. Quantitation of the bands in Fig. 3 (normalized to tubulin) indicates that FATP4 is reduced ∼50% by ezetimibe and ∼60% by the complete absence of NPC1L1. These data are consistent with the chain length- and saturation-dependent decrease in dietary fatty acid absorption by these mice.
Fig. 3.
Western blot analysis of proteins potentially important for fatty acid absorption. The protein from mucosal scrapings of the small intestine from control, ezetimibe-fed, and Npc1l1−/− mice were subjected to Western blot for analysis of expression of fatty acid transport protein 4 (FATP4), liver fatty acid binding protein (L-FABP), CD36, and NPC1L1. L-FABP was assayed in cytosolic fractions; all others were assayed in total cell membrane fractions. Mice were fasted 12 h before sample collection. Each blot is representative of at least 3 independent sample collections.
CD36 has also been reported to be important for fat absorption, especially for very long-chain fatty acids (12, 35). Western blots revealed a ∼35% decrease of CD36 (normalized to α-tubulin) in intestinal preparations from ezetimibe-treated mice but normal levels in preparations from Npc1l1−/− mice (Fig. 3). Thus it is possible that lower levels of CD36 may contribute to decreased fat absorption in drug-treated mice. However, recent studies (14) show that CD36 does not contribute to absorption of palmitate and stearate, which are the fatty acids most affected in our experiments. L-FABP is another protein that has been suggested to play a role in fatty acid transport and chylomicron production (4, 38). However, Western blots of cytosol from intestinal homogenates revealed no difference in the amount of L-FABP present in the different groups of mice (Fig. 3), indicating that this protein does not directly contribute to decreased fat absorption by Npc1l1−/− and ezetimibe-treated mice. Figure 3 also confirms the absence of NPC1L1 from knockout mice.
Lack of NPC1L1 function prevents diet-induced hyperglycemia.
Since obesity often correlates with hyperglycemia as well as higher plasma lipids, the effects of ezetimibe and NPC1L1 on glucose metabolism were also investigated. Line 1 of Table 3 summarizes the results from a cohort of C57BL/6J mice from Jackson Laboratories that were fed diabetogenic diet (lard based, BioServ) with or without ezetimibe. After 13 wk, fasting plasma glucose was significantly elevated in mice fed the diabetogenic diet (179 ± 27 mg/dl) but not in mice fed diabetogenic diet with ezetimibe (129 ± 19 mg/dl). As expected, plasma cholesterol was also elevated by the diabetogenic diet but remained normal when ezetimibe was included (data not shown). Lines 2 and 3 of Table 3 summarize results from a cohort of control and Npc1l1−/− mice bred in house (from the same colony) and fed either basal or diabetogenic diet (coconut oil-based, Research Diets) for 8 wk. As in the previous study, plasma glucose of Npc1l1−/− mice was not changed by the diabetogenic diet compared with basal diet, whereas plasma glucose of control mice was elevated. Maintenance of normal glycemia by ezetimibe-treated and Npc1l1−/− mice, despite consuming these calorie-dense diets, suggests that blocking function of the NPC1L1 pathway protects against diet-induced diabetes as well as hypercholesterolemia.
Table 3.
Diet effects on blood glucose of control and Npc1l1−/− mice
| Chow/Basal | Diabetogenic | Diabetogenic With Ezetimibe | |
|---|---|---|---|
| C57BL/6J | 146±17 | 179±27* | 129±19 |
| Control | 113±13 | 144±34† | n.d. |
| Npc1l1−/− | 102±18 | 102±28† | n.d. |
Values are means ± SD, in mg/dl. C57BL/6J cohort of mice were from Jackson Laboratories, 13 wk on diet, n = 4–6,
P < 0.03 vs. chow and diabetogenic diet + ezetimibe; control and Npc1l1−/− cohorts of mice were bred in-house, 10 wk on diet, n = 6 each,
P = 0.06. n.d., Not determined.
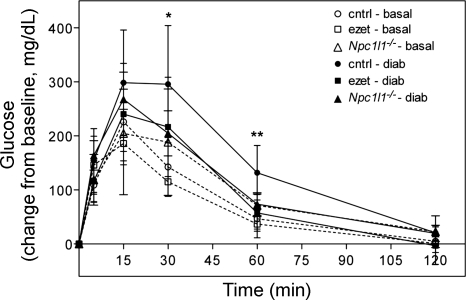
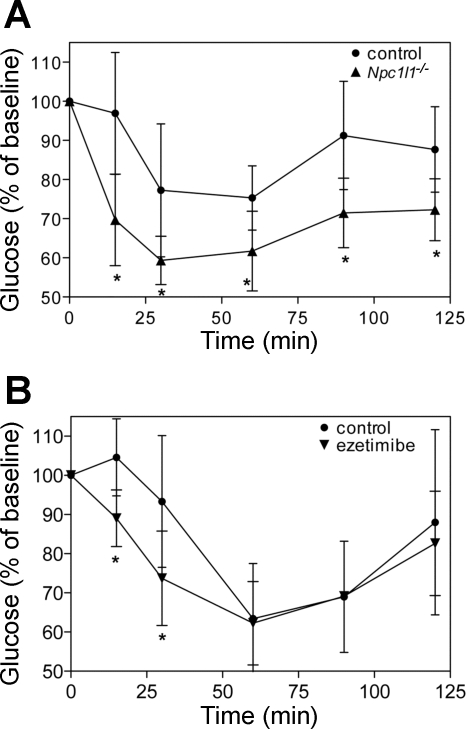
To further investigate this difference in diet-induced hyperglycemia, glucose tolerance and insulin sensitivity were measured. Glucose tolerance tests were performed on the three groups of mice fed either a basal diet or high-fat diet for 10 wk. After an overnight fast (8–10 h), baseline glucose was measured and the glucose tolerance test was initiated with an intraperitoneal injection of glucose (2 g/kg body wt). Blood glucose was monitored at 5, 15, 30, 60, and 120 min thereafter. As shown in Fig. 4, there were no differences in glucose tolerance between the groups fed basal diet (dotted lines), suggesting that ezetimibe treatment and NPC1L1 inactivation do not directly affect glucose metabolism in mice. However, when the animals were fed the diabetogenic diet for 10 wk, a significant difference in glucose tolerance was observed between groups. Control mice exhibited a loss of glucose tolerance, as depicted by the delayed glucose clearance curve in Fig. 4 (solid line, solid circles). However, glucose tolerance was maintained in ezetimibe-treated and Npc1l1−/− mice despite the diabetogenic diet (Fig. 4, solid lines, solid squares and triangles). Insulin secretion during the glucose tolerance test was also measured but did not differ between groups. In addition to glucose tolerance, insulin sensitivity was also assessed. Insulin tolerance tests were performed on Npc1l1−/− and control mice after 6 wk of high-fat diet. As shown in Fig. 5A, control mice exhibited a diet-induced loss of insulin sensitivity whereas Npc1l1−/− mice retained a robust response. Insulin tolerance tests were also performed on a separate group of mice fed high-fat diet with or without ezetimibe for 3 wk. Consistent with previous data, ezetimibe-treated mice were also more insulin sensitive than control mice after the dietary challenge (Fig. 5B).
Fig. 4.
Ezetimibe-treated and Npc1l1−/− mice are protected from diet-induced glucose intolerance. Mice were subjected to a glucose tolerance test while fed basal chow (dotted lines, open symbols) and again after 10 wk of diabetogenic diet (solid lines, solid symbols). After baseline glucose was measured, tests were initiated by intraperitoneal injection of 2 g glucose/kg body wt. Blood glucose was monitored from the tail vein for 2 h thereafter (n = 6–8 per group). *P < 0.05 control basal vs. control diabetogenic, **P < 0.05 control diabetogenic vs. ezetimibe diabetogenic and Npc1l1−/− diabetogenic.
Fig. 5.
Npc1l1−/− and ezetimibe-treated mice are protected from diet-induced loss of insulin sensitivity. A: control and Npc1l1−/− mice were subjected to an insulin tolerance test after 6 wk of Western diet. After baseline glucose was measured, animals were given insulin (0.75 U/kg) by intraperitoneal injection and glucose was monitored for 2 h thereafter (n = 8–10 per group). Groups were significantly different, *P < 0.01, at all times after time 0. B: insulin sensitivity was similarly measured in different groups of mice fed Western diet with or without ezetimibe for 3 wk (n = 8 per group). Groups were significantly different, *P < 0.02, at 15 and 30 min.
DISCUSSION
It is well recognized that NPC1L1 plays a key role in cholesterol absorption and that the drug ezetimibe potently inhibits this process (2, 8, 11, 16), although the exact mechanisms involved are poorly understood (1, 28, 30). Studies of Npc1l1−/− mice have focused primarily on diminished intestinal cholesterol absorption (2, 8, 11) and other functions for NPC1L1 have not been thoroughly investigated. However, the NPC1L1 protein is found in liver and other tissues as well (2, 8). Similarly, the notion that ezetimibe's overall effect on plasma cholesterol derives solely from its action in the intestine has not been rigorously proven even though the drug is known to be present at low levels in circulation (15, 37). The present report shows that the NPC1L1 pathway also affects dietary fat absorption, primarily that of long-chain saturated fatty acids, possibly by influencing the amount of FATP4 present in enterocyte membranes. Furthermore, blocking NPC1L1 function with ezetimibe treatment or eliminating the protein by gene ablation confers resistance to diet-induced obesity and diabetes.
The differences in weight gain between control vs. drug-treated and gene knockout mice were not due to differences in food intake. The latter was carefully monitored by two different methods in independent experiments and no differences were detected. However, decreased fat absorption by Npc1l1−/− and ezetimibe-treated mice results in decreased calorie intake from the same amount of food. Although the decrease in total fat absorbed by Npc1l1−/− and ezetimibe-treated mice compared with control mice does not appear dramatic, this decrease may account for some of the difference in weight gain between groups over the period of time they were fed the high-fat, high-calorie diets. The amount of weight not gained because of reduced fat absorption by the Npc1l1−/− and ezetimibe-treated mice can be estimated from the fat composition of the diet (36% by weight), the amount eaten per day (2.2 g), and the difference in overall fat absorption (∼7%). This calculation predicts that Npc1l1−/− and ezetimibe-treated mice would gain ∼0.06 g/day less than control mice, which could account for ∼50% of the observed difference in weight between groups after several weeks. Thus decreased fat absorption may not account for all of the difference in weight gained by these groups. Another possibility, not addressed by the experiments reported here, is that systemic effects of ezetimibe and NPC1L1 function in other tissues may modulate overall metabolic rates in mice. Feeding diets enriched in unsaturated fatty acids, such as in safflower oil, might distinguish between these possibilities since absorption should be nearly equivalent between the groups.
The effects of NPC1L1 and ezetimibe on fat absorption were unexpected, given the reported specificity of the drug and protein for cholesterol absorption (11, 47). However, the method employed in this study differs markedly from those used in previous reports in that it measures absorption of actual dietary fat under normal feeding conditions rather than by following acute absorption of an artificial bolus “meal” as is typical of many studies. Previous measurements of fat absorption in monkeys (46), hamsters (45, 47), rats (47), and mice (11) used a test bolus of oleate, linoleate, or myristate. Another important advantage of the sucrose-polybehenate method used in this study is that it allows simultaneous measurement of all fatty acids present in the diet (25). Thus, in agreement with these previous studies, we also show a limited decrease in absorption of these fatty acid species in the absence of NPC1L1 function (Table 2). In contrast, the method employed in the present study revealed a marked decrease in absorption of palmitate and stearate that could not have been detected by other methods.
It is well documented that FATP4 exhibits a distinct substrate preference for very-long-chain (>18 carbon) and long-chain saturated fatty acids in vitro and in cell culture (17, 20, 41). Furthermore, analysis of FATP4 knockout mice indicates that it is absolutely required for uptake of these fatty acids by skin cells (26). Although its role in intestinal fatty acid absorption has not been firmly established, cultured enterocytes from neonatal FATP4 knockout mice manifest impaired uptake of very-long-chain and long-chain saturated fatty acids (17, 20). Furthermore, it is the only member of this family highly expressed by enterocytes (41). The coordinate decrease in saturated fat absorption and ∼50% decreased intestinal FATP4 protein in mice with impaired NPC1L1 function is consistent with a primary role for this enzyme in absorption of these fatty acids. This notion is further supported by the report that saturated fatty acid uptake by enterocytes heterozygous for the FATP4 gene, which express ∼50% less protein than control cells, is diminished to the same extent as observed in this study (17). Together, these results support the model postulating that intracellular sequestration by CoA-thioesterification is a critical step of fatty acid absorption (21, 27, 34). Although consistent with free diffusion of fatty acids across cell membranes as part of this process (34), our studies do not exclude participation of a receptor/transporter. Decreased expression of FATP4 by the lack of NPC1L1 function is an intriguing finding that warrants further investigation into the underlying mechanism.
Decreased CD36 protein in intestinal scrapings from ezetimibe-treated mice could suggest a role for this transporter as well as FATP4 in saturated fat absorption. Although our data do not exclude the possibility that CD36 is involved, this seems less likely given the recent report showing reduced absorption of lignocerate (24:0) but normal absorption of palmitate and stearate by CD36 knockout mice (12). Furthermore we did not detect a decrease of CD36 in scrapings from Npc1l1−/− mice. Another factor that could potentially affect fatty acid absorption is a change in membrane fluidity resulting from diminished cholesterol in enterocytes lacking NPC1L1 function, especially during fat absorption. Changes in membrane composition might also affect the function or cellular location of transporters and other proteins important to the absorption process.
Hyperglycemia, insulin resistance, is a comorbidity of obesity. Thus maintenance of euglycemia and insulin sensitivity by Npc1l1−/− and ezetimibe-treated mice fed high-fat diets may derive, in part, from their total resistance to the diet-induced weight gain experienced by control mice. However, it is also possible that absence of NPC1L1 function systemically may affect glucose and/or fatty acid metabolism by tissues other than intestine, such as heart and muscle. Results of initial experiments with mice on basal diet are consistent with this hypothesis (not shown). Such systemic effects, if present, might be direct or indirect. For example, location of GLUT4 (glucose transporter 4) is sensitive to cholesterol levels in cells, with more being localized in the cell membrane when cholesterol is low (5, 32), a condition that might exist in the absence of NCP1L1 function (8, 39).
The experiments reported in this study used high-fat, high-calorie diets as a central component to explore the effects of NPC1L1 and ezetimibe on aspects of the metabolic syndrome other than hypercholesterolemia, specifically weight gain and hyperglycemia. Three novel and intriguing effects were revealed when mice were fed these diets: 1) diet-induced obesity was prevented, 2) saturated fat absorption was reduced, and 3) diet-induced hyperglycemia was prevented in ezetimibe-treated and Npc1l1−/− mice. Taken together, these results suggest that a clinically relevant, ezetimibe-sensitive and/or NPC1L1-mediated pathway has important physiological functions that extend well beyond facilitation of cholesterol absorption in the intestinal tract.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01DK76907 and R01HL78900, by U. S. Department of Agriculture Grant CSREES 2002-01135, and by American Heart Association Grant 0525340B (Great Rivers Affiliate).
Acknowledgments
Fat absorption was measured by the Cincinnati Mouse Metabolic Phenotyping Center NIH U24DK59630.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Altmann SW, Davis HR Jr, Yao X, Laverty M, Compton DS, Zhu LJ, Crona JH, Caplen MA, Hoos LM, Tetzloff G, Priestley T, Burnett DA, Strader CD, Graziano MP. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim Biophys Acta 1580: 77–93, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, Lipka LJ, Lebeaut AP, Yang B, Mellars LE, Cuffie-Jackson C, Veltri EP. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther 23: 1209–1230, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Besnard P, Niot I, Poirier H, Clement L, Bernard A. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol Cell Biochem 239: 139–147, 2002. [PubMed] [Google Scholar]
- 5.Blot V, McGraw TE. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J 25: 5648–5658, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Ryan DH. Drug treatment of the overweight patient. Gastroenterology 132: 2239–2252, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Carter CP, Howles PN, Hui DY. Genetic variation in cholesterol absorption efficiency among inbred strains of mice. J Nutr 127: 1344–1348, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 280: 12710–12720, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Davis HR Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 21: 2032–2038, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Davis HR Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE−/− mice. Arterioscler Thromb Vasc Biol 27: 841–849, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Davis HR Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 279: 33586–33592, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem 283: 13108–13115, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr 135: 2305–2312, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Ebine N, Demonty I, Jia X, Jones PJ. Plant stanol ascorbate esters reduce body weight gain through decreased energy absorption in hamsters. Int J Obes (Lond) 30: 751–757, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Ezzet F, Krishna G, Wexler DB, Statkevich P, Kosoglou T, Batra VK. A population pharmacokinetic model that describes multiple peaks due to enterohepatic recirculation of ezetimibe. Clin Ther 23: 871–885, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill K A, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimeno RE, Hirsch DJ, Punreddy S, Sun Y, Ortegon AM, Wu H, Daniels T, Stricker-Krongrad A, Lodish HF, Stahl A. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J Biol Chem 278: 49512–49516, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Goudriaan JR, Dahlmans VEH, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem 239: 199–202, 2002. [PubMed] [Google Scholar]
- 19.Grundy SM, Ahrens EH Jr, Salen G. Dietary beta-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J Lipid Res 9: 374–387, 1968. [PubMed] [Google Scholar]
- 20.Hall AM, Wiczer BM, Herrmann T, Stremmel W, Bernlohr DA. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J Biol Chem 280: 11948–11954, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton JA, Brunaldi K. A model for fatty acid transport into the brain. J Mol Neurosci 33: 12–17, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch D, Stahl A, Lodish HF. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci USA 95: 8625–8629, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann SM, Perez-Tilve D, Greer TM, Coburn BA, Grant E, Basford JE, Tschöp MH, Hui DY. Defective lipid delivery modulates glucose tolerance and metabolic response to diet in apolipoprotein E-deficient mice. Diabetes 57: 5–12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyer SP, Yao X, Crona JH, Hoos LM, Tetzloff G, Davis HR Jr, Graziano MP, Altmann SW. Characterization of the putative native and recombinant rat sterol transporter Niemann-Pick C1 Like 1 (NPC1L1) protein. Biochim Biophys Acta 1722: 282–292, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Jandacek RJ, Heubi JE, Tso P. A novel, noninvasive method for the measurement of intestinal fat absorption. Gastroenterology 127: 139–144, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Jia Z, Moulson CL, Pei Z, Miner JH, Watkins PA. Fatty acid transport protein 4 is the principal very long chain fatty acyl-CoA synthetase in skin fibroblasts. J Biol Chem 282: 20573–20583, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kamp F, Hamilton JA. How fatty acids of different chain length enter and leave cells by free diffusion. Prostaglandins Leukot Essent Fatty Acids 75: 149–159, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Knopfel M, Davies JP, Duong PT, Kvaerno L, Carreira EM, Phillips MC, Ioannou YA, Hauser H. Multiple plasma membrane receptors but not NPC1L1 mediate high-affinity, ezetimibe-sensitive cholesterol uptake into the intestinal brush border membrane. Biochim Biophys Acta 1771: 1140–1147, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Kosoglou T, Meyer I, Veltri EP, Statkevich P, Yang B, Zhu Y, Mellars L, Maxwell SE, Patrick JE, Cutler DL, Batra VK, Affrime MB. Pharmacodynamic interaction between the new selective cholesterol absorption inhibitor ezetimibe and simvastatin. Br J Clin Pharmacol 54: 309–319, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labonte ED, Howles PN, Granholm NA, Rojas JC, Davies JP, Ioannou YA, Hui DY. Class B type I scavenger receptor is responsible for the high affinity cholesterol binding activity of intestinal brush border membrane vesicles. Biochim Biophys Acta 1771: 1132–1139, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy P, Fried M, Santini F, Finer N. The comparative effects of bariatric surgery on weight and type 2 diabetes. Obes Surg 17: 1248–1256, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu P, Leffler BJ, Weeks LK, Chen G, Bouchard CM, Strawbridge AB, Elmendorf JS. Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am J Physiol Cell Physiol 286: C317–C329, 2004. [DOI] [PubMed] [Google Scholar]
- 33.McArthur MJ, Atshaves BP, Frolov A, Foxworth WD, Kier AB, Schroeder F. Cellular uptake and intracellular trafficking of long chain fatty acids. J Lipid Res 40: 1371–1383, 1999. [PubMed] [Google Scholar]
- 34.Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci 119: 4678–4688, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Ntanios FY, Jones PJ. Dietary sitostanol reciprocally influences cholesterol absorption and biosynthesis in hamsters and rabbits. Atherosclerosis 143: 341–351, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Patrick JE, Kosoglou T, Stauber KL, Alton KB, Maxwell SE, Zhu Y, Statkevich P, Iannucci R, Chowdhury S, Affrime M, Cayen MN. Disposition of the selective cholesterol absorption inhibitor ezetimibe in healthy male subjects. Drug Metab Dispos 30: 430–437, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Pohl J, Ring A, Hermann T, Stremmel W. Role of FATP in parenchymal cell fatty acid uptake. Biochim Biophys Acta 1686: 1–6, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Repa J, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res 46: 779–789, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Rubio MA, Gargallo M, Isabel Millán A, Moreno B. Drugs in the treatment of obesity: sibutramine, orlistat and rimonabant. Public Health Nutr 10: 1200–1205, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell 4: 299–308, 1999. [DOI] [PubMed] [Google Scholar]
- 42.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106: 1943–1948, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Toth PP, Davidson MH. Simvastatin plus ezetimibe: combination therapy for the management of dyslipidaemia. Expert Opin Pharmacother 6: 131–139, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Van Bennekum A, Werder M, Thuahnai ST, Han CH, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry 44: 4517–4525, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes 50: 1330–1335, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Van Heek M, Compton DS, Davis HR. The cholesterol absorption inhibitor, ezetimibe, decreases diet-induced hypercholesterolemia in monkeys. Eur J Pharmacol 415: 79–84, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol 134: 409–417, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wasan KM, Holtorf L, Subramanian R, Cassidy SM, Pritchard PH, Stewart DJ, Novak E, Moghadasian MH. Assessing plasma pharmacokinetics of cholesterol following oral coadministration with a novel vegetable stanol mixture to fasting rats. J Pharm Sci 90: 23–28, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wasan KM, Najafi S, Peteherych KD, Pritchard PH. Effects of a novel hydrophilic phytostanol analog on plasma lipid concentrations in gerbils. J Pharm Sci 90: 1795–1799, 2001. [DOI] [PubMed] [Google Scholar]
- 50.Waseem T, Mogensen KM, Lautz DB, Robinson MK. Pathophysiology of obesity: why surgery remains the most effective treatment. Obes Surg 17: 1389–1398, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem 281: 6616–6624, 2006. [DOI] [PubMed] [Google Scholar]