Abstract
Niemann-Pick C1-like 1 (NPC1L1) facilitates the uptake of sterols into the enterocyte and is the target of the novel cholesterol absorption inhibitor, ezetimibe. These studies used the Golden Syrian hamster as a model to delineate the changes in the relative mRNA expression of NPC1L1 and other proteins that regulate sterol homeostasis in the enterocyte during and following cessation of ezetimibe treatment and also to address the clinically important question of whether the marked inhibition of cholesterol absorption alters biliary lipid composition. In hamsters fed a low-cholesterol, low-fat basal diet, the abundance of mRNA for NPC1L1 in the small intestine far exceeded that in other regions of the gastrointestinal tract, liver, and gallbladder. In the first study, female hamsters were fed the basal diet containing ezetimibe at doses up to 2.0 mg·day−1·kg body wt−1. At this dose, cholesterol absorption fell by 82%, fecal neutral sterol excretion increased by 5.3-fold, and hepatic and intestinal cholesterol synthesis increased more than twofold, but there were no significant changes in either fecal bile acid excretion or biliary lipid composition. The ezetimibe-induced changes in intestinal cholesterol handling were reversed when treatment was withdrawn. In a second study, male hamsters were given a diet enriched in cholesterol and safflower oil without or with ezetimibe. The lipid-rich diet raised the absolute and relative cholesterol levels in bile more than fourfold. This increase was largely prevented by ezetimibe. These data are consistent with the recent finding that ezetimibe treatment significantly reduced biliary cholesterol saturation in patients with gallstones.
Keywords: enterocyte, cholesterol absorption, gallbladder bile, liver, cholesterol gallstone disease
the liver plays a central role in the maintenance of sterol balance across the whole body because not only is it the organ that receives most of the cholesterol absorbed by the small intestine, but also it is the site for the degradation and excretion of cholesterol through the bile (40). The liver also actively synthesizes cholesterol but the rate at which it does so varies widely depending on numerous factors and, in particular, the amount of cholesterol being delivered to it from the small intestine (40). It is well documented that dietary or pharmacological manipulation of the enterohepatic flux of either cholesterol or bile acids can potentially cause marked changes in the rate at which the liver synthesizes cholesterol, converts cholesterol to bile acids, incorporates cholesterol into very low-density lipoproteins, esterifies and stores cholesterol, or secretes unesterified cholesterol directly into bile (18, 22, 40). Such changes in the intrahepatic handling of cholesterol can, in turn, lead to clinically significant shifts in the circulating low-density lipoprotein-cholesterol (LDL-C) concentration and in the degree of biliary cholesterol saturation (15, 43, 48).
The intestinal absorption of cholesterol has long been a key target in the management of dyslipidemia primarily because of the substantial quantity of cholesterol that reaches the liver daily from the small bowel (5, 35). In the average adult consuming a typical Western diet, ∼1,200–1,700 mg of cholesterol enters the lumen of the small intestine each day (18). Approximately 300–500 mg of this cholesterol comes from the diet and the remainder is derived largely from bile (19). Given that in humans fractional cholesterol absorption averages ∼50%, the amount of intestinally derived cholesterol delivered to the liver throughout the day amounts to hundreds of milligrams (8, 34). This occurs through what is essentially a triphasic process that begins with a series of events within the lumen that culminate in the micellar solubilization of cholesterol and its movement up to the brush border membrane of the absorptive enterocyte (41). The second phase entails the transport of cholesterol, as well as noncholesterol sterols, across this limiting membrane facilitated by Niemann-Pick C1-Like 1 (NPC1L1) (1, 13). Within the enterocyte, cholesterol is esterified and subsequently incorporated, along with other lipids and apolipoprotein B48, into nascent chylomicrons. These latter processes together constitute the third phase of sterol absorption. Although the cellular and molecular events involved in each of these phases are generally well defined, there are nevertheless significant gaps in our knowledge of how cholesterol movement within the enterocyte is facilitated (49). Furthermore, although NPC1L1 clearly plays a fundamental role in sterol absorption, there is currently not unanimous agreement that it is this protein that facilitates the uptake of sterols across the brush border membrane (38).
Although the primary focus of research on the molecular control of cholesterol absorption has been to find more effective ways of modifying the plasma lipoprotein composition to lessen an individual's risk of developing atherosclerosis, the question of how changes in chylomicron cholesterol uptake by the liver might also alter the rate of biliary cholesterol secretion, and thus a person's predisposition to cholesterol gallstone formation, has not been as widely investigated. Most of the studies that have been carried out in animal models such as the baboon, squirrel monkey, Golden Syrian hamster, mouse, and prairie dog, as well as in humans, have shown that increases in dietary cholesterol intake result in a rise in biliary cholesterol content (2, 3, 14, 21, 24, 27, 28, 50, 52). However, the extent to which this happens varies markedly depending on the type of model and the dietary formulation that were utilized. Comparatively, much less is known about the converse situation involving the use of inhibitors that diminish the amount of cholesterol reaching the liver from the small bowel. Studies with agents that interfere with the intraluminal phase of absorption such as β-sitosterol, sucrose polyester (a nonabsorbable fat substitute), and surfomer (AOMA, a copolymer of maleic acid and an 18-carbon α-olefin) all showed either no change or a modest reduction in biliary cholesterol saturation (6, 10, 11). The impact of agents that act at the intracellular phase of sterol absorption has not been widely studied. However, the focus of attention has now shifted to ezetimibe, an entirely new class of inhibitors that, in milligram doses, markedly reduces cholesterol absorption by inhibiting NPC1L1 activity in the brush border membrane (17). The dose of ezetimibe needed to block cholesterol absorption varies between species because of wide differences in the affinity with which ezetimibe binds to NPC1L1 (20). In the LDL receptor knockout mouse fed a lipid-rich diet, the degree of increase in biliary cholesterol content was blunted substantially by the addition of ezetimibe to the diet (29). Similar findings have been recently reported for mice harboring constitutive hepatic liver X receptor activity and for gallstone-susceptible C57L mice fed a lithogenic diet (45). Ezetimibe has also been found to have beneficial effects in a murine model of cholecystosteatosis (26). There are also now newly published data showing that ezetimibe treatment lowered the degree of biliary cholesterol saturation in small groups of gallstone patients and overweight subjects without gallstones (51).
In the present studies the Golden Syrian hamster has been utilized as an alternate model to determine the impact of ezetimibe treatment on biliary lipid composition during periods of low and high dietary cholesterol intake and to delineate the concomitant biochemical and molecular changes in sterol metabolism within the small intestine and liver. Previous studies have established that this small animal has features of cholesterol metabolism that more closely resemble those seen in the human and nonhuman primate than do species like the mouse and rat (44). Utilizing this hamster model, the data demonstrate that ezetimibe, by blocking the transport of excess chylomicron cholesterol to the liver, was highly effective in preventing an otherwise pathological increase in hepatic cholesterol storage and biliary cholesterol content. The data also show for the first time that the ezetimibe-induced changes in the expression of multiple genes involved in cholesterol handling by the enterocyte are quickly reversed when treatment is withdrawn.
MATERIALS AND METHODS
Animals and diets.
Female and male Golden Syrian hamsters (Mesocricetus auratus) were obtained from either Charles River Laboratories, Wilmington, MA, or, for one experiment, from Harlan Sprague Dawley, Indianapolis, IN, in the weight range of 100–110 g. They were housed individually in plastic cages containing wood shavings and maintained in a room with alternating 12-h periods of light and darkness. The hamsters were adapted to these conditions for 2 wk, during which time they were fed a pelleted form of a cereal-based rodent diet (Wayne Lab Blox no. 8604; Harlan Teklad, Madison, WI). This was defined as the basal diet. It had cholesterol content of 0.02% (wt/wt) and a total lipid content of 4–5% (wt/wt). In some studies the meal form of the basal diet was enriched with cholesterol (1.0% wt/wt) and safflower oil (10% wt/wt). In the initial ezetimibe dose-response experiments, the hamsters were fed the basal diet containing only ezetimibe at levels that provided approximate doses of 0, 0.5, 1.0, and 2.0 mg·day−1·kg body wt−1 (based on the consumption of 73 g of diet·day−1·kg body wt−1) (44). In the studies with the lipid-rich diet, the ezetimibe was provided in the diet at a single dose of 1.0 mg·day−1·kg body wt−1. This dose was shown by other investigators to normalize combined dyslipidemia in obese, hyperinsulinemic male hamsters (47). Depending partly on the objective of the study, the hamsters were fed their respective experimental diet ad libitum for either 10, 21, or 34 days. All animals were studied in the fed state toward the end of the dark phase of their light cycle. All studies were approved by the Institutional Animal Care and Use Committee of The University of Texas Southwestern Medical Center.
Intestinal cholesterol absorption, fecal bile acid and neutral sterol excretion, and biliary lipid composition.
The details of the methods used for measuring these parameters are described previously (32). Intestinal cholesterol absorption was determined as a fractional value (percentage) by a fecal dual-isotope ratio method (32). For tissue, plasma, and bile harvesting, the hamsters were anesthetized and exsanguinated from the abdominal aorta. The liver was exposed and the gallbladder accessed. Bile was taken from the gallbladder via a 1-ml plastic syringe fitted with a 25-gauge, 5/8-in. needle. The bile was then transferred to a microfuge tube, after which aliquots were diluted in methanol for lipid composition analysis. The molar ratio of cholesterol in gallbladder bile was expressed as a percentage value. The rate of fecal bile acid excretion was taken as a measure of the rate of bile acid synthesis (44).
mRNA isolation and qRT-PCR gene expression analysis.
Hamsters were anesthetized with isoflurane (Baxter Healthcare, Deerfield, IL) and exsanguinated into heparinized syringes via the abdominal aorta. Small intestines were removed, flushed with ice-cold PBS, then cut longitudinally. The intestinal mucosae were removed by gentle scraping, immediately frozen in liquid nitrogen, and stored at −85°C. Total RNA was isolated from tissue samples by use of RNA STAT-60 (Tel-Test, Friendswood, TX). RNA concentrations were determined by absorbance at 260 nm. Quantitative RT-PCR (qRT-PCR) was performed by using an Applied Biosystems Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA) as described previously (25). Briefly, total RNA was treated with DNase I (RNase-free) (Roche Diagnostics, Indianapolis, IN) and reverse transcribed with random hexamers by use of SuperScript II (Invitrogen, Carlsbad, CA) to generate cDNA.
Primers for each gene were obtained from the literature (16) or were designed with PrimerExpress software (PerkinElmer Life and Analytical Sciences, Boston, MA) and validated by analysis of template titration and dissociation curves (46) (see Table 1 for primer sequences). The gene sequence for hamster ACAT2 was not yet available, so a partial cDNA was cloned by reverse transcription-PCR using hamster RNA as template and the following primers: forward, 5′-ACTTCATTGATGAGGGCAGG; reverse, 5′-CCCAGGACGAAGCAGAAGA. These cloning primers were designed according to regions of highest sequence similarity between the human (NM_003578), mouse (NM_146064), and rat (NM_153728) ACAT2 genes. The resulting 871-bp product was sequenced and found to share 89% nucleotide identity with the mouse ACAT2 mRNA (NM_146064), and this novel sequence was subjected to the Primer Express algorithm to identify qRT-PCR primers for measuring hamster ACAT2 mRNA levels.
Table 1.
Quantitative real-time PCR primers used to measure hamster RNA levels
| Gene | Primer Sequences | NCBI Accession Number or Reference |
|---|---|---|
| NPC1L1 | 5′-CCTGACCTTTATAGAACTCACCACAGA-3′ | DQ897680, Ref. 16 |
| 5′-GGGCCAAAATGCTCGTCAT-3′ | ||
| ABCA1 | 5′-ATAGCAGGCTCCAACCCTGAC-3′ | Ref. 16 |
| 5′-GGTACTGAAGCATGTTTCGATGTT-3′ | ||
| ABCG5 | 5′-TGATTGGCAGCTATAATTTTGGG-3′ | Ref. 16 |
| 5′-GTTGGGCTGCGATGGAAA-3′ | ||
| ABCG8 | 5′-TGCTGGCCATCATAGGGAG-3′ | Ref. 16 |
| 5′-TCCTGATTTCATCTTGCCACC-3′ | ||
| HMG-CoA synthase | 5′-CCTATGACTGCATTGGGCG-3′ | L00326 |
| 5′-CCCAGACTCCTCAAACAGCTG-3′ | ||
| HMG-CoA reductase | 5′-ACCATCTGTATGATGTCAATGAACA-3′ | X00494 |
| 5′-GCTCAATACGTCCTCTTCAAATTTT-3′ | ||
| Cyclophilin | 5′-CAAATGCTGGACCAAACACA-3′ | X17105 |
| 5′-CAGTCTTGGCGGTGCAGAT-3′ | ||
| ACAT2 | 5′-CCGAGATGCTTCGATTTGGA-3′ | See materials and methods |
| 5′-GTGCGGTAGTAGTTGGAGAAGGA-3′ |
NCBI, National Center for Biotechnology Information; NPC1L1, Niemann-Pick C1-like 1; ABCA1, adenosine triphosphate-binding cassette transporter A1; ABCG5, ABC transporter G5; ABCG8, ABC transporter G8; HMG CoA synthase, 3-hydroxy-3-methylglutaryl coenzyme A synthase; HMG-CoA reductase, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; ACAT2, acyl CoA:cholesterol acyltransferase 2.
Each qRT-PCR reaction contained (final volume of 10 μl): 25 ng of reverse-transcribed RNA, each primer at 150 nM, and 5 μl of 2× SYBRgreen PCR master mix (Applied Biosystems), and each sample was analyzed in triplicate. Data from the PCR reactions were analyzed by the comparative cycle number determined at threshold (CT) method (User Bulletin no. 2, PerkinElmer Life and Analytical Sciences) with cyclophilin as the invariant control gene. In most cases, mRNA levels in each experiment are expressed relative to those obtained for the wild-type mice fed the basal diet and reflect means ± SE. Otherwise, levels are expressed relative to the group with the least abundance of mRNA.
Tissue cholesterol synthesis.
The rate of cholesterol synthesis in the liver and entire small intestine was measured in vivo using [3H]water as described earlier and calculated as the nanomoles of [3H]water incorporated in digitonin-precipitable sterols per hour per gram wet weight of tissue (32).
Cholesterol concentration in plasma and liver and liver plasma enzyme activities.
Plasma total cholesterol concentration was measured by an enzymatic and colorimetric assay using a reagent mixture from Roche Diagnostics (Indianapolis, IN; catalog no. 11875523). Aliquots of liver tissue were digested in ethanolic potassium hydroxide, and the total cholesterol concentration was determined by gas chromatography (32). Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities were measured by a commercial laboratory. Hepatic triacylglycerol concentrations were determined as described (29).
Statistical analysis of data.
All data are reported as means ± SE for the specified number of individual animals. Differences between mean values were tested for statistical significance (P < 0.05) by an unpaired, two-tailed Student's t-test. All statistical analyses were performed with GraphPad Prism software (GraphPad Software, San Diego, CA).
RESULTS
Accelerated fecal loss of neutral but not acidic sterols accompanies ezetimibe-induced block of cholesterol absorption.
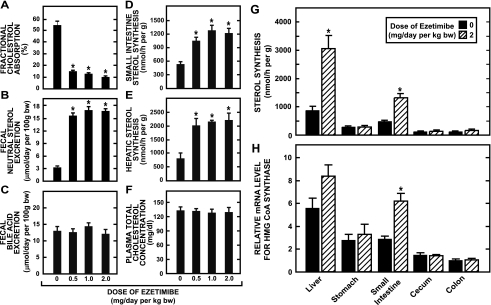
The data in Fig. 1 illustrate the magnitude of change in several parameters of cholesterol metabolism in female hamsters fed a basal diet containing ezetimibe at various levels, the highest of which provided a dose of ∼2 mg·day−1·kg body wt−1. At this dose, fractional cholesterol absorption fell by 82% and fecal neutral sterol excretion increased 5.3-fold but bile acid excretion remained unchanged (Fig. 1, A, B, and C, respectively). In addition, there was also a 2.3- and 2.8-fold increase in sterol synthesis in the small intestine (Fig. 1D) and liver (Fig. 1E), respectively. The plasma total cholesterol concentration remained unchanged across all doses of ezetimibe (Fig. 1F), as did the plasma activities of both ALT and AST. At ezetimibe doses of 0, 0.5, 1.0, and 2.0 mg·day−1·kg body wt−1, the plasma ALT activities (units/l) were 96 ± 13, 77 ± 11, 137 ± 30, and 100 ± 14, respectively, and the corresponding AST activities (units/l) were 48 ± 12, 33 ± 2, 45 ± 9, and 49 ± 10, respectively. The marked compensatory increase in sterol synthesis in the small intestine and liver seen with ezetimibe treatment was not found in other regions of the gastrointestinal tract, as demonstrated by both the rate of incorporation of [3H]water into sterols (Fig. 1G) and the relative mRNA level for 3-hydroxy-3-methylglutaryl (HMG) CoA synthase (Fig. 1H).
Fig. 1.
Parameters of cholesterol metabolism in female Golden Syrian hamsters fed a basal diet containing ezetimibe. Two experiments were conducted. In the first, fractional cholesterol absorption, fecal neutral sterol excretion, fecal bile acid excretion, intestinal sterol synthesis, hepatic sterol synthesis, and plasma total cholesterol concentration were measured as described in materials and methods in hamsters that were fed ad libitum for 10 days a basal low-cholesterol chow diet containing ezetimibe at levels that provided doses of 0, 0.5, 1.0, or 2.0 mg·day−1·kg body wt−1 (A–F). In the second experiment, the rate of sterol synthesis in the liver and different regions of the gastrointestinal tract was measured in hamsters that were fed for 34 days a low-cholesterol chow diet containing ezetimibe at doses of either 0 or 2 mg·day−1·kg body wt−1 (G). The relative mRNA level for 3-hydroxy-3-methylglutaryl (HMG) CoA synthase in the same tissues was determined in separate matching groups of hamsters (H). Here, each value was expressed relative to the mRNA level in the colon of hamsters not given ezetimibe, which was arbitrarily set at 1.0. All values are means ± SE of data from 5–7 animals in each group. *Significantly different from corresponding value for matching group not given ezetimibe, P < 0.05.
Expression of mRNA for NPC1L1 is highly localized in the small intestine in hamsters.
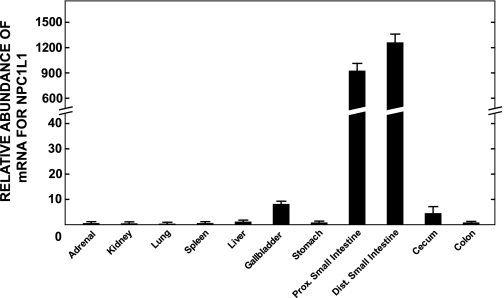
The data in Fig. 1 prompted us to compare the level of expression of mRNA for NPC1L1, the target of ezetimibe (17), in the liver, gallbladder, and various regions of the gastrointestinal tract of hamsters fed the basal diet alone. As shown in Fig. 2, the abundance of mRNA for NPC1L1 in the small intestine far exceeded that seen in the other regions of the gastrointestinal tract, the liver and gallbladder, and various other extrahepatic organs. The abundance of NPC1L1 mRNA in the gallbladder was greater than it was in the liver but still <1% of the level expressed in the small intestine.
Fig. 2.
Relative abundance of Niemann-Pick C1-like 1 (NPC1L1) in various tissues of male Golden Syrian hamsters fed a basal low-cholesterol diet. The relative mRNA level of NPC1L1 in each tissue was measured as described in materials and methods and expressed relative to the level found in the colon, which was arbitrarily set at 1.0. Values are means ± SE of data from 4 animals.
Response of the small intestine to acute withdrawal of ezetimibe treatment.
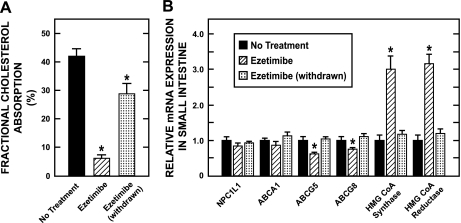
The main objective of this experiment was to determine how the small intestine responded when ezetimibe treatment was suddenly discontinued. In evaluating the data shown in Fig. 3, it should be emphasized that in the case of the group of hamsters subjected to treatment withdrawal, they had already been off ezetimibe for 3 days at the time they, and the other groups of hamsters, were dosed with labeled sterols for the measurement of fractional cholesterol absorption. Hence, although the mRNA data for the hamsters taken off ezetimibe reflect measurements made 6 days after drug treatment had stopped, the fractional absorption data reflect what was happening in the small intestine over the fourth, fifth, and sixth days following cessation of treatment. The data in Fig. 3A demonstrate that within this short time frame, the marked inhibition of cholesterol absorption by ezetimibe had largely been reversed. Similarly, the relative mRNA level for several proteins, which either fell (ABCG5 and G8) or increased (HMG CoA synthase and reductase) during treatment, had returned to normal values within 6 days of ezetimibe withdrawal (Fig. 3B).
Fig. 3.
Fractional cholesterol absorption and the relative expression level of mRNA for various proteins in the small intestine of female Golden Syrian hamsters after withdrawal of ezetimibe treatment. As described in materials and methods, female hamsters were given 1 of 3 treatments over a 10-day period. One group received only the basal diet, while a second group was fed the same diet containing ezetimibe (2 mg·day−1·kg body wt−1). A third group received the diet with ezetimibe for 10 days but was started on this diet 3 days ahead of the other group. These hamsters were switched back to the basal diet alone 3 days before they, and the other 2 groups, were dosed intragastrically with labeled sterols for the measurement of fractional cholesterol absorption (A). For all 3 groups, stools were collected for 3 days after dosing. The hamsters were then terminated and the small intestine was removed for the measurement of the relative expression level of mRNA for various proteins (B). Values are means ± SE of data from 4 animals (no treatment) and 7 animals in each of the ezetimibe and ezetimibe (withdrawn) groups. *Significantly different from corresponding value for group not given ezetimibe, P < 0.05.
Lipid composition of gallbladder bile was unchanged in hamsters given ezetimibe with a low-cholesterol, low-fat diet.
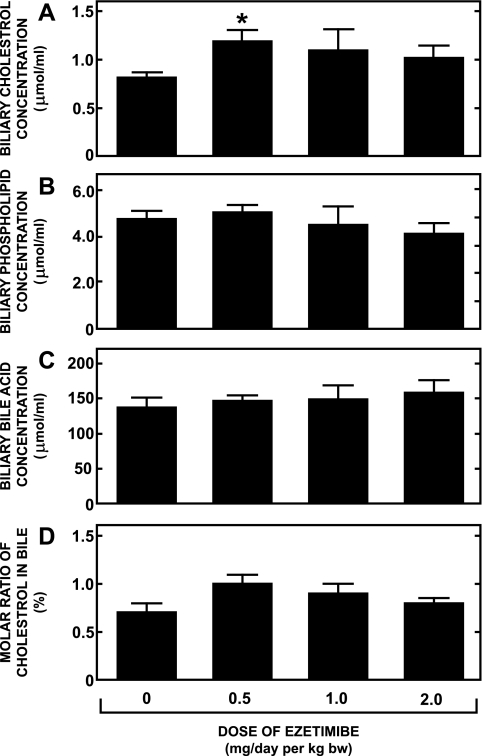
In many of the hamsters used in the preceding studies, gallbladder bile was harvested. The absolute concentrations of cholesterol, phospholipid, and bile acid in the bile from hamsters fed the basal diets containing ezetimibe at doses up to and including 2 mg·day−1·kg body wt−1 are given in Fig. 4, A, B, and C, respectively. Although there was a trend toward marginally higher cholesterol concentrations in the bile of the hamsters given ezetimibe, this trend was not dose related. Moreover, when the data for biliary cholesterol content were expressed as a molar percent of total biliary lipid content, none of the values for the ezetimibe-treated hamsters was significantly different from that for the group given the basal diet alone (Fig. 4D).
Fig. 4.
Biliary lipid composition in female Golden Syrian hamsters fed a basal diet containing ezetimibe. Gallbladder bile was harvested from female Golden Syrian hamsters that were fed for 21–28 days a basal low-cholesterol chow diet containing ezetimibe at levels that provided doses of 0, 0.5, 1.0, and 2.0 mg·day−1·kg body wt−1. The absolute concentrations of cholesterol, phospholipid and bile acid in bile extracts were measured as described in materials and methods (A–C). These data were, in turn, used to calculate the molar ratio of cholesterol in each bile sample (D). Values are means ± SE of data from 9 or 10 animals in each group. *Significantly different from corresponding value for group not given ezetimibe, P < 0.05.
Diet-induced cholesterol accumulation in liver and plasma was prevented by ezetimibe.
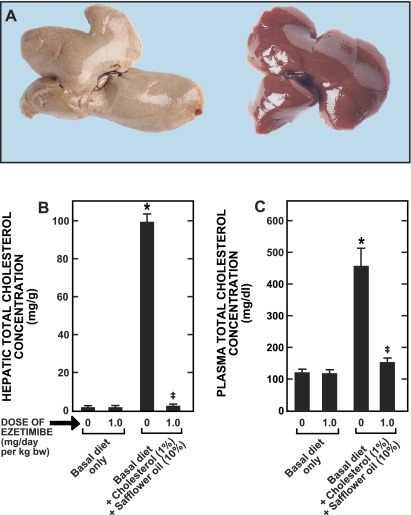
Western diets typically contain substantial amounts of triacylglycerol and cholesterol, particularly relative to endogenous synthesis rates. Thus, to examine the effect of this drug in a more relevant situation, female hamsters were fed for 28 days a diet containing cholesterol (1% wt/wt) and safflower oil (10% wt/wt) without or with ezetimibe at a dose of just 1 mg·day−1·kg body wt−1. The livers depicted in Fig. 5A are representative of what was found in hamsters fed the lipid-rich diet without ezetimibe (left) or with ezetimibe (right). The lipid-rich diet alone resulted in massive accumulation of cholesterol in both the liver (Fig. 5B) and plasma (Fig. 5C). The livers from the untreated hamsters had a total cholesterol concentration of 99 ± 4 mg/g and, relative to body weight, were 50% larger than the livers of hamsters given the lipid-rich diet with ezetimibe. The total cholesterol concentration in the livers from the treated animals averaged 2.5 ± 0.2 mg/g. The pale appearance of the livers from the untreated animals presumably reflected the accumulation of cholesteryl esters because hepatic total triacylglycerol concentrations averaged ∼5–6 mg/g irrespective of whether the lipid-rich diet contained ezetimibe.
Fig. 5.
Appearance of livers and hepatic and plasma total cholesterol concentrations in female hamsters fed a lipid-rich diet without or with ezetimibe. Female Golden Syrian hamsters were fed for 28 days either a basal diet alone or the same diet enriched with cholesterol and safflower oil without or with ezetimibe at a dose of 1.0 mg·day−1·kg body wt−1. The livers depicted (A) are representative of what was found in other hamsters fed the lipid-rich diet without ezetimibe (left) or with ezetimibe (right). Hepatic and plasma cholesterol levels (B and C, respectively) were measured as described in materials and methods. Values are means ± SE of data from 6 or 7 animals in each group. *Significantly different from corresponding value for group fed basal diet without ezetimibe, P < 0.05. ‡Significantly different from value for group fed lipid-rich diet without ezetimibe, P < 0.05.
Changes in hepatic relative mRNA levels for multiple proteins induced by lipid-rich diet were attenuated by ezetimibe.
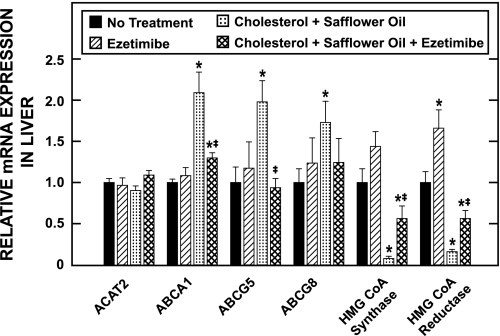
The relative mRNA level for multiple proteins was determined in the livers of the hamsters used in the study described in Fig. 5 and these are shown in Fig. 6. The mRNA for ACAT2 was essentially the same in all four experimental groups. In contrast, the level of mRNA expression for ABCA1, ABCG5, and ABCG8 was significantly elevated whereas that for HMG CoA synthase and HMG CoA reductase was markedly reduced in hamsters given the lipid-rich diet alone. These changes in message levels either did not occur or were far less pronounced in the hamsters given ezetimibe with the high-fat, high-cholesterol diet.
Fig. 6.
Relative level of expression of mRNA for various proteins in the livers of female hamsters fed a basal or lipid-rich diet without or with ezetimibe. The relative mRNA level of expression for various proteins was determined in the livers of the female hamsters used for the study described in the legend to Fig. 5. For each protein, the mRNA level was expressed relative to the level found in the group fed the basal diet alone, which was arbitrarily set at 1.0. Values are means ± SE of data from 6 or 7 animals in each group. *Significantly different from corresponding value for group fed basal diet without ezetimibe, P < 0.05. ‡Significantly different from corresponding value for group fed lipid-rich diet without ezetimibe, P < 0.05.
Diet-induced increase in biliary cholesterol content was prevented by ezetimibe.
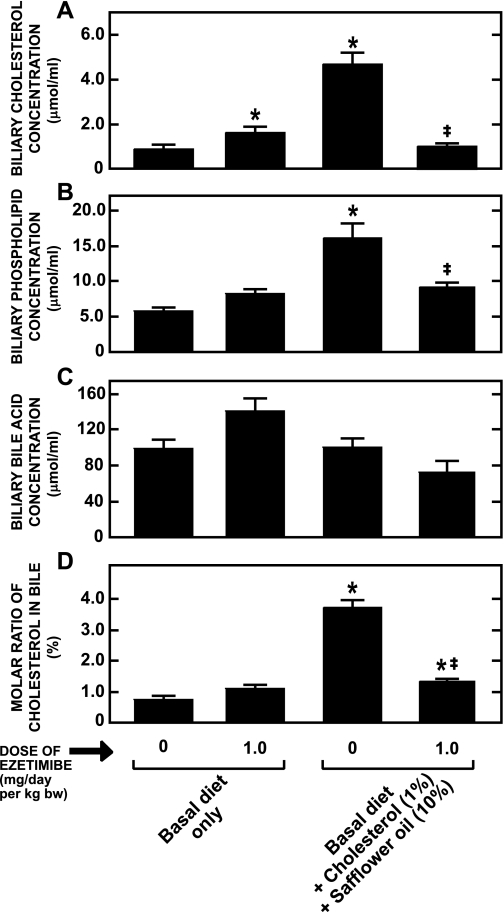
In the study with the female hamsters fed the lipid-rich diet (Figs. 5 and 6) the lipid composition of gallbladder bile was also determined (data not shown). The cholesterol, safflower oil-enriched diet caused only a modest rise in the molar ratio of cholesterol in the bile (1.4 ± 0.2 vs. 0.8 ± 0.2% in matching hamsters given the basal diet alone). In the group fed the lipid-rich diet with ezetimibe, the molar ratio of cholesterol was 0.9 ± 0.1%. However, it is known that the male of this species responds much more dramatically to dietary lipids (3). Consequently, these studies were repeated in male hamsters given the diets enriched with cholesterol and safflower oil. As shown by the data in Fig. 7A, this dietary formulation did raise the cholesterol concentration in the gallbladder bile 5.2-fold. There was also a 2.4-fold increase in the phospholipid concentration (Fig. 7B) but no change in the bile acid concentration (Fig. 7C). Thus, although ezetimibe treatment alone did not change the molar ratio of cholesterol in the bile, the lipid-rich diet alone did increase this ratio 4.6-fold (Fig. 7D). When this diet was given with ezetimibe, the increase in biliary cholesterol saturation was greatly diminished. Thus increasing the flow of cholesterol into the liver of the male animal markedly increased the rate of secretion of sterol by the liver into bile and this effect was nearly completely obviated by the administration of ezetimibe.
Fig. 7.
Biliary lipid composition in male Golden Syrian hamsters fed a basal or lipid-rich diet without or with ezetimibe. Gallbladder bile was harvested from male Golden Syrian hamsters that were fed the same diets as were used in the study described in the legend for Fig. 6, and biliary lipid concentrations (A–C) and molar ratio of cholesterol (D) were measured as described in materials and methods. Values are means ± SE of data from 7 or 8 animals in each group. *Significantly different from corresponding value for group fed basal diet without ezetimibe, P < 0.05. ‡Significantly different from corresponding value for group fed lipid-rich diet without ezetimibe, P < 0.05.
DISCUSSION
The discovery and development of the novel sterol absorption inhibitor, ezetimibe, has provided not only an effective new strategy for the management of primary hypercholesterolemia in the general population (5), but also a treatment for sitosterolemia, a rare disorder resulting from mutations in either ABCG5 or G8 that lead to the hyperabsorption of dietary sterols and premature atherosclerosis (30, 31). More recently, ezetimibe was found to be effective also in ameliorating liver dysfunction in a murine model of Niemann-Pick type C (NPC) disease, a multisystem disorder resulting from mutations in the NPC1 gene that encodes a protein involved in intracellular cholesterol transport (7). Those studies showed that the sooner in life mice deficient in npc1 received daily treatment with ezetimibe, the better was the improvement in their plasma liver enzyme levels. This benefit correlated directly with the degree of reduction in hepatic cholesterol accumulation that resulted from the ezetimibe-induced inhibition of intestinal cholesterol absorption and the diminished delivery of chylomicron cholesterol to the liver. The efficacy of ezetimibe in treating liver dysfunction in humans with NPC disease has yet to be determined. The underlying objective of the present studies was to investigate whether ezetimibe might also be potentially useful in the prevention and treatment of yet another disorder of cholesterol metabolism, cholelithiasis. The premise for doing so stems from the results of studies performed in several animal models, including primates, and to some extent in humans, which together show that biliary cholesterol content is determined in part by the rate of hepatic chylomicron cholesterol clearance (2, 3, 14, 21, 24, 27, 28, 50, 52).
Two interrelated points regarding the design of the present studies warrant emphasis here. One concerns the selection of the Golden Syrian hamster as the model for these experiments, and the other has to do with the types of dietary formulations to which ezetimibe was added. Golden Syrian hamsters manifest significant gender differences in some aspects of cholesterol metabolism such as the basal rate of cholesterol synthesis and the level of cholesteryl ester in the liver (33). However, both male and female hamsters normally produce bile that has a very low degree of cholesterol saturation, with the molar ratio of cholesterol usually being only ∼1% or less (3, 43). This is also the case with the dog (4, 23), which was the first species in which biliary cholesterol content was measured following treatment with ezetimibe.1 This comparison between the hamster and dog is emphasized here because of the finding that biliary cholesterol content increased in dogs given ezetimibe for 1 mo. Despite this, treatment of dogs with ezetimibe at a very high dose (300 mg·day−1·kg body wt−1) for 1 yr did not result in gallstone formation or any other adverse hepatobiliary effects.1 It should be noted that these findings were made in dogs maintained on a low-cholesterol, low-fat commercial canine diet (personal communication from Harry R. Davis at the Schering-Plough Institute). Thus the second point to be made here concerns the type of diet that is used when cholesterol modifying agents like ezetimibe are being tested in animal models. Ideally, such studies should be conducted in animals given both their conventional regimens, as well as formulations that more closely mimic the diets typically consumed by humans. The Golden Syrian hamster was chosen as a model for the present studies, not only because it normally produces bile with a low degree of cholesterol saturation as dogs do but, moreover, because it is a species in which profound changes in biliary cholesterol saturation can be induced by specific dietary formulations (3, 43). There are also a number of inherent similarities in several aspects of cholesterol metabolism between humans and the Golden Syrian hamster that made this species an appropriate model for these studies (44).
Four conclusions can be drawn from the initial set of experiments with hamsters given ezetimibe at different doses while being fed a basal, low-cholesterol, low-fat diet. The first is that, although there were dramatic increases in intestinal and hepatic sterol synthesis (Fig. 1, D and E), and fecal neutral sterol excretion (Fig. 1B), there were no consistent changes in biliary lipid composition (Fig. 4, A–D). There was a trend toward higher absolute cholesterol concentrations in the bile of the hamsters given ezetimibe, but this was not dose related (Fig. 4A). It should be emphasized that these effects of ezetimibe on sterol balance are not unique to this class of absorption inhibitor. For example, in hamsters given diets containing surfomer (α-olefin maleic acid), a polymeric nonabsorbable agent that inhibits cholesterol absorption by making the surface environment of the brush border membranes of the enterocytes more hydrophilic, there is a marked stimulation of intestinal and hepatic sterol synthesis (39, 42), just as there is with ezetimibe. This is accompanied by a modest reduction in the relative cholesterol content of the bile (39), an effect also reported for a small number of human subjects given surfomer (11). Taken together, these findings demonstrate that the marked compensatory increase in cholesterol synthesis in the liver that occurs when cholesterol absorption is inhibited does not drive biliary cholesterol secretion.
The second conclusion concerns the lack of change in plasma total cholesterol concentrations (Fig. 1F) under circumstances in which there was a profound reduction in intestinal cholesterol absorption (Fig. 1A) and a markedly accelerated rate of fecal neutral sterol excretion (Fig. 1B). Presumably, in the ezetimibe-treated hamsters, the compensatory increase in intestinal and hepatic sterol synthesis (Fig. 1, D and E) fully balanced the additional loss of sterol from the body, so that there was no detectable impact on circulating plasma cholesterol levels in the face of a major reduction in cholesterol absorption. The lowest dose of ezetimibe given to the hamsters (0.5 mg·day−1·kg body wt−1) corresponds to about four times the dose typically prescribed to humans (10 mg once daily). In subjects with a very low dietary cholesterol intake, this dose of ezetimibe reduced fractional cholesterol absorption by 58% and increased fecal neutral sterol excretion by 81% (9). There was also a 72% increase in whole body cholesterol synthesis. Despite this, plasma LDL-C levels in these subjects fell, on average, by 17%. Thus, in these individuals, the compensatory increase in cholesterol synthesis, which presumably reflected increases mainly by the liver and small bowel, did not fully balance the increased loss of sterol from the body, thus leading to a fall in circulating LDL-C levels. Although no effect of ezetimibe on plasma cholesterol levels was seen in hamsters fed the basal diet, there was clearly a marked reduction when dietary cholesterol intake was raised (Fig. 5C).
The third conclusion relates to the changes in the relative level of expression of mRNA for a number of key proteins involved in regulating sterol homeostasis in the enterocyte during, and following cessation of treatment with ezetimibe. It is particularly noteworthy that the relative mRNA level for NPC1L1 remained almost unchanged, irrespective of whether the hamsters were on or off ezetimibe treatment (Fig. 3B). This was also true for the transcript level for ABCA1. In contrast, the mRNA level for ABCG5 and G8 fell significantly when ezetimibe was given but returned to baseline values after withdrawal of treatment. Clearly, the reduced level of mRNA for ABCG5 and G8 reflected an ezetimibe-induced deficit of cholesterol within the enterocyte, a condition that also triggered a marked induction of local cholesterol synthesis as evidenced not only by the increased mRNA levels for HMG CoA synthase and HMG CoA reductase but also by direct measurements of the rate of small bowel sterol synthesis in vivo (Fig. 1, D and G). Although these data clearly attest the impact of blocking cholesterol uptake into the enterocyte at the brush border membrane, they also demonstrate that once ezetimibe treatment ceases there is a rapid return toward normal levels of cholesterol absorption (Fig. 3A) and the pathways that govern cholesterol homeostasis in the enterocyte (Fig. 3B).
The fourth conclusion concerns the relative expression of NPC1L1 mRNA in the liver and extrahepatic organs of the Golden Syrian hamster compared with what has been reported for other animal models (1, 36) and humans (1, 12). Although such comparative data remain limited and are based on mRNA rather than protein or activity measurements, it appears that in humans there is a much greater relative expression of mRNA for NPC1L1 in liver compared with other tissues than is the case in the few animal species in which this has been investigated. On the basis of the present data, the Golden Syrian hamster appears to be more like the rat, mouse, and miniature pig in that the dominant site of expression of NPC1L1 message is the small bowel, with comparatively little being present in the liver (Fig. 2; Refs. 1, 36). In the hamster, NPC1L1 mRNA was detected in the gallbladder at levels higher than those seen in the liver but still trivial compared with the level of expression in the small intestine. The pathophysiological significance of NPC1L1 in the liver of humans is not well understood, but studies with hepatocytes from African Green monkeys reveal that the protein localizes in the canalicular membrane (54). Furthermore, other data from a transgenic mouse model show that hepatic overexpression of human NPC1L1 in the liver resulted in a dramatic decrease in biliary cholesterol concentration and, moreover, that ezetimibe treatment of these mice essentially normalized biliary cholesterol concentration (37). These findings have been interpreted to mean that in humans NPC1L1 may function in regulating the amount of cholesterol entering the bile (53).
Taken together, these various observations raise the clinically important question of what the long-term impact of using ezetimibe, either in combination with statins or in some cases as monotherapy, for managing hypercholesterolemia might be on biliary cholesterol content and an individual's predisposition to cholesterol gallstone formation. The results of the present studies with ezetimibe treatment of hamsters fed a cholesterol- and fat-enriched diet (Fig. 5–7) make two points that bear directly on this question. One is that by placing a major block on absorption at the level of cholesterol uptake by the enterocyte, the dramatic and adverse changes in intrahepatic cholesterol metabolism and biliary cholesterol content associated with consumption of the high-cholesterol, high-fat diet are greatly attenuated (Fig. 5–7). The other is that although there was a detectable increase in the cholesterol concentration in the bile when hamsters fed the low-cholesterol basal diet were given ezetimibe, the corresponding increase associated with feeding the lipid-rich diet alone was far greater (Fig. 7A). More important, however, was the finding that ezetimibe treatment, given concurrently with this diet, resulted in a reduction in the absolute (Fig. 7A) and relative (Fig. 7D) cholesterol content of the bile to values at or near those seen in matching hamsters given the basal diet alone. Herein lies the potential relevance of these data in the hamster to the question of long-term ezetimibe treatment and biliary cholesterol levels in humans. Based on our current understanding of all the factors that can impact the level of biliary cholesterol saturation, it seems that any increase in biliary cholesterol levels that might arise from an effect of ezetimibe directly on the activity of NPC1L1 on the canalicular surface of hepatocytes would be more than offset by the marked reduction in the inflow of chylomicron cholesterol to the liver and the potential that this has to drive up biliary cholesterol content. Added to this is the point that ezetimibe is most widely used as an add-on to statins, which are known to reduce biliary cholesterol content (38). That aside, the present data, together with the results of a recent study demonstrating a favorable effect of short-term ezetimibe treatment on biliary cholesterol levels in humans (51), suggest that a clinical trial designed to investigate the potential of ezetimibe for reducing biliary cholesterol saturation in populations with a predisposition to cholelithiasis is now warranted.
GRANTS
This research was supported by National Institutes of Health Grants R37 HL09610 (J. M. Dietschy, S. D. Turley), T32 DK07745 (G. Quan), and GM07062 (M. A. Valasek); the Moss Heart Fund (J. M. Dietschy); and the American Heart Association, Texas Affiliate (J. J. Repa).
DISCLOSURES
From time to time, Dr. Dietschy has served as a consultant for Merck/Schering-Plough Pharmaceuticals. Dr. Turley has served as a member of the Speaker's Bureau for Merck & Co., Schering-Plough, and Merck/Schering-Plough Pharmaceuticals.
Acknowledgments
We thank Sean Campbell, Brian Griffith, Thien Tran, and Amanda Fletcher for excellent technical assistance and Kerry Foreman for preparation of the manuscript. The ezetimibe used in these studies was kindly provided by Harry R. Davis at the Schering-Plough Research Institute.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Information contained in the animal pharmacology section of the package insert for Zetia (ezetimibe) tablets.
REFERENCES
- 1.Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SPN, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303: 1201–1204, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Amigo L, Quiñones V, Mardones P, Zanlungo S, Miquel JF, Nervi F, Rigotti A. Impaired biliary cholesterol secretion and decreased gallstone formation in apolipoprotein E-deficient mice fed a high-cholesterol diet. Gastroenterology 118: 772–779, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Andersen JM, Cook LR. Regulation of gallbladder cholesterol concentration in the hamster. Role of hepatic cholesterol level. Biochim Biophys Acta 875: 582–592, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Ballesta MC, Martinez-Victoria E, Mañas M, Seiquer I, Huertas JR, Mataix FJ. Effect of dietary fat composition on biliary cholesterol saturation index in dogs. Arch Int Physiol Biochim Biophys 101: 3–7, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Bays HE, Neff D, Tomassini JE, Tershakovec AM. Ezetimibe: cholesterol lowering and beyond. Expert Rev Cardiovasc Ther 6: 447–470, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Begemann F, Bandomer G, Herget HJ. The influence of β-sitosterol on biliary cholesterol saturation and bile acid kinetics in man. Scand J Gastroenterol 13: 57–63, 1978. [DOI] [PubMed] [Google Scholar]
- 7.Beltroy EP, Liu B, Dietschy JM, Turley SD. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J Lipid Res 48: 869–881, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bosner MS, Lange LG, Stenson WF, Ostlund RE Jr. Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. J Lipid Res 40: 302–308, 1999. [PubMed] [Google Scholar]
- 9.Clarenbach JJ, Reber M, Lütjohann D, von Bergmann K, Sudhop T. The lipid-lowering effect of ezetimibe in pure vegetarians. J Lipid Res 47: 2820–2824, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Crouse JR, Grundy SM. Effects of sucrose polyester on cholesterol metabolism in man. Metabolism 28: 994–1000, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Crouse JR, Grundy SM, Johnson JH. Effects of AOMA on cholesterol metabolism in man. Metabolism 31: 733–739, 1982. [DOI] [PubMed] [Google Scholar]
- 12.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 280: 12710–12720, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Davis HR, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SPN, Lam M-H, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 279: 33586–33592, 2004. [DOI] [PubMed] [Google Scholar]
- 14.DenBesten L, Conn WE, Bell. The effect of dietary cholesterol on the composition of human bile. Surgery 73: 266–273, 1973. [PubMed] [Google Scholar]
- 15.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 34: 1637–1659, 1993. [PubMed] [Google Scholar]
- 16.Field FJ, Born E, Mathur SN. Stanol esters decrease plasma cholesterol independently of intestinal ABC sterol transporters and Niemann-Pick C1-like 1 protein gene expression. J Lipid Res 45: 2252–2259, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, MacIntyre DE, Ogawa A, O'Neill KA, Iyer SPN, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1). Proc Natl Acad Sci USA 102: 8132–8137, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy SM Absorption and metabolism of dietary cholesterol. Annu Rev Nutr 3: 71–96, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Metzger AL. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology 62: 1200–1217, 1972. [PubMed] [Google Scholar]
- 20.Hawes BE, O'Neill KA, Yao X, Crona JH, Davis HR Jr, Graziano MP, Altmann SW. In vivo responsiveness to ezetimibe correlates with Niemann-Pick C1 like-1 (NPC1L1) binding affinity: comparison of multiple species NPC1L1 orthologs. Mol Pharmacol 71: 19–29, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Ho KJ Comparative studies on the effect of cholesterol feeding on biliary composition. Am J Clin Nutr 29: 698–704, 1976. [DOI] [PubMed] [Google Scholar]
- 22.Insull W Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J 99: 257–273, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Kelly RE, Abedin MZ, Fonkalsrud EW, Masuda H, Huang E-J, Saunders K, Bjerke HS, Roslyn JJ. Early effects of colectomy and endorectal pullthrough operation on biliary lipid composition. J Surg Res 49: 111–115, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Kern F Effects of dietary cholesterol on cholesterol and bile acid homeostasis in patients with cholesterol gallstones. J Clin Invest 93: 1186–1194, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurrasch DM, Huang J, Wilkie TM, Repa JJ. Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol 389: 3–15, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Mathur A, Walker JJ, Al-Azzawi HH, Lu D, Swartz-Basile DA, Nakeeb A, Pitt HA. Ezetimibe ameliorates cholecystosteatosis. Surgery 142: 228–233, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Mott GE, Jackson EM, McMahan CA. Effects of dietary cholesterol, type of fat, and sex on bile lipid composition of adult baboons. Am J Clin Nutr 56: 511–516, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Osuga T, Portman OW, Tanaka N, Alexander M, Ochsner AJ III. The effect of diet on hepatic bile formation and bile acid metabolism in squirrel monkeys with and without cholesterol gallstones. J Lab Clin Med 88: 649–661, 1976. [PubMed] [Google Scholar]
- 29.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res 46: 779–789, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Salen G, Starc T, Sisk CM, Patel SB. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology 130: 1853–1857, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Salen G, von Bergmann K, Lütjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation 109: 966–971, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res 39: 1833–1843, 1998. [PubMed] [Google Scholar]
- 33.Spady DK, Turley SD, Dietschy JM. Dissociation of hepatic cholesterol synthesis from hepatic low-density lipoprotein uptake and biliary cholesterol saturation in female and male hamsters of different ages. Biochim Biophys Acta 753: 381–392, 1983. [DOI] [PubMed] [Google Scholar]
- 34.Sudhop T, Lütjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation 106: 1943–1948, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Sudhop T, von Bergmann K. Cholesterol absorption inhibitors for the treatment of hypercholesterolaemia. Drugs 62: 2333–2347, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PHR, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res 48: 699–708, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest 117: 1968–1978, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turley SD Role of Niemann-Pick C1-Like 1 (NPC1L1) in intestinal sterol absorption. J Clin Lipidol 2: S20–S28, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turley SD, Daggy BP, Dietschy JM. Cholesterol-lowering action of psyllium mucilloid in the hamster: sites and possible mechanisms of action. Metabolism 40: 1063–1073, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Turley SD, Dietschy JM. The metabolism and excretion of cholesterol by the liver. In: The Liver: Biology and Pathobiology, edited by Arias IM, Jakoby WB, Popper H, Schachter D, and Shafritz DA. New York: Raven, 1988, p. 617–641.
- 41.Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr Opin Lipidol 14: 233–240, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Turley SD, Herndon MW, Dietschy JM. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J Lipid Res 35: 328–339, 1994. [PubMed] [Google Scholar]
- 43.Turley SD, Spady DK, Dietschy JM. Alteration of the degree of biliary cholesterol saturation in the hamster and rat by manipulation of the pools of preformed and newly synthesized cholesterol. Gastroenterology 84: 253–264, 1983. [PubMed] [Google Scholar]
- 44.Turley SD, Spady DK, Dietschy JM. Regulation of fecal bile acid excretion in male Golden Syrian hamsters fed a cereal-based diet with and without added cholesterol. Hepatology 25: 797–803, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Uppal H, Zhai Y, Gangopadhyay A, Khadem S, Ren S, Moser JA, Xie W. Activation of liver X receptor sensitizes mice to gallbladder cholesterol crystallization. Hepatology 47: 1331–1342, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Valasek MA, Repa JJ. The power of real-time PCR. Adv Physiol Educ 29: 151–159, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Van Heek M, Austin TM, Farley C, Cook JA, Tetzloff GG, Davis HR Jr. Ezetimibe, a potent cholesterol absorption inhibitor, normalizes combined dyslipidemia in obese hyperinsulinemic hamsters. Diabetes 50: 1330–1335, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Volger OL, van der Boom H, de Wit ECM, van Duyvenvoorde W, Hornstra G, Plat J, Havekes LM, Mensink RP, Princen HMG. Dietary plant stanol esters reduce VLDL cholesterol secretion and bile saturation in apolipoprotein E*3-Leiden transgenic mice. Arterioscler Thromb Vasc Biol 21: 1046–1052, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Wang DQH Regulation of intestinal cholesterol absorption. Annu Rev Physiol 69: 221–248, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Wang DQH, Zhang L, Wang HH. High cholesterol absorption efficiency and rapid biliary secretion of chylomicron remnant cholesterol enhance cholelithogenesis in gallstone-susceptible mice. Biochim Biophys Acta 1733: 90–99, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQH. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 134: 2101–2110, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang HH, Wang DQH. Reduced susceptibility to cholesterol gallstone formation in mice that do not produce apolipoprotein B48 in the intestine. Hepatology 42: 894–904, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Yu L The structure and function of Niemann-Pick C1-like 1 protein. Curr Opin Lipidol 19: 263–269, 2008. [DOI] [PubMed] [Google Scholar]
- 54.Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem 281: 6616–6624, 2006. [DOI] [PubMed] [Google Scholar]