Abstract
Alcoholic fatty liver is associated with inhibition of sirtuin 1 (SIRT1) and AMP-activated kinase (AMPK), two critical signaling molecules regulating the pathways of hepatic lipid metabolism in animals. Resveratrol, a dietary polyphenol, has been identified as a potent activator for both SIRT1 and AMPK. In the present study, we have carried out in vivo animal experiments that test the ability of resveratrol to reverse the inhibitory effects of chronic ethanol feeding on hepatic SIRT1-AMPK signaling system and to prevent the development of alcoholic liver steatosis. Resveratrol treatment increased SIRT1 expression levels and stimulated AMPK activity in livers of ethanol-fed mice. The resveratrol-mediated increase in activities of SIRT1 and AMPK was associated with suppression of sterol regulatory element binding protein 1 (SREBP-1) and activation of peroxisome proliferator-activated receptor γ coactivator α (PGC-1α). In parallel, in ethanol-fed mice, resveratrol administration markedly increased circulating adiponectin levels and enhanced mRNA expression of hepatic adiponectin receptors (AdipoR1/R2). In conclusion, resveratrol treatment led to reduced lipid synthesis and increased rates of fatty acid oxidation and prevented alcoholic liver steatosis. The protective action of resveratrol is in whole or in part mediated through the upregulation of a SIRT1-AMPK signaling system in the livers of ethanol-fed mice. Our study suggests that resveratrol may serve as a promising agent for preventing or treating human alcoholic fatty liver disease.
Keywords: alcoholic liver steatosis, lipid metabolism, sirtuin 1, AMP-activated kinase, acetylation, sterol regulatory element-binding protein 1c, peroxisome proliferator-activated receptor γ coactivator α
accumulation of fat in the liver in response to chronic alcohol consumption increases the risk of developing progressive liver injuries such as alcoholic steatohepatitis, fibrosis, and cirrhosis. Hence it is important to develop specific pharmacological or nutritional agents to treat steatosis and prevent the development of more severe forms of liver injury.
Considerable progress has been made in recent years toward better understanding the molecular mechanisms underlying the development of alcoholic fatty liver. Chronic ethanol exposure causes enhanced lipogenesis and impaired fatty acid oxidation by targeting key transcriptional regulators of genes controlling these metabolic processes, including sterol regulatory element binding protein 1 (SREBP-1) and peroxisome proliferator-activated receptor γ (PPARγ) coactivator α (PGC-1α) (12, 13, 22–24, 48, 54–56, 58). More fundamentally, ethanol-mediated impairment of sirtuin 1 (SIRT1) and AMP-activated kinase (AMPK) has been reported to be responsible for the reduction in PGC-1α and the increase in SREBP-1 activities in the livers of several alcohol-fed animal models (13, 31, 32, 49, 56, 57, 59).
Mounting evidence suggests that SIRT1 and AMPK are two critical signaling molecules controlling the pathways of hepatic lipid metabolism. SIRT1 is a NAD+-dependent class III protein deacetylase that regulates lipid metabolism by deacetylation of modified lysine residues on histones and various transcriptional regulators (4, 41, 44). AMPK plays a central role in the regulation of lipid metabolism by switching on fatty acid oxidation pathways and inhibiting lipid synthesis (34). Therefore, SIRT1 and AMPK represent attractive targets in the development of therapies to prevent alcoholic fatty liver disease.
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a polyphenol found in a wide variety of plant species including grapes, red wines, berries, and peanuts (2). Resveratrol has been identified as a potent agonist of SIRT1 (5, 21, 25). In mammalian cells, resveratrol promotes cellular expression of SIRT1 protein and dramatically stimulates SIRT1 activity (10). However, the molecular mechanism by which resveratrol induces SIRT1 activation in vivo remains to be elucidated. Several lines of investigation have demonstrated that resveratrol modulates lipid metabolism mainly through activation of SIRT1 signaling (3, 29, 36, 40). For instance, in adipose tissue, modulation of SIRT1-PPARγ signaling pathways by resveratrol triggered lipolysis and loss of fat (36). In muscle, resveratrol induced PGC-1α activity through SIRT1, leading to an altered gene expression patterns that favor mitochondrial fatty acid oxidation and utilization in mice (19, 29). We recently reported that resveratrol abolished ethanol-mediated activation of SREBP-1 signaling in cultured hepatoma cells (59). Resveratrol and several of its structurally related polyphenols have also been shown to act as potent AMPK activators (60). More importantly, resveratrol treatment reduces lipid accumulation by stimulating SIRT1-AMPK signaling in cultured hepatocytes and in livers of animals (3, 20).
Several studies have demonstrated the protective effects of resveratrol against alcoholic liver injury in animals (6, 27, 28). Although the antioxidant effects of resveratrol have been proposed to contribute to its beneficial effects, the underlying molecular mechanism is not completely understood. In the present study, we investigated the effect of resveratrol treatment on ethanol-induced fatty liver in mice and explored the potential molecular mechanisms through which the protective effects of resveratrol may work.
MATERIALS AND METHODS
Animal studies.
Six- to 8-wk-old male C57BL/6J mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed individually in stainless steel wire-bottom cages in a temperature- and humidity-controlled room (23°C and 50% relative humidity) with a 12:12-h yellow light-dark cycle. As we previously described, liquid diets were based on the Lieber-DeCarli formulation and provide 1 kcal/ml (prepared by Dyets, Bethlehem, Philadelphia, PA) (15, 55, 56, 59). Mice (6–8 wk old) were divided into six dietary groups to test the effects of two doses of resveratrol in the setting of a low-fat diet: 1) low-fat diet alone [LF, 10% of calories as fat (6% cocoa butter and 4% safflower oil)]; 2) low-fat diet plus 200 mg·kg body wt−1·day−1 resveratrol (R200); 3) low-fat diet plus 400 mg·kg body wt−1·day−1 resveratrol (R400); 4) low-fat diet plus ethanol (E; identical to the control LF diet but with ethanol added to account for 29% of total calories); 5) low-fat diet plus ethanol and 200 mg·kg−1·day−1 resveratrol (E+R200); and 6) low-fat diet plus ethanol and 400 mg·kg−1·day−1 resveratrol (E+R400). In the latter two groups, resveratrol (Orchid Chemicals & Pharmaceuticals) was added to the diets for the last 2 wk of the study. The doses, 200 and 400 mg·kg body wt−1·day−1, for resveratrol treatment were based on a published study (29). All the liquid diets were freshly prepared from powder, and resveratrol was added just before mixing with a blender. The dietary and nutritional intake of control mice were matched to those of the ethanol-fed mice by pair feeding the same volume of isocaloric liquid diet for 4 wk. Ethanol was introduced gradually into the liquid diet; after the animals were accommodated to the liquid diet, they were given ethanol 9.5% of the total calories for 2 days, then 18.9% for 3 days, and finally 29%. For animals on an ethanol-containing diet, animal cages were placed on heating pads to maintain body temperature since ethanol consumption can induce hypothermia. Food intake of each mouse in all six groups was daily recorded. Resveratrol intake was monitored by total food intake. After 4 wk of liquid diet feeding, the animals were euthanized, at which time blood, liver tissue, and adipose tissues were collected. The studies were approved by the Institutional Animal Care Use Committees of University of South Florida.
Measurement of hepatic histology and triglyceride contents.
Sections of the liver were stained with Oil Red O or hematoxylin and eosin (H&E) as previously reported (15, 55). Total hepatic cholesterol and triglyceride levels were measured as described previously (15, 55, 58).
Assays from mouse serum.
The blood alcohol level's of mice were determined using an Alcohol test kit (Pointe Scientific, Canton, MI) on serum samples collected at the time of euthanasia. Ethanol concentrations were determined by measuring the absorbance at 340 nm resulting from the reduction of NAD+ to NADH by alcohol dehydrogenase. Serum levels of alanine aminotransferase (ALT) were determined by using a kit from Sigma-Aldrich (St. Louis, MO). Plasma β-hydroxybutyrate (β-OHB) was measured by a β-hydroxybutyrate LiquiColor procedure (SanBio Laboratory, Boerne, TX). Serum levels of adiponectin were determined by use of a commercial ELISA kit from R&D Systems (Minneapolis, MN). Plasma triglyceride and cholesterol levels were determined via the Sigma Diagnostics Triglyceride and Infinity Cholesterol Reagent.
Total RNA isolation and qRT-PCR.
Total RNA was prepared from mouse liver or adipose tissue using an RNAeasy Total RNA kit (Qiagen). Reverse transcription of 2–5 μg liver total RNA to cDNA was performed using the StrataScript qPCR cDNA synthesis kit (Stratagene). Real-time quantitative polymerase chain reaction (qRT-PCR) amplification was performed in an iCycler Spectrofluorometric thermal cycler (Bio-Rad Laboratories, Hercules, CA) using RT2 SYBR Green qPCR Master Mix and primer sets optimized for tested targets for SYBR Green-based real-time PCR from SuperArray bioscience (Frederick, MD). The following primer sets were purchased and used: SIRT1 (PPM05054A), GPAT1 (PPM33295A), FAS (PPM03816E), SCD1 (PPM05664E), ACCα (PPM05109E), ME (PPM 05495A), MCAD (PPM25604A), AOX (PPM04407A), CPT1a (PPM25930B), FOXO1 (PPM03381B), PGC-1α (PPM03360E), adiponectin (PPM05260A), AdipoR1 (PPM 35710A), AdipoR2 (PPM 38032E), TNF-α (PPM 03113E), PPARγ (PPM 05108B), and GAPDH (PPM02946E). The relative amount of target mRNA was calculated using the comparative cycle threshold (Ct) method by normalizing target mRNA Ct to those for GAPDH.
Western blot analysis.
Immunoblot analyses were performed using 30–50 μg liver nuclear extracts or whole liver extracts separated by electrophoresis in a 10, 8, or 5% SDS-polyacrylamide gel and transferred to nitrocellulose filters. SIRT1 or SREBP-1 was visualized by using antibodies from Santa Cruz. Nonspecific proteins were used as loading controls to normalize the signal obtained for liver nuclear protein extracts. N-acetyl-leucinal-leucinal-norleucinal (25 μg/ml) (Calbiochem, San Diego, CA) was present in all the procedures of nSREBP-1c analysis.
Measurements of phosphorylation levels of hepatic AMPK and ACC.
At the time of euthanasia, the liver samples were immediately freeze-clamped with tongs and cooled to the temperature of liquid nitrogen (56, 58). Tissues were ground to a powder under liquid nitrogen and homogenized. Western blots were performed using the liver homogenate. AMPKα, phospho-AMPKα, AMPKβ, phospho-AMPKβ, and phospho-ACC were visualized by use of antibodies (Cell Signaling Technology, Danvers, MA). Polyclonal rabbit anti-β-actin antibody (Sigma) was used to normalize the signal obtained for total liver protein extracts.
PGC-1α acetylation assays.
PGC-1α protein was immunoprecipitated with liver nuclear extracts by an anti-PGC-1α antibody (Calbiochem). PGC-1α levels and acetylation were detected via specific antibodies for PGC-1α and acetyl lysine (Cell Signaling) (57, 59).
Lipid peroxidation.
The lipid peroxidation product in liver was determined according to published methods based on the formation of the thiobarbituric reactive substances and expressed as the extent of malondialdehyde production (27, 28).
Data analysis.
Data are presented as means ± SD. All data were analyzed by two-way ANOVA followed by post hoc testing with Fisher protected least squares difference. Differences between means were considered significant at P < 0.05.
RESULTS
Resveratrol treatment ameliorated alcoholic liver steatosis in mice.
As we have previously published (15, 55–57, 59), we have consistently observed the development of the typical histological and biochemical features of liver steatosis in mice fed an ethanol-containing low-fat diet for 4 wk. It is also worthwhile to note that this low-fat, ethanol-feeding mouse model has provided an excellent in vivo system for investigating the effects of ethanol feeding on lipid metabolism pathways mediated by SIRT1, AMPK, SREBP-1, and PPARα in animal liver (15, 55–57, 59). Therefore, mice were fed a low-fat diet with ethanol (29% of the total calories) using the modified Lieber-DeCarli liquid diet and a pair-feeding protocol for 4 wk. For the final 2 wk of feeding, four groups of mice were given a dose of either 200 or 400 mg·kg−1·day−1 of resveratrol with or without ethanol in their diets.
During the 4-wk feeding period, food intake was similar in all groups. Ethanol or resveratrol administration had no apparent effect on the health status of the mice. In the last 2 wk of feeding, there were no significant differences in the average food intake among the various groups (data not shown).
The initial body weights were similar in all groups. At the end of the feeding period, an average increase in the body weight of 1–2 g was observed in control, ethanol-fed, and resveratrol-treated mice (Table 1). Surprisingly, when the ethanol-fed mice were cotreated with resveratrol, there was a nearly 3-g decrease in the body weight compared with the pair-fed control mice (Table 1). Accordingly, the liver weights were also significantly decreased in these two groups (Table 1). Blood alcohol determinations revealed a mean value of 52.3 ± 12 mg/dl among the mice administered ethanol, which was similar to the blood ethanol levels in ethanol fed mice cotreated with resveratrol (E+R200: 43.9 ± 9 mg/dl; E+R400: 53.1 ± 16 mg/dl).
Table 1.
Selected parameters in mice fed ethanol and resveratrol
| Parameters | Control | R200 | R400 | Ethanol | E+R200 | E+R400 |
|---|---|---|---|---|---|---|
| Starting weight, g | 22.9±1.4 | 22.3±1.8 | 23.1±1.4 | 22.1±1.6 | 22.9±1.0 | 22.2±1.6 |
| Final weight, g | 25.2±0.8a | 25.2±1.6a | 24.1±0.7a | 25.0±1.7a | 21.9±1.2b† | 21.5±2.0b† |
| Liver weight, g | 1.21±0.1a | 1.19±0.2a | 1.21±0.1a | 1.23±0.1a | 1.01±0.1b* | 0.98±0.1b† |
| Liver weight/body weight | 0.048±0.13 | 0.047±0.13 | 0.05±0.14 | 0.049±0.06 | 0.046±0.08 | 0.046±0.05 |
| Plasma triglycerides, mg/dl | 81±27b | 58±14c | 59±15c | 102±31a | 94±16b | 72±18b |
| Plasma cholesterol, mg/dl | 100±22 | 96±27 | 96±23 | 84±19 | 110±15 | 97±14 |
| Plasma β-OHB, mg/dl | 2.5±1.1c | 3.4±2.4c | 5.0±2.1b* | 6.2±3.4b* | 21.5±1.1a† | 19.3±5.5a† |
| Liver MDA, nmol/mg protein | 3.3±0.5a | 3.2±0.7a | 3.4±0.9a | 3.6±0.2a | 3.3±0.8a | 2.9±0.4b |
Results are expressed as means ± SD of 4–8 mice. Six- to 8 wk-old C57/BL6J male mice were divided into 6 groups as follows: 1) low-fat pair-fed control (Control); 2) control diet supplemented with resveratrol (200 mg·kg−1·day−1) (R200); 3) control diet supplemented with resveratrol (400 mg·kg−1·day−1) (R400); 4) ethanol-containing diet (29% of total calories); 5) ethanol-containing diet supplemented with resveratrol (200 mg·kg−1·day−1) (E+R200); and 6) ethanol-containing diet supplemented with resveratrol (400 mg·kg−1·day−1) (E+R400). Resveratrol was added to the diet for the last 2 wk of the feeding study. The animals were euthanized after 4 wk. β-OHB, β-hydroxybutryate; MDA, malondialdehyde. Means without a common letter differ,
P < 0.05;
P < 0.001.
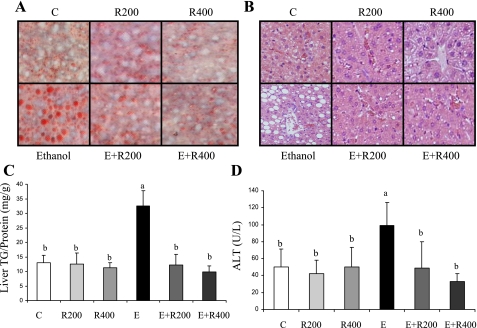
Histological analysis of the liver revealed accumulation of lipid droplets in the livers of ethanol-fed animals, whereas lipid droplets were rare in livers of the control animals or mice treated with resveratrol alone (Fig. 1, A and B). When the ethanol-fed mice were coadministered resveratrol, the histology showed no accumulation of hepatic lipid droplets (Fig. 1, A and B). Quantification of hepatic lipid contents was concordant with the histological findings (Fig. 1C). Ethanol feeding increased the liver triglyceride levels by ∼2.5-fold compared with pair-fed control mice. Resveratrol treatment alone did not alter the amount of hepatic triglyceride. However, when resveratrol was coadministered for the last 2 wk of the ethanol feeding, the hepatic triglyceride content was decreased to the level of controls (Fig. 1C). Liver cholesterol contents remained unchanged in all groups (data not shown). Furthermore, plasma ALT was modestly elevated in the ethanol-fed mice but was reduced to the level of control by coadministration of resveratrol (Fig. 1D). Collectively, these results demonstrate that resveratrol treatment protected against the development of alcoholic liver steatosis in mice.
Fig. 1.
Resveratrol ameliorated alcoholic liver steatosis in mice. Oil red O staining of liver sections (A), hematoxylin and eosin (H&E) stain ×20 (B), hepatic triglyceride (TG) content (C), and plasma alanine aminotransferase level (D) of mice fed a low-fat control diet (C), 200 mg·kg−1·day−1 resveratrol (R200), or 400 mg·kg−1·day−1 resveratrol (R400) with (E+R200, E+R400) or without ethanol (E). All data are expressed as means ± SD; n = 5–8 animals. Means without a common letter differ, P < 0.05.
Resveratrol treatment upregulated SIRT1 in the livers of ethanol-fed mice.
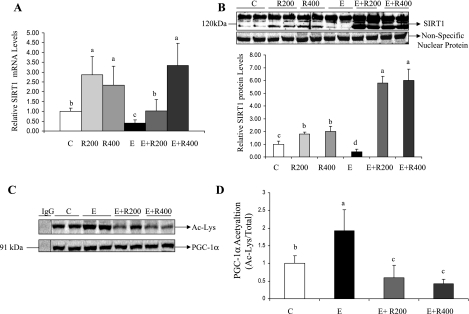
To dissect the molecular mechanisms underlying resveratrol's amelioration of alcoholic fatty liver, we examined the effect of resveratrol on hepatic SIRT1 signaling. As shown in Fig. 2, A and B, ethanol feeding reduced the mRNA and protein levels of hepatic SIRT1 by ∼50% compared with control animals. Previously, we showed that ethanol feeding (27.5% of total calories) did not significantly alter the SIRT1 mRNA expression in the livers of mice (59). The higher alcohol intake (29% of total calories) in the present study may lead to enhanced metabolism of ethanol in the liver and subsequently more pronounced effects on SIRT1 mRNA levels. Resveratrol treatment alone significantly increased mRNA and protein levels of SIRT1 by ∼2.5-fold. When ethanol-fed mice were given resveratrol, the SIRT1 mRNA expression levels were increased up to approximately threefold (Fig. 2A).
Fig. 2.
Resveratrol upregulated sirtuin 1 (SIRT1) in the livers of ethanol-fed mice. Hepatic SIRT1 mRNA (A), SIRT1 protein levels (B), acetylation of peroxisome proliferator-activated receptor γ (PPARγ) coactivator α (PGC-1α; C), and relative PGC-1α acetylation (D) of mice fed a low-fat diet, 200 mg·kg−1·day−1 resveratrol, or 400 mg·kg−1·day−1 resveratrol with or without ethanol. Hepatic nuclear SIRT1 protein levels were determined by use of an anti-SIRT1 antibody. A nonspecific nuclear protein band was used to confirm equal loading and to normalize the data. PGC-1α was immunoprecipitated from liver extracts then immunoblotted with either an anti-acetylated lysine (Ac-Lys) antibody to determine the extent of PGC-1α acetylation or with a PGC-1α antibody to determine the total amount of PGC-1α. All data are expressed as means ± SD; n = 4–8 animals. Means without a common letter differ, P < 0.05.
Interestingly, the increase in the abundance of SIRT1 protein was much more pronounced in the groups administered ethanol plus resveratrol, in which SIRT1 levels were approximately fivefold higher than in ethanol-fed animals, approximately fourfold higher than in controls, and approximately twofold higher than in mice treated with resveratrol alone (Fig. 2B). The apparent lack of correlation between SIRT1 mRNA and protein levels in resveratrol or resveratrol plus ethanol groups suggests that, in addition to transcriptional regulation, the SIRT1 expression may also be regulated at posttranscriptional levels in these mice.
We further determined hepatic SIRT1 deacetylase activity by evaluating the acetylation status of PGC-1α, a marker of in vivo SIRT1 activity (3, 29). As shown in Fig. 2C, the ratio of acetylated PGC-1α to total PGC-1α protein in the ethanol-fed mice was significantly higher than the controls. Resveratrol treatment alone modestly reduced acetylation levels of PGC-1α (data not shown). When ethanol-fed mice were given resveratrol, the acetylation levels of PGC-1α were greatly diminished, indicating that hepatic SIRT1 enzymatic activity was increased by resveratrol (Fig. 2, C and D).
Resveratrol stimulated AMPK activity in the livers of ethanol-fed mice.
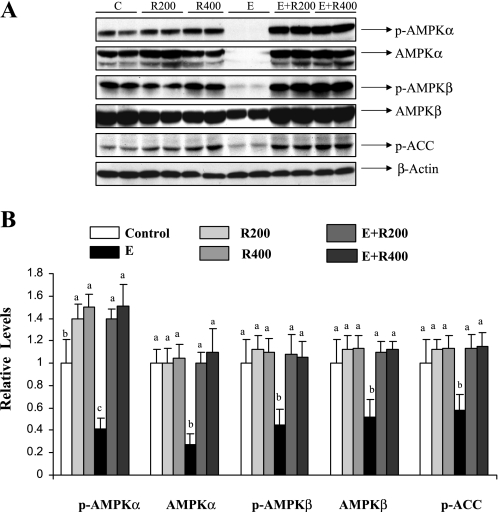
We examined the effect of resveratrol on ethanol-mediated inhibition of hepatic AMPK signaling. Compared with pair-fed control mice, ethanol feeding reduced phosphorylated AMPKα and AMPKβ by nearly 65 or 60%, with a concomitant decrease in total protein levels of AMPKα and AMPKβ (Fig. 3). Resveratrol supplementation blocked the inhibition of both phosphorylated and total protein levels of AMPKα or AMPKβ in the livers of ethanol-fed mice (Fig. 3). Moreover, resveratrol-mediated activation of AMPK was accompanied by increased phosphorylation of acetyl-CoA carboxylase (ACC), a downstream indicator of AMPK activity (Fig. 3).
Fig. 3.
Resveratrol stimulated hepatic AMPK activity in ethanol-fed mice. A: Western blots were performed using anti-phosphorylated-AMPKα (anti-p-AMPKα), anti-AMPKα, anti-p-AMPKβ, anti-AMPKβ, and anti-phosphorylated acetyl CoA carboxylase (P-ACC) antibodies from liver extracts of mice fed a low-fat diet, 200 mg·kg−1·day−1 resveratrol, or 400 mg·kg−1·day−1 resveratrol with or without ethanol. β-Actin protein band was used to confirm equal loading and to normalize the data. B: relative levels of p-AMPKα, AMPKα, p-AMPKβ, AMPKβ, and P-ACC. Western blots were quantified by a PhosphorImager and MultiAnalyst (Bio-Rad) software analysis. All data are expressed as means ± SD; n = 4–8 animals. Means without a common letter differ, P < 0.05.
It is important to note that total hepatic AMPK protein expression was robustly reduced by ethanol feeding in mice (Fig. 3). We previously reported that ethanol feeding decreased AMPKα protein levels by nearly 40% whereas the protein expression of the AMPKβ subunit was only slightly reduced (56). The higher ethanol intake in the present study may explain the increased inhibitory effects on AMPK protein expression.
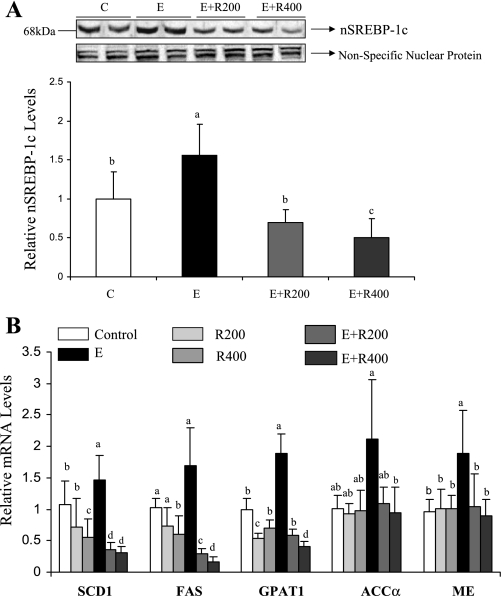
Resveratrol suppressed SREBP-1 activity and reduced the mRNA levels of SREBP-1 regulated genes encoding lipogenic enzymes in the livers of ethanol-fed mice.
SREBP-1 is regulated by SIRT1-mediated deacetylation as well as by AMPK activation (56, 59). Therefore, we examined the effect of resveratrol on ethanol-increased processing of hepatic nuclear SREBP-1c (nSREBP-1c) in mice. As shown in Fig. 4A, the amount of nSREBP-1c protein was significantly increased in the livers of ethanol-fed mice compared with control mice. However, resveratrol supplementation to ethanol-fed mice dramatically reduced hepatic nSREBP-1c protein levels, whereas resveratrol treatment alone only slightly reduced them (data not shown). Consistent with our previous findings (55), ethanol feeding led to significant increases in mRNA levels of several SREBP-1-regulated lipogenic enzymes, including mitochondrial glycerol-3-phosphate acyltransferase (GPAT1), stearoyl-coenzyme A desaturase 1 (SCD1), and malic enzyme (ME) compared with the controls (Fig. 4B). Ethanol feeding also increased the mRNA levels of fatty acid synthase (FAS) and ACCα but did not reach statistical significance. Although resveratrol alone modestly reduced mRNA levels of those enzymes, the resveratrol supplementation to ethanol-fed mice substantially suppressed mRNAs of FAS, GPAT1, SCD1, ACCα, and ME to levels significantly lower than that in controls or ethanol-treated mice (Fig. 4B).
Fig. 4.
Resveratrol suppressed sterol regulatory element-binding protein 1 (SREBP-1) activity and reduced the mRNA levels of SREBP-1 regulated genes encoding lipogenic enzymes in the livers of ethanol-fed animals. Representative Western blots of nuclear SREBP-1c (nSREBP-1c) protein (A) and relative mRNA levels of SREBP-regulated lipogenic enzymes (B) in the livers of mice fed a low-fat control diet, 200 mg·kg−1·day−1 resveratrol, or 400 mg·kg−1·day−1 resveratrol with or without ethanol. A nonspecific nuclear protein band in nuclear extracts was used to confirm equal loading and to normalize the data. All data are expressed as means ± SD; n = 5–8 animals. Means without a common letter differ, P < 0.05.
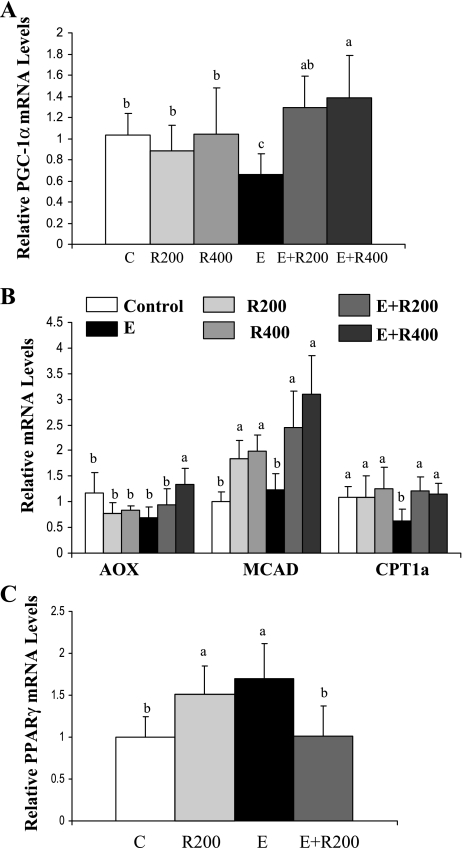
Resveratrol increased PGC-1α activity and enhanced expression of genes regulated by PGC-1α in the livers of ethanol-fed mice.
Both SIRT1 and AMPK positively regulate PGC-1α activity (30, 41, 43). We investigated whether resveratrol treatment relieves ethanol's inhibition of PGC-1α signaling. As shown in Fig. 5A, ethanol feeding significantly reduced mRNA expression of PGC-1α. However, PGC-1α mRNA levels were restored to control levels upon resveratrol treatment (Fig. 5A). We further determined whether the increased PGC-1α resulted in altered mRNA expression of enzymes involved in fatty acid oxidation by analyzing hepatic mRNA levels of several PGC-1α targets. As shown in Fig. 5B, mRNAs for acyl-CoA oxidase (AOX) and mitochondrial medium-chain acyl-CoA dehydrogenase (MCAD) were unchanged in the ethanol-fed group. However, ethanol feeding significantly reduced carnitine palmitoyltransferase 1a (CPT1a) mRNA expression compared with control. More importantly, in ethanol-fed mice, resveratrol induced mRNAs of AOX, MCAD, and CPT1a to levels significantly higher than that in control or ethanol-fed mice (Fig. 5B).
Fig. 5.
Resveratrol increased PGC-1α activity and enhanced expression of genes regulated by PGC-1α in the livers of ethanol-fed mice. Relative mRNA levels of PGC-1α (A), relative mRNA levels of PGC-1α-regulated fatty acid oxidation enzymes (B), and relative mRNA levels of PPARγ (C) in the livers of mice fed a low-fat control diet, 200 mg·kg−1·day−1 resveratrol, or 400 mg·kg−1·day−1 resveratrol with or without ethanol. All data are expressed as means ± SD; n = 5–8 animals. Means without a common letter differ, P < 0.05.
SIRT1 is known to regulate PPARγ (36), which has been implicated in the development of both alcoholic and nonalcoholic fatty liver (11, 39). We found that ethanol feeding significantly increased PPARγ mRNA levels by ∼70% and that resveratrol prevented this increase (Fig. 5C).
We further examined whether the activation of SIRT1-AMPK-PGC-1α signaling by resveratrol and ethanol led to increased hepatic fatty acid oxidation capacity. Consistent with our previous observations (15, 58), ethanol feeding modestly increased the plasma β-OHB levels, suggesting that some elevated hepatic free fatty acids in ethanol-fed mice might be converted to ketone bodies (Table 1). However, resveratrol supplementation to ethanol-fed mice dramatically increased the plasma β-OHB levels by approximately eightfold compared with controls and about threefold compared with ethanol-fed mice, suggesting that resveratrol with ethanol was capable of inducing hepatic fatty acid oxidation, promoting generation of ketone bodies, and preventing ethanol-induced liver steatosis.
Resveratrol upregulated adiponectin and increased mRNA expression of hepatic adiponectin receptors in ethanol-fed mice.
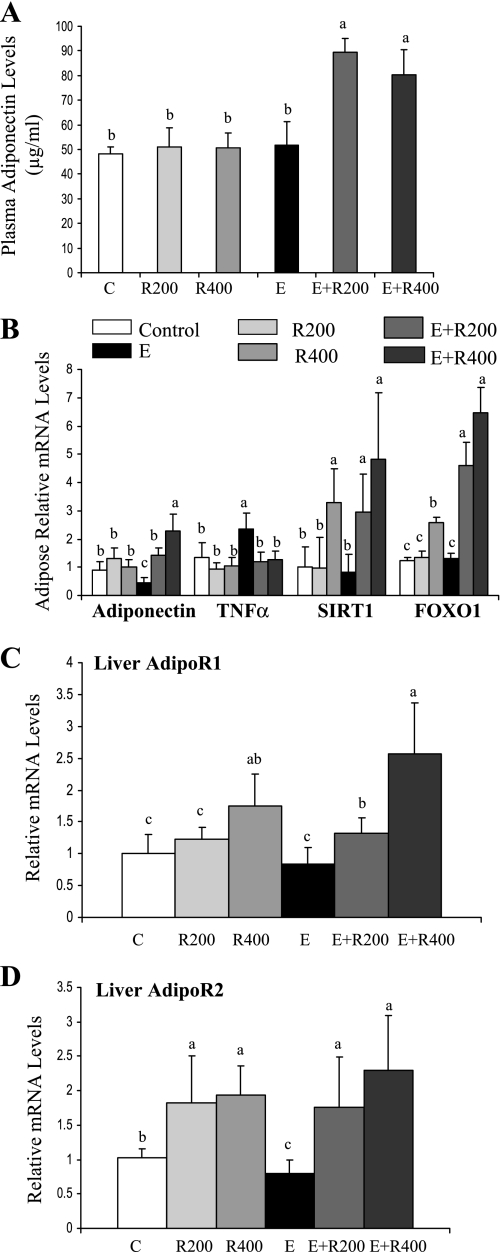
Adiponectin plays a vital role in alcoholic fatty liver, and SIRT1 is involved in regulating the expression and secretion of adiponectin (8, 13, 37, 38, 42, 47, 53, 58). Thus we investigated whether adiponectin might play a role in the prevention of alcoholic liver steatosis by resveratrol. As shown in Fig. 6A, the circulating adiponectin levels were similar in control, resveratrol-treated, and ethanol-fed mice. However, resveratrol supplementation to ethanol-fed mice caused a significant increase (∼2-fold) in serum adiponectin concentrations.
Fig. 6.
Resveratrol upregulated adiponectin and enhanced hepatic AdipoR1/R2 mRNA expression in ethanol-fed mice. Plasma adiponectin concentrations (A); relative adipose mRNA levels of adiponectin, TNF-α, SIRT1, and FOXO1; B); relative mRNA levels of AdipoR1 (C); and relative mRNA levels of AdipoR2 (D) of mice fed a low-fat control diet, 200 mg·kg−1·day−1 resveratrol, or 400 mg·kg−1·day−1 resveratrol with or without ethanol. All data are expressed as means ± SD; n = 4–6 animals. Means without a common letter differ, P < 0.05.
Adiponectin is expressed and secreted by adipose tissue. We examined mRNAs of adiponectin and several possible adiponectin regulators in adipose tissues. As shown in Fig. 6B, mRNA expression of adiponectin was significantly reduced ∼60% by ethanol feeding and was restored or increased by resveratrol supplementation of the ethanol diet. Interestingly, the ethanol-mediated reduction in adiponectin mRNA did not translate into a decrease in serum adiponectin protein. These results agree with a study showing that chronic ethanol exposure regulates serum adiponectin levels through a mechanism independent of adiponectin mRNA (8).
In adipose tissue, there was an inverse relationship between mRNA expression levels of adiponectin and TNF-α in each group (Fig. 6B). Hepatic TNF-α mRNA levels were unchanged in all of the groups (data not shown). Transcription of adiponectin in adipose tissue can be regulated by multiple factors besides TNF-α including SIRT1, PPARγ, and forkhead transcription factor O 1 (FOXO1) (37, 38). Although ethanol feeding did not affect the levels of SIRT1 or FOXO1 mRNAs, coadministration of resveratrol with ethanol significantly increased them both (Fig. 6B). Adipose PPARγ mRNA expression was not affected by ethanol feeding or resveratrol treatment (data not shown), suggesting that PPARγ might be not involved in the regulation of adiponectin by resveratrol and ethanol.
We further examined the effect of resveratrol on adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2) in the livers of ethanol fed-mice. Ethanol feeding significantly reduced mRNA expression of AdipoR2, whereas the inhibitory effect of ethanol feeding on AdipoR1 mRNA did not reach significance (Fig. 6, C and D). Although resveratrol treatment alone significantly increased mRNA levels of AdipoR1 and AdipoR2, resveratrol supplementation of ethanol diets exacerbated the effect (Fig. 6, C and D).
Resveratrol attenuated oxidative stress in the liver of ethanol-fed mice.
We examined whether resveratrol protects alcoholic liver through its well-known antioxidant properties. As shown in Table 1, chronic ethanol administration only slightly induced oxidative stress, as judged by the increase in the levels of hepatic malondialdehyde (MDA) (a lipid peroxidation product). However, resveratrol supplementation to ethanol-fed mice effectively reduced MDA to levels significantly lower than that in controls.
DISCUSSION
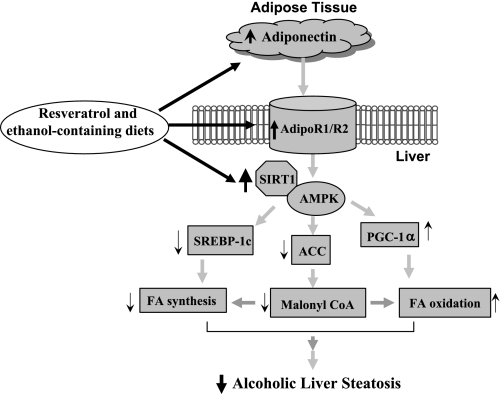
In the present paper, we demonstrated that resveratrol treatment upregulated SIRT1 and stimulated AMPK activity in the livers of chronically ethanol-fed mice. The activation of the SIRT1-AMPK signaling system by resveratrol correlated with reduced levels of hepatic nSREBP-1c protein and decreased mRNAs of SREBP-1c regulated lipogenic enzymes. Moreover, in the ethanol-fed mice, resveratrol induced the transcriptional coactivator PGC-1α activity and restored mRNA levels of several PGC-1α target genes encoding fatty acid oxidation enzymes. In parallel, circulating adiponectin levels and mRNA expression of hepatic AdipoR1 and R2 were markedly elevated by chronic ethanol feeding with resveratrol coadministration. Taken together, our findings suggest that resveratrol alleviates alcoholic fatty liver by coordinating multiple lipid metabolism signaling pathways, which in turn result in enhanced oxidation of fatty acids, reduced lipid synthesis, and prevention of hepatic lipid accumulation in mice (Fig. 7).
Fig. 7.
Proposed mechanism for the protective effects of resveratrol against alcoholic fatty liver in mice. AdipoR, adiponectin receptor; FA, free fatty acids.
Both SIRT1 and AMPK have been implicated in development of alcoholic fatty liver in several animal models (18, 31, 32, 56, 57, 59). The present study strongly suggests that resveratrol exerts its protective action against ethanol-induced liver steatosis by turning on the hepatic SIRT1-AMPK signaling system. Our findings further support the concept that dysregulation of the hepatic SIRT1-AMPK signaling system, in response to chronic ethanol exposure, represents a crucial mechanism that impairs multiple signaling pathways of lipid metabolism.
Growing evidence suggests an association between SIRT1 and AMPK signaling (3, 17, 20, 52). A recent study demonstrated that resveratrol-activated SIRT1 acts upstream of AMPK signaling via modulation of LKB-1, an upstream AMPK kinase, suggesting that stimulation of SIRT1/LKB1/AMPK signaling may serve as a key mechanism for the lipid-lowering effect of resveratrol in hepatic cells and in animal liver (20). It is not entirely clear whether the protective effect of resveratrol against alcoholic fatty liver is mediated primarily through activation of SIRT1 or through the subsequent (or parallel) increase of AMPK activity. Although the effect of ethanol on LKB1 has not been reported, it is possible that LKB1 may serve as a critical signaling link for the effect of the resveratrol on the SIRT1-AMPK signaling system in ethanol-fed animals. Further investigation of mice with liver-specific knockout of either SIRT1 or AMPK will be necessary to reveal the extent to which resveratrol and ethanol modulate hepatic lipid metabolism via SIRT1-LKB1-AMPK signaling.
Accumulating evidence has shown that chronic ethanol feeding targets two key transcriptional regulators, namely SREBP-1 and PGC-1α (12, 13, 22–24, 55, 56). Our findings suggest that the SIRT1-AMPK axis may be the upstream source of resveratrol-mediated activation of PGC-1α and reduction in SREBP-1 activity. It will be of great interest to determine whether or not stimulation of SIRT1 or AMPK alone by resveratrol is sufficient to erase ethanol's effect on these key downstream signaling molecules.
Admittedly, we cannot conclude unequivocally that the SIRT1-AMPK signaling pathway is solely responsible for resveratrol's effects. Indeed, we and others have demonstrated that resveratrol treatment inhibits hepatic oxidative damage induced by chronic ethanol feeding, suggesting that the positive effects of resveratrol may be mediated through its antioxidant properties (6, 27, 28). Interestingly, oxidative stress downregulates SIRT1 by triggering SIRT1 mRNA decay and reducing SIRT1 protein levels (1, 52). Thus it is possible that resveratrol-mediated reduction of hepatic oxidative stress generated by ethanol metabolism may be responsible, at least in part, for the robust increase in hepatic SIRT1 abundance.
It is intriguing that the increase in the abundance of hepatic SIRT1 protein was much more pronounced in the mice administered ethanol plus resveratrol. It has been reported that the increase in SIRT1 protein levels upon nutrient depletion in cultured cells is due to stabilization of SIRT1 protein mediated through proteasomal degradation pathway-independent unknown mechanisms (26). A major metabolite of ethanol, acetaldehyde, has been shown to form adducts with a number of proteins in the livers of animals (51). It is tempting to speculate that ethanol may stabilize hepatic SIRT1 protein in mice cotreated with resveratrol by stimulating the formation of acetaldehyde adducts, and subsequently, inhibition of SIRT1 degradation.
A surprising discovery was that resveratrol markedly increased circulating adiponectin levels in ethanol-fed mice. Altered expression of adiponectin is associated with alcoholic fatty liver in several animal models (8, 13, 42, 47, 53). Adiponectin has been shown to stimulate hepatic AMPK activity and subsequently to activate PGC-1α and inhibit SREBP-1 (58). A separate study demonstrated that adiponectin signaling increases SIRT1 protein expression levels in primary human myotubes (9). We have also observed that treatment of rat liver Kupffer cells with globular adiponectin increased SIRT1 protein levels (J. M. Ajmo and M. You, unpublished observations). Therefore, it is likely that upregulation of hepatic SIRT1 protein levels and activation of AMPK by resveratrol in ethanol-fed mice is partially mediated through increased serum adiponectin concentrations. An additional unexpected finding was that resveratrol reduced body weights of mice fed ethanol. Hypoadiponectinemia has been associated with obesity in rodents and humans (7). It is possible that, in ethanol-fed mice, resveratrol-mediated elevated circulating adiponectin levels contributed to the significant reduction in body weights.
The precise mechanism by which resveratrol dramatically induces adiponectin gene expression and its circulating protein levels in ethanol-fed mice remains to be elucidated. In adipocytes, adiponectin and TNF-α suppress each other's gene expression (13, 47, 53, 58). Our data showed that adipose mRNAs of adiponectin and TNF-α were indeed inversely related, suggesting that resveratrol may inhibit TNF-α and thereby increase adiponectin expression. SIRT1 is also known to induce adiponectin gene transcription and secretion in adipocytes via activating FOXO1 (37, 43). We observed that resveratrol significantly increased mRNA expression levels of both SIRT1 and FOXO1 in the adipose tissues of ethanol-fed mice. In this scenario, upregulation of adiponectin by resveratrol may be partially mediated through modulating the SIRT1/FOXO1 pathway.
Circulating adiponectin influences hepatic lipid metabolism by binding to the receptors AdipoR1 and R2. To date, very few data are available regarding the effect of chronic ethanol exposure on hepatic AdipoR1 and R2. In ethanol-fed Yucatan micropigs, hepatic AdipoR1 mRNA expression was found to be diminished (13). Our present data showed that hepatic AdipoR2 was significantly downregulated by chronic ethanol feeding in mice. AdipoR2 gene expression is upregulated by a PPARγ agonist both in vivo and in vitro (45). However, we observed that chronic ethanol feeding significantly increased the PPARγ mRNA levels in the mouse liver, suggesting that it is unlikely that the suppression of AdipoR2 mRNA in response to chronic ethanol feeding involves PPARγ. FOXO1 induces the gene expression levels of AdipoR1 and R2 (50). Our present study provides evidence that resveratrol increases gene expression of AdipoR1 and R2 in mouse liver, implying that SIRT1 signaling may be involved in regulation of AdipoR1 and R2. Indeed, SIRT1 positively regulates FOXO1 activity (16, 35). It is tempting to postulate that ethanol downregulates AdipoR1 and R2 by modulating the hepatic SIRT1-FOXO1 axis.
It is intriguing that several effects of resveratrol in mice were dramatically enhanced with the inclusion of ethanol in the diet. It appears that ethanol potentiates the beneficial effects of resveratrol in this animal model. Naturally occurring polyphenols, particularly resveratrol, have long been associated with the myriad of health benefits associated with red wine consumption in humans (33). However, it has been suggested that humans would most likely need to drink large quantities of red wine to obtain a sufficient amount of resveratrol for its biological effects to occur. Our study provides evidence for the first time to our knowledge that consuming alcohol along with concentrated resveratrol could be a more potent and efficient way of eliciting the health benefits of resveratrol.
Given that both ethanol and resveratrol have a myriad of effects in vivo, the mechanisms underlying the potentiation of resveratrol activity in the presence of ethanol remain unknown. In addition, the bioavailability and pharmacokinetics of resveratrol are highly complex. For instance, the known extremely rapid clearance rate of resveratrol from the circulation has been suggested to limit resveratrol's bioavailability and therapeutic potential in rodents and humans (14, 33). It is tempting to speculate that chronic ethanol feeding may enhance resveratrol's activity by slowing its clearance from mouse serum, thus increasing its bioavailability. The application of HPLC and gas chromatography together with mass spectrometry will be required to determine whether concentrations of plasma resveratrol or its metabolites are altered by ethanol feeding in mice. Moreover, it is important to note that the knowledge of solubility, absorption, pharmacokinetics, bioavailability, biodistribution, metabolism of resveratrol and its interaction with other dietary factors remains incomplete. Ethanol feeding may influence one or several of these processes resulting in altered resveratrol activity. The interaction of ethanol and resveratrol warrants detailed investigation in the future.
In summary, the present study suggests that resveratrol or similar activators of hepatic SIRT1-AMPK signaling system may serve as novel and promising nutritional or pharmacological therapeutic agents in treating human alcoholic fatty liver disease.
GRANTS
This study was supported by National Institute on Alcoholism and Alcohol Abuse Grants AA-015951 and AA-013623 (to M. You).
Acknowledgments
We thank Dr. Qi Cao for outstanding technical and intellectual contributions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abdelmohsen K, Pullmann R Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell 25: 543–557, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5: 493–506, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6: 298–305, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem 280: 17187–17195, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Bujanda L, García-Barcina M, Gutiérrez-de Juan V, Bidaurrazaga J, de Luco MF, Gutiérrez-Stampa M, Larzabal M, Hijona E, Sarasqueta C, Echenique-Elizondo M, Arenas JI. Effect of resveratrol on alcohol-induced mortality and liver lesions in mice. BMC Gastroenterol 6: 1–9, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chehab FF Obesity and lipodystrophy—where do the circles intersect? Endocrinology 149: 925–934, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Sebastian BM, Nagy LE. Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab 292: E621–E628, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E; CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med 4: e76, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto N, Takei Y, Hirose M, Konno A, Shibuya T, Matsuyama S, Suzuki S, Kitamura KI, Sato N. Prevention of ethanol-induced liver injury in rats by an agonist of peroxisome proliferator-activated receptor-gamma, pioglitazone. J Pharmacol Exp Ther 306: 846–854, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol 289: G54–G63, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Esfandiari F, You M, Villanueva JA, Wong DH, French SW, Halsted CH. S-adenosylmethionine attenuates hepatic lipid synthesis in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res 31: 1231–1239, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry 68: 2986–3008, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem 278: 27997–28004, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem 280: 20589–20595, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14: 661–673, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García-Villafranca J, Guillén A, Castro J. Ethanol consumption impairs regulation of fatty acid metabolism by decreasing the activity of AMP-activated protein kinase in rat liver. Biochimie 90: 460–466, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J 26: 1913–1923, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Jeong WI, Osei-Hyiaman D, Park O, Liu J, Bátkai S, Mukhopadhyay P, Horiguchi N, Harvey-White J, Marsicano G, Lutz B, Gao B, Kunos G. Paracrine activation of hepatic CB(1) receptors by stellate cell-derived endocannabinoids mediates alcoholic fatty liver. Cell Metab 7: 227–235, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology 124: 1488–1499, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol 45: 717–724, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem 280: 17038–17045, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kanfi Y, Peshti V, Gozlan YM, Rathaus M, Gil R, Cohen HY. Regulation of SIRT1 protein levels by nutrient availability. FEBS Lett 582: 2417–2423, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol 41: 236–239, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaâ S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci 80: 1033–1039, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 127: 1109–1122, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun 340: 291–295, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun 370: 44–48, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Lieber CS, Leo MA, Wang X, Decarli LM. Alcohol alters hepatic FoxO1, p53, and mitochondrial SIRT5 deacetylation function. Biochem Biophys Res Commun 373: 246–252, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Wang Y, Lam KS, Xu A. Moderate wine consumption in the prevention of metabolic syndrome and its related medical complications. Endocr Metab Immune Disord Drug Targets 8: 89–98, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Misra P AMP activated protein kinase: a next generation target for total metabolic control. Expert Opin Ther Targets 12: 91–100, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Nakae J, Cao Y, Daitoku H, Fukamizu A, Ogawa W, Yano Y, Hayashi Y. The LXXLL motif of murine forkhead transcription factor FoxO1 mediates Sirt1-dependent transcriptional activity. J Clin Invest 116: 2473–2483, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429: 771–776, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281: 39915–39924, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Qiang L, Wang H, Farmer SR. Adiponectin secretion is regulated by SIRT1 and the endoplasmic reticulum oxidoreductase Ero1-L alpha. Mol Cell Biol 27: 4698–4707, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahimian R, Masih-Khan E, Lo M, van Breemen C, McManus BM, Dubé GP. Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 224: 29–37, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, Puigserver P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA 104: 12861–12866, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song Z, Zhou Z, Deaciuc I, Chen T, McClain CJ. Inhibition of adiponectin production by homocysteine: a potential mechanism for alcoholic liver disease. Hepatology 47: 867–879, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Subauste AR, Burant CF. Role of FoxO1 in FFA-induced oxidative stress in adipocytes. Am J Physiol Endocrinol Metab 293: E159–E164, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6: 307–319, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Han R, Wang Z, Chen Y. Regulation of adiponectin receptors in hepatocytes by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Diabetologia 49: 1303–1310, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Suwa M, Nakano H, Kumagai S. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol 95: 960–968, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-α production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol 290: G998–G1007, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomita K, Azuma T, Kitamura N, Nishida J, Tamiya G, Oka A, Inokuchi S, Nishimura T, Suematsu M, Ishii H. Pioglitazone prevents alcohol-induced fatty liver in rats through up-regulation of c-Met. Gastroenterology 126: 873–885, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats. Alcohol Clin Exp Res 29: 240S–240S, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K, Kubota N, Terauchi Y, Froguel P, Nakae J, Kasuga M, Accili D, Tobe K, Ueki K, Nagai R, Kadowaki T. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279: 30817–30822, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology 23: 872–880, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Wu A, Ying Z, Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur J Neurosci 23: 2573–2580, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest 112: 91–100, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin HQ, Kim M, Kim JH, Kong G, Kang KS, Kim HL, Yoon BI, Lee MO, Lee BH. Differential gene expression and lipid metabolism in fatty liver induced by acute ethanol treatment in mice. Toxicol Appl Pharmacol 223: 225–233, 2007. [DOI] [PubMed] [Google Scholar]
- 55.You M, Fischer M, Deeg MA, Crabb DW. Ethanol induces fatty acid synthesis pathways by activation of sterol regulatory element-binding protein (SREBP). J Biol Chem 277: 29342–29347, 2002. [DOI] [PubMed] [Google Scholar]
- 56.You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology 127: 1798–1808, 2004. [DOI] [PubMed] [Google Scholar]
- 57.You M, Cao Q, Liang X, Ajmo JM, Ness GC. Mammalian sirtuin 1 is involved in the protective action of dietary saturated fat against alcoholic fatty liver in mice. J Nutr 138: 497–501, 2008. [DOI] [PubMed] [Google Scholar]
- 58.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42: 568–577, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol 294: G892–G898, 2008. [DOI] [PubMed] [Google Scholar]
- 60.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, Hou X, Jiang B, Wierzbicki M, Verbeuren TJ, Cohen RA. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes 55: 2180–2191, 2006. [DOI] [PubMed] [Google Scholar]