Abstract
Serotonin or 5-hydroxytryptamine (5-HT) influences numerous functions in the gastrointestinal tract. We previously demonstrated that 5-HT treatment of Caco-2 cells inhibited Na+/H+ exchangers (NHE) and Cl−/OH− exchange activities via distinct signaling mechanisms. Since regulation of several ion transporters such as NHE3 is influenced by intact cytoskeleton, we hypothesized that 5-HT modifies actin cytoskeleton and/or brush-border membrane architecture via involvement of signaling pathways. Ultrastructural analysis showed that 5-HT (0.1 μM, 1 h) treatment of Caco-2 cells caused the apical membrane to assume a convex dome shape that was associated with shortening of microvilli. To examine whether these cellular architecture changes are cytoskeleton driven, we analyzed actin cytoskeleton by fluorescence microscopy. 5-HT induced basal stress fibers with prominent cortical actin filaments via 5-HT3 and 5-HT4 receptor subtypes. This induction was partially attenuated by chelation of intracellular Ca2+ and PKCα inhibition (Go6976). In vitro assays revealed that PKCα interacted with actin and this association was increased by 5-HT. Our data provide novel evidence that 5-HT-induced signaling via 5-HT3/4 receptor subtypes to cause Ca2+ and PKCα-dependent regulation of actin cytoskeleton may play an important role in modulation of ion transporters that contribute to pathophysiology of diarrheal conditions associated with elevated levels of 5-HT.
Keywords: microvilli, serotonin and actin, 5-HT and curvature, stress fibers, ion transporter, cytoskeleton
serotonin (5-HT) is an important hormone and neurotransmitter that influences diverse functions in the gastrointestinal tract. High levels of 5-HT have been implicated in several diarrheal conditions associated with inflammatory bowel diseases or carcinoid syndrome (3, 5, 23, 37). We have previously shown that 5-HT inhibits Na+/H+ exchange (NHE) and Cl−/OH− exchange activities in human intestinal epithelial cells via 5-HT4 and 5-HT3/4 receptor subtypes, respectively (16, 31). The 5-HT-mediated inhibition in NHE2 and NHE3 activity occurred via a signal transduction pathway involving sequential activation of c-Src kinases, PLCγ1 and Ca2+-dependent PKCα (16). In contrast, 5-HT inhibited Cl−/OH− exchange activity via a Ca2+-independent pathway involving Src-kinase induced activation of PKCδ (31). Furthermore, recent studies have shown that 5-HT causes cytoskeletal reorganization via 5-HT4 receptors to induce pulmonary artery smooth muscle cell migration (10). However, nothing is known regarding the regulation of major cytoskeletal elements such as actin by 5-HT in intestinal epithelial cells.
A number of ion transporters such as members of NHE gene family have been shown to interact with the cytoskeleton (40). Microfilaments have been suggested to maintain basal activity of NHE3 and have also been implicated in transducing regulatory signals from receptors or hormones. For example, binding of NHE3 to the cytoskeletal binding proteins is an important factor for its inhibition by cAMP/protein kinase A-, Ca2+-, or cGMP/PKG-mediated pathways (4, 22, 24, 36, 39). Increasing evidence suggests that distinct signaling molecules interact with cytoskeleton to mediate physiological functions (14). In addition, a role for the actin cytoskeleton has emerged during clathrin- and caveolin-mediated endocytosis and exocytosis (29, 34). Our previous studies suggested that 5-HT induced inhibition of NHE activity involved Vmax changes indicating the involvement of membrane trafficking events (16). Hence, elucidating the role of 5-HT in cytoskeleton modification and/or cellular architecture warrants further investigations.
In light of 5-HT being an important signaling molecule, the aim of the present studies was to assess whether 5-HT causes modification in brush-border membrane architecture and cellular cytoskeletal proteins in the human intestinal epithelial cells. Our studies showed for the first time that 5-HT treatment of human intestinal epithelial monolayers altered the apical membrane curvature and induced prominent actin cytoskeletal reorganization. The underlying mechanisms of 5-HT-induced actin cytoskeletal reorganization required 5-HT3/4 receptor subtypes as well as Ca2+- and PKCα-dependent pathways. Additionally, PKCα was shown to interact with F-actin, and this association was significantly increased by 5-HT treatment. These studies have potential significance in increasing our understanding of 5-HT-mediated effects on intestinal ion transport processes in general and NHE3 in particular. Furthermore, identifying distinct mechanisms by which 5-HT modulates cytoskeleton or cellular architecture is crucial for defining various effects of 5-HT such as modulation of intestinal electrolyte absorption, secretion or motility.
MATERIALS AND METHODS
5-HT was obtained as creatinine sulfate complex from Sigma Chemical (St. Louis, MO). Caco-2 cells were obtained from ATCC (American Type Culture Collection, Manassas, VA). Bisindolylmaleimide (BIM), Go6976, BAPTA-AM, and rottlerin were obtained from Biomol (Plymouth Meeting, PA). 5-HT4 agonist 2-[1-(4-pieronyl)piperazinyl] benzothiazole and 5-HT3 agonist (m-chlorophenylbiguanide) were procured from Tocris (Ellisville, MO). PKC isoform antibodies were obtained from Santa Cruz (Santa Cruz, CA). Anti-actin antibody was from Sigma Chemical. All other chemicals were of at least reagent grade and were obtained from Sigma Chemical or Fisher Scientific (Pittsburgh, PA), etc.
Cell culture.
Caco-2 cells were grown routinely in 75-cm2 plastic flasks at 37°C in a 5% CO2-95% air environment. The culture medium consisted of DMEM, 20% FBS, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Cells reached confluence after 4–5 days in culture and were used for these studies between passages 25 and 55. Caco-2 cells were plated on Transwell inserts (Costar, Corning, NY) at a density of 4 × 104 cells/Transwell and used 12–14 days postplating. Cells were treated with 5-HT (0.1 μM) from either the apical side or the basolateral side for 60 min. In separate set of experiments, Caco-2 cells were pretreated with different inhibitors of PKC, Ca2+ (BAPTA-AM), in the Caco-2 medium for 1 h before the addition of 5-HT and then coincubated along with 5-HT for another 1 h as indicated in various figure legends.
Quantitative analysis of phalloidin binding.
Quantitative analysis of phalloidin binding was used to determine F-actin content as previously described (33). Cell monolayers were fixed with 3% paraformaldehyde in Hanks’ buffered saline solution (HBSS) at indicated times after 5-HT treatment. After fixation, the cell monolayer were washed twice with HBSS, permeabilized for 15 min in HBSS with 0.5% saponin (wt/vol), and incubated with 0.5 mM Alexa Fluor 488-conjugated phalloidin (Invitrogen) for 1 h at room temperature. Unbound phalloidin was removed by five washes in HBSS. Bound Alexa Fluor 488-conjugated phalloidin was then extracted quantitatively by incubation for 18 h at 4°C (in the dark) in 100% methanol. The fluorescent intensity of the extracted Alexa Fluor 488-conjugated phalloidin was measured on an Ultrospec 3300 fluorescent plate reader (Molecular Dynamics, Sunnyvale, CA).
Electron microscopy.
Caco-2 cells were grown in 35-mm tissue culture dishes, washed with phosphate-buffered saline and initially fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate (pH 7.4) buffer. The cells were postfixed with 1% osmium tetroxide and dehydrated in a series of ethanol solutions. After embedding in epoxy resin Lx112, cells were remounted on aluminum stubs for proper orientation of cutting plane (cross section and whole face). Ultramicrotome thick sections of 1 μm were stained with toluidine blue. Thin sections 87 nm were placed on 200 mesh copper grids and stained with 2% uranyl acetate and Reynolds lead citrate. The sections were examined utilizing a JEOL 1220 transmission electron JEM-1220 electron microscope (JEOL USA, Peabody, MA). Digital micrographs were taken with a Gatan micrograph charge-coupled device camera (Digital Micrograph 2.5 CCD Digital Camera/Software Gatan, Pleasanton, CA).
Immunofluorescence staining.
For staining of actin, Caco2 cells grown on Transwell inserts were washed twice in 1 × PBS containing 1 mM CaCl2, pH 7.4, and then fixed with 2% paraformaldehyde. Cells were then permeabilized with 0.08% saponin, washed and blocked in 5% normal goat serum for 30 min to 2 h at 25°C. Cells were then stained with rhodamine-phalloidin (Molecular Probes) diluted 1:60 and Hoechst 33342 (Invitrogen) for 60 min. For staining of PKCα, Caco-2 cells grown on Transwell inserts were treated with 5-HT (0.1 μM, 1 h) from either the apical or basolateral side and then incubated with anti-PKCα antibody followed by incubation with Alexa 488-conjugated goat anti-rabbit IgG, Alexa 568-conjugated phalloidin, and Hoechst 33342 for 60 min. Microscopy was performed with a Carl Zeiss LSM 510 laser scanning confocal microscope equipped with a ×63 water-immersion objective. Beams of 488 and 534 nm from an Ag-Kr laser and 361 nm from a UV laser were used for excitation. Green and red fluorescence emissions were detected through LP505 and 585 filters, respectively. The two different fluorochromes were scanned sequentially by using the multitracking function to avoid any bleedthrough among these fluorescent dyes. Series sections were taken at the z direction and orthogonal sections were made in a Z stack.
Image processing and analysis.
Images were processed postacquisition with Autodeblur 9 (AutoQuant Imaging, Watervliet, NY). For presentation of 3D projections, deconvolved stacks were imported into AutoVisualize 9 (AutoQuant Imaging) and 3D hardware-generated maximum volume projections were created by using matched settings for corresponding images. Well-oriented electron micrographs were used to quantify brush-border membrane curvature (33). To trace the shape of the brush-border membrane, the curved path between the most apical cell-cell contact sites within same cell was drawn by placing hinges at the base of each microvillus. The length ratio between the curved path and the distance between the two ends of the path were calculated. Forty segments were measured for each condition examined.
Actin spin-down assay.
Interaction of PKCα with actin was investigated utilizing commercially available actin spin-down assay kit from Cytoskeleton. Briefly, 50 μg of recombinant PKCα (rPKCα) reconstituted in 50 μl of actin compatible buffer (100 mM KCl, pH 7.0) was used for F-actin binding reactions as per instructions in the kit (Cytoskeleton). BSA (1 μM) was used as a control. After incubation of PKCα and F-actin for 30 min at room temperature, the F-actin separation was performed by centrifuging the tubes at 150,000 g for 1.5 h at 24°C. The supernatant was carefully removed and 10 μl of 5 × Laemmli reducing sample buffer was added. The pellet was resuspended in 60 μl of Laemmli reducing sample buffer. Both supernatant and pellet were run on 10% SDS-PAGE and transferred to nitrocellulose membranes for Western blot analysis using PKCα antibodies.
Immunoprecipitation and immunoblot analysis.
After treatment of Caco-2 cells with 5-HT (0.1 μM for 60 min), cells were washed with phosphate-buffered saline, and lysates were prepared in 20 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, and 1 × complete protease inhibitor mixture. The cells were homogenized by passing 10 times through a 26-gauge needle. The lysate was centrifuged at 5,000 rpm for 5 min at 4°C. The protein content of the resulting supernatant was adjusted to contain 0.5–2 mg in 200 μl of lysis buffer and incubated with the polyclonal antibody against specific isoforms PKC (2 μg) overnight at 4°C. After incubation, immune complexes were precipitated using protein G plus agarose beads. The immunoprecipitates were collected by centrifugation and washed four times with the lysis buffer. Proteins were separated by electrophoresis on 8 or 12% SDS-polyacrylamide gels and transblotted to nitrocellulose membranes. To detect association with actin, the protein-bound nitrocellulose membranes were incubated with monoclonal anti-actin antibody (1:15,000 dilution for 1 h). The blots were stripped and reprobed with specific PKC isoform antibody (1:800 dilution) to normalize for immunoprecipitated input (PKC isoforms). The membranes were washed with the wash buffer containing 1 × PBS and 0.1% Tween 20 for 15 min with agitation, during which time the wash buffer was changed every 5 min. Finally, the membranes were probed with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG antibody (1:2,000 dilution), and bands were visualized with enhanced chemiluminescence detection reagents.
Statistical analysis was performed by paired Student's two-tailed t-test. P < 0.05 was considered statistically significant.
RESULTS
5-HT alters brush-border architecture.
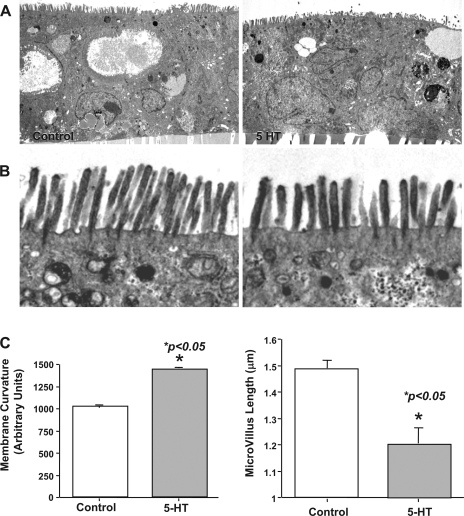
In our initial studies to examine the potential effects of 5-HT on human intestinal epithelial cell architecture, we performed ultrastructural analysis utilizing Caco-2 as an in vitro cell model. Cell monolayers grown on Transwell inserts were treated with 5-HT (0.1 μM, 1 h) and subjected to transmission electron microscopy (TEM). 5-HT treatment of Caco-2 cells caused modification in the cellular architecture that was especially apparent at the apical membrane. The brush-border membrane assumed a convex dome shape (Fig. 1A) that was associated with shortening of microvilli (Fig. 1B). The quantitative measurements of microvillus length and brush-border membrane curvature were performed by morphometric analysis. There was a significant increase (P < 0.05) in membrane curvature in response to 5-HT treatment compared with control (Fig. 1C). Furthermore, the microvillus length was decreased ∼30% in response to 5-HT treatment compared with control intestinal epithelium (Fig. 1C). These results suggested that brush-border ultrastructure is altered in human intestinal epithelial cells in response to 5-HT treatment.
Fig. 1.
Transmission electron microscopy (TEM) of control and 5-HT-treated Caco-2 cells. Caco-2 cells grown on filter inserts were treated with 5-HT (0.1 μM for 60 min) and processed for TEM as described in materials and methods. A: formation of convex curvature on the apical surface was observed in 5-HT-treated cells (right) compared with control monolayers. Magnification ×2,500. B: microvillus length was decreased in 5-HT-treated Caco 2 monolayers (left). Magnification ×8,000. C: apical membrane curvature and microvillus length were measured by morphometric analysis. Measurements were done on randomly chosen fields from 30 different images of several control and treated samples from 3 different occasions. Error bars indicate the SE of the mean values.
5-HT modifies actin cytoskeleton.
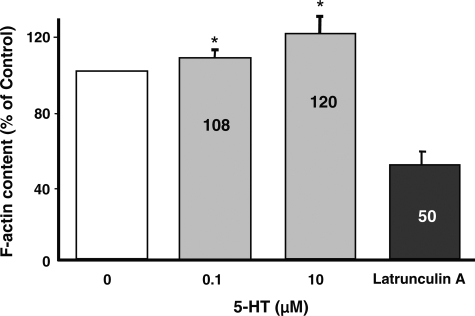
Previous studies suggest that epithelial ion transport function in intestinal epithelia may be differentially influenced by dynamic alterations in F-actin (22, 35). Also, our TEM data showed alterations in cell shape changes, which could be actin cytoskeleton driven. Therefore, to examine whether 5-HT influences actin cytoskeleton organization, F-actin content was assessed by quantitative analysis of phalloidin binding (33). Latrunculin A was used as a positive control and showed a decrease in F-actin content. Within 1 h of 5-HT treatment, phalloidin binding significantly increased with 0.1 and 10 μM 5-HT concentrations, respectively (Fig. 2). This was not due to an increase in actin synthesis, since SDS-PAGE immunoblot analyses showed no significant change in total actin content in response to 5-HT treatment (not shown). These studies indicated that 5-HT causes reorganization of the actin cytoskeleton.
Fig. 2.
F-actin content in Caco-2 cells. Quantitative analysis of phalloidin binding was used to determine F-actin content in cells treated with different concentrations of 5-HT (0.1 μM to 10 μM) for 1 h. 5-HT significantly increased F-actin content. Results are shown as means ± SE of 3 determinations. *P < 0.05 vs. control.
5-HT induces prominent basal stress fibers.
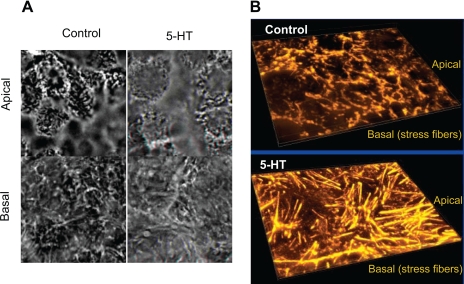
To examine how 5-HT influences F-actin assembly, morphological assessment of F-actin utilizing fluorescent microscopy was performed in control and 5-HT-treated cells. Treatment of Caco-2 monolayers with 5-HT resulted in induction of prominent basal stress fibers, which correlates with increase in actin polymerization (Fig. 3A). Examination of the apical surface indicated subtle curvature changes, which were more obvious in the TEM (Fig. 1). The basal stress fibers are clearly distinguished in the 3D projections created from the series of images of F-actin taken from apical to basolateral surface as shown in Fig. 3B.
Fig. 3.
Morphological assessment of F-actin by confocal microscopy. Control Caco-2 monolayers or treated with 5-HT were stained with phalloidin. In 5-HT-treated monolayers, a dramatic reorganization of F-actin was observed such that prominent stress fibers were induced at the basal surface (A). Images were converted on 3D projections generated using Autovisualize (B). Representative results of 5–6 different experiments performed on different occasions are shown.
5-HT sidedness and receptor subtypes mediating stress fiber assembly.
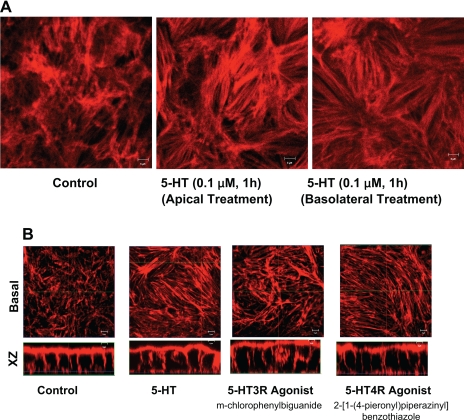
Since enterochromaffin cells release 5-HT both from the apical and basolateral side (12, 15, 30); we investigated whether sidedness plays a role in 5-HT-mediated effects on stress fiber assembly. Results as depicted in Fig. 4A demonstrated that induction in stress fiber assembly was observed when 5-HT was applied from either the apical side or the basolateral side of the Caco-2 cells. These studies correlate with our previous functional studies showing that 5-HT inhibits NHE activity when applied from either the apical or basolateral side (16).
Fig. 4.
5-HT sidedness and role of 5-HT receptor subtypes. Caco-2 cells grown on filter inserts were treated with 5-HT (0.1 μM) either from apical side or basolateral side followed by staining with phalloidin. Horizontal x-y image at the basolateral surface shows that 5-HT treatment of Caco-2 cells resulted in induction of stress fibers when applied form either the apical side or basolateral side (A). Caco-2 cells were incubated with 5-HT4 receptor agonist 2-[1-(4-pieronyl)piperazinyl]benzothiazole (10 μM) or 5-HT3 receptor-specific agonist (m-chlorophenylbiguanide) in the cell culture medium for 60 min. Both 5-HT3 and 5-HT4 agonists mimicked the effects of 5-HT in inducing stress fiber assembly (B). Data are representative of 5 different experiments performed on different occasions. Scale bars represent 10 μm.
Our previous studies demonstrated that 5-HT modulates Na+ and Cl− transport in human intestinal epithelial cells via involvement of 5-HT4 and 5-HT3/4 receptor subtypes, respectively (16, 31). Therefore, we examined which receptor subtype(s) is involved in stress fiber formation. For these studies, Caco-2 cells were treated apically with 5-HT3 and 5-HT4 receptor-specific agonists for 1 h, followed by staining with actin phalloidin. Both 5-HT3 and 4 receptor agonists mimicked the effects of 5-HT in inducing stress fibers (Fig. 4B).
Role of signal transduction pathways.
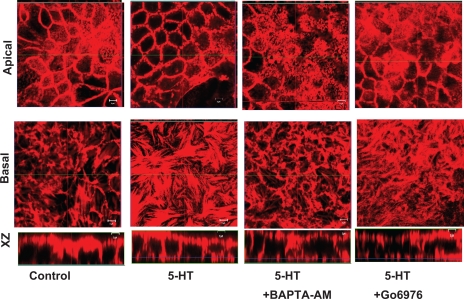
5-HT is well known to induce various signaling pathways (16, 31). Therefore, it was important to investigate the role of signal transduction pathways in 5-HT induced stress fiber reorganization. For these studies, Caco-2 cells were pretreated with specific inhibitors of signaling pathways and then treated in the absence or presence of 5-HT followed by labeling with actin phalloidin. As represented in Fig. 5, inhibition of intracellular Ca2+ by BAPTA-AM (10 μM), PKC by BIM (0.5 μM) (not shown) or conventional PKC isoforms by Go6976 partially prevented the increases in stress fiber reorganization. Also, treatment of inhibitor alone to Caco-2 cells had no effect on actin reorganization (not shown). These results indicated that 5-HT-mediated increase in Ca2+ and conventional PKC isoforms are involved in 5-HT-mediated modulation of actin cytoskeleton.
Fig. 5.
Ca2+ and conventional PKCs (cPKCs) partially mediate the effects of 5-HT. Caco-2 cells were preincubated with the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM (10 μM), or the cPKC inhibitor (Go6976, 10 nM) in the cell culture medium and then coincubated with 0.1 μM of 5-HT for another 1 h. The induction of stress fiber assembly by 5-HT was partially blocked by chelation of Ca2+ or cPKC inhibition. Data are representative of 5 different experiments performed on different occasions. Scale bars represent 5 μm.
Activation of PKCα and δ.
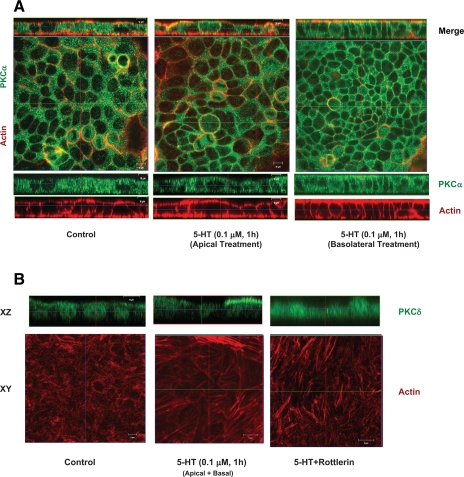
Our previous studies revealed that 5-HT activates both PKCα and PKCδ in human intestinal epithelial cells (16, 31). To ascertain the role of PKCα and δ in stress fiber reorganization, we examined the activation of both PKCα and δ isoforms in response to 5-HT treatment of Caco-2 cells by confocal microscopy. As shown in Fig. 6A, in control cells, most of PKCα staining (green) was localized to the cytoplasm. However, treatment with 5-HT either from the apical or basolateral side caused the translocation of PKCα from cytoplasm to the membrane compartments. Colocalization of PKCα (green) with actin (red) was observed in 5-HT-treated cells (Fig. 6A). Similarly, 5-HT caused activation of PKCδ as evidenced by translocation of the cytoplasmic/nuclear staining in untreated cells to the membranes in response to 5-HT. Interestingly, unlike PKCα, which was translocated to both apical and basolateral membranes, PKCδ (green) was translocated specifically to the apical membranes (Fig. 6B). We then asked whether PKCδ signaling pathway is involved in 5-HT-mediated stress fiber induction at the basal surface. However, inhibition by rottlerin (PKCδ inhibitor at 10 μM) was ineffective in preventing stress fiber formation (Fig. 6B). These results suggest that differential activation of PKCα and PKCδ to the distinct membranes might explain the involvement of PKCα but not PKCδ in the induction of basal stress fiber assembly by 5-HT in Caco-2 cells.
Fig. 6.
Activation of PKCα and PKCδ by 5-HT. A: Caco-2 cells grown on filter inserts were treated with 5-HT (0.1 μM) from either the apical side or the basolateral side followed by staining with phalloidin (red) and PKCα (green). Horizontal (x-y) and vertical (x-z) sections show mostly cytoplasmic staining of PKCα in the control cells. 5-HT treatment from both apical and basolateral sides resulted in translocation of PKCα from cytoplasm to both apical and basolateral membranes colocalizing with actin. Scale bars represent 10 μm. B: Caco-2 cells grown on filter inserts were pretreated with specific PKCδ inhibitor (rottlerin) and then treated with 5-HT (0.1 μM) both from the apical and basolateral sides followed by staining with phalloidin actin (red) and PKCδ (green) (B). Horizontal (x-y) images show basal stress fibers in control and 5-HT-treated cells in the absence or presence of rottlerin. The x-z sections show corresponding translocation of PKCδ upon activation. Although most of the staining of PKCδ is noted in the nuclear region in control cells, upon activation by 5-HT, PKCδ is seen translocated preferentially to the apical membranes. However, 5-HT-mediated induction of basal stress fibers remained unaltered in the presence of rottlerin. Scale bars represent 10 μm.
In vitro interaction of PKCα with F-actin.
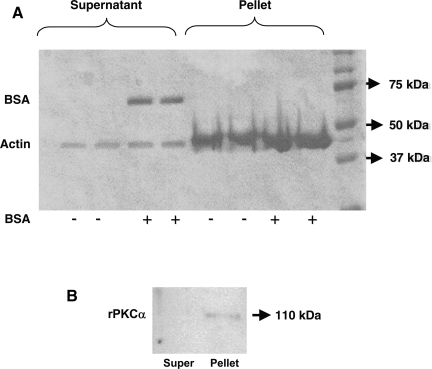
PKC isoforms are also known to be associated with a range of cytoskeletal components. Previous studies have shown that PKCδ binds to F-actin through its C2 domain (26). However, the direct interaction of PKCα with actin has not been reported. As a direct evidence of PKCα interaction with F-actin, an in vitro actin spin-down assay was performed utilizing recombinant PKCα. In this assay, F-actin binding proteins cosediment with actin filaments and form a pellet at the bottom of the centrifugation tube, whereas F-actin severing proteins, G-actin binding proteins, or non-actin-binding proteins remain in the supernatant. Bovine serum albumin (BSA) was used as a negative control (non-actin-binding protein) and was only found in the supernatant fraction (Fig. 7A). Our results as shown in Fig. 7B suggested that rPKCα was detected mostly in the pelleted fractions, indicating its association with actin under basal conditions.
Fig. 7.
Involvement of PKCα in 5-HT-mediated effects and interaction with actin cytoskeleton. In vitro actin spin-down assay was performed to assess interaction of PKCα with actin. Coomassie blue-stained gel showing that BSA is detected in the supernatant (non-actin-binding) fraction but not in the pelleted (actin binding) (A). Binding of recombinant PKCα (rPKCα) (50 μg) with F-actin was performed in vitro and the mixture was spun at 59,000 g for 1.5 h. Pelleted and supernatant fractions were subjected to SDS-PAGE followed by Western blotting with anti-PKC antibodies. Recombinant PKCα was detected in pelleted fraction indicating its association with F-actin (B).
5-HT increases association of PKCα with actin.
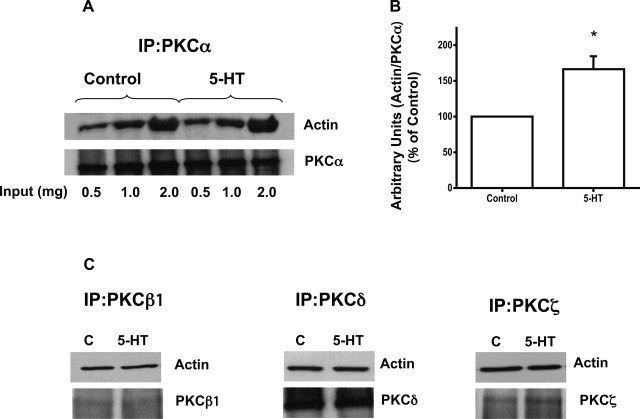
To gain further insight into mechanisms of 5-HT induced stress fiber assembly formation, coimmunoprecipitation studies were performed. Lysates from control and 5-HT-treated cells (0.5, 1.0, and 2 mg protein) were immunoprecipitated with different PKC isoform antibodies and immunoblotted with antibodies against actin. Figure 8A shows that 5-HT treatment increased the association of PKCα with actin. Densitometric analysis of actin association by normalizing to the PKCα values showed that 5-HT treatment increased the association of PKCα with actin by ∼1.8 fold (Fig. 8B). The interaction of PKCδ, PKCζ, or PKCβ1, however, was not altered (Fig. 8C). These studies suggest that 5-HT-mediated increased PKCα interactions with actin may be involved in modulation of the basal stress fiber assembly in Caco-2 cells.
Fig. 8.
5-HT increases association of PKCα with actin. Varying concentrations of protein lysates (0.5 mg-2.0 mg protein) from the control and 5-HT-treated cells (0.1 μM, 1 h) were immunoprecipitated (IP) with PKC-specific antibodies or control IgG and the immunoprecipitates were blotted with anti-actin and anti-PKC specific isoform antibodies. Increased association of PKCα was seen with actin (A). Densitometric analysis showing association as arbitrary units of actin normalized to PKCα (B). Coimmunoprecipitation assays utilizing different PKC isoform antibodies with actin are shown (C). Representative blots from 3–5 different experiments are shown. *P < 0.005 vs. control.
DISCUSSION
5-HT has been shown to exhibit numerous effects on mammalian physiology in both health and disease. In the gut, 5-HT plays an important role in the regulation of the gut motility; absorption of sugars, amino acids, and electrolytes; and fluid transport via specific 5-HT receptor subtypes (2, 3, 16, 21, 28, 31, 32). We previously suggested that 5-HT-mediated inhibition of NHE3 and Cl−/OH− exchange activities might contribute to the pathophysiology of diarrheal diseases associated with 5-HT (16, 31). Since actin cytoskeleton influences the optimal function of many transporters including NHE3 (22), we investigated whether 5-HT is capable of modulating actin cytoskeleton and brush-border membrane architecture. Our present studies provide novel data that 5-HT treatment of human intestinal Caco-2 cells caused alterations in brush-border membrane architecture and actin cytoskeleton. We also show that 5-HT induced Ca2+ and PKCα signaling pathway mediates the actin cytoskeleton reorganization via the participation of both 5-HT3 and 5-HT 4 receptor subtypes. Also, the effects of 5-HT on induction of stress fibers were observed when applied from either the apical or basolateral side.
Ultrastructural analysis by TEM to understand the plasma membrane architecture revealed that 5-HT-treated cells displayed a small but significant decrease in microvillus length. Microvilli are actin-rich membrane protrusions present in all sensory and transporting epithelial cell types. The apical microvillus surface of the intestinal epithelial cell is supported by an extensive cytoskeletal apparatus composed of actin filaments and associated binding proteins. Interestingly, our studies showed that total F-actin content in 5-HT-treated cells was increased correlating with increase in cortical actin and induction of basal stress fiber reorganization. In this regard, previous studies have shown NHE3 activity to be inhibited by actin-disrupting agents such as latrunculin A and cytochalasin B as well F-actin stabilizing agents such as jasplakinolide (22). It was implied from these studies that NHE3 activity is not a simple function of the cellular F-actin content but an intact F-actin network is required for optimal NHE3 function. Therefore, the induction of basal stress fiber assembly by 5-HT might underlie the 5-HT-mediated effects on intestinal ion transport processes such as inhibition of NHE3 or Cl−/OH− exchangers. Furthermore, our results showed for the first time that 5-HT treatment of intestinal epithelial cells promoted convex dome shape of the brush-border membrane. Interestingly, recent studies have shown that changes in plasma membrane curvature (convex shaped) resulting from hypoosmolar exposure caused stimulation of NHE3 isoform activity (1). In contrast, our data with TEM suggest that 5-HT induced convex dome shape may be associated with inhibition of NHE3 (16). Therefore, cell volume changes are not likely involved in the observed membrane curvature alterations and inhibition of NHE3 by 5-HT. It is more likely that convex deformation of the apical membrane per se does not regulate the NHE3 activity but is a critical marker of cytoskeletal reorganization that impacts association of NHE3 with other plasma membrane proteins or accessory factors resulting in either stimulation as shown previously (1) or inhibition of NHE3 activity as implied in present studies. Thus our studies suggest that redistribution of F-actin from microvilli and the cortical ring to basal stress fibers may imply a more global change in cellular function. Moreover, PKCα activation is closely linked to both actin reorganization and NHE3 inhibition. We and others have clearly shown that PKCα activation can downregulate NHE3 activity by multiple mechanisms (8, 16, 24, 25).
Whether cytoskeleton plays a role in 5-HT induced modification of membrane architecture is not known. Interestingly, morphological assessment of F-actin utilizing fluorescent microscopy revealed that basal stress fibers were induced in response to 5-HT treatment. Previous studies from our laboratory have shown that 5-HT induces tyrosine phosphorylation that activates downstream targets such as PLCγ1 and PKCδ (16, 31). PLCγ1 activation was further shown to increase intracellular Ca2+ and activate the PKCα isoform in Caco-2 cells (16). Our present studies revealed that both Ca2+ and PKCα were partially involved in 5-HT-mediated effects on actin cytoskeleton reorganization. Functional involvement of PKC in this process appears to be isoform selective as PKCδ was not involved in stress fiber formation by 5-HT.
PKC isoforms are known to be associated with a wide range of cytoskeletal elements (7, 20). For example, PKCδ directly binds F-actin through its C2 domain, and these interactions were shown to be important in regulating actin redistribution in neutrophils (26). PKCα has been implicated in actin remodeling during cell motility, phagocytosis, and neurite overgrowth as well as acting as a ligand and substrate for actin-associated proteins such as filamin (19, 38). However, a direct interaction of PKCα with actin has not been defined. In present studies, analysis of interaction between recombinant PKCα and actin was done via an in vitro actin spin-down assay. Our data showed that majority of recombinant PKCα was found to bind to F-actin. Interestingly, immunoprecipitation assays suggested that 5-HT specifically increased the association of PKCα (but not other PKC isoforms) with actin. Therefore, although both PKCα and PKCδ are activated in response to 5-HT treatment; however, only PKCα was partially involved in inducing stress fiber formation. These findings highlight the complexity of signaling pathways and actin interactions that underlie the molecular events leading to stress fiber formation. In this regard, previous studies suggest that the subcellular localization of inactive PKC isoforms and their dynamic translocation to other subcellular compartments upon activation might determine the specificity of regulation of various biological processes as well as define a unique physiological role for each PKC isoform (6, 11, 17, 27). Our studies, for the first time, demonstrated differences in localization of PKCα and δ isoforms to the antipodal membranes of Caco-2 cells in response to 5-HT treatment. Notably, PKCδ was only translocated to the apical membranes, whereas PKCα was observed on both apical and basolateral membranes upon activation by 5-HT. Previous studies of Song et al. (35) demonstrated that PKCα and PKCɛ translocate to the basal but not apical membranes upon activation. This difference in PKCα translocation may represent differences in the epithelial cell type studied. Song et al. utilized T84 cells to demonstrate that activation of PKCα antagonized the ability of PKCɛ to disassemble F-actin and stimulate basolateral membrane endocytosis.
The membrane-associated F-actin cytoskeleton has widely been proposed as the regulatory site of membrane trafficking events (29). Our previous studies suggested that 5-HT might be involved in modulating endocytic/exocytic processes since alterations in Vmax values of NHE activity were observed in response to 5-HT treatment (16). Similarly, membrane deformation is known to be essential for various cellular processes such as cell division, locomotion, organelle biogenesis, and creation of the high-curvature vesicles and tubules that mediate cargo transport along the endocytic and exocytic pathways (18). The observed decrease in microvillus length along with deformation in membrane curvature in response to 5-HT might suggest initiation of membrane trafficking events such as endocytosis (9, 13) that might underlie effects of 5-HT on ion transporters in human intestinal epithelial cells. In this regard, our previously published studies showed that 5-HT (0.1 μm for 1 h) inhibited the activities of apical ion exchangers including NHE2, NHE3, and Cl−/OH− exchange but did not affect the activities of the apical sodium-dependent bile acid transporter or d-glucose uptake. Our studies also showed that the basolaterally expressed NHE1 isoform was not affected by 5-HT. Furthermore, 5-HT was shown to inhibit NHE and Cl−/OH− exchange activities via distinct signaling pathways (16, 31), indicating that the effects of 5-HT are through specific pathways. Our present findings showed that 5-HT influences actin cytoskeleton via Ca2+ and PKCα mediated signaling pathways, which are also involved in inhibition of NHE3 but not of Cl−/OH− exchange activity by 5-HT. These studies indicate potential mechanisms by which activation of 5-HT-mediated specific signaling mechanisms may trigger distinct physiological responses, some of which may be partly related to downstream events such as modification of cellular cytoskeleton.
In summary, our results demonstrated that 5-HT treatment of Caco-2 cells induces alterations in brush-border membrane architecture and basal stress fiber reorganization. Actin cytoskeletal reorganization occurred via 5-HT3/4 receptor subtypes and was partially dependent on Ca2+ and PKCα-dependent pathways. Further studies are warranted to directly link the basal stress fiber assembly formation with the apical membrane curvature changes that might underlie the pathophysiology of irritable colon or diarrheal conditions associated with elevated levels of 5-HT.
GRANTS
These studies were supported by the Department of Veterans Affairs and the National Institute of Diabetes and Digestive and Kidney Diseases Grants P01 DK-067887 (P. K. Dudeja, K. Ramaswamy, J. R. Turner), DK-54016 (P. K. Dudeja), DK-33349 (K. Ramaswamy) DK-09930 (W. A. Alrefai) and DK-074459 (R. K. Gill).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexander RT, Malevanets A, Durkan AM, Kocinsky HS, Aronson PS, Orlowski J, Grinstein S. Membrane curvature alters the activation kinetics of the epithelial Na+/H+ exchanger, NHE3. J Biol Chem 282: 7376–7384, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Arruebo MP, Mesonero JE, Murillo MD, Alcalde AI. Effect of serotonin on d-galactose transport across the rabbit jejunum. Reprod Nutr Dev 29: 441–448, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Bjorck S, Ahlman H, Dahlstrom A, Phillips SF, Kelly KA. Serotonergic regulation of canine enteric motility (measured as electrical activity) and absorption: physiologic and morphologic evidence. Acta Physiol Scand 133: 247–256, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem 280: 16642–16650, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Challacombe DN, Dawkins PD, Baker P. Increased tissue concentrations of 5-hydroxytryptamine in the duodenal mucosa of patients with coeliac disease. Gut 18: 882–886, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen ZZ, McGuire JC, Leach KL, Cambier JC. Transmembrane signaling through B cell MHC class II molecules: anti-Ia antibodies induce protein kinase C translocation to the nuclear fraction. J Immunol 138: 2345–2352, 1987. [PubMed] [Google Scholar]
- 7.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 268: 233–239, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cory GO, Cullen PJ. Membrane curvature: the power of bananas, zeppelins and boomerangs. Curr Biol 17: R455–R457, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Day RM, Agyeman AS, Segel MJ, Chevere RD, Angelosanto JM, Suzuki YJ, Fanburg BL. Serotonin induces pulmonary artery smooth muscle cell migration. Biochem Pharmacol 71: 386–397, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekker LV, Palmer RH, Parker PJ. The protein kinase C and protein kinase C related gene families. Curr Opin Struct Biol 5: 396–402, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara A, Zinner MJ, Jaffe BM. Intraluminal release of serotonin, substance P, and gastrin in the canine small intestine. Dig Dis Sci 32: 289–294, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature 419: 361–366, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Forgacs G, Yook SH, Janmey PA, Jeong H, Burd CG. Role of the cytoskeleton in signaling networks. J Cell Sci 117: 2769–2775, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Fujimiya M, Okumiya K, Kuwahara A. Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol 108: 105–113, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Gill RK, Saksena S, Tyagi S, Alrefai WA, Malakooti J, Sarwar Z, Turner JR, Ramaswamy K, Dudeja PK. Serotonin inhibits Na+/H+ exchange activity via 5-HT4 receptors and activation of PKCα in human intestinal epithelial cells. Gastroenterology 128: 962–974, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Halsey DL, Girard PR, Kuo JF, Blackshear PJ. Protein kinase C in fibroblasts. Characteristics of its intracellular location during growth and after exposure to phorbol esters and other mitogens. J Biol Chem 262: 2234–2243, 1987. [PubMed] [Google Scholar]
- 18.Holthuis JCM Regulating membrane curvature. In: Regulatory Mechanisms of Intracellular Membrane, edited by Keränen S and Jäntti J. Berlin: Springer, 2004, p. 39–64.
- 19.Hryciw DH, Pollock CA, Poronnik P. PKC-α-mediated remodeling of the actin cytoskeleton is involved in constitutive albumin uptake by proximal tubule cells. Am J Physiol Renal Physiol 288: F1227–F1235, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Keenan C, Kelleher D. Protein kinase C and the cytoskeleton. Cell Signal 10: 225–232, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kellum JM, Budhoo MR, Siriwardena AK, Smith EP, Jebraili SA. Serotonin induces Cl− secretion in human jejunal mucosa in vitro via a nonneural pathway at a 5-HT4 receptor. Am J Physiol Gastrointest Liver Physiol 267: G357–G363, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Kurashima K, D'Souza S, Szaszi K, Ramjeesingh R, Orlowski J, Grinstein S. The apical Na+/H+ exchanger isoform NHE3 is regulated by the actin cytoskeleton. J Biol Chem 274: 29843–29849, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Kyosola K, Penttila O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol 12: 363–367, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKCα which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol 285: C1527–C1536, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Zhang H, Cheong A, Leu S, Chen Y, Elowsky CG, Donowitz M. Carbachol regulation of rabbit ileal brush border Na+-H+ exchanger 3 (NHE3) occurs through changes in NHE3 trafficking and complex formation and is Src dependent. J Physiol 556: 791–804, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Lluch G, Bird MM, Canas B, Godovac-Zimmerman J, Ridley A, Segal AW, Dekker LV. Protein kinase C-delta C2-like domain is a binding site for actin and enables actin redistribution in neutrophils. Biochem J 357: 39–47, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masmoudi A, Labourdette G, Mersel M, Huang FL, Huang KP, Vincendon G, Malviya AN. Protein kinase C located in rat liver nuclei. Partial purification and biochemical and immunochemical characterization. J Biol Chem 264: 1172–1179, 1989. [PubMed] [Google Scholar]
- 28.Mesonero JE, Arruebo MP, Alcalde AI. Study of the inhibitory effect of serotonin on sugar intestinal transport. Rev Esp Fisiol 46: 309–310, 1990. [PubMed] [Google Scholar]
- 29.Noda Y, Sasaki S. The role of actin remodeling in the trafficking of intracellular vesicles, transporters, and channels: focusing on aquaporin-2. Pflügers Arch 456: 737–745, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Racke K, Schworer H. Regulation of serotonin release from the intestinal mucosa. Pharmacol Res 23: 13–25, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Saksena S, Gill RK, Tyagi S, Alrefai WA, Sarwar Z, Ramaswamy K, Dudeja PK. Involvement of c-Src and protein kinase C delta in the inhibition of Cl−/OH− exchange activity in Caco-2 cells by serotonin. J Biol Chem 280: 11859–11868, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Salvador MT, Rodriguez-Yoldi MC, Alcalde AI, Rodriguez-Yoldi MJ. 5-HT receptor subtypes involved in the serotonin-induced inhibition of l-leucine absorption in rabbit jejunum. Life Sci 61: 309–318, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119: 2095–2106, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song JC, Rangachari PK, Matthews JB. Opposing effects of PKCalpha and PKCepsilon on basolateral membrane dynamics in intestinal epithelia. Am J Physiol Cell Physiol 283: C1548–C1556, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Szaszi K, Kurashima K, Kaibuchi K, Grinstein S, Orlowski J. Role of the cytoskeleton in mediating cAMP-dependent protein kinase inhibition of the epithelial Na+/H+ exchanger NHE3. J Biol Chem 276: 40761–40768, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JH Serotonin and the alimentary tract. Res Commun Chem Pathol Pharmacol 2: 687–781, 1971. [PubMed] [Google Scholar]
- 38.Tigges U, Koch B, Wissing J, Jockusch BM, Ziegler WH. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase Cα. J Biol Chem 278: 23561–23569, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Weinman EJ, Steplock D, Donowitz M, Shenolikar S. NHERF associations with sodium-hydrogen exchanger isoform 3 (NHE3) and ezrin are essential for cAMP-mediated phosphorylation and inhibition of NHE3. Biochemistry 39: 6123–6129, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. [DOI] [PubMed] [Google Scholar]