Abstract
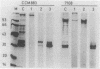
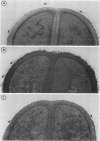
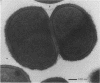
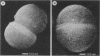
A 95-kDa protein was isolated from Staphylococcus saprophyticus 7108 grown on dialysis membranes placed on the surface of brain heart infusion agar. Strain CCM883 did not produce this protein. Ultrathin sections revealed the presence of very thin, tuftlike, 50- to 75-nm-long structures on the surface of strain 7108, whereas strain CCM883 was comparably smooth. The surface material could be removed by digestion with proteinase K, suggesting that the surface structures contain protein. High-resolution scanning electron microscopy showed a thick layer of surface material on strain 7108, whereas strain CCM883 appeared smooth. The 95-kDa protein was purified by Sephacryl S-300 chromatography, and an antiserum was raised in rabbits. This antiserum was used in immunogold labeling experiments, which showed that the protein is associated with the surface structures. Our experiments thus demonstrate the presence of a fibrillar protein on the surface of S. saprophyticus (Ssp for S. saprophyticus surface-associated protein).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almeida R. J., Jorgensen J. H. Comparison of adherence and urine growth rate properties of Staphylococcus saprophyticus and Staphylococcus epidermidis. Eur J Clin Microbiol. 1984 Dec;3(6):542–545. doi: 10.1007/BF02013615. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Simpson W. A., Ofek I., Hasty D. L., Dale J. B., Whitnack E. Attachment of Streptococcus pyogenes to mammalian cells. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S670–S677. doi: 10.1093/clinids/5.supplement_4.s670. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheung A. L., Fischetti V. A. Variation in the expression of cell wall proteins of Staphylococcus aureus grown on solid and liquid media. Infect Immun. 1988 May;56(5):1061–1065. doi: 10.1128/iai.56.5.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espersen F., Clemmensen I. Isolation of a fibronectin-binding protein from Staphylococcus aureus. Infect Immun. 1982 Aug;37(2):526–531. doi: 10.1128/iai.37.2.526-531.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein extracted by nonionic detergent. I. Properties of the antiphagocytic and type-specific molecules. J Exp Med. 1976 Jul 1;144(1):32–53. doi: 10.1084/jem.144.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989 Jul;2(3):285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli D., Wirth R., Wanner G. Identification of aggregation substances of Enterococcus faecalis cells after induction by sex pheromones. An immunological and ultrastructural investigation. Arch Microbiol. 1989;151(6):486–490. doi: 10.1007/BF00454863. [DOI] [PubMed] [Google Scholar]
- Gatermann S., John J., Marre R. Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect Immun. 1989 Jan;57(1):110–116. doi: 10.1128/iai.57.1.110-116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Marre R. Cloning and expression of Staphylococcus saprophyticus urease gene sequences in Staphylococcus carnosus and contribution of the enzyme to virulence. Infect Immun. 1989 Oct;57(10):2998–3002. doi: 10.1128/iai.57.10.2998-3002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatermann S., Marre R., Heesemann J., Henkel W. Hemagglutinating and adherence properties of Staphylococcus saprophyticus: epidemiology and virulence in experimental urinary tract infection of rats. FEMS Microbiol Immunol. 1988 Dec;1(3):179–185. doi: 10.1111/j.1574-6968.1988.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Gunnarsson A., Mårdh P. A., Lundblad A., Svensson S. Oligosaccharide structures mediating agglutination of sheep erythrocytes by Staphylococcus saprophyticus. Infect Immun. 1984 Jul;45(1):41–46. doi: 10.1128/iai.45.1.41-46.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley P. S., Carter P. L., Fielding J. Streptococcus salivarius strains carry either fibrils or fimbriae on the cell surface. J Bacteriol. 1984 Jan;157(1):64–72. doi: 10.1128/jb.157.1.64-72.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm E., Lundell-Etherden I. Slime production by Staphylococcus saprophyticus. Infect Immun. 1991 Jan;59(1):445–448. doi: 10.1128/iai.59.1.445-448.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Haemagglutination by Staphylococcus saprophyticus and other staphylococcal species. Acta Pathol Microbiol Scand B. 1979 Feb;87B(1):45–50. doi: 10.1111/j.1699-0463.1979.tb02401.x. [DOI] [PubMed] [Google Scholar]
- Hovelius B., Mårdh P. A. Staphylococcus saprophyticus as a common cause of urinary tract infections. Rev Infect Dis. 1984 May-Jun;6(3):328–337. doi: 10.1093/clinids/6.3.328. [DOI] [PubMed] [Google Scholar]
- Hussain M., Hastings J. G., White P. J. Isolation and composition of the extracellular slime made by coagulase-negative staphylococci in a chemically defined medium. J Infect Dis. 1991 Mar;163(3):534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- Johnson J. R. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991 Jan;4(1):80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambe D. W., Jr, Ferguson K. P., Keplinger J. L., Gemmell C. G., Kalbfleisch J. H. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can J Microbiol. 1990 Jul;36(7):455–463. doi: 10.1139/m90-080. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Jonsson P., Mårdh P. A. Hemagglutination by Staphylococcus aureus. Studies on strains isolated from bovine mastitis. APMIS. 1989 Feb;97(2):175–180. [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Teti G., Chiofalo M. S., Tomasello F., Fava C., Mastroeni P. Mediation of Staphylococcus saprophyticus adherence to uroepithelial cells by lipoteichoic acid. Infect Immun. 1987 Mar;55(3):839–842. doi: 10.1128/iai.55.3.839-842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallmark G., Arremark I., Telander B. Staphylococcus saprophyticus: a frequent cause of acute urinary tract infection among female outpatients. J Infect Dis. 1978 Dec;138(6):791–797. doi: 10.1093/infdis/138.6.791. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., Jacobs T. Cell wall-associated protein antigens of Streptococcus salivarius: purification, properties, and function in adherence. Infect Immun. 1982 Oct;38(1):233–242. doi: 10.1128/iai.38.1.233-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]