Abstract
Pulmonary vascular remodeling, a major cause for the elevated pulmonary vascular resistance in patients with pulmonary arterial hypertension (PAH), is partially due to increased proliferation of pulmonary arterial smooth muscle cells (PASMC) in the media, resulting in vascular wall thickening. Platelet-derived growth factor (PDGF) is a potent mitogen that may be involved in the progression of PAH. Blockade of PDGF receptors has been demonstrated to have therapeutic potential for patients with severe pulmonary hypertension. Prednisolone is an immunosuppressant shown to have anti-inflammatory and antiproliferative effects on PASMC. This study was designed to investigate whether PDGF and prednisolone affect human PASMC proliferation by regulating the nuclear translocation of NF-κB (a transcription factor composed of 2 subunits, p50 and p65). Treatment of human PASMC with PDGF (10 ng/ml) significantly increased nuclear translocation of p50 and p65 subunits. Inhibition of NF-κB activation or nuclear translocation of p50/p65 significantly attenuated PDGF-induced PASMC proliferation (determined by [3H]thymidine incorporation). In the presence of prednisolone (200 μM), the PDGF-induced nuclear translocation of p50 and p65 subunits was markedly inhibited (P < 0.05 vs. the cells treated with PDGF alone). These results indicate that PDGF-induced nuclear translocation of NF-κB may play an important role in stimulating PASMC proliferation (and/or enhancing PASMC survival), whereas prednisolone may exert anti-inflammatory and antiproliferative effects on PASMC by inhibiting NF-κB nuclear translocation.
Keywords: proliferation, pulmonary arterial hypertension, immunosuppressant, therapy
pulmonary vascular remodeling is a major contributor to the elevated pulmonary vascular resistance in patients with pulmonary arterial hypertension. Increased growth factors (e.g., PDGF) and enhanced inflammatory responses (characterized with cytokines and chemokines that can stimulate or activate NF-κB) have recently been demonstrated to be important causes for the development of pulmonary vascular remodeling in patients with idiopathic pulmonary arterial hypertension (IPAH) and chronic thromboembolic pulmonary hypertension (26, 29). Prednisolone is an anti-inflammatory and immunosuppressive drug widely used for treatment of bronchial asthma, chronic obstructive pulmonary disease, autoimmune disease, and leukemia (2, 6, 17, 23, 28). Prednisolone is also routinely used postoperatively in patients with IPAH who have undergone lung transplantation (24, 42). The use of immunosuppressants to treat pulmonary hypertension has shown promising beneficial effects in some patients (4, 19). In addition to its immunosuppressive effects, prednisolone has been shown to inhibit PDGF-stimulated proliferation of pulmonary artery smooth muscle cells (PASMC) from patients with IPAH (26). The antiproliferative effect of prednisolone is believed to be due, in part, to increased expression of p27, an inhibitor of cyclin-dependent kinase (26).
Our laboratory has previously demonstrated that PDGF can stimulate PASMC proliferation by upregulation of canonical transient receptor potential channels (TRPC) and enhancement of receptor-mediated increase in cytosolic Ca2+ concentration ([Ca2+]cyt) via activation of STAT3 and c-Jun (37, 40). Furthermore, upregulation of TRPC channels and cell proliferation in human PASMC by the inflammatory mediator, tumor necrosis factor (TNF)-α, was shown to involve nuclear translocation of NF-κB, a transcription factor known to exert antiapoptotic effects (38, 39).
Since excessive proliferation of PASMC is a contributory factor to pulmonary vascular remodeling in patients with pulmonary arterial hypertension, it is plausible that immunoresponsive pathways suppressed by prednisolone contribute to this response. Indeed, this may involve inhibition of PDGF-mediated proliferative effects and attenuation of NF-κB nuclear translocation. Therefore, the aim of this study was to test the hypothesis that interaction with PDGF-mediated nuclear transduction of NF-κB is one of the pathways by which prednisolone inhibits PASMC proliferation.
MATERIALS AND METHODS
Cell culture.
Human PASMC from normal subjects were purchased from Cambrex (East Rutherford, NJ) and cultured in a humidified 5% CO2 atmosphere at 37°C in DMEM (Invitrogen, Carlsbad, CA) with 10% FBS. After reaching 70% confluency, the cells were subcultured following trypsin/EDTA (0.05%/0.02%) treatment. For experiments, PASMC were grown in 10% FBS containing DMEM to ∼80% confluence. Then, on day 1, the culture media were replaced with low-serum culture media (DMEM, 0.1% FBS), and the cells were cultured for another 48 h. On day 3, PDGF-BB (10 ng/ml; Sigma, St. Louis, MO) with or without prednisolone (200 μM, Sigma) was added to the cultures for 24 h. For experiments testing the effect of NF-κB inhibitors on PDGF-mediated PASMC proliferation, tyrrolidine dithiocarbamate (PDTC; 50 μM, Sigma) or 4-methyl-N1-(3-phenylpropyl)benzene-1,2-diamine (JSH-23; 20 μM, EMD Chemicals, Gibbstown, NJ) was added to the media for 24 h on day 3. For experiments involving TNF-α (Biomol International), cells were treated with 30 ng/ml for 30 min. In some experiments, PASMC were prepared from transplant lung tissues. Human lung tissues were retrieved after approval from the Human Ethics Committee and the Institutional Review Board of the University of California, San Diego was obtained. Documented written informed consent was obtained from all patients before the procedure. PASMC were isolated using enzymatic dissociation from pulmonary arteries isolated from both IPAH patients (tissues obtained at lung transplantation) and from control patients (non-IPAH). Peripheral arterial segments (<1 mm in outer diameter) were used. Isolated cells were cultured in smooth muscle growth media (Cambrex) containing 10% FBS and 0.1 mg/ml kanamycin in a humidified 5% CO2 atmosphere at 37°C.
Western blot analysis.
The cells were gently washed twice in cold PBS, scraped into 0.3 ml of lysis buffer [1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 100 μg/ml phenylmethylsulfonyl fluoride, and protease inhibitors], and incubated for 30 min on ice. The cell lysates were then sonicated and centrifuged at 12,000 rpm for 10 min, and the insoluble fraction was discarded. Cytoplasmic and nuclear extraction was performed using the protocol by Jobin et al. (16). The cytoplasmic fraction was extracted in cytoplasmic extraction buffer (10 mM Tris·HCl, 60 mM KCl, 1 mM EDTA, 0.4% NP-40, 1 mM dithiothreitol, and protease inhibitors) and concentrated by centrifugation at 2,500 rpm for 3 min. Pellets were resuspended in nuclear extraction buffer (50 mM Tris·HCl, 420 mM NaCl, 1.5 mM MgCl2, 25% glycerol, and protease inhibitors), incubated for 10 min on ice, and then concentrated by centrifugation at 12,000 rpm for 10 min. Protein concentrations were determined by DC Protein assay (Bio-Rad Laboratories, Hercules, CA) using BSA as a standard. For experiments, protein suspensions were electrophoretically separated on SDS-PAGE gels (4–20% or 10%), and protein bands were transferred onto nitrocellulose membranes by electroblot. Blots were incubated overnight at 4°C with primary antibodies including anti-NF-κB (p65 and p50; Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-p44/42 MAP kinase (Cell Signaling Technology, Danvers, MA), anti-TRPC1, TRPC3, and TRPC6 (Alomone Labs). The primary antibodies were detected by either donkey anti-rabbit or donkey anti-mouse secondary antibodies conjugated to horseradish peroxidase. Blots were developed using the SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology, Rockford, IL). Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Millipore, Billerica, MA) or anti-histone H2A + H4 antibody (Abcam, Cambridge, MA) was used to normalize for loading and transfer artifacts.
Immunofluorescence staining.
PASMC on 25-mm cover slips were fixed in 4% paraformaldehyde in PBS for 10 min and then washed 3× in PBS. They were blocked in PBS containing 2% BSA, 0.1% Triton X-100, and FBS. Cells were exposed to rabbit anti-p65 antibody or mouse anti-p50 antibody for 2 h, washed 3×10 min in PBS, and incubated for 45 min at room temperature with secondary antibodies diluted in blocker, including Alexa Fluor 594 goat anti-rabbit IgG or Alexa Fluor 488 goat anti-mouse IgG (Invitrogen). Cells were incubated in DAPI (5 μM, Invitrogen) staining solution for 5 min and washed in PBS before being mounted in anti-fade mounting solution. The imaging was performed on a Leica DMRB microscope and captured using a Leica DFC300 camera (Leica Microsystems, Bannockburn, IL) and Openlab software (Improvision). The staining intensity of the p65 and p50 was quantified by using Image J software (National Institutes of Health, Bethesda, MD).
Measurement of [Ca2+]cyt.
Cells on 25-mm cover slips were placed in a recording chamber on the stage of an inverted Nikon microscope (Eclipse/TE 200) with the TE-FM epifluorescence attachment. Cytoplasmic Ca2+ ([Ca2+]cyt) in single PASMC was measured using the membrane-permeable, Ca2+-sensitive fluorescent indicator fura-2-AM (Invitrogen). Cells were loaded at room temperature (22–24°C) for 30 min using a Modified Krebs Solution (MKS) containing 4 μM fura-2-AM. The loaded cells were then washed with MKS for 30 min to remove excess extracellular dye and allow intracellular esterases to cleave cytosolic fura-2-AM into active fura-2. Fura-2 fluorescence was detected as 510-nm-wavelength light emission with excitation wavelengths of 340 and 380 nm by use of the digital fluorescence imaging system from Intracellular Imaging (Cincinnati, OH). In all experiments, multiple cells were imaged in a single field, and one arbitrarily chosen peripheral cytosolic area from each cell was spatially averaged. [Ca2+]cyt was expressed as fura-2 fluorescence emission ratio excited at 340 and 380 nm (F/F0).
RT-PCR.
Total RNA was isolated from cultured PASMC using TRIzol (Invitrogen) method. One microgram of RNA was treated with DNase I (Invitrogen) before being reverse transcribed to cDNA using Superscript II reverse transcriptase (Invitrogen). The sense and antisense primers were specifically designed from the coding regions of TRPC1, TRPC3, TRPC4, TRPC6, and GAPDH (Table 1). The fidelity and specificity of the sense and antisense oligonucleotides were examined using the BLAST program. PCR was performed by a DNA thermal cycler (MyCycler, Bio-Rad Laboratories) using the Platinum PCR Supermix (Invitrogen). The PCR products were electrophoresed through a 1.5% agarose gel, and amplified cDNA bands were visualized by GelStar nucleic acid stain (Lonza, Switzerland). To semiquantify the PCR products, an invariant mRNA of GAPDH was used as an internal control. The net intensity values of cDNA bands (from electrophoretically separated PCR products) measured by a Kodak electrophoresis documentation system were normalized to the net intensity values of the GAPDH signals; the ratios are expressed as arbitrary units for quantitative comparison.
Table 1.
Oligonucleotide sequences of the primers used for RT-PCR
| Standard Names (Accession No.) | Size, bp | Predicted Sense/Antisense | Location (nt.) | Gene (Chrom.) |
|---|---|---|---|---|
| TRPC1 (X89066) | For regular PCR: | 3q23 | ||
| 369 | 5′-GATTTTGGAAAATTTCTTGGGATGT-3′/ | 1659–2027 | ||
| 5′-TGATAGCAAATCATGAAGACAAA-3′ | ||||
| For real-time PCR: | ||||
| 108 | 5′-GCACCTGCTTTGCTTTGTTC-3′/ | 1819–1926 | ||
| 5′-TGACAGCTCCCACAAAGGAC-3′ | ||||
| TRPC3 (Y13758) | For regular PCR: | 4q27 | ||
| 318 | 5′-TGACTTCCGTTGTGCTCAAATATG-3′/ | 1909–2226 | ||
| 5′-CCTTCTGAAGCCTTCTCCTTCTGC-3′ | ||||
| For real-time PCR: | ||||
| 203 | 5′-CGCTGCCCAATATCACAGTT-3′/ | 1255–1457 | ||
| 5′-GAAGATGGACAGCATCCCAA-3′ | ||||
| TRPC4 (U40983) | For regular PCR: | 13q13.3 | ||
| 415 | 5′-TCTGCAAATATCTCTGGGAAGAATGC-3′/ | 2370–2784 | ||
| 5′-AAGCTTTGTTCGTGCAAATTTCCATTC-3′ | ||||
| For real-time PCR: | ||||
| 161 | 5′-CTCGGATCCCATCACTTCAG-3′/ | 790–950 | ||
| 5′-GGCGAGAGTTCTGATTCTGCT-3′ | ||||
| TRPC6 (AJ006276) | For regular PCR: | 11q22.1 | ||
| 438 | 5′-CTTTGAGGAGGGCAGAACACTT-3′/ | 2684–3121 | ||
| 5′-AGGAGTTCATAGCGGAGACTTG-3′ | ||||
| For real-time PCR: | ||||
| 117 | 5′-CAGACAATGGCGGTCAAGTT-3′/ | 1626–1742 | ||
| 5′-TGGTCCACGCATTATCTTCC-3′ | ||||
| GAPDH (AF261085) | For regular PCR: | 12p13.31 | ||
| 243 | 5′-GACAACGAATTTGGCTACAGC-3′/ | 1091–1333 | ||
| 5′-GATGGTACATGACAAGGTGC-3′ | ||||
| For real-time PCR: | ||||
| 108 | 5′-ATGGGGAAGGTGAAGGTCG-3′/ | 149–256 | ||
| 5′-GGGGTCATTGATGGCAACAATA-3′ |
The accession numbers in GenBank for the sequence used to design the primers are shown in parentheses. Chrom., chromosome.
Real-time RT-PCR was performed by Opticon 2 (MJ Research, Waltham, MA) using a qPCR Mastermix Plus SYBRGreen I kit (Eurogentec). Twenty-five nanograms of cDNA and 0.5 μM sense/antisense primers were used. Cycle conditions were 95°C for 10 min (1 cycle), 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s (45 cycles). Real-time data were normalized to RNA expression level of GAPDH.
Proliferation assay.
Cell number was determined using a hemocytometer. Cell count in each of the four 1-mm3 corner squares in the hemocytometer was averaged to calculate total cell number per milliliter in cell suspension. Cell number, normalized by the surface area of the wells (cells/cm2), was used to compare cell growth rate. Cell viability was determined using trypan blue solution.
[3H]thymidine incorporation assay was performed to assess DNA synthesis. PASMC were seeded in 12-well plates and serum-starved for 48 h. On day 3, 1 μCi [3H]thymidine was added to the conditioned media. Twenty-four hours later, cells were washed with cold PBS once, washed twice with cold 7.5% trichloroacetic acid, and then lysed with 0.5 M NaOH. The radioactivity was measured in a liquid scintillation counter.
Statistical analysis.
The composite data are expressed as means ± SE. Differences between groups were examined for statistical significance using Student's t-test or one-way ANOVA. Differences between controls and drug-treated cells were examined for statistical significance using Fisher's protected least significant differences test. Differences were considered to be statistically significant when P < 0.05.
RESULTS
PDGF induces nuclear translocation of NF-κB.
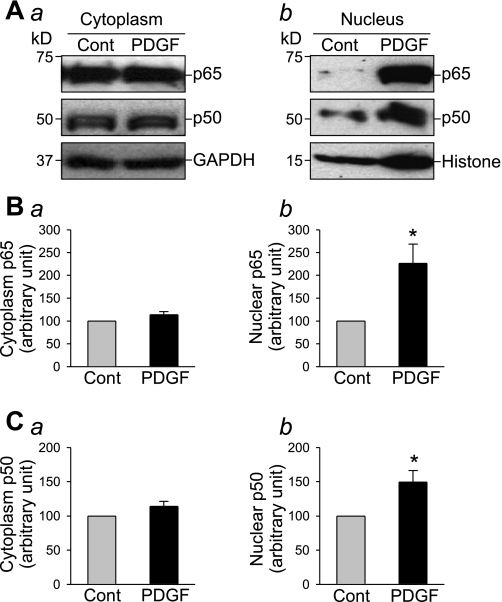
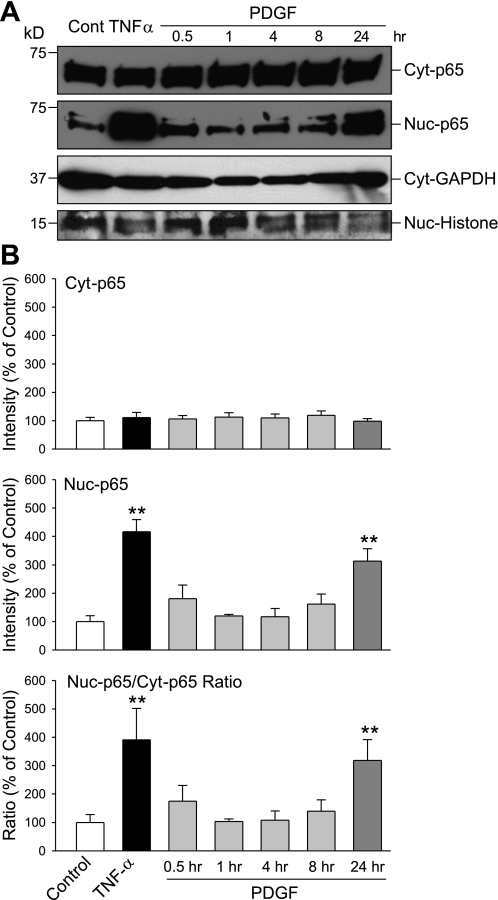
Activation of PDGF receptors in the surface membrane of human PASMC by PDGF may stimulate cell proliferation via intracellular signaling cascades, in addition to activating STAT3 and c-Jun as we previously reported (37, 40). PDGF is also known to cause nuclear translocation of NF-κB, a transcription factor that exerts proliferative and antiapoptotic effect on many cell types (1, 7, 14). As shown in Fig. 1, exposure of human PASMC to PDGF (10 ng/ml) had little effect on expression level of NF-κB subunits p65 and p50 in the cytoplasm, but significantly increased the p65 and p50 protein levels in nuclei (Fig. 1). However, the time course of the effect of PDGF was very different from that of the proinflammatory cytokine, TNF-α. Treatment of human PASMC with TNF-α (30 ng/ml) for 0.5 h caused a significant increase of p65 protein levels in the nuclear fraction. Although treatment with PDGF (10 ng/ml) increased p65 level in the nuclei at 30 min, a similar increase to TNF-α-treated cells in p65 protein level was induced only at 24 h (Fig. 2). These results suggest that PDGF induces PASMC proliferation via multiple pathways, and the enhanced nuclear translocation of NF-κB (p65 and p50) during chronic exposure to PDGF may be one pathway in the mitogenic effect of PDGF.
Fig. 1.
PDGF induces nuclear translocation of NF-κB in pulmonary arterial smooth muscle cells (PASMC). Western blot analysis (A) shows the expression level of p65 and p50 in the cytoplasm (Aa) and in the nuclei (Ab) in PASMC cultured in 0.1% FBS-DMEM with (PDGF) or without (Cont) PDGF (10 ng/ml) for 24 h. Summarized data (n = 3 experiments) show the average protein level of p65 (B) and p50 (C) (normalized to the protein level of GAPDH or histone) corresponding to the bands shown in the Western blots. *P < 0.05 vs. control (Cont).
Fig. 2.
PDGF-induced nuclear translocation of p65 in PASMC occurs over 24 h. A: Western blot analysis shows the expression level of p65 in the cytoplasmic (Cyt-p65) and nuclear (Nuc-p65) fraction in PASMC before (Control, 0 h) and after incubation in the presence of TNF-α (30 ng/ml) for 0.5 h or PDGF (10 ng/ml) for 0.5, 1, 4, 8, and 24 h. Protein levels of internal controls, GAPDH and histone, are also shown. B: summarized data (means ± SE, n = 3 experiments) show the average protein level of p65 in the cytoplasmic (Cyt-p65, top) and nuclear (Nuc-p65, middle) fraction and the ratio of cytoplasmic and nuclear p65 (Nuc-p65/Cyt-p65 Ratio, bottom). **P < 0.01 vs. control.
Inhibition of NF-κB attenuates PDGF-induced PASMC proliferation.
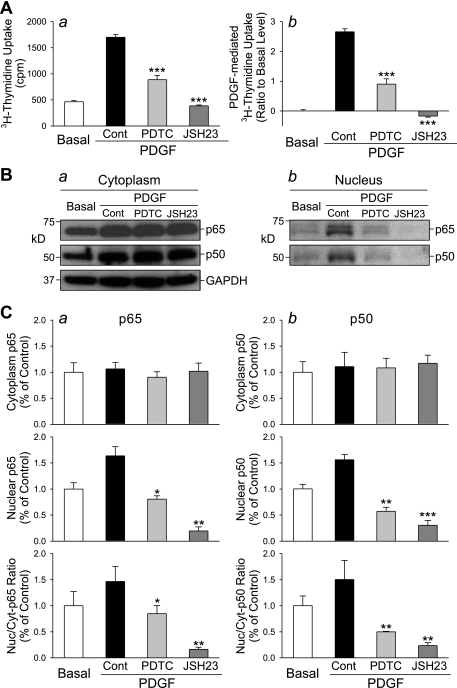
To further confirm the involvement of NF-κB in PDGF-mediated PASMC proliferation, we treated the cells with two different NF-κB inhibitors, PDTC and JSH-23 (18, 21, 30, 32). As shown in Fig. 3, inhibition of NF-κB nuclear translocation with 50 μM PDTC or with 20 μM JSH-23 significantly decreased PDGF-induced [3H]thymidine uptake in human PASMC (Fig. 3A). Western blot experiments using cytoplasmic and nuclear proteins show that PDTC and JSH-23 both markedly inhibited the nuclear translocation of p65 and p50 (Fig. 3B). These data indicate that NF-κB nuclear translocation is one of the pathways for PDGF to induce proliferative effect on human PASMC.
Fig. 3.
Inhibition of NF-κB nuclear translocation suppresses PDGF-induced PASMC proliferation. Growth-arrested PASMC were incubated in 0.1% FBS-DMEM, PDGF (10 ng/ml), PDGF+PDTC, and PDGF+JSH-23 for 24 h before [3H]thymidine uptake was measured. A: [3H]thymidine incorporation (a), measured as counts per minute (cpm), and PDGF-induced [3H]thymidine uptake (b, normalized to the basal level) in control cells (Basal, incubated in 0.1% FBS-DMEM) and cells treated with PDGF (10 ng/ml, PDGF-Cont), PDGF+PDTC (50 μM), and PDGF+JSH-23 (20 μM). ***P < 0.001 vs. PDGF-Cont. B: Western blot analysis shows the expression level of p65 and p50 in the cytoplasmic (a) and nuclear (b) fraction in PASMC before (Basal) and after incubation in the presence of PDGF (10 ng/ml, PDGF-Cont), PDGF+PDTC (50 μM), and PDGF+JSH-23 (20 μM). C: summarized data (means ± SE, n = 3 experiments) show the averaged protein levels of p65 (left) and p50 (right) in the cytoplasmic (Cyt-p65 and Cyt-p50, top) and nuclear (Nuc-p65 and Nuc-p50, middle) fraction and the ratio of cytoplasmic and nuclear p65 and p50 (bottom). *P < 0.05, **P < 0.01 vs. PDGF-Cont.
Inhibitory effect of prednisolone on PDGF-induced NF-κB nuclear translocation.
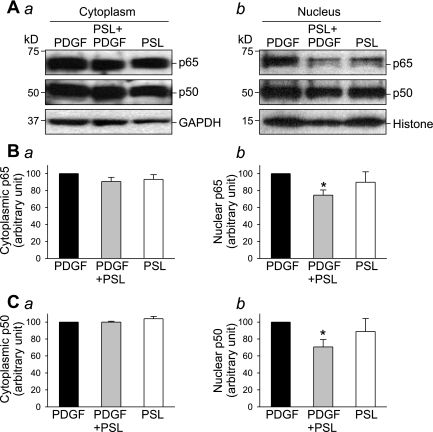
Prednisolone is an immunosuppressant that has been shown to exert antiproliferative and anti-inflammatory effects on PASMC (26), vascular smooth muscle cells (36), and cardiomyocytes (20, 34). In human PASMC treated with PDGF, concurrent treatment with prednisolone (200 μM) significantly inhibited PDGF-induced nuclear translocation of NF-κB subunits (p65, 80.9 ± 8.1%, P < 0.05; p50, 74.8 ± 8.2%, P < 0.05) (Fig. 4). The cytosolic fraction of p65 and p50, however, remains unaffected by prednisolone.
Fig. 4.
Inhibitory effect of prednisolone on PDGF-induced NF-κB nuclear translocation. Growth-arrested PASMC were incubated with or without PDGF (10 ng/ml) and/or prednisolone (200 μM) for 24 h. Western blot analysis (A) shows the effect of PDGF and prednisolone on expression level of p65 and p50 in the cytoplasmic (a) and nuclear (b) fraction. Summarized data (n = 3, panels a; n = 6, panels b) show the averaged protein level of p65 (B) and p50 (C) (normalized to the protein level of GAPDH or histone) corresponding to the bands in the Western blots above. *P < 0.05 vs. PDGF.
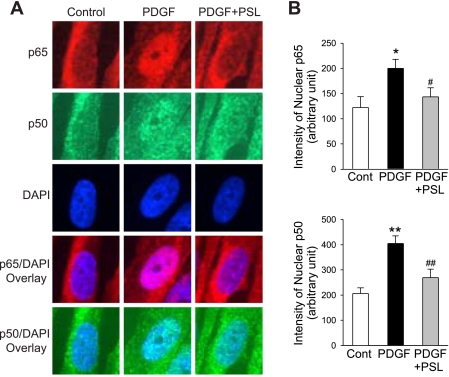
Consistent with the protein expression, a significant inhibitory effect of prednisolone on the PDGF-induced NF-κB nuclear translocation determined by immunofluorescence staining was observed. As shown in Fig. 5, the fluorescence intensity of p65 and p50 in the nuclear area was significantly increased in cells treated with PDGF [from 122.2 ± 6.5 to 199.9 ± 5.1 arbitrary units (a.u.) for p65 and from 206.4 ± 6.7 to 404.8 ± 8.4 a.u. for p50; P < 0.05]. However, concurrent treatment of the cells with PDGF and prednisolone significantly reduced the increase of p65 (from 199.9 ± 5.1 to 143.7 ± 5.2 a.u., P < 0.05) and p50 (from 404.8 ± 8.4 to 269.0 ± 9.8 a.u., P < 0.01).
Fig. 5.
Immunofluorescent imaging shows a prednisolone-mediated suppression of PDGF-mediated nuclear translocation of p65 and p50. A: representative immunofluorescence staining of p65 and p50 in PASMC. DAPI staining was performed to indicate cell nuclei. Overlay of the images (p65 or p50 with DAPI) identifies nuclear translocation of p65 and p50 in PASMC treated with (PDGF), without (Control) PDGF (10 ng/ml), and with both PDGF and prednisolone (PDGF+PSL). B: summarized immunofluorescent staining of p65 and p50. The fluorescence intensity of p65 and p50 in the nuclear area was significantly increased in cells treated with PDGF, and concurrent treatment of the cells with PDGF and prednisolone significantly reduced the p65 and p50 in the nuclear area. All fluorescent images were taken at ×100 magnification. *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ##P < 0.01 vs. PDGF bars.
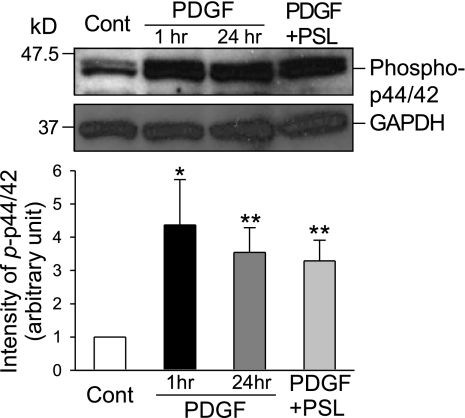
In addition to augmenting NF-κB nuclear translocation, PDGF also activated the MAP kinase pathway by stimulating p44/42 phosphorylation. Treatment with PDGF for 1 h significantly increased phosphorylation of p44/42 MAP kinase by 4.4 ± 1.4-fold (P < 0.05); extended treatment for 24 h increased phosphorylated p44/42 MAP kinase by 3.5 ± 0.7-fold (P < 0.01, Fig. 6). Notably, concurrent treatment with prednisolone for 24 h had little effect on the PDGF-induced p44/42 MAP kinase activation (or phosphorylation) (Fig. 6). These results indicate that, although PDGF activates multiple intracellular signaling cascades, prednisolone selectively inhibits the PDGF-mediated NF-κB nuclear translocation.
Fig. 6.
Prednisolone has minimal effect on MAP kinase pathway activated by PDGF. Western blot analysis (top) shows PDGF activation of the MAP kinase pathway by stimulating p44/42 phosphorylation in PASMC treated with (PDGF) or without (Cont) PDGF for 1 or 24 h. Concurrent treatment with prednisolone for 24 h (PDGF+PSL) has little effect on the PDGF-induced p44/42 MAP kinase phosphorylation. Summarized data (n = 3 experiments, bottom) shows the average protein level of phosphorylated p44/42 MAP kinase (normalized to the protein level of GAPDH) corresponding to the bands shown in the Western blots. *P < 0.05, **P < 0.01 vs. control.
Effects of prednisolone on store-operated Ca2+ entry and TRPC expression.
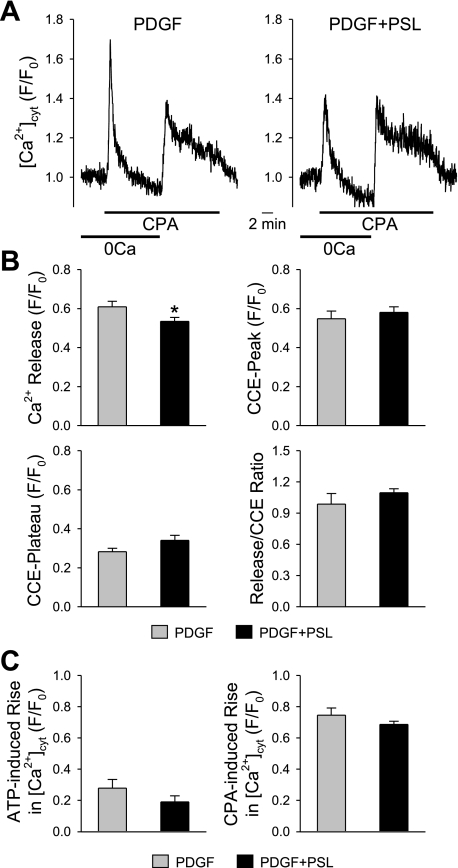
As PDGF can stimulate PASMC proliferation by upregulation of TRPC, the effects of prednisolone on store-operated Ca2+ entry were investigated. Ca2+ influx was measured as a change in [Ca2+]cyt using the membrane-permeable fluorescent probe fura-2. Representative traces showing the time course of changes in [Ca2+]cyt in PASMC treated only with PDGF (10 ng/ml) or with prednisolone (200 μM) and PDGF are shown in Fig. 7A. The cyclopiazonic acid (CPA; 10 μM)-induced rise in [Ca2+]cyt, due to Ca2+ mobilization from the sarcoplasmic reticulum (SR), is reduced by treatment with prednisolone (from 0.61 ± 0.03 to 0.55 ± 0.02 F/F0, P < 0.05) (Fig. 7B). However, prednisolone did not show significant effect on the kinetics of the increases in [Ca2+]cyt or the capacitative Ca2+ entry (CCE) characteristics (Fig. 7B). The “CCE-plateau” shown in Fig. 7B is calculated by subtracting the fluorescence intensity level before restoration of extracellular Ca2+ (or at the end of 0Ca solution) from the level right before washout of CPA. ATP is also a potent mitogen that plays an important role in the development of pulmonary vascular wall remodeling (10, 41). Extracellular application of ATP (5 mM) did not affect the increase in [Ca2+]cyt in PASMC treated with PDGF in the presence of prednisolone compared with those treated only with PDGF (Fig. 7C). The percentage of cells responding to ATP was 50.0% for PDGF treatment and 31.3% for concurrent treatment with prednisolone and PDGF. It is worth noting that all the cells treated with ATP were responsive to CPA when treated after ATP.
Fig. 7.
Prednisolone does not significantly alter cyclopiazonic acid (CPA)-induced changes in [Ca2+]cyt in PASMC. A: representative traces show the [Ca2+]cyt changes in PASMC treated with PDGF (PDGF) or with PDGF and prednisolone (PDGF+PSL). B: summarized data show the CPA-induced rise in [Ca2+]cyt due to Ca2+ mobilization from the sarcoplasmic reticulum (top left), the rise in [Ca2+]cyt due to capacitative Ca2+ entry (CCE) (top right), CCE-plateau (bottom left), and the ratio of the peak of CPA-induced rise to CCE-peak in PASMC treated only with PDGF (n = 32 cells) and with both PDGF and prednisolone (n = 37 cells). *P < 0.05 vs. control. C: summarized data showing the ATP (5 mM)-induced rise in [Ca2+]cyt (left) and the rise induced by CPA (right) in PASMC treated with PDGF only (n = 26 cells) or with both PDGF and prednisolone (n = 48 cells).
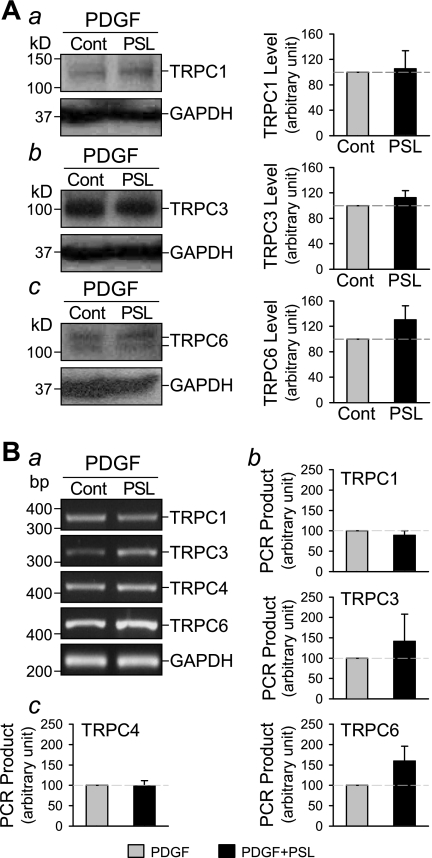
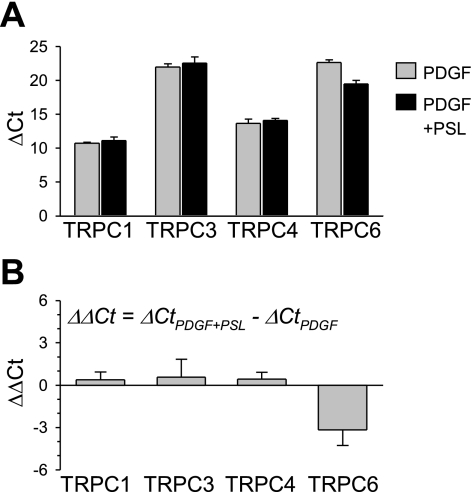
Consistent with the results on store-induced Ca2+ influx, prednisolone does not affect expression level of TRPC in PDGF-treated PASMC. Western blot analysis revealed no significant effect of prednisolone on the protein expression level of TRPC1, TRPC3, and TRPC6 in PASMC cultured with PDGF for 24 h (Fig. 8A). Summarized data show an averaged protein level of TRPC1, TRPC3, and TRPC6 of 105.3 ± 28.2%, 112.8 ± 10.7%, and 130.4 ± 21.9% compared with expression level in PASMC treated only with PDGF, respectively. Messenger RNA expression of TRPC1, TRPC3, TRPC4, and TRPC6 was also examined by regular (Fig. 8B) and real-time (Fig. 9) RT-PCR. There was no significant difference of TRPC expression levels between PDGF-treated cells and prednisolone and PDGF-treated cells (Figs. 8 and 9).
Fig. 8.
Prednisolone does not affect expression level of TRPC1/3/4/6 in PDGF-treated PASMC. A: Western blot analysis (left) shows the expression levels of TRPC 1, 3, and 6 in PASMC cultured in the presence of PDGF for 24 h. Summarized data (n = 3, right) show the average protein level of TRPC1, 3, and 6 (normalized to the protein level of GAPDH) corresponding to the bands shown on the left. B: representative images of RT-PCR products (a) show the effect of concurrent treatment with prednisolone (PSL; 200 μM) on expression level of TRPC 1, 3, 4, and 6 in PASMC cultured with PDGF for 24 h. Summarized data (n = 3) show the average mRNA levels of TRPC1, 3, and 6 (b) and TRPC4 (c) (normalized to the mRNA level of GAPDH) corresponding to the bands shown at a.
Fig. 9.
Comparison of mRNA expression of TRPC1/3/4/6 channels in control and prednisolone-treated PASMC. Summarized real-time RT-PCR data (means ± SE, n = 3 experiments) show the average mRNA levels of TRPC1, 3, 4, and 6 (measured as ΔCt, A) in PASMC treated with PDGF (gray bars) and PDGF+prednisolone (PSL; black bars), and prednisolone-mediated changes in TRPC1, 3, 4, and 6 transcripts (measured as ΔΔCt, B) in PASMC.
Inhibitory effect of prednisolone on TNF-α-induced NF-κB nuclear translocation.
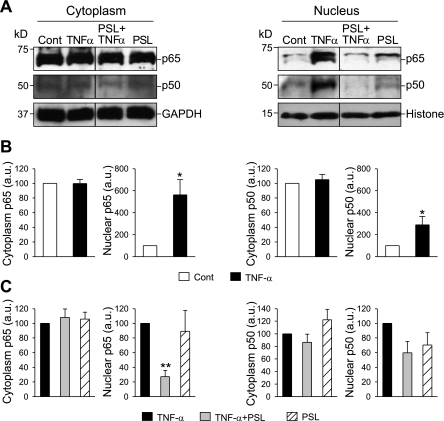
Treatment of human PASMC with TNF-α (30 ng/ml) for 0.5 h had little effect on the expression level of p65 and p50 in the cytoplasm but caused a significant increase of p65 and p50 protein levels in nuclei (p65, 561.5 ± 138.2%; p50, 288.3 ± 77.6%, P < 0.05) (Fig. 10, A and B). In PASMC treated with TNF-α and prednisolone, TNF-α-induced nuclear translocation of NF-κB (p65) was significantly inhibited (27.2 ± 8.7% of cells treated only with TNF-α, P < 0.01) (Fig. 10, A and C). Little effect of prednisolone on expression level of p65 and p50 in the cytoplasm was observed.
Fig. 10.
Prednisolone inhibits TNF-α-induced nuclear translocation of NF-κB in PASMC. Growth-arrested PASMC were incubated with or without TNF-α for 0.5 h and/or prednisolone for 24 h. A: Western blot analysis shows changes in the expression level of p65 and p50 in the cytoplasm (left) and in the nuclear fraction (right) of cells treated with TNF-α, TNF-α with prednisolone, or prednisolone alone. B and C: summarized data (n = 3 experiments) show the average protein level of p65 (left) and p50 (right), normalized to the protein level of GAPDH or histone. *P < 0.05 vs. control, **P < 0.01 vs. TNF-α.
Antiproliferative effect of prednisolone.
In PASMC cultured in 0.1% FBS-DMEM, treatment with PDGF (10 ng/ml for 24 h) induced a 1.4-fold increase in cell number. The addition of prednisolone, at a concentration that caused an inhibitory effect on nuclear translocation of NF-κB, significantly inhibited PDGF-induced proliferation of PASMC by more than 20% (20.5 ± 3%, P < 0.05). These results indicate that prednisolone attenuates PDGF-mediated PASMC proliferation, at least in part, by inhibiting nuclear translocation of NF-κB.
DISCUSSION
PDGF is a potent mitogen likely to be involved in the pathogenesis of IPAH (3, 13). It has previously been shown to be significantly increased in lung biopsies for IPAH patients (15, 31), and blockade of PDGF receptors with imatinib mesylate attenuates pulmonary hypertension significantly (11, 31). Previously, it has been reported that prednisolone, a drug that has both anti-inflammatory and immunosuppressive effects (20, 34, 36), inhibits PDGF-induced proliferation in PASMC from patients with IPAH (26). It is important to fully understand the mechanisms by which prednisolone regulates PDGF-induced PASMC proliferation to understand the potential involvement of immunoresponsive pathways in the vascular remodeling associated with pulmonary hypertension. The current research indicates that prednisolone inhibits PDGF-induced nuclear translocation of NF-κB, preventing its stimulation of PASMC proliferation.
Prednisolone is a glucocorticoid (GC), known to affect NF-κB activation (35), a transcription factor controlling many genes important for immunity, inflammation, cell proliferation, and apoptosis. Our data show that, in proliferating PASMC, treatment with PDGF caused nuclear translocation of NF-κB verified by significantly increased p65 and p50 protein levels in nuclei (Figs. 1–3). In human smooth muscle cells, the major component of NF-κB is present as a heterodimer of a 65-kDa (p65) and a 50-kDa (p50) subunit bound to either IκBα or IκBβ (5). In quiescent cells, NF-κB resides in the cytosol in an inactive form. Phosphorylation of the IκB inhibitory protein and its subsequent degradation by a proteasome-dependent pathway results in activation of NF-κB (27). NF-κB dimers then translocate to the nucleus and promote transactivation of target genes and release cytokines and adhesion molecules (8, 9). This study also indicates that prednisolone significantly inhibits this PDGF-stimulated nuclear translocation of NF-κB (Figs. 4 and 5). In addition, prednisolone also inhibited TNF-α-induced nuclear translocation of NF-κB (Fig. 10). Prednisolone therefore has potent inhibitory effects on PASMC NF-κB nuclear translocation induced by inflammatory mediators. Such an effect on NF-κB is potentially very important as NF-κB is already indicated in the proliferation of vascular smooth muscle cells (22, 25).
The MAP kinase pathway is also known to be activated by PDGF. In our studies, activation of the MAP kinase pathway by PDGF was verified by detection of p44/42 phosphorylation. However, cotreatment with prednisolone did not significantly affect the PDGF-induced p44/42 activation/phosphorylation (Fig. 6), indicating that, although PDGF activates multiple intracellular signaling cascades, prednisolone selectively inhibits the PDGF-mediated NF-κB nuclear translocation.
In mouse carotid artery smooth muscle cells, however, Mehrhof et al. (22) demonstrated that PDGF- or serum-mediated proliferation was not affected in a knockin mouse model expressing the NF-κB superrepressor IκBαΔN (cIκBαΔN). The difference between our data and the observations by Mehrhof et al. (22) may be related to the difference of species (mouse vs. human) and/or the difference of systemic artery and pulmonary artery. It has been demonstrated that systemic and pulmonary arterial smooth muscle cells may respond differently to the same stimuli.
PDGF may additionally stimulate PASMC proliferation by upregulation of TRPC, enhancing receptor-mediated increases in [Ca2+]cyt, as shown previously by our laboratory (37, 40). The regulation of cell cycle progression and cell proliferation depends on elevated [Ca2+]cyt. Store-operated Ca2+ (SOC) influx through TRPCs is known to contribute to proliferative mechanisms in PASMC (12, 33, 40, 41). TRPC1, TRPC3, TRPC4, and TRPC6 are TRPC isoforms that have been implicated in forming receptor-operated Ca2+ and/or SOC channels in human PASMC. As prednisolone induces cell cycle arrest and inhibits proliferation, its regulatory effect on cell proliferation may be associated with reduced CCE via downregulated TRPC (26). However, prednisolone did not show any significant effect on changes in [Ca2+]cyt corresponding to CCE. Only the rise in [Ca2+]cyt due to Ca2+ mobilization from the SR in PASMC treated with PDGF was slightly decreased (Fig. 7). Protein and mRNA quantification verified these calcium experiments. Our results, therefore, suggest that prednisolone may regulate PDGF-stimulated [Ca2+]cyt in PASMC via an alternative pathway to that shown to involve TRPC.
In conclusion, the results of the current study indicate that PDGF-induced nuclear translocation of NF-κB may play an important role in stimulating PASMC proliferation and that prednisolone may exert anti-inflammatory and antiproliferative effects on PASMC by inhibiting NF-κB nuclear translocation. Furthermore, the inhibitory effects of prednisolone on NF-κB expression may have a therapeutic benefit to patients with pulmonary vascular remodeling, and further study is necessary to fully characterize such mechanisms in, for example, cells and tissues from patients with pulmonary hypertension.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-054043, HL-064945, and HL-066012.
Acknowledgments
We thank Dr. Hideo Kobayashi and Jenny Dolkas in Dr. Robert R. Myers' laboratory for providing technical assistance in fluorescence microscopy experiments, and Dr. Fiona Murray in Dr. Paul A. Insel's laboratory for providing technical assistance in real-time RT-PCR experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, Royer HD, Grinstein E, Greiner A, Scheidereit C, Dorken B. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest 100: 2961–2969, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes PJ Inhaled glucocorticoids for asthma. N Engl J Med 332: 868–875, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ PDGF signaling in pulmonary arterial hypertension. J Clin Invest 115: 2691–2694, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellotto F, Chiavacci P, Laveder F, Angelini A, Thiene G, Marcolongo R. Effective immunosuppressive therapy in a patient with primary pulmonary hypertension. Thorax 54: 372–374, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourcier T, Sukhova G, Libby P. The nuclear factor κB signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem 272: 15817–15824, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Buhl R, Farmer SG. Current and future pharmacologic therapy of exacerbations in chronic obstructive pulmonary disease and asthma. Proc Am Thorac Soc 1: 136–142, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Duckett CS, Perkins ND, Leung K, Agranoff AB, Nabel GJ. Cytokine induction of nuclear factor κB in cycling and growth-arrested cells. Evidence for cell cycle-independent activation. J Biol Chem 270: 18836–18840, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Ye RD, Malik AB. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 281: L1037–L1050, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Garg AK, Aggarwal BB. Reactive oxygen intermediates in TNF signaling. Mol Immunol 39: 509–517, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem 277: 44638–44650, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Ghofrani HA, Seeger W, Grimminger F. Imatinib for the treatment of pulmonary arterial hypertension. N Engl J Med 353: 1412–1413, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol 280: H746–H755, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79: 1283–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Hoshi S, Goto M, Koyama N, Nomoto K, Tanaka H. Regulation of vascular smooth muscle cell proliferation by nuclear factor-κB and its inhibitor, IκB. J Biol Chem 275: 883–889, 2000. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, Capron F, Galanaud P, Duroux P, Simonneau G, Emilie D. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur Respir J 11: 554–559, 1998. [PubMed] [Google Scholar]
- 16.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of IκB alpha degradation in human colonic epithelial cells. J Immunol 158: 226–234, 1997. [PubMed] [Google Scholar]
- 17.Laan RF, Jansen TL, van Riel PL. Glucocorticosteroids in the management of rheumatoid arthritis. Rheumatology (Oxford) 38: 6–12, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Lavie J, Dandre F, Louis H, Lamaziere JM, Bonnet J. Vascular cell adhesion molecule-1 gene expression during human smooth muscle cell differentiation is independent of NF-κB activation. J Biol Chem 274: 2308–2314, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Levy NT, Liapis H, Eisenberg PR, Botney MD, Trulock EP. Pathologic regression of primary pulmonary hypertension in left native lung following right single-lung transplantation. J Heart Lung Transplant 20: 381–384, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Liakopoulos OJ, Teucher N, Muhlfeld C, Middel P, Heusch G, Schoendube FA, Dorge H. Prevention of TNFα-associated myocardial dysfunction resulting from cardiopulmonary bypass and cardioplegic arrest by glucocorticoid treatment. Eur J Cardiothorac Surg 30: 263–270, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Liu SF, Ye X, Malik AB. Inhibition of NF-κB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 100: 1330–1337, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Mehrhof FB, Schmidt-Ullrich R, Dietz R, Scheidereit C. Regulation of vascular smooth muscle cell proliferation: role of NF-κB revisited. Circ Res 96: 958–964, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Mills JA Systemic lupus erythematosus. N Engl J Med 330: 1871–1879, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Morton JM, Williamson S, Kear LM, McWhinney BC, Potter J, Glanville AR. Therapeutic drug monitoring of prednisolone after lung transplantation. J Heart Lung Transplant 25: 557–563, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Obata H, Biro S, Arima N, Kaieda H, Kihara T, Eto H, Miyata M, Tanaka H. NF-κB is induced in the nuclei of cultured rat aortic smooth muscle cells by stimulation of various growth factors. Biochem Biophys Res Commun 224: 27–32, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa A, Nakamura K, Matsubara H, Fujio H, Ikeda T, Kobayashi K, Miyazaki I, Asanuma M, Miyaji K, Miura D, Kusano KF, Date H, Ohe T. Prednisolone inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. Circulation 112: 1806–1812, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78: 773–785, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med 354: 166–178, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez O, Marcos E, Perros F, Fadel E, Tu L, Humbert M, Dartevelle P, Simonneau G, Adnot S, Eddahibi S. Role of endothelium-derived CC chemokine ligand 2 in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 176: 1041–1047, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, Yamamoto H, Imanaka-Yoshida K, Shimpo H, Mizoguchi A, Maruyama K, Komada Y. A nuclear factor-κB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 132: 1265–1274, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, Sydykov A, Lai YJ, Weissmann N, Seeger W, Grimminger F. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest 115: 2811–2821, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin HM, Kim MH, Kim BH, Jung SH, Kim YS, Park HJ, Hong JT, Min KR, Kim Y. Inhibitory action of novel aromatic diamine compound on lipopolysaccharide-induced nuclear translocation of NF-κB without affecting IκB degradation. FEBS Lett 571: 50–54, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS, Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 283: L144–L155, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Toyozaki T, Saito T, Takano H, Yorimitsu K, Kobayashi S, Ichikawa H, Takeda K, Inagaki Y. Expression of intercellular adhesion molecule-1 on cardiac myocytes for myocarditis before and during immunosuppressive therapy. Am J Cardiol 72: 441–444, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Unlap T, Jope RS. Inhibition of NF-κB DNA binding activity by glucocorticoids in rat brain. Neurosci Lett 198: 41–44, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Voisard R, Seitzer U, Baur R, Dartsch PC, Osterhues H, Hoher M, Hombach V. Corticosteroid agents inhibit proliferation of smooth muscle cells from human atherosclerotic arteries in vitro. Int J Cardiol 43: 257–267, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Yu Y, Fantozzi I, Remillard CV, Landsberg JW, Kunichika N, Platoshyn O, Tigno DD, Thistlethwaite PA, Rubin LJ, Yuan JX. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA 101: 13861–13866, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Y, Safrina O, Landsberg JW, Vangala N, Nicholson A, Rubin LJ, Cahalan MD, Yuan JX. A functional single-nucleotide polymorphism (−254 C to G) in the TRPC6 gene is associated with idiopathic pulmonary arterial hypertension. Circulation 114: II-130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Safrina O, Platoshyn O, Cahalan MD, Rubin LJ, Yuan JX. Enhancement of TRPC6-mediated Ca2+ entry in pulmonary artery smooth muscle cells of idiopathic pulmonary arterial hypertension patients. Circulation 116: II-59-60, 2007. [Google Scholar]
- 40.Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, Yuan JX. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol 284: C316–C330, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Remillard CV, Fantozzi I, Yuan JX. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 287: C1192–C1201, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Zuckermann A, Reichenspurner H, Birsan T, Treede H, Deviatko E, Reichart B, Klepetko W. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a 2-center prospective randomized trial. J Thorac Cardiovasc Surg 125: 891–900, 2003. [DOI] [PubMed] [Google Scholar]