Abstract
We have recently described a putative receptor for lung surfactant protein-A (SP-A) on rat type II pneumocytes. The receptor, P63, is a 63-kDa type II transmembrane protein. Coincubation of type II cells with P63 antibody (Ab) reversed the inhibitory effect of SP-A on secretagogue-stimulated surfactant secretion from type II cells. To further characterize SP-A interactions with P63, we expressed recombinant P63 protein in Escherichia coli and generated antibodies to P63. Immunogold electron microscopy confirmed endoplasmic reticulum and plasma membrane localization of P63 in type II cells with prominent labeling of microvilli. Binding characteristics of iodinated SP-A to type II cells in the presence of P63 Ab were determined. Binding (4°C, 1 h) of 125I-SP-A to type II cells demonstrated both specific (calcium-dependent) and nonspecific (calcium-independent) components. Ab to P63 protein blocked the specific binding of 125I-SP-A to type II cells and did not change the nonspecific SP-A association. A549 cells, a pneumocyte model cell line, expressed substantial levels of P63 and demonstrated specific binding of 125I-SP-A that was inhibited by the P63 Ab. The secretagogue (cAMP)-stimulated increase in calcium-dependent binding of SP-A to type II cells was blocked by the presence of P63 Ab. Transfection of type II cells with small interfering RNA to P63 reduced P63 protein expression, attenuated P63-specific SP-A binding, and reversed the ability of SP-A to prevent surfactant secretion from the cells. Our results further substantiate the role of P63 as an SP-A receptor protein localized on the surface of lung type II cells.
Keywords: lung, receptor, small interfering RNA, transmembrane protein, secretion
surfactant protein-A (SP-A), the most abundant surfactant-associated protein in the bronchoalveolar fluid, is a calcium-dependent lectin composed of 18 protein subunits of ∼28 kDa or higher depending on glycosylation status (32). This protein has several functions including the regulation of surfactant turnover (2, 25). Lung surfactant is the phospholipid-rich lipid-protein mixture produced by type II pneumocytes in the alveoli that is responsible for lowering the surface tension in the lung to maintain normal function. Through receptor-mediated specific binding of SP-A to alveolar type II cells, SP-A enhances the uptake of surfactant-like liposomes and profoundly inhibits the secretion of surfactant lipids from the pneumocytes (3, 29, 43). Inhibitors of the budding of clathrin-coated vesicles from the cell surface blocked the uptake of SP-A by cells in culture and the intact lung, consistent with a clathrin-coated pit-dependent clearance pathway for SP-A (24, 36, 39, 44).
In view of compelling evidence for the SP-A-mediated regulation of pneumocyte lipid metabolism in isolated cells, the protein was assumed to have an important role in surfactant turnover in the intact lung as well. However, mice lacking SP-A (SP-A −/−) generated with gene knockout technology demonstrated normal rates of synthesis, secretion, and uptake of surfactant lipids, although some structural and functional abnormalities in the properties of surfactant were described (21, 22). On the other hand, our further investigations using an isolated perfused intact lung system demonstrated that SP-A was necessary for the increase in lung surfactant clearance in response to physiological challenges such as secretagogue treatment or CO2-induced hyperventilation (23). Uptake of labeled dipalmitoylphosphatidylcholine (DPPC) liposomes doubled with exposure to secretagogues in the wild-type mice lungs but remained unchanged in the SP-A −/− lungs. Recently, we examined the pathways of surfactant lipid removal from the alveolar space by the isolated lungs of wild-type and SP-A −/− mice in more detail utilizing inhibitors of receptor-mediated/clathrin coated pit-dependent endocytosis and actin-mediated endocytosis (2). We found that the clathrin/SP-A receptor-mediated pathway accounted for 66% or 81% of the clearance of surfactant phospholipid from the normal wild-type mouse lung under basal or secretagogue-stimulated conditions, respectively. The SP-A −/− mouse compensated for the loss of SP-A by upregulation of alternate actin-dependent clearance pathways. These nonclathrin nonreceptor mechanisms are capable of maintaining basal rates of surfactant uptake but, unlike the clathrin-mediated pathways, cannot respond to challenge. Notably, SP-A binding to type II cells isolated from wild-type and SP-A −/− mice was similar, suggesting that SP-A receptors were present in the SP-A gene-targeted mice and were available if SP-A was added with the liposomes. In fact, the abnormal phenotype was “rescued” by the addition of SP-A to the surfactant liposomes instilled into the perfused lung, restoring the secretagogue-mediated enhanced clearance of liposomes in the SP-A −/− mice (2). Taken together, the data indicate that, in stark contrast to the conclusion that SP-A may not play a role in phospholipid turnover in vivo, SP-A and its receptor may be critically important for regulation of the clearance of surfactant phospholipid from the lung.
Several candidate SP-A receptor proteins have been described, but definitive evidence for a cell surface receptor for SP-A on type II pneumocytes that regulates turnover of phospholipid is lacking (for review, see Ref. 26). Recently, we identified an alveolar type II cell surface protein that satisfies the criteria for an SP-A receptor protein (17). Using chemical cross-linking approaches, we identified a 63-kDa transmembrane protein on the surface of type II cells linked to SP-A. Subsequent studies identified the protein as P63/cytoskeleton-associated protein 4 (CKAP4). P63, a 63-kDa protein, is a type II transmembrane protein with a 106-aa-long cytosolic tail at the NH2 terminus and a transmembrane domain and an extracytoplasmic domain of 474 amino acids (aa) at the COOH terminus (37, 38). Originally described as an endoplasmic reticulum (ER) resident protein that mediates the association between ER membranes and microtubules in African green monkey kidney cells, recent reports have identified P63 as a receptor for tissue plasminogen activator on smooth muscle cells and a receptor for antiproliferative factor on epithelial cells (11, 33, 37). In type II pneumocytes, we found P63 to be localized in intracellular structures compatible with the ER and on the plasma membrane (17). The protein interacted with SP-A in vivo as immunoprecipitation of type II cell lysates with either SP-A antibody or P63 antibody also precipitated P63 or SP-A, respectively. Inhibition of SP-A interaction with type II cells by P63 antibody blocked the biological activity of SP-A, namely the ability to inhibit phosphatidylcholine (PC) secretion from type II cells (17). The present investigation provides further evidence that P63 is a functional receptor for SP-A. We demonstrate that P63 antibody abrogated the specific calcium-dependent binding of SP-A to P63 on pneumocytes. In addition, lowering P63 protein expression using small interfering RNA (siRNA) techniques reduced specific SP-A interactions with type II cells.
MATERIALS AND METHODS
Cell Culture
Type II pneumocytes.
All animal protocols adhered to the guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pennsylvania Animal Care and Use Committee (IACUC). Alveolar type II cells were isolated from adult male Sprague-Dawley rat lungs as described previously (8, 9, 13). Briefly, perfused lungs were digested with elastase, minced, and filtered. The cell preparation was placed on IgG-coated Petri dishes for 1 h to remove macrophages. The nonadherent cells were plated on 35-mm dishes, 12-well plates, or 6-well dishes with Transwell membrane inserts in MEM with 10% FBS and used after overnight culture.
Cell lines.
A549 human lung adenocarcinoma cells and the L2 rat cell line (derived from alveolar epithelial cells) were from the American Type Culture Collection (Rockville, MD) and grown in MEM (Invitrogen) containing 10% FBS and 1% antibiotics.
Purification of SP-A
Native human SP-A was isolated from the bronchoalveolar lavage fluids of patients with alveolar proteinosis as previously described in detail (1, 20). The purity of the SP-A preparation was monitored by SDS-PAGE (30). Endotoxin levels were 0.5 pg endotoxin/1 μg SP-A protein as measured by the Limulus Amebocyte Lysate Test (Lonza, Walkersville, MD). Iodination of SP-A was performed according to the manufacturer's directions (Iodogen; Pierce, Rockford, IL) using 100 μg of SP-A and 100 μCi of Na125I. Iodinated protein was dialyzed against 5 mM Tris buffer. Specific activity for all preparations was approximately 200–400 counts per minute (cpm) per nanogram of protein. The TCA precipitability ranged from 90% to 97%. The iodinated proteins were stored at 4°C and used within 2–3 wk.
Production of P63 Antibody
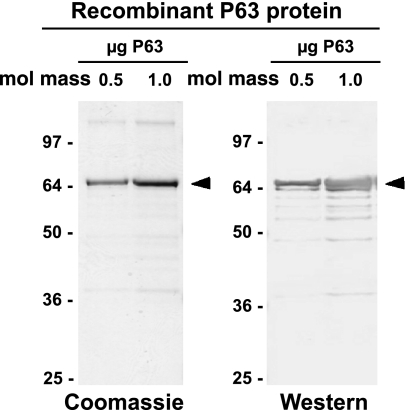
Plasmid containing human P63 DNA (a kind gift of Dr. Jack Rohrer, Ref. 38) was cloned into the pET28b plasmid in frame with the region encoding the NH2-terminal His tag between the NdeI and XhoI sites, without the natural termination codon, resulting in a COOH-terminal His tag as well. Robust expression of P63 was obtained in a Rosetta strain (Novagen, San Diego, CA) of Escherichia coli, which is supplemented with tRNAs that are common in eukaryotes but rare in prokaryotes. Cells were grown to an optical density at 595 nm (OD595) of 0.6, induced with 1 mM isopropyl β-d-thiogalactopyranoside (IPTG) for 3 h, and lysed with BugBuster reagent (Novagen). The P63 protein recovered from the supernatant fraction was purified using nickel-agarose affinity chromatography and standard protocols followed by slab gel electrophoresis. Figure 1, left, shows a Coomassie blue-stained P63 protein band in the SDS gel at ∼66 kDa, the predicted molecular mass of the human P63 (602 aa) with two 6-aa His tags (total 614 aa). Approximately 2 mg of P63 protein cut from several slab gels were employed for antibody production in rabbits using standard protocols (Strategic Biosolutions, Newark, DE). Immunoblotting procedures demonstrated that the newly generated antibody to human P63 recognized the isolated His-tagged human P63 protein that was used as the antigen (Fig. 1, right).
Fig. 1.
Recombinant P63, a 63-kDa type II transmembrane protein. Recombinant human P63 protein with a His tag on both ends was expressed in Escherichia coli, purified using nickel-agarose affinity chromatography and visualized using gel electrophoresis. A similar preparation of P63 run on slab gels was used for antibody (Ab) production in rabbits. Left: expressed P63 stained with Coomassie blue. Right: Western blot of the expressed P63 visualized using the rabbit P63 Ab. Arrowheads indicate the P63 protein band. Values under mol mass are in kilodaltons.
Preparation of Cell Lysates
Cells were scraped from culture dishes using ice-cold radioimmunoprecipitation assay buffer (RIPA; Upstate, Temecula, CA) and protease inhibitor cocktail containing 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, bestatin hydrochloride, N-(trans-epoxysuccinyl)-l-leucine 4-guanidinobutylamide, EDTA, and pepstatin A (Sigma, St. Louis, MO). Lysates were spun at 12,000 g for 15 min, and supernatants were stored at −20°C. Cell protein was quantitated using the Bradford protein assay (Bio-Rad, Hercules, CA) with BSA as the standard (5).
SDS-PAGE and Western Blotting
Cell lysates were resolved on 12% Tris-glycine gels (Invitrogen) by SDS-PAGE under reducing conditions (30). Proteins were electrophoretically transferred to nitrocellulose membrane using a Bio-Rad semidry apparatus (41, 42). Membranes were blocked with 5% nonfat dry milk in TBS with 0.1% Tween 20 (TBST) at room temperature for 1 h on a shaking platform and incubated overnight in 5% milk TBST buffer (4°C) containing either polyclonal anti-sera against P63 (kindly supplied by Drs. Jack Rohrer and Anja Schweizer, Univ. of Zurich, Zurich, Switzerland) or human recombinant P63 antibody (produced as described above). After rinsing with TBST, blots were incubated with the appropriate horseradish peroxidase-labeled secondary antibodies (Amersham, Arlington Heights, IL). Visualization of the protein bands on the Western blots using the Odyssey procedure was performed according to the manufacturer's instruction (LI-COR Biosciences, Lincoln, NE). Briefly, nitrocellulose membranes were blocked in Odyssey blocking buffer (room temperature, 1 h) and incubated at 4°C overnight with primary antibody in blocking buffer containing 0.1% Tween 20. After the membranes were washed in TBST (4 times), they were incubated (1 h, room temperature) with the appropriate secondary antibody conjugated to either IRDye 800 (green) or IRDye 700 (red) (Rockland Immunochemicals, Gilbertsville, PA) at a dilution of 1:4,000. Membranes were washed in TBST buffer five times, rinsed in PBS buffer, and scanned on the Odyssey infrared scanner.
Binding of SP-A to Cells
After overnight culture, cells were placed on ice and washed two times with ice-cold MEM and once with MEM containing 0.1% fatty acid-free BSA. Cells then were incubated at 4°C for 1 h with 1 mg/ml BSA and various concentrations of 125I-SP-A in either MEM or HBSS, which contained phenol red as a pH indicator. During the hour incubation at 4°C, the pH of the MEM was maintained using an airtight box flushed with 5% CO2 in air. To terminate the experiment, the media were removed, and the cells were washed once with incubation media containing 0.3% fatty acid-free BSA, twice with media containing 0.1% fatty acid-free BSA, and twice with PBS. Cells were dissolved in 0.2 N NaOH, and aliquots taken for protein determination (31) and radioactive counts (Beckman). Total binding of 125I-SP-A, which includes both specific and nonspecific binding, was measured. Nonspecific binding was determined by the “slope-peeling” method as described by Goldstein and Brown (16) for binding of low-density lipoprotein to its receptor. Specific binding was determined by subtraction of nonspecific SP-A binding from total binding (16, 28, 43). The effect of calcium on SP-A association with type II cells was determined by incubating the cells in HBSS without or with 1 mM calcium. 125I-SP-A was added at 4°C for 1 h, and the cells were harvested as described above. Calcium-dependent 125I-SP-A binding was determined by subtraction of the total SP-A bound in the presence of calcium (calcium-dependent and calcium-independent) from the SP-A bound in the absence of calcium (calcium-independent binding).
siRNA Knockdown of P63 Expression in Rat Alveolar Type II Cells
siRNAs for targeted disruption of rat P63/CKAP4 (GenBank acc. no. XM_343189) were designed by Ambion (Austin, TX). P63 siRNA (5′-AGUGGAAUCAGACUUGAAtt-3′) and negative control siRNA (Ambion) were transiently transfected using Nucleofector technology (Amaxa Biosystems, Gaithersburg, MD). Briefly, 3 × 106 freshly isolated type II cells were pelleted at 100 g at room temperature. The medium was aspirated, and 100 μl of Normal Human Bronchial Epithelial Cell kit (Amaxa Biosystems) Nucleofector solution was added to the cell pellets, mixed with 1 μg of siRNA duplexes, transferred to the cuvettes, and pulsed in the Amaxa Nucleofector II apparatus (Amaxa Biosystems). Based on preliminary experiments, program W001 was used for electroporation. Immediately after electroporation, cells were aspirated in prewarmed medium containing 10% FBS, held at 37°C for 10 min, and then transferred to 35-mm plastic dishes and incubated at 37°C for up to 72 h. Transfection efficiency with siRNA was estimated using indocarbocyanine (Cy3)-labeled GAPDH siRNA (Ambion) and fluorescence microscopy. Knockdown efficiency of P63 siRNA was assayed by Western blot analysis using P63 antibody and anti-β-actin antibody for loading normalization.
PC Secretion
To assay the effect of P63 siRNA on PC secretion, type II cells were electroporated with 1 μg of either P63 or negative control siRNA and grown for 72 h in MEM containing 10% FCS and 0.7 μCi per dish of [methyl-3H]choline (Amersham) to label cellular phospholipids and to allow time for a decrease in P63 protein. After 72 h, the cells were washed thoroughly and incubated for 30 min. One set of cells was harvested to serve as a “zero time” control. The remaining cells were treated with ATP (1 mM, Sigma) for 2 h to stimulate PC secretion. Cells were incubated without or with SP-A (0.2 μg/ml) before ATP stimulation. The media were removed and centrifuged to remove detached cells, methanol was added to the cell monolayer, and the cells were scraped from the dish. The cells and the media were extracted using the Bligh and Dyer method (4). The amount of phospholipid secretion was calculated as the percentage of lipid 3H cpm in the medium relative to the total lipid 3H cpm present in the cells and the medium.
Immunofluorescent Confocal Microscopy
Freshly isolated rat type II cells were grown on glass cover slips for 24 h and treated with SP-A (1 μg/ml, 2 h). For nonpermeabilized samples, cells then were rinsed with PBS and fixed with 2% paraformaldehyde for 20 min. For permeabilized samples, type II cells were permeabilized by fixation with either cold methanol-acetone (1:1 in volume) for 5 min or 4% paraformaldehyde for 60 min followed by permeabilization with 0.2% Triton in PBS for 30 min at 4°C. Next, all samples were washed, treated with sodium borohydride (0.2% in PBS, 10 min), and blocked with a mixture of 2% BSA and 5% normal goat serum for 1 h at room temperature. The samples were incubated with nonimmune rabbit IgG (control) or P63 antibody overnight at 4°C. The following day, the cells were washed and incubated for 1 h with the Alexa 488 (green)- or Alexa 594 (red)-labeled goat anti-rabbit IgG (Invitrogen-Molecular Probes) secondary antibody at room temperature for 1 h. The cells were mounted and viewed by confocal microscopy using a ×60 lens.
Immunoelectron Microscopy
Type II cells were plated on plastic or glass cover slips and incubated for 24 h at 37°C, washed, and incubated with SP-A (1 μg/ml, 2 h). The cells were fixed in 4% paraformaldehyde for 4 h, dehydrated with graded acetone (−20°C), and embedded in LR White hydrophilic resin and polymerized with UV illumination at −20°C for 2 or 3 days. Ultrathin sections (70 nm) were prepared with a diamond knife and stained with rabbit anti-p63 polyclonal antibody (15 μg/ml) overnight (4°C) followed by addition of goat-anti-rabbit IgG conjugated with 10- or 15-nm gold particles (Aurion) secondary antibody for 1 h at room temperature. The sections were imaged with a JEOL electron microscope model 100CX (Tokyo, Japan).
Statistics
Values are reported as means ± SE. Statistical comparisons were done with SigmaStat (Jandel Scientific) where statistical significance occurs at P values of <0.05. Multiple-group comparisons were performed by one-way ANOVA.
RESULTS
Western Blots and Immunocytochemistry with P63 Antibody
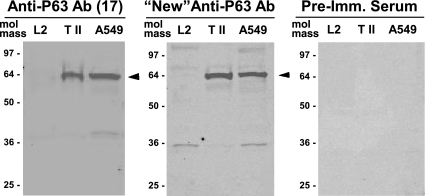
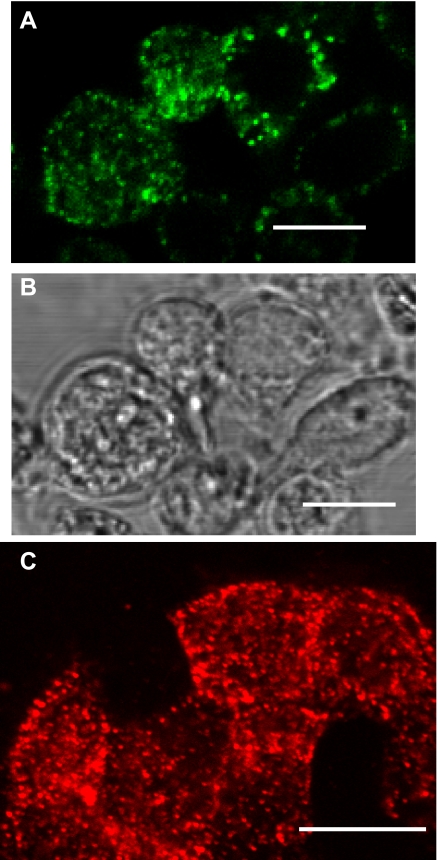
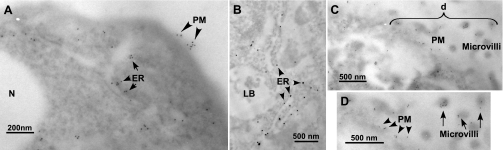
Polyclonal rabbit antibodies were raised against human recombinant P63 protein that had been expressed in E. coli (see materials and methods). Immunoblot techniques were used to compare this “new” antibody to another P63 antibody that we had used in our previous work (17). Both antibodies detected a protein band of ∼63 kDa in type II cells in agreement with our earlier studies (Fig. 2). In addition, the immunoblots showed a similar immunoreactive protein of ∼63 kDa in human A549 cell lysates. P63 was not found in L2 cells. The lack of specific binding of SP-A to L2 cells has been reported previously (28). Gels probed with preimmune sera were negative. Immunolocalization of P63 in both permeabilized and nonpermeabilized rat type II cells using the new antibody confirmed our previous work that P63 was localized on the cell surface (Fig. 3A) and present within punctate organelles showing an ER-like distribution (Fig. 3C). Immunogold electron microscopic analysis in Fig. 4 confirmed the ER (Fig. 4, A and B) and plasma membrane (Fig. 4, A, C, and D) localization of type II cell P63 protein. P63-immunoreactive gold particles were particularly prominent on type II cell microvilli (Fig. 4, C and D).
Fig. 2.
P63 protein is present in rat type II cells and human A549 cells. Western blot analysis was performed on whole cell lysates from the rat L2 and the human A549 alveolar epithelial cell lines and the freshly isolated type II cells (T II) after 24 h in culture. After gel electrophoresis and transfer to nitrocellulose, the blots were probed with P63 Ab used previously (left; Ref. 17), newly prepared P63 Ab (middle), and preimmune (Pre-Imm.) rabbit serum (right). Arrowheads indicate the P63 protein band.
Fig. 3.
Immunolocalization of P63 in isolated rat type II cells. Type II cells were either left unpermeabilized (A and B) or were permeabilized (C) and incubated with P63 Ab. A: immunofluorescence of Alexa 488 (green)-conjugated secondary Ab. B: phase contrast microscopy of cells in A. C: immunofluorescence of Alexa 594 (red)-conjugated secondary Ab. Scale bar = 10 μm.
Fig. 4.
P63 localization in type II cells at the electron micrographic level. Type II cells were incubated with surfactant protein-A (SP-A; 1 μg/ml) for 2 h, fixed, and incubated with P63 Ab followed by gold-conjugated goat anti-rabbit Ab. D is enlargement of area (“d”) shown in C. Gold particles (10-nm size in A, C, and D; 15-nm size in B) marking P63 proteins were found in structures consistent with endoplasmic reticulum (ER) (A, short arrows; B, arrowheads), plasma membrane (PM) [A, C, and D (A and D, arrowheads)], and on microvilli [C and D (D, long arrows)]. LB, lamellar body; N, nucleus.
Binding of SP-A by Type II Pneumocytes and A549 Cells
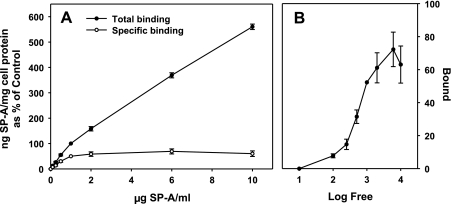
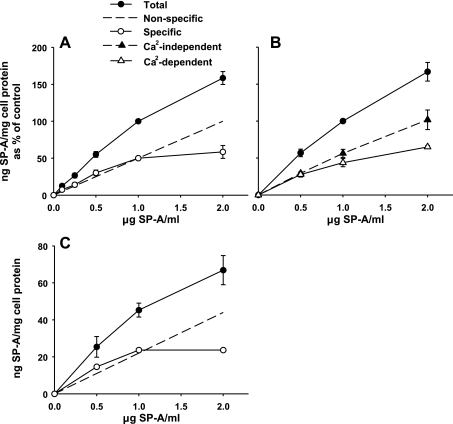
Primary cultures of rat type II cells were cultured overnight and incubated with increasing concentrations of human 125I-SP-A at 4°C for 1 h. Previous studies had shown that maximum binding had occurred by that time (24). The shape of the binding curves in each experiment was similar, but the total amount of SP-A bound to the type II cells differed between experiments. In this series of experiments, total binding ranged from 40 to 279 ng SP-A/mg cell protein at the SP-A concentration of 1 μg/ml with a mean of 104.5 ± 30.4 ng SP-A/mg cell protein (mean ± SE, n = 8). Thus the total binding data from each experiment were normalized to the values at 1 μg SP-A/ml and set equal to 100%. As shown in Fig. 5A, SP-A binding demonstrated biphasic characteristics with a high-affinity component between 0.1 and 1 μg/ml SP-A and a lower affinity, linear cell association at concentrations between 1 and 10 μg SP-A/ml. Specific high-affinity binding was calculated using the curve-peeling method of Goldstein and Brown (16) where the low-affinity values were subtracted from the total binding. Analysis of the specific binding data by Klotz plot (27) demonstrated that half-maximal binding occurred at 0.55 ± 0.01 μg SP-A/ml (Fig. 5B). Maximum specific binding averaged 68.8 ± 3.7 ng SP-A/mg cell protein (mean ± SE, n = 8) for the eight experiments or 5 ng SP-A per 106 cells (70 μg of cell protein per 106 type II cells). The data obtained between 0.1 and 2 μg SP-A/ml in Fig. 5A were replotted in Fig. 6A and compared with binding data performed with HBSS with or without calcium (Fig. 6B). The calcium-independent SP-A cell association (Fig. 6B) reflected the linear nonspecific binding in Fig. 6A, whereas the calcium-dependent binding (Fig. 6B) reflected the specific saturable binding curve (Fig. 6A).
Fig. 5.
Concentration-dependent binding of SP-A to type II cells. Increasing concentrations of 125I-SP-A were incubated with pneumocyte cells plated on 12-well plates at 4°C for 1 h followed by harvest with 2 N NaOH. A: SP-A binding to type II cells. Total binding (closed circles) was measured in the presence of calcium. Specific binding (open circles) was determined by the “slope-peeling” method (16) where the nonspecific portion of the binding curve is subtracted from the total binding curve. Data are means ± SE of 3–8 experiments performed in duplicate or triplicate expressed as a percentage of the nanograms of SP-A per milligram of cell protein bound at 1 μg of 125I-SP-A/ml. Total binding at 1 μg SP-A/ml (100% value) was 104.5 ± 30.4 ng SP-A/mg cell protein (mean ± SE, n = 8) with a range of 40–279 ng SP-A/mg cell protein. B: Klotz plot of the specific binding of SP-A. The log of the concentration of free SP-A (μg/ml) is plotted vs. the amount of SP-A specifically bound (ng SP-A/mg cell protein) to the cells normalized to the mean binding value at 1 μg SP-A/ml for the 8 experiments (104.5 ng SP-A/mg cell protein).
Fig. 6.
Binding characteristics of SP-A to type II pneumocytes and A549 cells. Increasing concentrations of 125I-SP-A were incubated at 4°C, 1 h. The cells were harvested with 2 N NaOH. A: type II cells. The total binding data of SP-A to type II cells (closed circles) at SP-A concentrations from 0.1 to 2 μg SP-A/ml are replotted from Fig. 5A. The specific binding (open circles) was determined by the slope-peeling method (16) where nonspecific portion of the binding curve (long, dashed line) is subtracted from the total binding curve. Data are the means ± SE of 3–8 experiments performed in duplicate or triplicate. B: type II cells. Binding was performed in HBSS with (total binding, closed circles) or without calcium (Ca2-independent, closed triangles). The calcium-dependent binding (open triangles) was determined by subtraction of the total binding from the calcium-independent binding. The data are the ng SP-A/mg cell protein expressed as a percentage of the binding at 1 μg SP-A/ml. 100% = 68.9 ± 18.9 ng SP-A/mg cell protein (mean ± SE, n = 3). C: A549 cells. Binding was performed in MEM. Total binding (closed circles) was measured. Specific binding (open circles) was determined by the slope-peeling method (16) as described in A. Data are ng SP-A/mg cell protein (means ± SE of 3 experiments performed in duplicate).
Next, the binding properties of SP-A to A549 cells were compared with that of type II cells. Increasing concentrations of 125I-SP-A were added to A549 cells at 4°C. The amount of SP-A bound to the A549 cell line was similar between experiments. The binding curves of SP-A with A549 cells (Fig. 6C) demonstrated the same biphasic characteristics with high-affinity binding as seen for type II cells (Fig. 6A). Total binding of SP-A to the cells as a function of the 125I-SP-A concentration in the media was replotted using the slope-peeling method of Goldstein and Brown (16) as described above. The specific binding curve for A549 cells had a maximum binding capacity (Bmax) of 36.9 ± 3.1 ng SP-A/mg cell protein and an apparent Kd of 0.4 ± 0.1 μg SP-A/ml.
Antibody to P63 Protein Inhibits Specific Binding of SP-A to Type II Cells
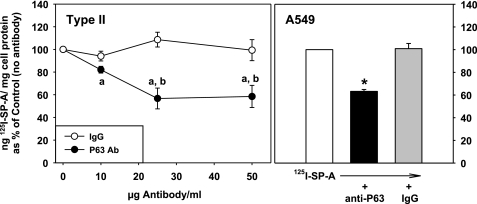
In our earlier work, we (17) found that coincubation of type II cells with P63 antibody and SP-A blocked the biological activity of SP-A on surfactant secretion from type II cells. To support our hypothesis that this effect of the P63 antibody was due to interference with SP-A-P63 protein receptor interactions, we performed competition experiments. Increasing concentrations of P63 antibody or nonimmune IgG were added to type II cells in the presence of a constant amount of 125I-SP-A (0.5 μg SP-A/ml) at 4°C for 1 h, and binding of 125I-SP-A was assayed. P63 antibody competitively inhibited the binding of 125I-SP-A to type II cells in a concentration-dependent manner with a maximum effect of 45% inhibition seen at 25 μg of P63 antibody protein/ml (Fig. 7, left). Higher antibody concentrations had no further effect. Nonimmune IgG had no effect on SP-A binding at all concentrations tested. P63 antibody was also effective at blocking the interaction of SP-A with A549 cells with no effect of IgG (Fig. 7, right).
Fig. 7.
Ab to P63 inhibits the binding of SP-A to lung epithelial cells. Type II cells: cells were incubated without or with increasing concentrations of P63 Ab or nonimmune IgG for 15 min followed by the addition of 125I-SP-A (1 μg/ml) for 1 h at 4°C. The cells were harvested, and the binding of SP-A (ng 125I-SP-A/mg cell protein) was expressed as a percentage of the binding in the absence of Ab (171 ± 45 ng 125ISP-A/mg cell protein), which was set equal to 100%. Data are means ± SE, n = 3. “a,” Statistically significant difference from no Ab (100%); “b,” statistically significant difference from IgG. P < 0.05. A549 cells. Cells were incubated with no additions or with P63 Ab or IgG (25 μg protein/ml) for 15 min followed by the addition of 125I-SP-A (0.5 μg/ml) for 1 h at 4°C. The binding of SP-A (ng 125I-SP-A/mg cell protein) was expressed as a percentage of the binding in the absence of Ab (27.6 ± 2.3 ng 125I SP-A/mg cell protein), which was set equal to 100%. Data are means ± SE, n = 3. *Statistically significant difference from either no Ab or nonimmune IgG. P < 0.05.
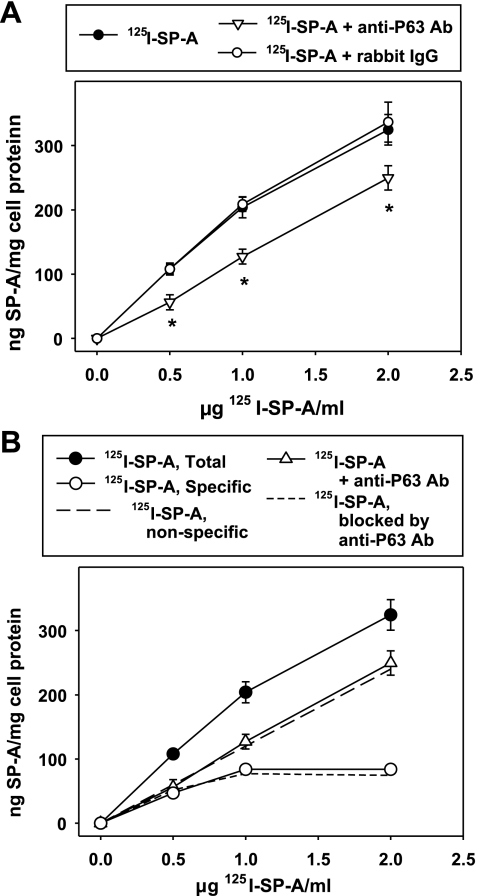
Since, at 0.5 μg SP-A/ml, the amount of nonspecific and specific binding are approximately equal (Fig. 6), we speculated that the reason the P63 antibody blocked only half of the binding of SP-A was that the antibody was effective only on specific SP-A-P63 receptor interactions. Thus the characteristics of the SP-A binding curves were determined in the presence of P63 antibody. Type II cells were exposed to increasing levels of SP-A alone or in combination with either P63 antibody or nonimmune rabbit IgG (25 μg protein/ml), and total binding of SP-A (1 h, 4°C) was determined. Figure 8A shows that nonimmune IgG had no effect on the interaction of SP-A with type II cells. In the presence of P63 antibody, the binding of SP-A to type II cells did not demonstrate specific receptor-mediated kinetics as binding was linearly related to SP-A concentration, a characteristic of a nonspecific process. In Fig. 8B, the total binding of SP-A to type II cells without antibody present was replotted into nonspecific (long dashed line) and specific (open circles) curves using the slope-peeling method of Goldstein and Brown (16). The extent of SP-A binding in the presence of P63 antibody is superimposable on the nonspecific binding process indicating only nonspecific cell association is occurring in the presence of the antibody. The amount of SP-A binding that was inhibited by the P63 antibody was calculated by subtracting the total amount of 125I-SP-A (ng/mg cell protein) bound in the presence of antibody from the total 125I-SP-A (ng/mg cell protein) bound in the absence of P63 antibody. The quantity of SP-A binding inhibited by P63 antibody was superimposable on the specific binding curve of SP-A (Fig. 8B). The data are consistent with the conclusion that the P63 antibody was blocking the specific binding of SP-A without affecting the nonspecific binding.
Fig. 8.
The specific binding of SP-A to type II cells is blocked by P63 Ab. Type II cells were incubated with P63 Ab or nonimmune IgG (25 μg protein/ml) for 15 min followed by addition of increasing concentrations of 125I-SP-A for 1 h at 4°C. A: total binding of SP-A. Data are means ± SE of 3 experiments performed in duplicate. *Statistically significant difference from binding of SP-A alone or with rabbit nonimmune IgG. B: binding of 125I-SP-A in A expressed as total, nonspecific, and specific binding. The nonspecific SP-A binding data reflects the binding of SP-A in the presence of P63 Ab. The specific SP-A binding curve can be superimposed on the amount of SP-A blocked by the presence of P63 Ab [determined by subtracting the binding of SP-A with anti-P63 Ab present (open triangles) from that without additions (closed circles)].
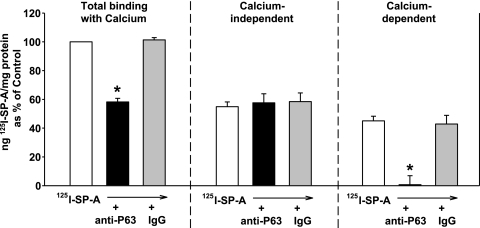
Given that the specific association of SP-A to type II cells requires calcium, binding studies were performed at 0.5 μg 125I SP-A/ml in the absence or presence of 1 mM calcium (28, 43). As shown in Fig. 9, the calcium-dependent SP-A binding to pneumocytes represented 45% of the total binding and was completely inhibited by inclusion of P63 antibody in the media, whereas nonimmune IgG had no effect. Neither antibody altered the calcium-independent association of SP-A with pneumocytes. The data are consistent with the curve-peeling analysis of the SP-A binding curves in Fig. 8, where, at 0.5 μg SP-A/ml, specific binding was 48% of the total binding; with previous work, where ∼50% of the uptake of SP-A by type II cells was clathrin-dependent receptor-mediated (24); and with published data of others (10).
Fig. 9.
The calcium-dependent binding of SP-A is inhibited by P63 Ab. Type II cells were incubated in HBSS without or with calcium (1 mM). Anti-P63 or nonimmune IgG was added for 15 min followed by the addition of 125I-SP-A (0.5 μg/ml) for 1 h at 4°C. The cells were harvested, and the calcium-dependent binding was determined by subtracting the binding in the absence of calcium (calcium-independent) from the total binding (with calcium). Data are the means ± SE (n = 3) expressed as a percentage of total binding with calcium (Control). Control = 111 ± 31 ng 125I-SP-A/mg cell protein. *Statistically significant difference from binding without additions or with IgG. P < 0.05.
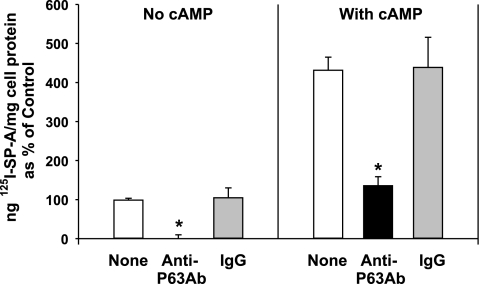
The ability of P63 antibody to inhibit specific binding of SP-A to type II cells was substrate-independent as coincubation of SP-A and P63 antibody with type II cells on Transwell membranes also inhibited the calcium-dependent SP-A binding (Fig. 10, left). We have shown that secretagogues stimulate the binding of SP-A to type II cells cultured on Transwell membranes by increasing the number of SP-A receptors sites on the cell surface (7, 8). With secretagogue treatment (0.1 mM cAMP), there was a fourfold elevation in calcium-dependent binding (Fig. 10, right), as shown previously (7, 8). The presence of P63 antibody in the media significantly attenuated the levels of calcium-dependent cAMP-stimulated binding of SP-A, whereas the presence of equal levels of IgG had no effect. Inclusion of P63 antibody in the binding study did not alter the calcium-independent association of type II cells with SP-A under either basal or secretagogue-stimulated conditions (data not shown).
Fig. 10.
The secretagogue-stimulated increase in calcium-dependent SP-A binding is attenuated by Ab directed against P63 protein. Type II cells were placed on Transwell membranes and incubated for 24 h in HBSS without or with calcium (1 mM). Anti-P63 or nonimmune IgG was added for 15 min followed by the addition of 125I-SP-A (0.5 μg/ml) for 1 h at 4°C. The cells were harvested, and the calcium-dependent binding was determined by subtracting the binding in the absence of calcium (calcium-independent) from the total binding with calcium. Data are the means ± SE of 3–9 experiments performed in triplicate expressed as percentage of the calcium-dependent binding (20.5 ± 0.8 ng 125I-SP-A/mg cell protein, n = 9), which was set equal to 100% (Control, no cAMP). *Statistically significant difference from binding without additions or with IgG. P < 0.05.
Inhibition of SP-A Biological Activity with siRNA Techniques
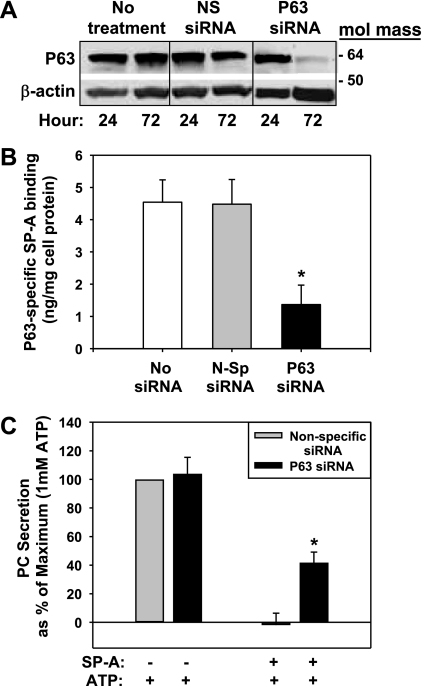
Further evidence for the identification of P63 as an SP-A receptor protein was provided by using specific siRNA directed against P63. Rat type II cells were transiently transfected with predesigned siRNA using the Amaxa Nucleofector kit. Targeting P63 mRNA at nucleotide positions 1554-1574 resulted in a >90% reduction in the expression of P63 polypeptide at 72-h posttransfection as detected by Western blot analysis (Fig. 11A). Cellular P63 content was unchanged between 24 and 72 h of culture in the untransfected cells or the cells transfected with nonspecific siRNA. β-Actin served as a normalization and gel loading control.
Fig. 11.
Small interfering RNA (siRNA) knockdown of P63 expression reduced type II cell interactions with SP-A. Type II cells were transfected with nonspecific (NS or N-Sp siRNA) or P63-specific siRNA (P63 siRNA). [3H]choline was added to some dishes to label phosphatidylcholine (PC). A: Western blot for P63. The cells were cultured for 24 or 72 h and harvested, and lysates were subjected to Western blotting procedures using either P63 or β-actin Ab. The latter served as a loading control. No treatment, no siRNA added. B: P63-specific SP-A binding. At 72 h posttransfection, the cells were incubated (4°C, 1 h) with 0.5 μg/ml 125I SP-A without or with 25 μg/mg anti-P63 polyclonal Ab to block specific binding of SP-A to the cells. The cells were harvested with 0.2 N NaOH and counted. The data are the ng 125I-SP-A/mg cell protein bound to the cells that were inhibited by the anti-P63 Ab (SP-A binding with anti-P63 Ab subtracted from binding without anti-P63 Ab), thus representing P63-specific binding of SP-A. The total binding of SP-A to untreated cells (no siRNA) in the absence of Ab was low (15.3 ± 1.2 ng 125I-SP-A/mg cell protein, n = 3), possibly due to the fact that transdifferentiation of type II to type I cells after 72 h of culture (6, 15) may have affected the cell surface density of P63. Data are the means ± SE of triplicate determinations. *Statistically significant difference from no siRNA and nonspecific siRNA, P < 0.05. C: PC secretion. After 72 h, the cells that were previously incubated with [3H]choline were exposed to ATP (1 mM) ± SP-A (0.2 μg/ml) for 1 h. 3H-PC was extracted from cells and media and analyzed. Basal secretion was subtracted from all data = 0% maximum. Data are expressed as a percentage of PC secretion that occurred with nonspecific siRNA + ATP alone (1.75% ± 0.21% PC secretion, mean ± SE, n = 3), which was set equal to 100% (maximum). Data are means ± SE of duplicate or triplicate samples from 3 experiments. *Statistically significant difference from nonspecific siRNA (+ SP-A + ATP), P < 0.05; n = 3.
A second group of transfected cells was used to measure the status of cell surface SP-A receptors. After 72 h, the cells were washed and 125I-SP-A (0.5 μg/ml) without or with P63 antibody (25 μg/ml) was added (4°C, 1 h). We had shown earlier that the P63 antibody blocks the specific calcium-dependent binding of SP-A (Figs. 8–10). Thus iodinated SP-A binding in the presence of the P63 antibody was subtracted from the binding, which occurred in the absence of antibody to determine P63-specific SP-A binding. The data indicate that only 33% of the P63-specific SP-A binding remained after P63 siRNA treatment and reduction of P63 protein compared with untransfected (no siRNA) control cells and with no effect of the nonspecific siRNA treatment (Fig. 11B).
A third group of cells were used to measure surfactant secretion and incubated with [3H]choline to label intracellular PC pools immediately after transfection. After 72 h, the 3H-labeled cells were washed to remove extracellular [3H]choline, ATP (1.0 mM) was added to stimulate PC secretion, and cells were incubated without or with SP-A (0.2 μg/ml). Figure 11C demonstrates that type II cells transfected with P63 siRNA responded to ATP to the same extent as cells transfected with nonspecific siRNA. The presence of SP-A completely blocked the ATP-stimulated PC secretion after nonspecific siRNA transfection (PC secretion = −2% ± 8% of maximum), as demonstrated previously for nontransfected cells (3). On the other hand, the reduction in P63 protein due to transfection with P63 siRNA significantly prevented SP-A-mediated inhibition of ATP-stimulated PC secretion, with PC secretion maintained at 42% ± 7% of maximum (mean ± SE, n = 3; Fig. 11C). The data are consistent with the conclusion that the presence of P63 in type II cells is important for SP-A-mediated blockage of PC secretion.
DISCUSSION
Evidence for receptors on alveolar type II cells with high affinity for SP-A has been provided by our group as well as others for cells grown under various cell culture conditions (1, 7, 28, 43). P63/CKAP4 has emerged as a candidate pneumocyte SP-A receptor (17). P63 is found on the plasma membrane of type II cells; it can be cross-linked to SP-A, demonstrating its close proximity to the surfactant protein; and antibodies to P63 block the ability of SP-A to inhibit phospholipid secretion from type II cells, a biological activity that relies on interactions of SP-A with a receptor (17, 32). The present work provides further evidence that P63/CKAP4 is a plasma membrane receptor protein for SP-A on type II cells. Immunoelectron microscopy clearly demonstrated the plasma membrane localization of P63. Importantly, competition experiments with antibody to P63 and iodinated SP-A showed that incubation of the pneumocytes with antibody to P63 blocked the saturable calcium-dependent binding of SP-A. Furthermore, the secretagogue-stimulated upregulation of specific SP-A binding also was inhibited by P63 antibody, consistent with the conclusion that it is the P63 membrane protein that is responsible for the enhanced SP-A binding. Finally, siRNA directed against P63 reduced P63 protein levels in type II cells, lowered P63-specific SP-A binding, and interfered with the biological activity of SP-A, which is dependent on SP-A/receptor interaction, specifically the inhibition of secretagogue-stimulated surfactant secretion. Thus preventing association between SP-A and P63 through the use of competing P63 antibody or siRNA techniques was effective in blocking the receptor-mediated interaction of SP-A with type II pneumocytes.
A newly generated anti-P63 rabbit antibody directed against full-length recombinant human P63 protein identified the human P63 antigen used as the immunogen as well as rat P63 in type II cells and human P63 in A549 cells in Western blots. In addition, immunofluorescence microscopy revealed labeling of P63 on the plasma membrane and in punctate organelles of type II cells, data supporting our previous results using a different P63 antibody (17). Together, these data validated the specificity of the new antibody for P63. Immunoelectron microscopy showed definitive localization of P63 in the ER, as reported by others (37), and on the plasma membrane, especially in the microvilli, in support of the light microscopic data and Western blots (17). The presence of P63 on microvilli is intriguing as microvilli provide additional surface area for type II cell-protein contact and are in the ideal location for interaction with SP-A in the surfactant fluid lining the alveolar space.
Previous work and the present report concluded that SP-A bound to type II cells via a specific cell surface receptor because the binding of SP-A was saturable and calcium-dependent, typical for many receptor-ligand interactions (7, 28, 43). The binding curve of SP-A to type II cells demonstrated both a saturable component and a nonsaturable process (7, 10, 28, 43). Using isolated type II cells in culture for 1 day, we found that the quantity of SP-A bound to the cells differed between experiments, although the concentration of SP-A required for half saturation of specific binding (0.55 μg SP-A/ml) was similar in each experiment. Thus the number of SP-A receptors on the cell surface was quite variable. Any analysis of the binding characteristics of SP-A is complicated by two factors. The first is the ability of SP-A to self-associate (19, 34), a property that may be contributing to the nonsaturable process observed. The difficulties associated with measuring binding kinetics in presence of protein oligomerization is well-recognized as discussed in relation to the studies of the nonsaturable association of the vitamin D binding protein to neutrophils (12). Second, SP-A isolated from alveolar proteinosis patients has been shown by gel filtration to be composed of a mixture of two oligomeric structures with molecular masses of 1.65 MDa for alveolar proteinosis protein-I (APP-I) and 0.93 MDa fro APP-II (18). Each has been shown to have unique type II cell binding characteristics (19). Since APP-II has a higher binding affinity for type II cells, for discussion purposes, we utilized the SP-A molecular mass of 0.93 MDa. There were ∼3,200 binding sites/type II cell (range 1,200–8,400) with half-maximal binding at 6 × 10−10 M. The apparent dissociation constant is comparable to other published data using rat SP-A (28, 43). The shape of the binding curve was similar to that reported by Wright et al. (43) using freshly isolated cells in suspension at 4°C, with high-affinity binding occurring at concentrations of SP-A less than 1 μg/ml and a low-affinity association at higher concentrations (from approximately 1 to 10 μg/ml SP-A). However, the receptor numbers in the present report are lower than those reported for unattached type II cells (40,000 binding sites per cell; Ref. 43) or in type II cells attached to dishes but incubated for 5 h at 37°C using trypsin-sensitivity as a measure of binding (135,000 binding sites per cell; Ref. 28). Possible explanations for differences in receptor number include the species source of SP-A (rat vs. human), cell culture conditions (in suspension vs. attached), and time and temperature of incubations (1 h at 4°C vs. 5 h at 37°C), to outline a few. Furthermore, we have preliminary evidence that incubation of type II cells at 37°C for 2 h with SP-A increased the levels of P63 on the cell surface, data that might partially explain the higher receptor numbers in the study performed at 37°C with SP-A for 5 h (28).
In the current study, we show that antibody to P63 blocks the specific saturable calcium-dependent binding of SP-A to type II cells regardless of the substrate, plastic dishes or Transwell membranes. In the presence of competing P63 antibody, the interaction of SP-A and type II cells became a calcium-independent, nonsaturable, linear process. Such data indicate that the anti-P63 polyclonal antibody recognized the precise site on the P63 transmembrane protein that was important for specific SP-A binding. Mutagenesis studies have identified regions in the carbohydrate recognition domain of the SP-A protein as the receptor binding domain. Substitutions of Glu195 and Arg197 in SP-A inhibit SP-A binding as do alterations in the calcium ligating residues of the carbohydrate binding site (for review, see Ref. 32). Although the critical part of the P63 protein necessary for SP-A binding is not yet known, the COOH-terminal portion of P63 located distal to the transmembrane domain is the probable candidate because, as a type II transmembrane protein, this portion of the P63 protein is predicted to be on the external cell surface, whereas the NH2 terminus is in the cytoplasm. The identification of the peptide sequence of this binding domain is of considerable interest and possible physiological importance as related peptides should prove to be useful in pharmaceutical control of the turnover of SP-A and its associated phospholipids in the lung.
The A549 epithelial cell line, generated from a human adenocarcinoma, proved to be a useful cell model for the study of P63 in pneumocytes. The amount of SP-A binding was fairly consistent between experiments indicating more stability in receptor numbers in the transformed cell line. A549 cells demonstrated specific binding of SP-A with saturation characteristics similar to those seen with type II cells. P63 antibody identified P63 protein in cell lysates of A549 cells using Western blot techniques. Finally, using P63 antibody in competition studies, we showed that the antibody competed with SP-A for binding to A549 cells, indicating that the P63 protein was functioning as a cell surface receptor for SP-A in this epithelial cell line.
We (8) have previously studied SP-A binding proteins on type II cell membranes. Using the antibody, A2R, we found several results similar to those we have described using the P63 antibody. The question arises as to whether the two antibodies are recognizing the same or similar receptor proteins alone or in a complex. Both antibodies label proteins on the cell surface plasma membrane and in punctate paranuclear granules of type II cells. Furthermore, both antibodies inhibited the binding of SP-A to type II cells plated on plastic or Transwell membranes (8). Another group demonstrated that A2R inhibited the ability of SP-A to block PC secretion (40), whereas we (17) demonstrated that P63 antibody had the same property. A2R also blocked the SP-A-stimulated uptake of liposomes (8), but that function has not yet been studied for the P63 antibody. It was of interest that ligand blots of proteins from type II cell plasma membranes probed with iodinated A2R or SP-A revealed two proteins in the range of 30–35 kDa and 55–65 kDa (8), especially since, in our recent cross-linking experiments, we found two proteins cross-linked to SP-A of 30 and 63 kDa, respectively (17). Because of the similarity in experimental results and the identification of two proteins of comparable molecular weights using different approaches, our current hypothesis is that the SP-A receptor consists of two protein subunits. We have identified the cross-linked 63-kDa protein as CKAP4/P63, the protein that remains the subject of current investigations, while studies of the 30-kDa protein also are ongoing. It remains a possibility that the latter may be the SP-A receptor responsible for the ability of SP-A to augment lipid uptake or for calcium-independent cell association of SP-A.
There is considerable published evidence supporting linkage between SP-A-receptor interactions and regulation of surfactant secretion (for review, see Ref. 32). The ability of SP-A to block phospholipid secretion from type II cells has been shown to be completely dependent on specific binding of SP-A with cell surface receptors (32). Removal of calcium or mutations of key amino acids in SP-A will destroy this biological activity of the surfactant protein (32). Previously, we showed that antibody to P63 interfered with this function of SP-A (17). In the present results, we demonstrate, through the use of siRNA techniques, that lowering pneumocyte P63 protein content reduces SP-A binding and the ability of SP-A to block secretion of phospholipid. Thus altering P63 protein levels had physiological consequences on the function of SP-A in controlling lung lipid metabolism, specifically the blocking of surfactant secretion.
SP-A has another biological function related to lung lipid metabolism, namely the reuptake of phospholipid from the alveolar space (14). The SP-A-dependent clathrin-mediated pathway for clearance of surfactant phospholipid from the alveolar space by the lung tissue has recently been recognized to be an important mechanism for the regulation of extracellular surfactant pool size. The clathrin-dependent uptake of surfactant is responsible for 30–70% of the uptake of surfactant lipids by the rat and mouse lung (2, 35). In the absence of SP-A, the gene-targeted mice maintain basal rates of lipid clearance by upregulation of an alternative actin-dependent pathway. Although this route can compensate for the lack of SP-A, it is insensitive to physiological stimuli, whereas the SP-A-receptor mediated mechanism is able to respond by a two- to fourfold elevation in lipid uptake on secretagogue treatment of mice or rat lungs, respectively (2, 35). Using type II cells plated on Transwell membranes, we had shown previously that incubation with secretagogues triggered an increase in the expression of SP-A receptors on the surface of the cells in culture (7, 8). The enhanced SP-A binding was saturable and calcium-dependent (7). In the present report, we found that this stimulated, calcium-dependent SP-A-type II cell interaction could be competed by the presence of P63 antibody. Those results are consistent with the conclusion that secretagogue-enhanced binding of SP-A to pneumocytes on Transwell membranes occurred due to an increase in P63 protein concentration on the cell surface. However, further experiments will be required to determine whether secretagogue-induced SP-A-dependent clearance of surfactant lipid from the lung and/or SP-A-stimulated uptake of liposomes into type II cells occurs via a P63-mediated process.
In conclusion, the interaction of SP-A with type II cells and subsequent biological consequences are dependent on the binding of SP-A to a specific protein receptor on the cell surface and internalization through the classical clathrin-coated pit pathway (24). We have identified a candidate SP-A receptor protein through cross-linking techniques, CKAP4/P63. This transmembrane protein was previously described as an ER-resident protein but has recently been identified as a receptor for the frizzled 8 protein-related antiproliferative factor on the surface of bladder epithelial cells and a receptor for tissue plasminogen activator on the surface of vascular smooth muscle cells (11, 33, 37). Our current work establishes the importance of this protein as a receptor for SP-A on the plasma membrane of lung type II cells. Antibody to P63 inhibited the specific calcium-dependent binding of SP-A to pneumocytes in competition studies. Furthermore, reducing the quantity of P63 protein interfered with SP-A binding and biological activity. The properties of this protein that allow it to act as a receptor for different ligands in such diverse cell types remain to be elucidated but may be related to a possible role as part of a receptor protein complex. Furthermore, the mechanisms that regulate synthesis of P63 or the movement of P63 from internal cellular compartments to the cell surface and whether it transports SP-A to intracellular compartments such as lamellar bodies are topics of interest. In any case, the evidence that P63 is a receptor for SP-A on type II cells continues to accumulate. The further characterization of this important SP-A receptor will contribute to the understanding of the maintenance of lung surfactant homeostasis.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-19737.
Acknowledgments
Preliminary results of portions of this work were presented at the 2008 American Thoracic Society meeting in Toronto, Canada. We thank Drs. Jack Rohrer and Anja Schweizer for the gift of the polyclonal P63 antibody and Ling Gao for excellent technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bates SR, Dodia C, Fisher AB. Surfactant protein A regulates uptake of pulmonary surfactant by lung type II cells on microporous membranes. Am J Physiol Lung Cell Mol Physiol 267: L753–L760, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bates SR, Dodia C, Tao JQ, Fisher AB. Surfactant protein-A plays an important role in lung surfactant clearance: evidence using the surfactant protein-A gene-targeted mouse. Am J Physiol Lung Cell Mol Physiol 294: L325–L333, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bates SR, Tao JQ, Notarfrancesco K, DeBolt K, Shuman H, Fisher AB. Effect of surfactant protein A on granular pneumocyte surfactant secretion in vitro. Am J Physiol Lung Cell Mol Physiol 285: L1055–L1065, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Med Sci 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M A rapid and sensitive method for quantitation of microgram quantities of proteins utilizing the principle of protein dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 6.Cheek JM, Evans MJ, Crandall ED. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp Cell Res 184: 375–387, 1989. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Bates SR, Fisher AB. Secretagogues increase the expression of surfactant protein A receptors on lung type II cells. J Biol Chem 271: 25277–25283, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Fisher AB, Strayer DS, Bates SR. Mechanism for secretagogue-induced surfactant protein A binding to lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 275: L38–L46, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Chinoy MR, Dodia C, Fisher AB. Increased surfactant internalization by rat type II cells cultured on microporous membranes. Am J Physiol Lung Cell Mol Physiol 264: L300–L307, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chroneos ZC, Abdolrasulnia R, Whitsett JA, Rice WR, Shepherd VL. Purification of a cell-surface receptor for surfactant protein A. J Biol Chem 271: 16375–16383, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Conrads TP, Tocci GM, Hood BL, Zhang CO, Guo L, Koch KR, Michejda CJ, Veenstra TD, Keay SK. CKAP4/p63 is a receptor for the frizzled-8 protein-related antiproliferative factor from interstitial cystitis patients. J Biol Chem 281: 37836–37843, 2006. [DOI] [PubMed] [Google Scholar]
- 12.DiMartino SJ, Kew RR. Initial characterization of the vitamin D binding protein (Gc-globulin) binding site on the neutrophil plasma membrane: evidence for a chondroitin sulfate proteoglycan. J Immunol 163: 2135–2142, 1999. [PubMed] [Google Scholar]
- 13.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 134: 141–145, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB Lung surfactant clearance and cellular processing. In: Lung Surfactant: Cellular and Molecular Processing, edited by Rooney SA and Landes RG. Austin, TX: Landes Bioscience, 1998, p. 165–189.
- 15.Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 311: 31–45, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein JL, Brown MS. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem 249: 5153–5162, 1974. [PubMed] [Google Scholar]
- 17.Gupta N, Manevich Y, Kazi AS, Tao JQ, Fisher AB, Bates SR. Identification and characterization of p63 (CKAP4/ERGIC-63/CLIMP-63), a surfactant protein A binding protein, on type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 291: L436–L446, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hattori A, Kuroki Y, Katoh T, Takahashi H, Shen HQ, Suzuki Y, Akino T. Surfactant protein A accumulating in the alveoli of patients with pulmonary alveolar proteinosis: oligomeric structure and interaction with lipids. Am J Respir Cell Mol Biol 14: 608–619, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Hattori A, Kuroki Y, Sohma H, Ogasawara Y, Akino T. Human surfactant protein A with two distinct oligomeric structures which exhibit different capacities to interact with alveolar type II cells. Biochem J 317: 939–944, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawgood S, Benson BJ, Hamilton RL Jr. Effects of surfactant-associated proteins and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry 24: 184–190, 1985. [DOI] [PubMed] [Google Scholar]
- 21.Ikegami M, Korfhagen TR, Bruno MD, Whitsett JA, Jobe AH. Surfactant metabolism in surfactant protein A-deficient mice. Am J Physiol Lung Cell Mol Physiol 272: L479–L485, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Ikegami M, Korfhagen TR, Whitsett JA, Bruno MD, Wert SE, Wada K, Jobe AH. Characteristics of surfactant from SP-A-deficient mice. Am J Physiol Lung Cell Mol Physiol 275: L247–L254, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Jain D, Dodia C, Bates SR, Hawgood S, Poulain FR, Fisher AB. SP-A is necessary for increased clearance of alveolar DPPC with hyperventilation or secretagogues. Am J Physiol Lung Cell Mol Physiol 284: L759–L765, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Jain D, Dodia C, Fisher AB, Bates SR. Pathways for clearance of surfactant protein A from the lung. Am J Physiol Lung Cell Mol Physiol 289: L1011–L1018, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Khubchandani KR, Snyder JM. Surfactant protein A (SP-A): the alveolus and beyond. FASEB J 15: 59–69, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol 43: 1293–1315, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Klotz IM Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science 217: 1247–1249, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Kuroki Y, Mason RJ, Voelker DR. Alveolar type II cells express a high-affinity receptor for pulmonary surfactant protein A. Proc Natl Acad Sci USA 85: 5566–5570, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroki Y, Mason RJ, Voelker DR. Chemical modification of surfactant protein A alters high affinity binding to rat alveolar type II cells and regulation of phospholipid secretion. J Biol Chem 263: 17596–17602, 1988. [PubMed] [Google Scholar]
- 30.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 31.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951. [PubMed] [Google Scholar]
- 32.McCormack FX Structure, processing and properties of surfactant protein A. Biochim Biophys Acta 1408: 109–131, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Razzaq TM, Bass R, Vines DJ, Werner F, Whawell SA, Ellis V. Functional regulation of tissue plasminogen activator on the surface of vascular smooth muscle cells by the type-II transmembrane protein p63 (CKAP4). J Biol Chem 278: 42679–42685, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Ruano ML, Garcia-Verdugo I, Miguel E, Perez-Gil J, Casals C. Self-aggregation of surfactant protein A. Biochemistry 39: 6529–6537, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Ruckert P, Bates SR, Fisher AB. Role of clathrin- and actin-dependent endocytotic pathways in lung phospholipid uptake. Am J Physiol Lung Cell Mol Physiol 284: L981–L989, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Ryan RM, Morris RE, Rice WR, Ciraolo G, Whitsett JA. Binding and uptake of pulmonary surfactant protein (SP-A) by pulmonary type II epithelial cells. J Histochem Cytochem 37: 429–440, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Schweizer A, Ericsson M, Bachi T, Griffiths G, Hauri HP. Characterization of a novel 63 kDa membrane protein. Implications for the organization of the ER-to-Golgi pathway. J Cell Sci 104: 671–683, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Schweizer A, Rohrer J, Jeno P, DeMaio A, Buchman TG, Hauri HP. A reversibly palmitoylated resident protein (p63) of an ER-Golgi intermediate compartment is related to a circulatory shock resuscitation protein. J Cell Sci 104: 685–694, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Stevens PA, Wissel H, Zastrow S, Sieger D, Zimmer KP. Surfactant protein A and lipid are internalized via the coated-pit pathway by type II pneumocytes. Am J Physiol Lung Cell Mol Physiol 280: L141–L151, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Strayer DS, Pinder R, Chander A. Receptor-mediated regulation of pulmonary surfactant secretion. Exp Cell Res 226: 90–97, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran H, Pankov R, Tran SD, Hampton B, Burgess WH, Yamada KM. Integrin clustering induces kinectin accumulation. J Cell Sci 115: 2031–2040, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Wright JR, Borchelt JD, Hawgood S. Lung surfactant apoprotein SP-A (26–36 kDa) binds with high affinity to isolated alveolar type II cells. Proc Natl Acad Sci USA 86: 5410–5414, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young SL, Wright JR, Clements JA. Cellular uptake and processing of surfactant lipids and apoprotein SP-A by rat lung. J Appl Physiol 66: 1336–1342, 1989. [DOI] [PubMed] [Google Scholar]