Abstract
The assembly of elastic fibers in tissues that undergo repeated cycles of extension and recoil, such as the lungs and blood vessels, is dependent on the proper interaction and alignment of tropoelastin with a microfibrillar scaffold. Here, we describe in vivo histopathological effects of neuraminidase-1 (Neu1) deficiency on elastin assembly in the lungs and aorta of mice. These mice exhibited a tight-skin phenotype very similar to the Tsk mouse. Normal septation of Neu1-null mice did not occur in neonatal mice, resulting in enlarged alveoli that were maintained in adults. The abnormal development of elastic fibers was remarkable under electron microscopy and confirmed by the overlapping distribution of elastin, fibrillin-1, fibrillin-2, and fibulin-5 (Fib-5) by the light microscopy immunostainings. Fib-5 fibers appeared diffuse and unorganized around the alveolar walls and the apex of developing secondary septal crests. Fibrillin-2 deposition was also abnormal in neonatal and adult lungs. Dispersion of myofibroblasts appeared abnormal in developing lungs of Neu1-null mice, with a random distribution of myofibroblast around the alveolar walls, rather than concentrating at sites of elastin synthesis. The elastic lamellae in the aorta of the Neu1-null mice were thinner and separated by hypertrophic smooth muscle cells that were surrounded by an excess of the sialic acid-containing moieties. The concentration of elastin, as measure by desmosine levels, was significantly reduced in the aorta of Neu1-null mice. Message levels for tropoelastin and Fib-5 were normal, suggesting the elastic fiber defects in Neu1-null mice result from impaired extracellular assembly.
Keywords: elastin, fibulin-5, fibrillin, extracellular matrix
elastin is synthesized in the cell as a soluble precursor, tropoelastin, which is transported to the cell surface bound to the 67-kDa elastin-binding protein, EBP, a recyclable molecular chaperone that facilitates the secretion of tropoelastin (6, 31, 32). EBP is an enzymatically inactive splice variant of lysosomal β-galactosidase (β-Gal) and was originally named short β-Gal (S-Gal) (31). The orderly release of tropoelastin into the extracellular matrix occurs at the cell surface, where the tropoelastin is then assembled onto a scaffold of microfibrils and cross-linked by lysyl oxidase into mature elastic fibers. These elastic fibers are composed of two major components: a core containing resilient polymer elastin and 10- to 12-nm microfibrils made up of several structural glycoproteins; fibrillins (FBN) 1, 2, and 3, fibulins 1, 3, and 5 (Fib-5), and MAGP (8, 9, 22, 29, 34, 36, 39, 45, 47). The role of these microfibrils in elastic fiber assembly has been well documented (45).
Neuraminidases (sialidases) are members of a superfamily of hydrolytic enzymes ubiquitously expressed in tissues of all animals, with a primary function of initiating hydrolysis of sialo-glucoconjugates through the removal of their terminal sialic acid residues (1, 37). In mammalian cells, four genetically distinct neuraminidases (sialidases), which differ in their tissue distribution, subcellular localization, and substrate specificity have been characterized. They have been localized to the lysosomes [neuraminidase-1 (Neu1); Refs. 19, 35], the cytosol (Neu2; Refs. 27, 38), the plasma membrane (Neu3, also known as ganglioside sialidase; Refs. 28, 44), and the lysosomes/mitochondria (Neu4; Refs. 29, 47). Neu1, which is expressed in all mammalian tissues and active mostly toward sialylated glycoproteins, is the only sialidase linked to a human disease (9, 34). Genetic mutations at the Neu1 locus are at the basis of the lysosomal storage disorder sialidosis, a severe systemic condition characterized by a broad spectrum of clinical manifestations affecting most of the organs and the nervous system (13).
In mammalian cells, Neu1 is an integral component of a lysosomal multiprotein complex that includes at least two other hydrolases: the glycosidase β-Gal and the carboxypeptidase protective protein/cathepsin A (PPCA) (3, 4, 30). By virtue of its interaction with PPCA, Neu1 is correctly compartmentalized and catalytically activated in lysosomes (4). In addition to their role in the intralysosomal catabolism of sialylated macromolecules, Neu1 and PPCA are targeted to the cell surface together with EBP as part of a protein complex, the elastin receptor (6, 17, 23, 32). It has been proposed that, within this complex, Neu1 has a role in the regulation or assembly of elastic fibers (6, 18), although studies in skin fibroblasts do not concur (42a).
A Neu1-null mouse has been generated that is an accurate model of sialidosis (10). These mice develop abnormalities typical of the early-onset forms of the disease in children and are characterized by growth retardation, severe nephropathy, diffuse edema, kyphosis of the spine, splenomegaly as a consequence of extramedullary hematopoiesis, and premature death. No specific abnormalities in elastin or other extracellular matrix proteins have been described in these mice to date. However, our (18) recent studies using neuraminidase inhibitors in cell culture and chick embryos provided strong evidence that neuraminidase activity was essential for normal elastic fiber assembly. We have now investigated the direct effect of a Neu1 deficiency on elastin metabolism in Neu1-null mice.
MATERIALS AND METHODS
Animals.
A breeding colony of mice heterozygous for the neuraminidase gene was established from the original colony (10). Crossing heterozygotes was necessary since the Neu1-null mice would not breed. Genotyping was performed as described previously (10). For the losartan experiment, the pregnant mothers were given losartan (0.6 g/l) in the drinking water starting on day 13 or 14 of pregnancy. After parturition, the mothers were provided the losartan-treated water until weaning at 28 days. Some of the weaned pups were continued on the losartan treatment for another 2 mo. Control littermates and Neu1-null mice were killed at various times during treatment, and the lungs and aorta were removed for histology and desmosine analysis. All animal research was approved by the University of Texas Health Science Center Animal Research Committee.
Biochemical assay.
The desmosine and hydroxyproline assays were performed on both fresh and fixed tissue from aorta and lung. The tissues were hydrolyzed in 6 N HCl for 24 h at 100°C. The hydrolysates were evaporated to dryness, redissolved in water, and analyzed for desmosine by RIA (42) and hydroxyproline by amino acid analysis. Protein content of the tissue hydrolysates was determined by a ninhydrin-based method (41).
Histology.
For histology, the tissues were fixed in Excell (American Master Tech, Lodi, CA). The lungs were fixed inflated under 20-cm pressure. Five-micrometer cross-sections of the aorta and lung were stained for elastin with Hart's elastic stain and counterstained with tartrazine. Sialomucins were detected with Alcian blue pH 2.5, counterstained with Kernechtrot solution. Immunohistochemistry was performed on paraffin-embedded sections of aorta and lung using a monoclonal anti-α-smooth muscle actin clone 1A4 (Sigma, St. Louis, MO), Fib-5 antibody from the laboratory of Elaine Davis, McGill University, and FBN-2 antibody from Lynn Saki, Shriners Hospital, Oregon. For electron microscopy, the descending thoracic aorta, lung, and skin were cut into small pieces and fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer containing 2.5% tannic acid, postfixed with 1% osmium tetroxide in the same buffer, dehydrated in ethanol, and embedded in Epon. This preparation gave high contrast of elastin when thin sections were stained with uranyl acetate and lead citrate.
Real-time PCR.
The hair was shaved from the back of the mice, and the skin was removed and immediately frozen in liquid nitrogen. Tissues were freeze-fractured and homogenized in 4 M guanidinium isothiocyanate buffer, layered over 5.7 M cesium chloride, and centrifuged overnight at 237,000 g to extract RNA. Concentration of RNA was measured, and purity was confirmed by spectroscopy. Reverse-transcription reactions were conducted with 2 μg of total RNA in a reaction volume of 20 μl. Each reaction contained 10 mM DTT, 0.5 mM dNTPs, 0.015 μg/μl random primers, 40 units of RNase inhibitor (Invitrogen, Carlsbad, CA), and 200 units of reverse transcriptase (Invitrogen). Primer sequences for amplifications were chosen using published cDNA sequences and the Primer Express program (Applied Biosystems, Foster City, CA). Primers were chosen such that the resulting amplicons would cross an exon junction thereby eliminating false positive signals from genomic DNA contamination. Primer sequences for Fbln5 were 162GGTCCAGGTCAAAGCCGTTT143 (GenBank BC006636). For tropoelastin, primers were 2433CTTTGGACTTTCTCCCATTTATCC2456 and 2596GGTCCCCAGAAGATCACTTTCTC2574 (GenBank U08210). SYBR Green was used for amplicon detection. Gene expression was normalized to expression of the housekeeping gene β2-microglobulin (B2M). Controls without template and without reverse-transcriptase reactions with water were run on each plate. All primer sets were tested to ensure that efficiency of amplification over a wide range of template concentrations was equivalent to that of B2M. PCR reactions were carried out in the ABI PRISM 7900HT sequence detection system (Applied Biosystems). The reverse-transcription product from 25-ng RNA was used as template, and reaction volumes (15 μl) contained 1× Master Mix (Applied Biosystems). Primer concentrations were 900 nM. Cycling conditions were 2 min at 50°C, followed by 10 min at 95°C, then 40 cycles of 15 s at 95°C, and 1 min at 60°C. A preprogrammed dissociation protocol was used after amplification to ensure that all samples exhibited a single amplicon. Levels of mRNA were determined using the ddCt method (Applied Biosystems) and expressed relative to the mean dCt from day 18 wild-type.
Statistical analysis.
Data are expressed as the mean with SD or in one instance as the mean with SE. Statistical significance was calculated using a computer software package (InStat; GraphPad, San Diego, CA).
RESULTS
Neu1-null mice exhibit a tight-skin phenotype.
One of the diagnostic abnormalities of Neu1-null mice was a very noticeable tight skin when pinched at the shoulder, which was virtually identical to the tight-skin phenotype described for homozygous Tsk mice, which have defects in collagen and elastin metabolism due to FBN-1 abnormalities (38). This tight-skin phenotype was 100% penetrant and more obvious in the Neu1-null males, since male mice have a thicker skin containing more collagen than their female counterparts (39). Male Neu1-null mice were unable to breed, and some developed genital prolapse. One female Neu1-null mouse became pregnant but died during parturition. Most Neu1-null pups were noticeably smaller than normal littermates before weaning but reached normal adult weights.
Neu1-null mouse lungs developed enlarged air spaces.
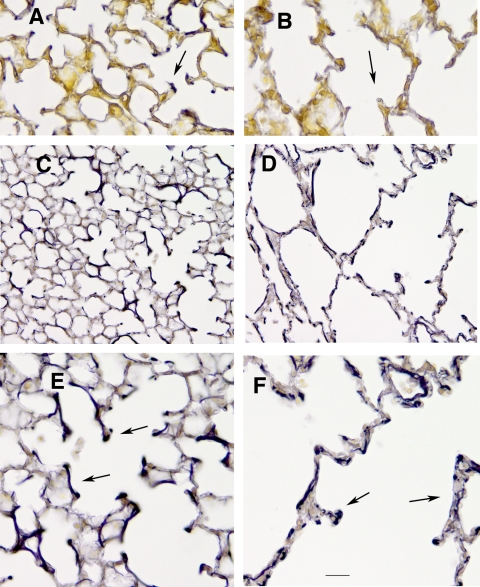
The lungs of Neu1-null mice were removed at various ages and compared with normal littermates histologically and biochemically. An emphysematous appearing lung with areas of dramatically enlarged air spaces was evident very early in neonatal development in the Neu1−/− mice and persisted throughout the life of the animals. Sections stained with Hart's elastin stain showed the appearance of elastic fibers at the apex of secondary crest of lungs from 5-day-old wild-type mice (Fig. 1A), but very little elastin was detected in 5-day-old Neu1−/− lungs (Fig. 1B). Even at this early age (5–7 days), the diminished alveolarization was evident in the affected mice that also displayed widespread emphysema-like lesions compared with their wild-type littermates (Fig. 1, C and D). At higher magnification, 7-day-old Neu1−/− lungs (Fig. 1F) showed abnormal structural organization with short, disrupted elastic fibers compared with solid, heavily stained fibers in the wild-type lungs (Fig. 1E). To assess the degree of alveolar involvement with age, we measured the mean linear intercepts of Neu1−/− and Neu1+/+ littermates (Fig. 2). Numbers of animals varied from 2 to 5 at each time point. The average mean linear intercept with SD for both groups between the ages of 7 days and 3 months was 25.8 ± 2.6 for wild-type mice and 53.5 ± 8.4 for Neu1−/− mice (P < 0.0001; n = 24 in each group). Although similar to the Tsk mouse (38), enlarged airways in the Neu1-null mice were not as uniformly involved, with areas of severe alveolar enlargement concentrated in the upper and lower regions of each lung. There was no evidence of increased numbers of inflammatory cells at any age during lung development in Neu1-null mice compared with their normal littermates.
Fig. 1.
Histology of neuraminidase-1 (Neu1)-null lungs. A and B: elastic fiber staining of 5-day control lungs (A) and Neu1-null littermate (B). Sections were stained with Hart's elastic stain and counterstained with tartrazine. Elastic fibers stain dark purple (arrows). C and D: control 7-day lung (C) compared with 7-day Neu1-null lung (D) illustrating the severe emphysema-like lesions in neonatal Neu1-null lungs. High magnification of 7-day control lungs (E) and Neu1-null lungs (F) depicting abnormal elastin deposition in the null lungs is shown (arrow). Bar: A and B = 22 μm; C and D = 44 μm; E and F = 11 μm.
Fig. 2.
Mean linear intercept (MLI) values as a function of age. Control lungs (solid line) and Neu1-null lungs (dashed line) are shown. Values represent the means from 2–5 mice at each time point.
Lungs from Neu1-null mice show defective myofibroblasts and microfibril distribution.
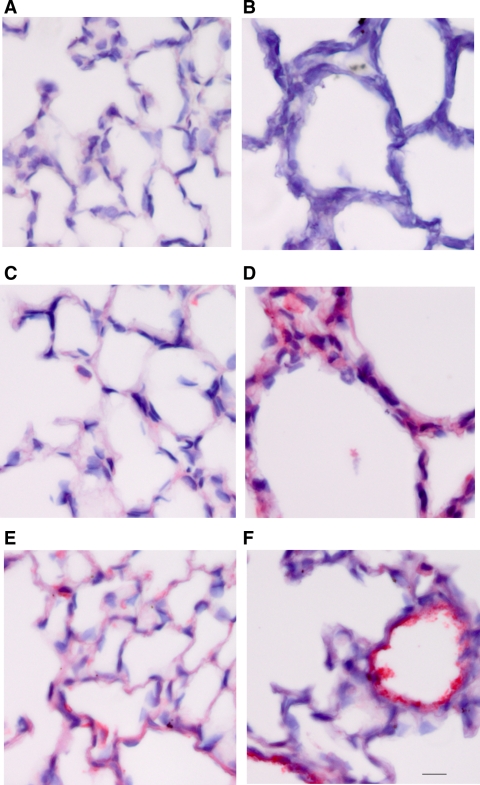
Immunostaining of lungs from 7-day-old wild-type mice with antibodies for α-actin revealed the typical concentration of myofibroblasts near the tips of the alveolar septa, the site of elastin synthesis and alveolar wall formation (Fig. 3A). In contrast, lungs from Neu1−/− mice displayed no area of concentration of myofibroblast as they tended to be disordered and dispersed around the alveolar walls (Fig. 3B). Likewise, immunostaining for Fib-5 showed normal distribution, particularly at the apex of developing secondary crest in 5-day-old wild-type lungs (Fig. 4A). In contrast, Fib-5 immunostaining was severely reduced in Neu1−/− lungs, and the fibers appeared disorganized (Fig. 4B). By day 7, control lungs showed well-developed alveoli with prominent Fib-5 staining around the air spaces and heavy, organized fibers surrounding the blood vessels (Fig. 4C). In the Neu1-null lungs, there was a failure of septation, resulting in abnormally large alveoli with Fib-5 dispersed in no apparent order around the alveolar walls (Fig. 4D). In control, young adult lungs (18 days), Fib-5 was prominently displayed around the alveolar walls and blood vessels (Fig. 4E), whereas in Neu1−/− lungs, Fib-5 was sparse and disrupted even in the small blood vessels (Fig. 4F). We also performed FBN-2 staining on sections from the same lung specimens (Fig. 5). Five-day-old wild-type lungs stained uniformly but very lightly around the alveolar walls (Fig. 5A). Alveolar walls were much thicker in the Neu1-null lungs, and very little if any staining for FBN-2 was evident (Fig. 5B). By 7 days of age, FBN-2 was visible as thin, well-organized fibers uniformly distributed around the alveolar walls of the control lung (Fig. 5C). In Neu1−/− mice of this age, FBN-2 was present in the alveolar walls but appeared diffuse and disorganized and not arranged in discrete fibers (Fig. 5D). In older animals (18 days), a well-organized pattern of FBN-2-positive fibers was observed around the alveolar walls and also as a fine lining around the endothelium of blood vessels of wild-type lungs (Fig. 5E), whereas the alveolar walls of the Neu1-null lungs were thicker than normal, and the FBN-2 staining was intense around the endothelium of blood vessels as well as the epithelial lining of airways (Fig. 5F) yet appeared diffuse and unorganized with no fibrillar structure (Fig. 5F).
Fig. 3.
Myofibroblast distribution in mouse lungs. Localization of myofibroblasts was determined by immunostaining for α-actin (red) in a 7-day control lung (A) and Neu1-null lung (B). Bar: A and B = 22 μm.
Fig. 4.
Fibulin-5 (Fib-5) immunostaining in mouse lung. Lung sections from 5-day-old mice showed a normal distribution of Fib-5 (red) in wild-type lungs, concentrating around the apex of developing secondary crest (A). Fib-5 immunostaining was less in the Neu1−/− lungs, and the fibers appeared disorganized (B). Control lungs at day 7 showed well-developed alveoli with prominent Fib-5 staining around the alveoli and heavy, organized fibers surrounded the blood vessels (C). Neu1-null lungs at 7 days were characterized by large alveoli and disordered Fib-5 around the alveolar walls (D). Fib-5 at 18 days was prominently displayed around the alveolar walls and blood vessels (E), whereas in Neu1−/− lungs, Fib-5 was sparse and disrupted, even in the small blood vessels (F). Bar = 22 μm.
Fig. 5.
Fibrillin-2 (FBN-2) immunostaining in mouse lung. Five-day-old wild-type lungs were uniformly and lightly stained for FBN-2 (red) around the alveolar walls (A). In 5-day Neu1-null lungs, the alveolar walls were much thicker, and very little if any staining for FBN-2 was evident (B). By 7 days of age, FBN-2 was visible as thin, well-organized fibers uniformly distributed around the alveolar walls of the control lung (C). FBN-2 was present in the alveolar walls of Neu1-null lungs but appeared diffuse and disorganized and not arranged in discrete fibers (D). At 18 days, a well-organized pattern of FBN-2-positive fibers was observed around the alveolar walls and around the endothelium of blood vessels of wild-type lungs (E). In Neu1-null lungs, the alveolar walls were thicker than normal, and although the FBN-2 staining was intense around the endothelium of blood vessels and epithelial lining of airways (F), it appeared diffuse and unorganized with no fibrillar structure (F). Bar = 22 μm.
Taken together, histomorphology and immunostaining of lung sections from wild-type and Neu1−/− mice indicate that Neu1−/− mice develop emphysematous lungs characterized by an abnormal distribution of myofibroblasts and severe disruption in the organization and distribution of FBN-2 and Fib-5, two proteins important for elastic fiber assembly.
Elastin concentration is reduced in aortas from Neu1-null mice.
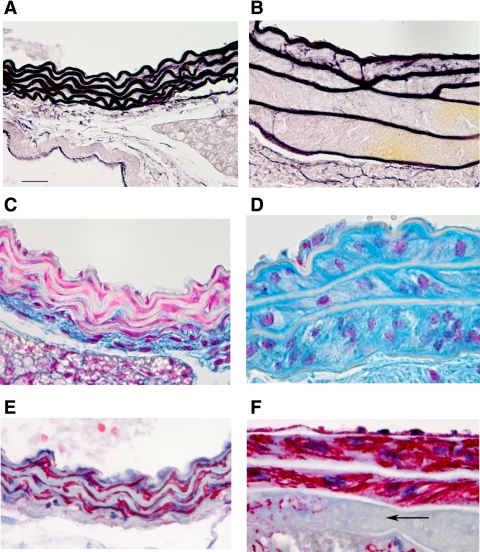
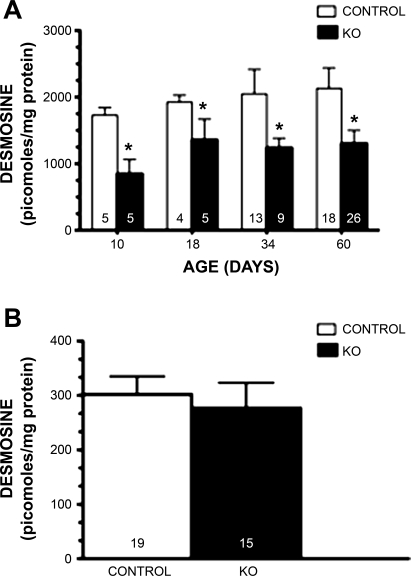
Elastic fiber staining of the aorta of control mice shows the typical closely aligned and wavy lamellae ∼7 μm in width with 2–3 μm of extracellular matrix between lamellae (Fig. 6A). Elastin lamellae from Neu1 aorta were usually thinner (3–5 μm) and rather straight with a significant separation of elastic lamellae, which on occasion distended to more than 10 times normal (Fig. 6B). Not all aortas were as severely separated, but every Neu1-null aorta showed significant lamellae separation. Staining for sialomucins with Alcian blue indicated that the area filling the space between the lamellae of Neu1-null mice (Fig. 6D) was much richer in sialic acid containing material (blue) compared with normal littermates (Fig. 6C). Elastin lamellae are viewed as nonstaining white bands lying parallel. Sialomucin staining in lungs was uniform, and we observed no consistent differences between control and Neu1-null lungs. This was true for neonates as well as adult mice. The interlamellar area in a normal mouse aorta contains smooth muscle cells (SMCs) as illustrated with an antibody to smooth muscle actin (Fig. 6E). This was true for the Neu1-null mouse aorta, however, due to the increased interlamellar space, there appeared to be more total smooth muscle present (Fig. 6F). On occasion, there were interlamellar areas where smooth muscle was completely absent (Fig. 6F, arrow). Elastin levels in the aorta of neonates and adult Neu1-null mice, as estimated by desmosine concentration, were significantly reduced compared with control mice of the same age (Fig. 7A). The lung, unlike the aorta, did not show significant differences in desmosine concentration between the Neu1-null and control littermates (Fig. 7B).
Fig. 6.
Histochemical staining of control and Neu1-null aorta for elastin, mucins, and smooth muscle. Sections stained for elastin with Hart's stain: control aorta (A) stained for elastin showing 5–6 elastic lamellae and a Neu1-null aorta (B) showing the abnormal separation between the elastic lamellae. C: control aorta stained for sialomucins with Alcian blue (blue) with elastic lamellae appearing opaque. D: Neu1-null aorta stained for sialomucins indicating the large mass of positive material between elastic lamellae. E and F: control aorta (E) immunostained for smooth muscle with an antibody to α-smooth muscle actin (red) showing the thin area between elastic lamellae and Neu1-null aorta (F) showing the increased mass of smooth muscle between elastic lamellae, whereas some areas were devoid of smooth muscle (arrow). Bar = 22 μm.
Fig. 7.
Desmosine levels of aorta and lung of control and Neu1-null (KO) littermates. Desmosine levels at different ages in control aorta (A) are shown in white bars, and desmosine levels for Neu1-null aorta in black. *P < 0.01. Lung desmosine (B) for control mice are shown in white bars and Neu1-null in black. Numbers of animals in each group are shown at the bottom of each bar.
Electron microscopy illustrates abnormal elastic fiber organization.
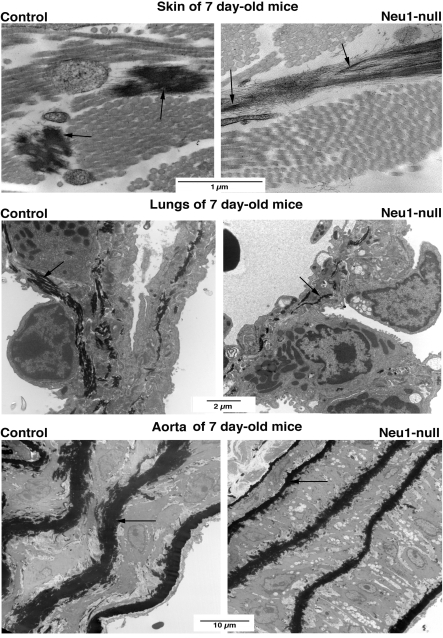
Electron microscopy confirmed that skin, lungs, and descending thoracic aortas of 7-day-old Neu1-null mice had significantly different organization of cells and extracellular matrix than their normal counterparts. The common feature detected in all the above-mentioned organs of Neu1−/− mice was the presence of vacuolated fibroblasts or SMCs that were surrounded by the loose extracellular matrix, usually more than normal number of collagen fibers and striking abnormalities of the elastic fibers system. In skin, the vacuolated fibroblasts were surrounded by dense bundles of collagen fibers of variable diameters. The elastic fibers were scarce, short, and highly immature. In fact, most of them consisted only of microfibrillar scaffold that was barely decorated with the single patches of the electron-dense elastin (Fig. 8, top, right). The electron microscopic analysis of the lung of Neu1−/− mice basically confirmed the aberrant structure of the lungs observed under light microscopy and additionally demonstrated that their alveolar septa contained fewer, thinner, and not entirely assembled elastic fibers compared with normal counterparts (Fig. 8, middle). Electron microscopy further demonstrated that grossly abnormal organization of aortic wall of the Neu1-deficient mice coincided with the presence of thinner and irregularly shaped elastic laminae that contained remarkably less electron-dense elastin than aortas of the age-matched control mice (Fig. 8, bottom).
Fig. 8.
Electron microscopy of skin, lung, and aorta of control and Neu1-null littermates. In the skin of Neu1-null mice (top, right), the scarce elastic fibers look highly immature. They mostly consist of parallel-oriented microfibrils, lightly decorated with single patches, electron-dense elastin (arrows). These immature fibers differ from the well-developed counterparts containing the electron-dense core (arrows) made of cross-linked elastin embedding the microfibrillar scaffold (top, left). In contrast to well-developed elastic fibers (containing electron-dense elastin) seen in the lungs of control mice (middle, left), lungs of Neu1-null mice (middle, right) demonstrate scarce, thin, and fragmented elastic fibers deposited by the vacuolated fibroblasts or smooth muscle cells (SMCs) (middle, right). The aortic wall of the Neu1-deficient mice (bottom, right) show grossly abnormal organization of vacuolated SMCs coinciding with the presence of thinner and irregularly shaped elastic laminae that contained remarkably less electron-dense elastin than aortas of the age-matched control mice (bottom, left).
α-1-Antitrypsin and blood glucose levels were reduced in Neu1-null mice.
α-1-Antitrypsin is a protease inhibitor, deficiency of which is associated with emphysema predominantly of the lower lungs. Blood glucose and α-1-antiprotease levels were measured in Neu1-null mice and compared with normal littermates. Blood glucose was significantly lower in Neu1-null mice before weaning 147 ± 20 mg/ml compared with 222 ± mg/ml for control mice (P < 0.05). In older adult mice, there was no difference between the null mice and controls. Serum α-1-antitrypsin levels in control and Neu1-null mice were measured in six neonates and six adult mice each. Maximum inhibitory capacity of control serum reached 55%, whereas the inhibitory capacity of serum from the deficient mice was significantly less at 45% (P < 0.01; Fig. 9). This was a consistent finding with similar results in both age groups (data shown are mean values from both age groups).
Fig. 9.
Serum α-1 antitrypsin levels of young and older mice. Percent inhibition of elastase with increasing concentrations of serum from control (solid line) and Neu1-null mice (dashed line). Each value represents the mean of 2 experiments of 6 mice each with the SE. OD, optical density.
Expression of tropoelastin and Fib-5 mRNA was normal in Neu1-null mice.
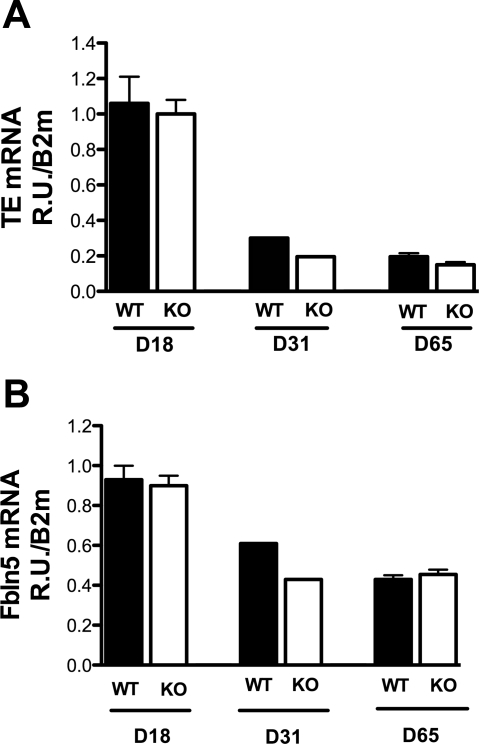
Since elastin and Fib-5 organization and staining patterns were abnormal in tissues from Neu1-null mice, mRNA levels of tropoelastin and Fib-5 were determined in adult and young wild-type and Neu1-null mice (Fig. 10). Although mRNA levels for both proteins were much higher in younger mice relative to older animals, expression of tropoelastin or Fib-5 was similar between Neu1−/− mice and wild-type+/+ littermates in all age groups (Fig. 10). These data suggest that abnormalities in elastic fibers in Neu1-null mice are not due to abnormal expression of Fib-5 or tropoelastin mRNA but that posttranslational modification of these proteins is more likely to be involved.
Fig. 10.
Expression of tropoelastin (TE) and fibulin-5 (Fbln5) mRNA in control and Neu1-null mice. mRNA for TE is shown in A for tissues from control (white bars) and Neu1-null (black bars) mice. In B, mRNA levels for fibulin-5 are shown for control (white bars) and Neu1-null animals (black bars). Age of mice was 18, 31, and 65 days (D). Values represent mean and SE of 6 mice at 18 and 31 days and 2 mice at 31 days. WT, wild-type.
Potential role of TGF-β signaling in defective elastogenesis in Neu1-null mice.
Studies by others have shown that errors in transforming growth factor-β (TGF-β) metabolism can cause phenotypic changes similar to the ones observed in the Neu1-null mouse (40). For example, increased TGF-β signaling is believed to play a major role in the development of emphysema in FBN-1-deficient mice, and the phenotype can be rescued by administration of anti-TGF-β antibodies or the drug losartan. To determine if TGF-β played a similar role in the matrix disorders observed in Neu1-null mice, we administered losartan to pregnant mothers and continued treatment for several months after parturition. Administration of losartan did not rescue any of the histological or biochemical defects observed in the Neu1-null mice. Specifically, histomorphometric features of lungs, aorta, and skin from 24 losartan-treated mice ranging from 7 to 60 days of age were identical to nontreated animals (data not shown).
DISCUSSION
Elastogenesis in most animal tissues is an early event that is initiated in the last trimester and continues at high levels during the prenatal period. During this developmental period, it is essential that other scaffolding proteins necessary for normal elastic fiber assembly are not only present, but also present in the correct geometric configuration. Altering the degree of normal glycosylation of these glycoproteins has the potential of impairing normal fiber assembly. In the present study, we have provided evidence that elastic fiber assembly in the lungs and aorta of Neu1-null mice is negatively impacted by the absence of Neu1 activity, an enzyme responsible for sialic acid removal from sialoglycoproteins in lysosomes and which is also transported to the cell surface as a component of the elastin receptor. Because mRNA levels for both tropoelastin and Fib-5 were normal in the Neu1-null mice, the defects we observed in pulmonary and vascular elastin most likely result from abnormal posttranslational modifications of these proteins that affect the proper assembly of the elastic fiber.
The decreased elastin content in the aortas of the Neu1-null mice was demonstrated by the highly significant loss in desmosine levels, a cross-link amino acid present only in mature elastic fibers. This decrease is apparently not due to progressive elastolysis, as the deficiency becomes evident in neonates and remains constant as the mice aged. Both light and electron microscopy indicated that, in addition to the presence of thinner and irregularly edged elastic laminae that lost their normal wavy appearance, the interlamellar spaces are filled with multiple vacuolated SMCs surrounded by a large amount of histochemically detected sialomucins. The thin elastic lamellae coupled with the increased volume of the extracellular space between lamellae accounts for the decreased elastin concentration. One would assume that the basic functioning of vessels demonstrating such pathological morphology should have been markedly impaired. Despite the severe distortion and thinning of the elastic lamellae, however, we did not have any indications of dissecting aneurysms in Neu1-null vessels.
The impaired elastic fiber formation and distribution is even more pronounced in lungs of Neu1-deficient mice and is associated with an alveolar enlargement that resembles emphysema. Since these abnormal alveoli are already present in neonates before the period of rapid alveolarization (normally occurring between 7 and 14 days of age) and do not worsen with age, we conclude that they are caused by a developmental problem due to the impaired primary elastogenesis and not to enzymatic degradation of previously deposited elastic fibers.
We also observed that the distribution of myofibroblasts in the developing lungs of the Neu1-null mice is disorganized. Instead of the normal location near the tips of the alveolar septa where alveolar wall formation is initiated, they appear randomly scattered around the alveolar wall. Alveolar myofibroblasts are interstitial contractile cells that are essential for secondary septation and that share many features of SMCs, including the production of elastin-rich extracellular matrix (5, 12, 43). Elastin expression is initiated early during the pseudoglandular stage of lung development, and, shortly after birth, elastin synthesis is upregulated with elastic fibers concentrating at the apex of developing secondary septal crests. Under normal conditions, the elastic fibers form rings around the alveolar openings and line the alveolar walls (6). This sequence of events is required for secondary septation to occur. The reason why Neu1 is required for normal myofibroblast location is not clear, but it has been recently proposed that it could modify specific domains of FBN-1 and FBN-2, interacting with lung fibroblast integrins to direct their migration (25). It is therefore possible that a deficiency of Neu1 activity may have a direct effect on signaling processes directing cell migration that ultimately results in defective alveolar formation. However, it is equally plausible that the observed displacement of the myofibroblasts could be secondary to the primarily impaired elastic fiber formation that normally guides or immobilizes myofibroblasts. The latter interpretation seems to be the most probable since similar delayed lung septation and abnormal alveolar enlargements have been reported in elastin-null mice (46) and in transgenic mice deficient in another component of the elastin receptor, PPCA, that also display impaired elastic fiber assembly (6).
Fib-5 localizes at the site of elastogenesis during alveolarization and maintains this close association throughout lung development. In the lungs from Neu1-null mice, we have identified defects in both Fib-5 and FBN-2, early in life and most notably at sites of elastic fiber formation. It is difficult to say whether the lower amounts and disorganization of Fib-5 and FBN-2 in the Neu1-null lungs is a primary defect resulting from the altered tropoelastin assembly on microfibrillar scaffold or whether accumulation of sialylated moieties extracellularly may also disrupt the initial formation of microfibrils.
α-1-Antitrypsin is a major protease inhibitor that assists in protecting the lung from elastin catabolism and is particularly important during acute lung injury and inflammation (16, 21). α-1-Antitrypsin is initially synthesized as a highly sialylated protein containing 7–8 sialic acid residues per mole of protein (2) and requires partial desialylation for proteinase blocking activity. We examined the possibility that the Neu1-null mice would have a deficiency in α-1-antitrypsin inhibitory capacity that might contribute to the observed lung damage. However, only a 10% reduction in total serum inhibitory capacity of α-1-antiprotease was detected in the Neu1-null mice compared with their wild-type counterparts. Whether this decrease bears a physiological significance is still unclear.
Abnormal storage and release of TGF-β by extracellular matrix proteins has been documented as a cause of elastin-related defects mediated through structural defects in FBN-1 (7). FBN-1-deficient mice demonstrate a failure of alveolar septation associated with elevated TGF-β signaling leading to an emphysematous appearance in the lung (33). This condition could be corrected by the administration of TGF-β-neutralizing antibodies. Association with FBN-1 stabilizes latent TGF-β complexes, whereas destabilization of FBN-1 leads to increased levels of active TGF-β (20). Mutations of FBN-1 in Marfan syndrome and the tight-skin mouse model (11, 40) lead to many of the phenotypic changes we have observed in the Neu1-null mice, which, to our knowledge, are the only other mutant mouse developing a tight skin. Although the molecular basis of this unique phenotype are not yet understood, it is thought to be related to destabilization of FBN-1 and resultant effects on TGF-β activity. In fact, addition of TGF-β blocking antibody or the TGF-β antagonists losartan, an angiotensin II type 1 receptor blocker, was shown to be an effective therapeutic means of preventing the pulmonary and vascular defects in a mouse model of Marfan syndrome (15). Since many of the pulmonary, vascular, and skin changes in Neu1-deficient mice resembled those observed in Marfan syndrome, we rationalized that lack of normal desialylation of extracellular matrix proteins in Neu1-null mice could lead to a similar defect in TGF-β activation. This appeared not to be the case, as administration of losartan prepartum and continued administration of losartan for several months after birth had no effect on the vascular, pulmonary, or tight-skin defects observed in these mice.
In summary, we have documented that elastin metabolism is severely disrupted in neuraminidase-1 deficiency. In both the aorta and lung, organs where elastin has an important physiological role, elastic fiber deposition was altered. At least two of the microfiber components required for normal assembly, Fib-5 and FBN-2, were also negatively affected. Since the initial expression of both elastin and Fib-5 gene products were not affected, we suggest that the elastic fiber pathology observed in Neu1-null mice is not due to primary defects in the production of elastic fiber components but reflected their impaired extracellular assembly.
GRANTS
This work was supported, in part, by Flight Attendants Medical Research Institute (B. Starcher); National Institutes of Health (NIH) Grant R01 GM060950, the Assisi Foundation, and the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude (A. d'Azzo); NIH Grant AG028048 (P. W. Keller); and the Canadian Institutes of Health Research Grant PG 13920 and the Heart and Stroke Foundation of Canada Grants NA 4381 and CI 4198 (A. Hinek).
Acknowledgments
We acknowledge the generous gifts of antibodies from Dr. Elaine Davis (Fib-5) and Lynn Saki (FBN-2).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Achyuthan KE, Achyuthan AM. Comparative enzymology, biochemistry and pathophysiology of human exo-alpha-sialidases (neuraminidases). Comp Biochem Physiol B Biochem Mol Biol 129: 29–64, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bolmer SD, Kleinerman J. Galactosamine-induced alpha 1-antitrypsin deficiency in rats. Alterations in plasma glycoproteins and alpha 1-antitrypsin carbohydrate composition. Am J Pathol 126: 209–219, 1987. [PMC free article] [PubMed] [Google Scholar]
- 3.Bonten EJ, Arts WF, Beck M, Covanis A, Donati MA, Parini R, Zammarchi E, d'Azzo A. Novel mutations in lysosomal neuraminidase identify functional domains and determine clinical severity in sialidosis. Hum Mol Genet 9: 2715–2725, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bonten EJ, D'Azzo A. Lysosomal neuraminidase. Catalytic activation in insect cells is controlled by the protective protein/cathepsin A. J Biol Chem 275: 37657–37663, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Brody JS, Vaccaro C. Postnatal formation of alveoli: interstitial events and physiologic consequences. Fed Proc 38: 215–223, 1979. [PubMed] [Google Scholar]
- 6.Caciotti A, Donati MA, Bardelli T, d'Azzo A, Massai G, Luciani L, Zammarchi E, Morrone A. Primary and secondary elastin-binding protein defect leads to impaired elastogenesis in fibroblasts from GM1-gangliosidosis patients. Am J Pathol 167: 1689–1698, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charbonneau NL, Ono RN, Corson GM, Keene DR, Sakai LY. Fine tuning of growth factor signals depends on fibrillin microfibril networks. Birth Defects Res C Embryo Today 72: 37–50, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cleary EG, Gibson MA. Elastin-associated microfibrils and microfibrillar proteins. Int Rev Connect Tissue Res 10: 97–209, 1983. [DOI] [PubMed] [Google Scholar]
- 9.D'Azzo A, Andria G, Strisiuglioi P, Galjard H. The Metabolic and Molecular Bases of Inherited Disease (8th ed.), edited by Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, and Vogelstein B. New York: McGraw-Hill, 2001, p. 3811–3826.
- 10.De Geest N, Bonten E, Mann L, de Sousa-Hitzler J, Hahn C, d'Azzo A. Systemic and neurologic abnormalities distinguish the lysosomal disorders sialidosis and galactosialidosis in mice. Hum Mol Genet 11: 1455–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, Stetten G, Meyers DA, Francomano CA. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352: 337–339, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda Y, Ferrans VJ, Crystal RG. The development of alveolar septa in fetal sheep lung. An ultrastructural and immunohistochemical study. Am J Anat 167: 405–439, 1983. [DOI] [PubMed] [Google Scholar]
- 13.Gopaul KP, Crook MA. The inborn errors of sialic acid metabolism and their laboratory investigation. Clin Lab 52: 155–169, 2006. [PubMed] [Google Scholar]
- 14.Green MC, Sweet HO, Bunker LE. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol 82: 493–512, 1976. [PMC free article] [PubMed] [Google Scholar]
- 15.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiemstra PS Novel roles of protease inhibitors in infection and inflammation. Biochem Soc Trans 30: 116–120, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Hinek A, Keeley FW, Callahan J. Recycling of the 67-kDa elastin binding protein in arterial myocytes is imperative for secretion of tropoelastin. Exp Cell Res 220: 312–324, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Hinek A, Pshezhetsky AV, von Itzstein M, Starcher B. Lysosomal sialidase (neuraminidase-1) is targeted to the cell surface in a multiprotein complex that facilitates elastic fiber assembly. J Biol Chem 281: 3698–3710, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Igdoura SA, Gafuik C, Mertineit C, Saberi F, Pshezhetsky AV, Potier M, Trasler JM, Gravel RA. Cloning of the cDNA and gene encoding mouse lysosomal sialidase and correction of sialidase deficiency in human sialidosis and mouse SM/J fibroblasts. Hum Mol Genet 7: 115–121, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278: 2750–2757, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Janoff A, Carp H. Proteases, antiproteases, and oxidants: pathways of tissue injury during inflammation. Monogr Pathol 23: 62–82, 1982. [PubMed] [Google Scholar]
- 22.Kielty CM Elastic fibers in health and disease. Expert Rev Mol Med 8: 1–23, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Malvagia S, Morrone A, Caciotti A, Bardelli T, d'Azzo A, Ancora G, Zammarchi E, Donati MA. New mutations in the PPBG gene lead to loss of PPCA protein which affects the level of the beta-galactosidase/neuraminidase complex and the EBP-receptor. Mol Genet Metab 82: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Markova MS, Zeskand J, McEntee B, Rothstein J, Jimenez SA, Siracusa LD. A role for the androgen receptor in collagen content of the skin. J Invest Dermatol 123: 1052–1056, 2004. [DOI] [PubMed] [Google Scholar]
- 25.McGowan SE, Holmes AJ, Mecham RP, Ritty TM. Arg-Gly-Asp-containing domains of fibrillins-1 and -2 distinctly regulate lung fibroblast migration. Am J Respir Cell Mol Biol 38: 435–445, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Mecham RP Elastic fibers. In: The Lung: Scientific Foundations, edited by Crystal RG, Weibel ER, and Barns PJ. Philadelphia, PA: Lippincott-Raven, p. 729–736.
- 27.Miyagi T, Hata K, Hasegawa A, Aoyagi T. Differential effect of various inhibitors on four types of rat sialidase. Glycoconj J 10: 45–49, 1993. [DOI] [PubMed] [Google Scholar]
- 28.Monti E, Bassi MT, Papini N, Riboni M, Manzoni M, Venerando B, Croci G, Preti A, Ballabio A, Tettamanti G, Borsani G. Identification and expression of NEU3, a novel human sialidase associated to the plasma membrane. Biochem J 349: 343–351, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monti E, Bassi MT, Bresciani R, Civini S, Croci GL, Papini N, Riboni M, Zanchetti G, Ballabio A, Preti A, Tettamanti G, Venerando B, Borsani G. Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics 83: 445–453, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Morreau H, Galjart NJ, Gillemans N, Willemsen R, van der Horst GT, d'Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem 264: 20655–20663, 1989. [PubMed] [Google Scholar]
- 31.Morreau H, Galjart NJ, Willemsen R, Gillemans N, Zhou XY, d'Azzo A. Human lysosomal protective protein. Glycosylation, intracellular transport, and association with beta-galactosidase in the endoplasmic reticulum. J Biol Chem 267: 17949–17956, 1992. [PubMed] [Google Scholar]
- 32.Morrone A, Bardelli T, Donati MA, Giorgi M, Di Rocco M, Gatti R, Parini R, Ricci R, Taddeucci G, D'Azzo A, Zammarchi E. Beta-galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum Mutat 15: 354–366, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Pattison S, Pankarican M, Rupar CA, Graham FL, Igdoura SA. Five novel mutations in the lysosomal sialidase gene (NEU1) in type II sialidosis patients and assessment of their impact on enzyme activity and intracellular targeting using adenovirus-mediated expression. Hum Mutat 23: 32–39, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Pshezhetsky AV, Richard C, Michaud L, Igdoura S, Wang S, Elsliger MA, Qu J, Leclerc D, Gravel R, Dallaire L, Potier M. Cloning, expression and chromosomal mapping of human lysosomal sialidase and characterization of mutations in sialidosis. Nat Genet 15: 316–320, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez F, Carta L, Lee-Arteaga S, Liu C, Nistala H, Smaldone S. Fibrillin-rich microfibrils - structural and instructive determinants of mammalian development and physiology. Connect Tissue Res 49: 1–6, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Saito N, Yu RK. Biology of Sialic Acids, edited by Rosemberg A. New York: Plenum Press, 1995, p. 261–313.
- 38.Sato K, Miyagi T. Genomic organization and the 5′-upstream sequence of the rat cytosolic sialidase gene. Glycobiology 5: 511–516, 1995. [DOI] [PubMed] [Google Scholar]
- 39.Sicot FX, Tsuda T, Markova D, Klement JF, Arita M, Zhang RZ, Pan TC, Mecham RP, Birk DE, Chu ML. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol Cell Biol 28: 1061–1067, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siracusa LD, McGrath R, Ma Q, Moskow JJ, Manne J, Christner PJ, Buchberg AM, Jimenez SA. A tandem duplication within the fibrillin 1 gene is associated with the mouse tight skin mutation. Genome Res 6: 300–313, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Starcher B A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal Biochem 292: 125–129, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Starcher B, Conrad M. A role for neutrophil elastase in the progression of solar elastosis. Connect Tissue Res 31: 133–140, 1995. [DOI] [PubMed] [Google Scholar]
- 42a.Tatano Y, Takeuchi N, Kuwahara J, Sakuraba H, Takahashi T, Takada G, Itoh K. Elastogenesis in cultured dermal fibroblasts from patients with lysosomal β-galactosidase, protective protein/cathepsin A and neuraminidase-1 deficiencies. J Med Invest 53: 103–112, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Viscardi R, Manimtim W, He JR, Hasday JD, Sun CC, Joyce B, Pierce RA. Disordered pulmonary myofibroblast distribution and elastin expression in preterm infants with Ureaplasma urealyticum pneumonitis. Pediatr Dev Pathol 9: 143–151, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Wada T, Yoshikawa Y, Tokuyama S, Kuwabara M, Akita H, Miyagi T. Cloning, expression, and chromosomal mapping of a human ganglioside sialidase. Biochem Biophys Res Commun 261: 21–27, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 81: 229–240, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi K, Hata K, Koseki K, Shiozaki K, Akita H, Wada T, Moriya S, Miyagi T. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochem J 390: 85–93, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 415: 168–171, 2002. [DOI] [PubMed] [Google Scholar]