Abstract
Fibroblasts are key structural cells that can be damaged by cigarette smoke. Cigarette smoke contains many components capable of eliciting oxidative stress, which may induce heme oxygenase (HO)-1, a cytoprotective enzyme. There are no data on HO-1 expression in primary human lung fibroblasts after cigarette smoke extract (CSE) exposure. We hypothesized that human lung fibroblasts exposed to cigarette smoke would increase HO-1 though changes in intracellular glutathione (GSH). Primary human lung fibroblasts were exposed to CSE, and changes in HO-1 expression and GSH levels were assessed. CSE induced a time- and dose-dependent increase in expression of HO-1, but not HO-2 or biliverdin reductase, in two different primary human lung fibroblast strains, a novel finding. This induction of HO-1 paralleled a decrease in intracellular GSH, and a sustained reduction in GSH resulted in a dramatic increase in HO-1. Treatment with the antioxidants N-acetyl-l-cysteine or GSH reduced the expression of HO-1 induced by CSE. We also examined the signal transduction mechanism responsible for HO-1 induction. Nuclear factor erythroid-derived 2, like 2 (Nrf2) was not involved in HO-1 induction by CSE. Activator protein-1 (AP-1) is a redox-sensitive transcription factor shown in other systems to regulate HO-1 expression. CSE exposure resulted in nuclear accumulation of c-Fos and c-Jun, two key AP-1 components. Reduction of c-Fos and c-Jun nuclear translocation by SP-600125 attenuated the CSE-induced expression of HO-1. These data support the concept that changes in the cellular redox status brought on by cigarette smoke induce HO-1 in fibroblasts. This increase in HO-1 may help protect against cigarette smoke-induced inflammation and/or cell death.
Keywords: oxidative stress; activator protein-1; biliverdin reductase; chronic obstructive pulmonary disease; nuclear factor erythroid-derived 2, like 2
heme oxygenase (HO) enzymes catalyze the rate-limiting step in the oxidative degradation of heme to form equimolar amounts of ferrous iron, carbon monoxide (CO), and biliverdin (60, 69). Biliverdin is subsequently converted to bilirubin by biliverdin reductase (BVR). Two isozymes of HO have been well-characterized: an inducible form, HO-1, and the constitutive form, HO-2. Under basal condition, HO-1 occurs at low to undetectable levels in most tissues, but its expression is rapidly increased in response to a variety of environmental stimuli, particularly those that produce oxidative stress and generate reactive oxygen species (49, 60).
Cigarette smoke contains many compounds capable of eliciting oxidative stress, yielding an estimated 1017 oxidant molecules per puff (14). Oxidative stress caused by cigarette smoke leads to bronchial and alveolar inflammation and lung cell death. This lung inflammation and cell death may lead to chronic obstructive pulmonary disease (COPD; chronic bronchitis and emphysema) and lung cancer in susceptible individuals. Cigarette smoke induces HO-1 expression in several cell types, including alveolar epithelial cells (23, 65), macrophages (5), and mouse embryonic fibroblasts (33, 43). We (41, 42) and others (10, 12) have identified human pulmonary fibroblasts as an important target of cigarette smoke. Fibroblasts are the main cell type in the lung interstitium and are vital in the production of extracellular matrix for tissue maintenance and repair. They provide structural support to the alveolar compartment and are potent producers of proinflammatory mediators (42). We recently reported (6) that fibroblasts from different human beings vary in their susceptibility to cigarette smoke-induced cell death, a feature that is proportional to the ability of the cell to regulate intracellular glutathione (GSH) levels. GSH is the principal antioxidant in the lung (31, 54). GSH homeostasis is essential for normal lung function, and alterations in GSH levels can submit cells to oxidative stress (53). GSH levels decrease after cigarette smoke exposure (6, 53). Induction of HO-1 in response to GSH depletion has been shown in the mouse (58) and rat (49) liver; this GSH-related increase in HO-1 is proposed to have antiapoptotic and/or antioxidant properties (57, 60, 64, 72) that would serve to counteract the loss of intracellular GSH. We hypothesized that cigarette smoke would induce HO-1 in lung fibroblasts because of alterations in intracellular GSH levels.
The molecular mechanisms involved in the induction of HO-1 are diverse and include transcription factors such as nuclear factor-κB (NF-κB), mitogen-activated protein kinases (MAPKs), activator protein-1 (AP-1) and nuclear factor, erythroid-derived 2, like 2 (Nrf2) (4, 33, 38, 39, 74). These transcription factors are sensitive to conditions of oxidative stress, particularly Nrf2. Nrf2 readily translocates to the nucleus in epithelial cells in response to cigarette smoke (34), and Nrf2 deficiency exacerbates cigarette smoke-induced emphysema (28, 55). We speculated that this induction of HO-1 by cigarette smoke would involve redox-sensitive transcription factors, particularly Nrf2. Here, we demonstrate that cigarette smoke induces HO-1 expression because of a sustained decrease in intracellular GSH levels. Importantly, Nrf2 was not responsible for the induction of HO-1 by cigarette smoke in human lung fibroblasts. Blockade of AP-1 and NF-κB partially attenuated CSE-induced HO-1. These new findings suggest that there are cell-specific differences in the regulation of HO-1 expression. The upregulation of HO-1 may have a protective role in lung fibroblasts to counteract oxidative stress caused by cigarette smoke.
MATERIALS AND METHODS
Chemicals
N-acetyl-l-cysteine (NAC), glutathione reduced ethyl ester, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), β-reduced nicotinamide adenine dinucleotide phosphate (β-NADPH), glutathione reductase, 5-sulfosalicylic acid, hemin, and dl-buthionine-[S,R]-sulfoximine (BSO) were purchased from Sigma (St. Louis, MO). SP-600125 was purchased from Axxora (San Diego, CA). The ERK1/2 inhibitor U0126 was obtained from Cell Signaling Technology (Danvers, MA). The NF-κB inhibitor SC-514 was purchased from Calbiochem (Gibbstown, NJ).
Cell Culture
Primary human fetal lung fibroblasts (HFL-1) and A549 epithelial cells were purchased from the American Type Culture Collection (Manassas, VA). The primary human neonatal lung fibroblast strain L828 was established by us as previously described (22) from lung biopsies of morphologically normal lung by a tissue explant technique (7). Mouse lung fibroblasts (MLFs) and the primary adult human lung fibroblast strain CH2 were derived in the same manner. These cells were previously identified as fibroblasts by their morphology, adherent nature, expression of vimentin and types I and III collagen, and lack of expression of cytokeratin, α-smooth muscle actin, factor VIII, and CD45 (7). All cells were cultured in minimum essential medium (MEM) supplemented with 2 mM glutamine (Invitrogen, Carlsbad, CA) and 10% fetal bovine serum (FBS) (Hyclone Labs, Logan UT). Cells were maintained at 37°C and incubated in humidified 5% CO2-95% air. Fibroblasts were studied at passage 15 or below.
Preparation of Cigarette Smoke Extract
Research grade cigarettes (1R3F) with a filter were obtained from the Kentucky Tobacco Research Council (Lexington, KT). Cigarette smoke extract (CSE) was prepared by bubbling smoke from two cigarettes into 20 ml of serum-free MEM with a modification of the method developed by Carp and Janoff (11) and as previously described by us (6, 41, 42). The pH of the MEM was adjusted to 7.4, and the medium was sterile filtered with a 0.45-μm filter (25 mm Acrodisc; Pall, Ann Arbor, MI). The CSE (called 100%) was prepared immediately before use. To ensure consistency in the CSE between experiments, measurements of optical density were taken at a wavelength of 320 nm immediately after preparation of the CSE. An optical density of 0.65 was considered to represent 100% CSE. This CSE preparation was diluted to the appropriate concentration in serum-free MEM.
Western Blot Analysis
Equivalent numbers of primary human lung fibroblasts were grown to confluence and serum starved for 24 h. Cells were then treated with varying percentages of CSE for selected times. Total cellular protein was prepared from fibroblasts with 1% IGEPAL lysis buffer supplemented with a protease inhibitor cocktail (leupeptin, aprotinin, pepstatin, and PMSF; Sigma). Cell lysates were centrifuged (14,000 g, 4°C for 10 min) to remove debris, and protein quantitation was performed with the bicinchoninic acid (BCA) method according to the manufacturer's instructions (Pierce, Rockford, IL). Five micrograms of total cellular protein was fractionated on 10% SDS-PAGE gels, electroblotted onto Immun-blot polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA), and blocked with 5% nonfat dry milk in 0.1% Tween 20 (in PBS) overnight at 4°C. Antibodies against HO-1 (1:5,000), HO-2 (1:5,000), BVR (1:5,000; Stressgen Bioreagents, Victoria, BC, Canada), phospho-ERK1/2 (1:1,000; Cell Signaling Technology), and actin (1:20,000; Oncogene Research Products, San Diego, CA) were used to assess changes in protein levels following exposure of the fibroblasts to CSE. In some experiments, cells were pretreated with 10 μM U0126 for 2 h to inhibit ERK1/2 activation. To assess the effect of NF-κB inhibition on CSE-induced HO-1 expression, HFL-1 cells were pretreated for 1 h with SC-514 (20 μM) followed by cotreatment with CSE. The antibody against Nrf2 (R&D Systems, Minneapolis, MN) was used at 1:500. Protein was visualized by enhanced chemiluminescence (NEN Life Science Products, Boston, MA) and developed on Classic X-ray film (Laboratory Product Sales, Rochester, NY). Densitometric analysis of protein expression was performed with Kodak 1D Imaging Software (Kodak Scientific Imaging Systems, New Haven, CT); values are normalized to total actin.
Real-Time RT-PCR
After treatment with CSE, total RNA was isolated from human lung fibroblasts with the RNeasy RNA isolation kit according to the manufacturer's instructions (Qiagen, Crawley, UK). RNA was reverse transcribed to cDNA, and HO-1 and HO-2 mRNA were quantified with the following primers (24): HO-1: 5′-CAGGCAGAGAATGCTGAGTTC-3′ (sense) and 5′-GCTTCACATAGCGCTGCA-3′ (antisense); HO-2: 5′-GCAATGTCAGCGGAAGTGGAA-3′ (sense) and 5′-AAGTCACCTGAGGTGGTAGTT-3′ (antisense) (24); GAPDH: 5′-AGGTGAAGGTCGGAGTCAAC-3′ (sense) and 5′-TGGGTGGAATCATATTGGAAC-3′ (antisense). Cycle threshold values were determined with a standard curve and analyzed with Bio-Rad Icycler Software (Bio-Rad Laboratories). Values were normalized to GAPDH, and fold change was compared between untreated and CSE-treated fibroblasts.
Immunocytochemical Staining
Immunocytochemistry for HO-1 and HO-2.
To assess HO induction, fibroblasts (HFL-1) were seeded on eight-well glass chamber slides at a density of 1 × 104 cells/well, allowed to adhere for 24 h, and serum starved for 24 h. Cells were either left untreated or treated with 1% CSE or BSO (100 μM) for 24 h. After this, cells were washed once with PBS-Tween 20 (0.1%), fixed with 3% H2O2 for 15 min, and blocked with 5% normal goat serum. Antibodies against HO-1 and HO-2 were diluted in PBS-BSA (1:500) and incubated overnight at 4°C. To assess the level of nonspecific staining, cells were incubated under the same conditions with a rabbit IgG isotype antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Biotinylated anti-rabbit antibody was used for secondary binding (1:200). After application of the secondary antibody, the cells were incubated with streptavidin-horseradish peroxidase and antibody binding was visualized with the substrate 3-amino-9-ethylcarbazol (AEC; Zymed, South San Francisco, CA). Finally, cells were coverslipped in Immu-mount (Shanndon, Pittsburgh, PA), viewed with an Olympus BX51 microscope (New Hyde Park, NY), and photographed with a SPOT camera with SPOT RT software (New Hyde Park, NY).
Nrf2 and HO-1 immunofluorescence.
Human lung fibroblasts (HFL-1), MLFs, and epithelial cells (A549) were cultured on glass chamber slides as described above. After serum starvation for 24 h, cells were treated with CSE or hemin (5 μM) for 4 or 6 h; all three types of cells were treated at the same time with the identical stock reagents. Antibodies against HO-1 and Nrf2 (Santa Cruz Biotechnology; 1:200) were diluted in PBS-BSA. After secondary binding with biotinylated anti-rabbit antibody, cells were incubated with streptavidin-FITC. Cells were coverslipped in Vectashield and photographed. All photographs were taken at the same time with identical image settings.
c-Fos and c-Jun immunofluorescence.
After treatments, HFL-1 cells were washed in PBS-Tween 20 and nonspecific binding was blocked with 5% normal goat serum. Antibodies against c-Fos and c-Jun (1:200; Santa Cruz Biotechnology) were incubated overnight at 4°C, followed by incubation with the biotinylated secondary antibody and FITC-conjugated streptavidin as described above.
Measurement of Total Intracellular GSH
HFL-1 cells were grown to confluence in 25-cm2 cell culture flasks and treated with control medium, BSO, or varying percentages of CSE for 1, 3, 6, and 24 h. Measurements of intracellular GSH were as previously described (6, 52). Briefly, after treatments, monolayers of fibroblasts were washed with 2 ml of ice-cold PBS and scraped into 300 μl of ice-cold extraction buffer (0.1% Triton X-100-0.6% sulfosalicylic acid in 0.1 M phosphate buffer with 5 mM EDTA, pH 7.5). Cells were then sonicated (30 s), vortexed (20 s), and centrifuged (2,000 rpm for 5 min at 4°C). Determination of total intracellular levels of GSH was performed as originally described by Tietze (70) with DTNB-GSSG/glutathione reductase recycling (31). Results are in nanomoles of GSH per milligram of protein.
Statistical Analysis
Statistical analysis was performed with Statview V5.0 (SAS Institute, Cary, NC), and analysis of variance (ANOVA) and Fisher's post hoc test were used to assess differences between multiple treatment groups. P < 0.05 is considered to be statistically significant.
RESULTS
CSE Increases HO-1 Protein and mRNA Expression in Primary Lung Fibroblast Strains
Nothing is known about HO-1 expression in primary human lung fibroblasts. Therefore, we examined the expression of HO-1 and HO-2 in response to cigarette smoke in primary strains of human lung fibroblasts. To determine whether cigarette smoke could induce HO-1 expression, two primary strains of lung fibroblasts (both from different human beings) were left untreated (control medium) or were treated with increasing percentages of CSE (0.25–5%) for 24 h and HO-1 protein expression was examined by Western blot analysis. The basal level of HO-1 expression was low in both of the fibroblast strains tested (Fig. 1A). In response to CSE, HO-1 expression dose-dependently increased. A slight induction in HO-1 was observed in the HFL-1 and L828 fibroblast strains when these cells were exposed to 0.5% CSE. This expression continued to increase with increasing percentages of CSE, with the strongest induction occurring when the fibroblasts were exposed to 5% CSE (Fig. 1).
Fig. 1.
Cigarette smoke extract (CSE) induces a dose- and time-dependent increase in heme oxygenase (HO)-1 protein in human lung fibroblasts. Primary human lung fibroblast strains HFL-1 and L828 were treated with varying percentages of CSE, and HO-1, HO-2, and total actin levels were assessed by Western blot analysis. A: there was a dose-dependent increase in HO-1 protein expression in both HFL-1 and L828 human lung fibroblast strains. B: there was a time-dependent increase in HO-1 in response to 1% CSE, beginning between 2 and 8 h after exposure. The induction of HO-1 by 1% CSE declined by 48 h in the HFL-1 fibroblast strain but persisted in the L828 fibroblast strain. Western blots are representative of 3 independent experiments.
Next, the kinetics of HO-1 induction was examined in these fibroblast strains exposed to 1% CSE. We showed previously (42) that this percentage of CSE potently activates human lung fibroblasts but is nonapoptotic in these lung fibroblast strains (6). Induction of HO-1 following exposure to 1% CSE occurred between 2 and 8 h after exposure (Fig. 1B). The expression of HO-1 decreased between 24 and 48 h in the HFL-1 fibroblast strain. By contrast, in the L828 fibroblast strain, HO-1 expression persisted through 72 h of CSE exposure, the latest time point examined (Fig. 1B). The expression of HO-2 was constitutive in both fibroblast strains and did not change as a consequence of CSE exposure (Fig. 1B). Because both fibroblast strains exhibited similar increases in HO-1 in response to CSE at 24 h, the majority of the remaining experiments were conducted with the commercially available HFL-1 fibroblast strain, with supplementation of key data from the L828 fibroblast strain.
To investigate whether the increase in HO-1 expression also occurred at the mRNA level, we examined the induction of HO-1 and HO-2 mRNA in CSE-treated HFL-1 cells. Consistent with the expression of HO-1 protein, steady-state mRNA levels dramatically increased (≈12 fold) by 8 h after exposure to 2% CSE (Fig. 2). HO-1 mRNA levels decreased over time and by 24 h after exposure were similar to untreated mRNA levels. Steady-state HO-2 mRNA levels were unaffected by CSE exposure (data not shown).
Fig. 2.
Increased steady-state HO-1 mRNA levels in CSE-treated HFL-1 cells. HO-1 mRNA levels were measured in HFL-1 human lung fibroblasts exposed to 2% CSE for 30 min to 24 h by real-time PCR. A maximum 12-fold induction of HO-1 mRNA was detected after exposure to 2% CSE for 8 h. Cycle threshold values were normalized to GAPDH.
Human Lung Fibroblasts Express Biliverdin Reductase
We next examined the expression of BVR in both HFL-1 and L828 human lung fibroblast strains. Whether BVR is expressed in human pulmonary cells, including fibroblasts, or whether BVR expression changes in response to cigarette smoke, is unknown. We therefore performed Western blot analysis to assess BVR expression in primary lung fibroblasts exposed to CSE. BVR was constitutively expressed in both the HFL-1 and L828 fibroblast strains, with a band of ≈41 kDa (Fig. 3), the mass of human BVR (40, 48). Unlike HO-1, BVR expression was unchanged as a result of CSE exposure (Fig. 3).
Fig. 3.
Biliverdin reductase (BVR) is expressed in human lung fibroblasts. Human lung fibroblast strains HFL-1 and L828 were treated with control medium (−) or with increasing percentages of CSE for 24 h, and Western blot analysis was performed for BVR. BVR is constitutively expressed in human lung fibroblasts, where a band of ≈41 kDa was observed. BVR expression was not altered by exposure to CSE.
CSE-Induced HO-1 in HFL-1 Cells is a Result of a Sustained Decrease in Intracellular GSH
To first examine the link between GSH and HO-1, we used the GSH-depleting agent BSO to reduce intracellular GSH levels (25, 53). We treated HFL-1 cells with BSO (25, 50, and 100 μM) for 6 or 24 h and then examined GSH and HO-1 levels. Treatment with BSO dose-dependently decreased intracellular GSH levels in HFL-1 cells (Fig. 4A). Exposure to 50 and 100 μM BSO for 6 h, and 100 μM for 24 h, induced a significant decrease in GSH. Concomitant with the decrease in GSH, there was a corresponding increase in HO-1 protein expression (Fig. 4A), with HO-1 expression being strongly induced when there was a sustained decrease in GSH for 24 h. Immunocytochemical staining revealed similar changes in HO-1 expression. Here, treatment with either BSO (100 μM) or 1% CSE for 24 h resulted in an increase in HO-1 (Fig. 4B, left). HO-2 levels were constitutive and did not change as a result of either BSO or CSE exposure (Fig. 4B, center).
Fig. 4.
HO-1 is strongly induced in HFL-1 cells in response to sustained decreases in intracellular glutathione (GSH). Human lung fibroblast strain HFL-1 cells were treated with dl-buthionine-[S,R]-sulfoximine (BSO) or CSE, and GSH levels were assessed as described in materials and methods. HO levels were examined by Western blotting and immunocytochemistry. A: treatment with BSO significantly decreased GSH levels. Representative Western blot shows that there was a concomitant increase in HO-1 expression. Note that HO-1 expression is strongest when GSH levels are depleted for 24 h. Results are expressed as means ± SE; n = 3 or 4. *P < 0.05, statistical difference between untreated and treated cells. B: immunocytochemical staining: treatment with BSO (100 μM) or 1% CSE for 24 h increased HO-1, but not HO-2, expression. C: CSE induced a dose-dependent decrease in GSH and a corresponding increase in HO-1 expression (representative Western blot shown). Results are expressed as means ± SE; n = 3 or 4. *P < 0.05, statistical difference between untreated and CSE-treated cells.
To determine whether the CSE-induced increase in HO-1 correlated with decreased GSH levels, we treated HFL-1 fibroblasts with increasing percentages of CSE for 1, 3, 6, or 24 h and measured GSH and HO-1 levels. A small decrease in GSH occurred within 1 h of exposure with 1% CSE (Fig. 4C). HFL-1 fibroblasts were able to recover GSH levels, and at 6 h after exposure to 1% CSE GSH levels were significantly greater compared with untreated. HO-1 levels were also increased at the 24 h time point (Fig. 4C, compare lanes 1 and 5). Treatment with 2% CSE for 1 and 3 h caused a significant decrease in GSH (Fig. 4C); here, HO-1 levels were detectable 1 h after exposure (Fig. 4C, compare lanes 1 and 6). GSH levels recovered by 6 h, and by 24 h after exposure to 2% CSE the amount of intracellular GSH was significantly greater than that in non-CSE-treated fibroblasts. HO-1 increased slightly through 24 h (Fig. 4C, lanes 6–9). Similarly, both 5% and 10% CSE also induced an immediate (i.e., 1 h after exposure) and sustained decrease in GSH and an accompanying time-dependent increase in HO-1 (Fig. 4C). Thus the fibroblasts were unable to completely recover intracellular GSH levels. Although there was a time-dependent increase in GSH on exposure to 5% CSE, intracellular GSH remained significantly less than that in medium-treated cells (Fig. 4C). Not surprisingly, the HFL-1 cells were also unable to recover GSH levels when exposed to 10% CSE for up to 24 h. This sustained decrease in GSH (from 5% and 10% CSE exposure) also resulted in a more dramatic increase in HO-1 at 3, 6, and 24 h (Fig. 4C, lanes 11–13 and 15–17, respectively).
The increase in HO-1 in HFL-1 cells induced by low percentages of CSE (e.g., 1% CSE) could be augmented by GSH depletion. Fibroblasts were treated with BSO (100 μM, to deplete GSH) followed by treatment with both BSO and 1% CSE for 6 or 24 h. BSO and 1% CSE, which significantly decreased intracellular GSH content (Fig. 5A), synergized to significantly enhance HO-1 expression compared with treatment with 1% CSE alone (fold change of 1,172 ± 147 vs. 16 ± 3) (Fig. 5). The induction of HO-1 by BSO in conjunction with CSE was greater than that by treatment with BSO alone (Fig. 5B).
Fig. 5.
Depletion of GSH with BSO potentiates the CSE-induced increase in HO-1 expression in HFL-1 cells. HFL-1 fibroblasts were treated with 1% CSE or pretreated with BSO (100 μM) for 1 h followed by cotreatment with 1% CSE. After this, GSH levels and HO-1 expression were assessed as described in materials and methods. A: treatment with BSO (100 μM) in conjunction with 1% CSE for either 6 or 24 h significantly decreased GSH levels compared with cells treated with CSE alone. This decrease in GSH was accompanied by a dramatic increase in HO-1 expression compared with cells treated with CSE alone. *P < 0.05, statistical difference between untreated and CSE-treated cells. Results are expressed as means ± SE; n = 3 separate experiments. Representative Western blots are shown. B: densitometric analysis of HO-1 expression revealed that the increase in HO-1 was significantly greater (**P < 0.01) in HFL-1 fibroblasts treated for 24 h with 1% CSE + BSO (1,172 ± 147-fold) compared with treatment with BSO alone (283 ± 183-fold). CSE (1%) increased HO-1 by ∼15-fold. Fold increase is based on comparison to untreated fibroblasts, which was arbitrarily set at 1 (n = 2 or 3).
Treatment of HFL-1 Human Lung Fibroblasts with NAC and GSH Attenuates CSE-Induced HO-1 Expression
To determine whether application of GSH or NAC could attenuate the CSE-induced increase in HO-1 mRNA and protein, we treated HFL-1 cells with either GSH reduced ethyl ester (5 mM) or NAC (1 mM) followed by incubation with 1% or 2% CSE for 24 h. The increase in HO-1 protein expression following treatment with 1% or 2% CSE alone (Fig. 6A, lanes 4 and 7) was attenuated by treatment with either GSH or NAC (Fig. 6A). Similar results were obtained with the L828 lung fibroblast strain (data not shown). BVR expression was also unchanged as a result of antioxidant pretreatment in the L828 or HFL-1 fibroblast strains (data not shown).
Fig. 6.
CSE-induced increase in steady-state HO-1 mRNA and protein levels in HFL-1 cells are attenuated by antioxidants GSH and N-acetyl-l-cysteine (NAC). HFL-1 fibroblasts were treated with 0.5%, 1%, or 2% CSE and 2% CSE with either GSH or NAC, and HO-1 steady-state mRNA levels were assessed by real-time PCR and protein expression by Western blot analysis as described in materials and methods. A: HO-1 expression, induced by 1% or 2% CSE (24 h), was attenuated by treatment with the antioxidants GSH and NAC. The ability of GSH to dampen the HO-1 expression was less when cells were treated with 2% CSE. B: 2% CSE induced an increase in HO-1 mRNA; this effect was attenuated by treatment with either of the antioxidants GSH and NAC. Cycle threshold values were normalized to GAPDH.
To assess whether this occurred at the mRNA steady-state level, mRNA levels were analyzed by quantitative real-time PCR in HFL-1 cells. Here, both GSH and NAC reduced the induction of HO-1 (Fig. 6B), but not HO-2 (data not shown), mRNA compared with exposure to 2% CSE alone. Collectively, these data suggest that alterations in intracellular GSH content mediate the CSE-induced upregulation of HO-1 expression in human lung fibroblasts.
AP-1 and NF-κB, but Not Nrf2 or ERK1/2, Participate in the CSE-Induced Increase in HO-1 in HFL-1 Lung Fibroblasts
There is little information regarding Nrf2 expression in human lung fibroblasts. Therefore, we first assessed whether Nrf2 was expressed in primary lung fibroblasts by Western blot. Figure 7A demonstrates that Nrf2 is expressed in three fibroblast strains, all isolated from different human beings. Next, we assessed whether Nrf2 translocates to the nucleus in response to hemin in HFL-1 cells or MLFs. In untreated MLFs, Nrf2 was located in the cytoplasm, with little nuclear localization (Fig. 7B, MLF, left). There was little basal HO-1 expression (Fig. 7B, MLF, right). When these cells were treated with hemin for 6 h, there was a dramatic increase in nuclear Nrf2 (Fig. 7B, MLF, left, arrows); HO-1 was also increased (Fig. 7B, MLF, right). In HFL-1 cells, there was some nuclear distribution (Fig. 7B, HFL-1, left, arrows). However, hemin failed to increase nuclear Nrf2 (Fig. 7B, HFL-1, left) even though HO-1 expression increased (Fig. 7B, HFL-1, right).
Fig. 7.
Nuclear factor, erythroid-derived 2, like 2 (Nrf2) does not translocate to the nucleus in CSE-treated HFL-1 lung fibroblasts. HFL-1, mouse lung fibroblasts (MLFs), and A549 cells were cultured on glass chamber slides and treated with CSE (4 h) or hemin (5 μM; 6 h), and HO-1 and Nrf2 were assessed by immunofluorescence. Nrf2 expression in human lung fibroblasts was determined by Western blot analysis. A: Nrf2 is expressed in 3 human lung fibroblast strains. B: Nrf2 translocates to the nucleus in MLFs, but not HFL-1 cells, in response to hemin. Nrf2 was distributed predominantly through the cytoplasm in MLFs. On exposure to hemin, Nrf2 translocates to the nucleus (MLF, arrows) and HO-1 increases. In contrast, HLF-1 cells exhibit some nuclear Nrf2 that is not further increased in response to hemin. Note that HO-1 is increased. C: lack of Nrf2 nuclear translocation in response to CSE in HFL-1 cells. A549 epithelial cells exhibit cytoplasmic distribution of Nrf2 when cells are untreated (A549, left). There was a dose-dependent increase in nuclear Nrf2 in response to CSE, with strong Nrf2 within the nucleus when A549 cells were treated with 10% CSE for 4 h (epithelial cells, A549, right, arrows). MLF cells also had an increase in Nrf2 in response to CSE (fibroblasts, MLF, center and right, arrows). In contrast, there was no detectable increase in nuclear Nrf2 in HFL-1 cells after treatment with either 1% or 2% CSE. Images were photographed at ×20 magnification and insets at ×60 magnification.
To determine whether Nrf2 played a role in CSE-induced HO-1 in HFL-1 cells, we next treated A549, MLF, and HFL-1 cells with CSE and assessed nuclear translocation of Nrf2 by immunofluorescence. In both A549 and MLF, Nrf2 was predominantly cytoplasmic in cells treated with control medium (Fig. 7C). In A549 cells, CSE increased the expression of Nrf2 within the nucleus in a dose-dependent manner (Fig. 7C, center and right, arrows). Similar results were obtained for MLFs. Here, Nrf2 also translocated to the nucleus in response to CSE (Fig. 7C, fibroblasts, MLF, arrows). In contrast, exposure of HFL-1 cells to CSE at the same concentration as MLFs (i.e., 1% and 2%) failed to increase nuclear Nrf2 (Fig. 7C). Collectively, these data suggest that Nrf2 is not the dominant transcription factor involved in the induction of HO-1 by CSE in the human lung fibroblast strain HFL-1.
Because the ability of CSE to influence Nrf2 nuclear translocation was minimal in HFL-1 cells, we then assessed whether other transcription factors were regulating induction of HO-1 by CSE. We first used U0126, a selective pharmacological inhibitor of ERK1/2 (19). Treatment with 1% CSE for 15 min activated ERK1/2 (Fig. 8A, panel 1). U0126 prevented the phosphorylation of ERK1/2 induced by CSE (Fig. 8A, panel 1). Treatment with 10 μM U0126 alone for up to 24 h did not increase HO-1 expression in HFL-1 cells (Fig. 8A, panel 2). Inhibition of ERK1/2 activation by U0126 was not able to prevent the induction of HO-1 by low percentages of CSE. Here, 1% CSE increased HO-1 expression between 2 and 6 h (Fig. 8A, panel 3; compare with Fig. 1), and this increase persisted for 24 h. Treatment with U0126 did not dramatically attenuate the induction of HO-1. These data suggest that ERK1/2, despite activation by 1% CSE, is not a dominant transcriptional regulator of CSE-induced HO-1 expression in HFL-1 lung fibroblasts.
Fig. 8.
Inhibition of nuclear factor-κB (NF-κB), but not ERK1/2, partially reduces CSE-induced HO-1 expression. HFL-1 cells were treated with the ERK1/2 inhibitor U0126 or the NF-κB inhibitor SC-514 alone or in conjunction with CSE, and changes in protein expression were analyzed by Western blot as described in materials and methods. A: panel 1: U1026 attenuates basal and CSE-induced ERK1/2 phosphorylation. Cells were treated with 1% CSE for 15 min with and without U0126. Panel 2: U0126 does not induce HO-1 expression. Panel 3: 1% CSE increases HO-1 expression by 6 h. Inhibition of ERK1/2 activation does not dramatically alter the induction of HO-1 by CSE. B: HFL-1 cells were treated with SC-514 alone or in the presence of IL-1β or 2% CSE. IL-1β-induced Cox-2 was reduced by SC-514. CSE-induced HO-1 was also dampened by SC-514. Left: representative Western blot is shown. Right: densitometric analysis revealed a decrease in CSE-induced HO-1 expression. Values are expressed as means ± SE (n = 2). The change in HO-1 expression is based on the fold induction compared with untreated (which was arbitrarily set to 1).
We also assessed whether the induction of HO-1 by CSE would be influenced by NF-κB inhibition. HFL-1 cells were pretreated with SC-514, a selective inhibitor of NF-κB-dependent gene expression (32), followed by treatment with either 2% CSE or IL-1β. SC-514 was able to attenuate IL-1β-induced Cox-2 induction (Fig. 8B), suggesting that it was effective in blocking NF-κB-dependent gene expression. Western blot analysis revealed that SC-514 was also partially effective in blocking the induction of HO-1 by 2% CSE (Fig. 8B). Densitometric analysis revealed that SC-514 inhibited HO-1 by ∼45% (Fig. 8B).
Finally, we examined whether AP-1 was involved in the ability of CSE to induce HO-1 expression in HFL-1 cells. In fibroblasts that were treated with control medium, the expression of c-Fos and c-Jun was low (Fig. 9, A and B). After treatment with 1%, 2% (data not shown), or 5% (Fig. 9) CSE for 1 h, the expression of both AP-1 dimers was increased, with cells treated with 5% CSE exhibiting the strongest induction. The intracellular distribution of CSE-induced c-Fos and c-Jun was predominantly nuclear (Fig. 9, A and B, arrows), consistent with previous reports demonstrating the nuclear concentration of AP-1 (13, 59).
Fig. 9.
Inhibition of AP-1 nuclear accumulation with SP-600125 attenuates CSE-induced HO-1 expression in human lung fibroblast strain HFL-1 cells. A and B: HFL-1 fibroblasts were treated with 5% CSE for 1 h, and the expression of c-Fos (A) and c-Jun (B) was assessed by immunofluorescence. There was an increase in both AP-1 subunits in response to CSE. Nonspecific fluorescence was minimal, as evidenced by the low fluorescence intensity of the isotype control (not shown). Nuclei are stained with DAPI. CSE induced predominant nuclear expression of both AP-1 subunits (arrows). Pretreatment with SP-600125 (10 μM) dramatically attenuated the induction of both c-Fos and c-Jun by 5% CSE. Images are representative of 2 independent experiments. Magnification, ×60. C and D: HFL-1 cells were cultured in the presence or absence of SP-600125 (10 μM) in conjunction with 1%, 2%, or 5% CSE for 24 h. The level of HO-1 protein was assessed by Western blot. Densitometric values were normalized to actin and are expressed as fold change over untreated. Treatment with SP-600125 resulted in depressed HO-1 protein expression compared with CSE-treated cells. Results are expressed as means ± SE; n = 2 or 3 independent experiments.
The participation of AP-1 in the expression of HO-1 by CSE in HFL-1 lung fibroblasts was analyzed with the pharmacological inhibitor SP-600125 (9). Treatment with SP-600125 (10 μM) dramatically reduced the CSE-induced nuclear accumulation of both c-Fos and c-Jun, even at the highest percentage of CSE tested, 5% CSE (Fig. 9, A and B). To assess whether prevention of Fos/Jun nuclear accumulation would attenuate the CSE-induced increase in HO-1, HFL-1 fibroblasts were treated with SP-600125 alone or in conjunction with 1%, 2%, and 5% CSE for 24 h and HO-1 expression was analyzed by Western blot. Figure 9C demonstrates that treatment with 1%, 2%, and 5% CSE for 24 h dose-dependently increased HO-1 expression (compare with Fig. 1). Densitometric analysis revealed that there was ∼9-, 34-, and 260-fold induction (1%, 2%, and 5% CSE, respectively) compared with non-CSE-treated fibroblasts (Fig. 9D). Treatment with CSE along with 10 μM SP-600125 resulted in a partial reduction of HO-1 (Fig. 9, C and D). Collectively, these data suggest the involvement of AP-1 in the induction of HO-1 by CSE.
DISCUSSION
Oxidative stress, arising from an imbalance between oxidants and antioxidants, plays a key role in the pathogenesis of pulmonary disease (64). The induction of HO-1 is an important cellular event during conditions of oxidative stress and inflammation. The oxidation of heme by HO-1 generates ferrous iron, biliverdin, and CO, all of which have cytoprotective properties (21, 51, 64). In the lung, exposure to environmental toxicants is associated with increased HO-1 (46, 64, 65). Cigarette smoke contains ≈5,000 chemicals, many of which have oxidant activities. Cigarette smoke is also the principal cause of diseases such as COPD and lung cancer. HO-1 induction in response to cigarette smoke (or components of cigarette smoke) has been shown in endothelial cells (73) and alveolar epithelial cells (65). In the present study, we demonstrate for the first time that exposure of human lung fibroblasts to CSE results in a time- and dose-dependent increase in HO-1 mRNA and protein (Figs. 1 and 2). The induction of HO-1 occurred at percentages of CSE as low as 1%. This percentage of CSE has previously been shown by us to activate, but not induce, apoptosis in the two human lung fibroblast strains used in this study (6, 42).
CSE is widely used as a model system to study in vitro effects of tobacco smoke (34, 42, 50), but this is not without limitations. Although CSE contains many components inhaled by smokers (62), this feature makes it difficult to determine the component(s) of cigarette smoke mediating a given biological effect. Additionally, the generation of CSE in aqueous solutions (such as cell culture media) results in the collection of the water-soluble (particulate) components of whole cigarette smoke, which constitute only 5% (15). However, water-soluble components of cigarette smoke can readily reach both the systemic circulation (16) and interstitial cells such as fibroblasts (30), suggesting that compounds found in CSE may mimic in vivo situations. This is further supported by observations that in vivo smoke exposure can mimic in vitro CSE challenge (16, 50). Another limitation is the difficulty in predicting whether the concentrations of CSE (i.e., 1%) used in our studies are physiologically relevant. On the basis of levels of nicotine present in CSE (27), we speculate that exposure of fibroblasts to 1% CSE approximates what pulmonary interstitial cells might encounter in a regular smoker (2, 26, 27).
We speculated that the induction in HO-1 caused by CSE would be due to alterations in cellular redox (oxidation-reduction) status and, in particular, the level of intracellular GSH. GSH is the principal antioxidant in the lung (54), and exposure to cigarette smoke depletes GSH (6, 53); a sustained reduction in GSH is associated with enhanced CSE-induced cell death (6). The depletion of GSH induces HO-1 in brain (18) and liver (58) as well as skin fibroblasts (36). We used BSO, an inhibitor of GSH synthesis, to first demonstrate that reducing intracellular GSH resulted in an increase in HO-1 (Figs. 4 and 10). We next correlated the induction of HO-1 with the CSE-induced depletion of GSH. Treatment with lower percentages of CSE (i.e., 1% and 2%) resulted in a moderate increase in HO-1 (Fig. 4). There was a small, but not statistically significant, decrease in GSH when cells were treated with 1% CSE for 1 and 3 h; this was followed by a significant increase in GSH by 6 h. Similar results were obtained when cells were exposed to 2% CSE, where there was an initial decrease in GSH followed by a significant increase. These results are in agreement with a recent report by Rahman and colleagues (35), where in primary small airway epithelial cells cigarette smoke induced an initial decrease, followed by an increase, in GSH levels. We found that mRNA encoding the catalytic subunit of γ-glutamylcysteine ligase (GCL), the rate-limiting enzyme of GSH biosynthesis, was also upregulated after exposure to CSE (data not shown). This rebound effect is likely the result of the compensatory upregulation of GCL. At higher percentages of CSE, however, GSH levels were not able to recover (Fig. 4), possibly because of the overwhelming oxidant burden to the lung fibroblasts exerted by the CSE. This sustained reduction in GSH led to a robust increase in HO-1. Treatment with 1% CSE in conjunction with BSO also led to a sustained decrease in GSH and a dramatic increase in HO-1, compared with treatment with 1% CSE alone (Fig. 5). Finally, treatment of lung fibroblasts with exogenous GSH, as well as the GSH precursor NAC, dramatically reduced the induction of HO-1 by 1% and 2% CSE (Fig. 6). The administration of GSH reduced ethyl ester augments intracellular GSH levels in human lung fibroblasts (6), thus supporting our hypothesis that CSE induction of HO-1 is regulated by changes in intracellular GSH.
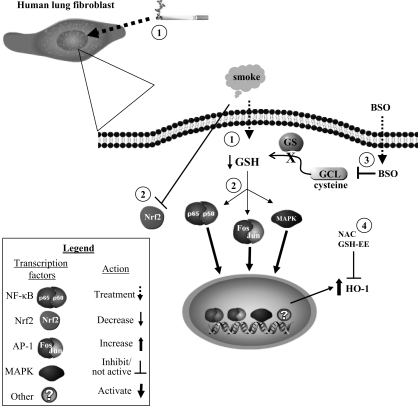
Fig. 10.
Summary of intracellular regulation of CSE-induced HO-1 expression in human lung fibroblasts. 1) Treatment of human lung fibroblasts with CSE reduces intracellular GSH, an important antioxidant. 2) This decrease in GSH triggers some redox-sensitive transcription factors, such as NF-κB, AP-1, and mitogen-activated protein kinase (MAPK). The induction of HO-1 by CSE was partially attenuated by inhibition of NF-κB and AP-1 signaling pathways. Interestingly, Nrf2 did not translocate to the nucleus in human lung fibroblasts in response to CSE. 3) BSO reduces intracellular GSH via inhibition of γ-glutamylcysteine ligase (GCL) activity, the rate-limiting enzyme in GSH biosynthesis. Reduction of GSH by BSO also increased HO-1 expression. 4) Treatment with antioxidants such as NAC and GSH (glutathione reduced ethyl ester, GSH-EE) attenuates the CSE-induced expression of HO-1. Collectively these data suggest that oxidative stress caused by cigarette smoke increased HO-1 expression in human lung fibroblasts by multiple signal transduction pathways. GS, glutathione synthetase.
Changes in the cellular redox status regulate signal transduction (68). The de novo synthesis of HO-1 can involve multiple signaling pathways, including MAPKs, AP-1, NF-κB, Nrf2, BACH-1, and phosphatidylinositol 3-kinase, among others (3, 29, 39, 61, 71). Many of these transcription factors are potently activated by cigarette smoke and are sensitive to alterations in cellular GSH (33, 42). We showed previously (42) that the doses of CSE used in the present study can activate MAPK, ERK1/2, and NF-κB pathways. However, pharmacological inhibition of ERK1/2 did not prevent the induction of HO-1 by CSE (Fig. 8). In addition, inhibition of NF-κB by the IKK-2 inhibitor SC-514 only partially reduced CSE-induced HO-1 (Fig. 8B). We also examined the ability of CSE to activate Nrf2. Nrf2 is proposed to be a principal inducer of antioxidant genes, including HO-1 (1). Exposure of NIH 3T3 mouse lung fibroblasts to CSE results in HO-1 induction through a dose-dependent increase in Nrf2 protein expression, translocation to the nucleus, and subsequent activation (33). In addition, Nrf2 is activated by cigarette smoke in human epithelial cells (34). However, the ability of CSE to activate Nrf2 in human lung fibroblasts is unknown. Interestingly, hemin (an inducer of both HO-1 expression and Nrf2 translocation; Refs. 45, 47) was unable to translocate Nrf2 to the nucleus in the human lung fibroblast strain HFL-1 (Fig. 7B). MLFs, by contrast, were responsive to hemin, and there was an increase in both HO-1 and nuclear expression of Nrf2 after hemin treatment (Fig. 7B). Despite the ability of CSE to induce Nrf2 nuclear translocation in primary MLFs generated by us as well as human A549 epithelial cells, exposure of human lung fibroblasts to doses of CSE that potently upregulate HO-1 did not result in a detectable increase in nuclear Nrf2 (Fig. 7C). These data indicate that Nrf2 is not the key transcription factor responsible for the CSE-induced increase in HO-1 in primary human lung fibroblasts (Fig. 10), a feature that reflects key differences in the signaling pathways between human and mouse cells as well as between epithelial cells and fibroblasts.
AP-1 is a redox-sensitive transcription factor consisting of Jun oncoproteins that homo- or heterodimerize with other Jun or Fos proteins (61). The participation of AP-1 in the induction of HO-1 is cell- and stimulus specific. Although AP-1 was not involved in tobacco smoke-induced HO-1 in the human premonocytic cell line U937 (20), cigarette smoke contains a variety of prooxidant compounds that regulate AP-1 protein expression and activation (44, 56). Therefore, we examined the ability of CSE to increase the expression of the two main AP-1 proteins, c-Fos and c-Jun, by immunofluorescence. Basal expression of c-Fos and c-Jun was low (Fig. 9, A and B, untreated). Treatment with CSE (1%, 2%, or 5%) for 1 h resulted in a dose-dependent increase in the nuclear expression of both AP-1 proteins (Fig. 9), indicating that cigarette smoke induces AP-1 in human lung fibroblasts, a novel finding.
AP-1 is a target of the c-Jun NH2-terminal kinase (JNK). SP-600125, a selective inhibitor of JNK (63) that reduced c-Fos and c-Jun nuclear accumulation, partially prevented the induction of HO-1 by CSE (Figs. 9 and 10). Cigarette smoke is a complex mixture containing >4,800 compounds, and it contains many molecules that are potent ligands for other receptor pathways, such as the aryl hydrocarbon receptor (17, 41). Thus it is likely that the upregulation of HO-1 in response to CSE in human lung fibroblasts involves more than one intracellular pathway, which would explain the lack of complete inhibition by SP-600125 as well as other pharmacological inhibitors used in this study.
The induction of HO-1 is believed to protect against oxidative stress. Indeed, the generation of bilirubin, iron, and CO from HO activity can protect against cell death (21, 64), and pharmacological induction of HO-1 is protective in a nonautoimmune arthritis mouse model (8). While modest HO-1 expression is cytoprotective, exacerbation of oxidative injury correlates with high HO-1 expression (37, 66, 67). We found that there was a dose-dependent increase in HO-1 expression in response to CSE (Figs. 1 and 4). Here, lower doses of CSE (i.e., 1% and 2%) resulted in modest HO-1 expression; these concentrations of CSE are nonapoptotic in HFL-1 fibroblasts (6). In contrast, 10% CSE robustly increases HO-1 expression (Fig. 4) and provokes oxidative stress-induced cell death (6). Fibroblasts are a vital structural component of the alveolar air space, and their ability to withstand CSE-mediated apoptosis may relate to the level of HO-1 induction. A better understanding of the role of HO-1 in pulmonary biology may provide new therapeutic opportunities to treat tobacco-related lung disease.
GRANTS
This research was supported by National Institutes of Health (NIH) Grants DE-011390, ES-01247, ES-07026, HL-075432, and HL-088325; NIH/NCRR-ULIRR024160-1; and a Parker B. Francis Fellowship (C. J. Baglole).
Acknowledgments
We thank Tse-Yao Wang for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des 9: 2499–2511, 2003. [DOI] [PubMed] [Google Scholar]
- 2.An Z, Wang H, Song P, Zhang M, Geng X, Zou MH. Nicotine-induced activation of AMP-activated protein kinase inhibits fatty acid synthase in 3T3L1 adipocytes: a role for oxidant stress. J Biol Chem 282: 26793–26801, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Andreadi CK, Howells LM, Atherfold PA, Manson MM. Involvement of Nrf2, p38, B-Raf, and nuclear factor-kappaB, but not phosphatidylinositol 3-kinase, in induction of hemeoxygenase-1 by dietary polyphenols. Mol Pharmacol 69: 1033–1040, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med 39: 227–236, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Atzori L, Caramori G, Lim S, Jazrawi E, Donnelly L, Adcock I, Barnes PJ, Chung KF. Effect of cigarette smoking on haem-oxygenase expression in alveolar macrophages. Respir Med 98: 530–535, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, Phipps RP. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: implications for emphysema. Am J Physiol Lung Cell Mol Physiol 291: L19–L29, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, Phipps RP. Isolation and phenotypic characterization of lung fibroblasts. Methods Mol Med 117: 115–127, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Benallaoua M, Francois M, Batteux F, Thelier N, Shyy JY, Fitting C, Tsagris L, Boczkowski J, Savouret JF, Corvol MT, Poiraudeau S, Rannou F. Pharmacologic induction of heme oxygenase 1 reduces acute inflammatory arthritis in mice. Arthritis Rheum 56: 2585–2594, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnevali S, Petruzzelli S, Longoni B, Vanacore R, Barale R, Cipollini M, Scatena F, Paggiaro P, Celi A, Giuntini C. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 284: L955–L963, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis 118: 617–621, 1978. [DOI] [PubMed] [Google Scholar]
- 12.Chen LJ, Zhao Y, Gao S, Chou IN, Toselli P, Stone P, Li W. Downregulation of lysyl oxidase and upregulation of cellular thiols in rat fetal lung fibroblasts treated with cigarette smoke condensate. Toxicol Sci 83: 372–379, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Chida K, Vogt PK. Nuclear translocation of viral Jun but not of cellular Jun is cell cycle dependent. Proc Natl Acad Sci USA 89: 4290–4294, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 64: 111–126, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clunes LA, Bridges A, Alexis N, Tarran R. In vivo versus in vitro airway surface liquid nicotine levels following cigarette smoke exposure. J Anal Toxicol 32: 201–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csiszar A, Labinskyy N, Podlutsky A, Kaminski PM, Wolin MS, Zhang C, Mukhopadhyay P, Pacher P, Hu F, de Cabo R, Ballabh P, Ungvari Z. Vasoprotective effects of resveratrol and SIRT1: attenuation of cigarette smoke-induced oxidative stress and proinflammatory phenotypic alterations. Am J Physiol Heart Circ Physiol 294: H2721–H2735, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dertinger SD, Nazarenko DA, Silverstone AE, Gasiewicz TA. Aryl hydrocarbon receptor signaling plays a significant role in mediating benzo[a]pyrene- and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis 22: 171–177, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Ewing JF, Maines MD. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem 60: 1512–1519, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273: 18623–18632, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Favatier F, Polla BS. Tobacco-smoke-inducible human haem oxygenase-1 gene expression: role of distinct transcription factors and reactive oxygen intermediates. Biochem J 353: 475–482, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1: 152–157, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Fries KM, Sempowski GD, Gaspari AA, Blieden T, Looney RJ, Phipps RP. CD40 expression by human fibroblasts. Clin Immunol Immunopathol 77: 42–51, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Fukano Y, Oishi M, Chibana F, Numazawa S, Yoshida T. Analysis of the expression of heme oxygenase-1 gene in human alveolar epithelial cells exposed to cigarette smoke condensate. J Toxicol Sci 31: 99–109, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Goh BJ, Tan BT, Hon WM, Lee KH, Khoo HE. Nitric oxide synthase and heme oxygenase expressions in human liver cirrhosis. World J Gastroenterol 12: 588–594, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith OW, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem 254: 7558–7560, 1979. [PubMed] [Google Scholar]
- 26.Holden WE, Maier JM, Malinow MR. Cigarette smoke extract increases albumin flux across pulmonary endothelium in vitro. J Appl Physiol 66: 443–449, 1989. [DOI] [PubMed] [Google Scholar]
- 27.Hoshino Y, Mio T, Nagai S, Miki H, Ito I, Izumi T. Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol 281: L509–L516, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka T, Ishii Y, Itoh K, Kiwamoto T, Kimura T, Matsuno Y, Morishima Y, Hegab AE, Homma S, Nomura A, Sakamoto T, Shimura M, Yoshida A, Yamamoto M, Sekizawa K. Nrf2-deficient mice are highly susceptible to cigarette smoke-induced emphysema. Genes Cells 10: 1113–1125, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 39: 355–364, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii T, Matsuse T, Igarashi H, Masuda M, Teramoto S, Ouchi Y. Tobacco smoke reduces viability in human lung fibroblasts: protective effect of glutathione S-transferase P1. Am J Physiol Lung Cell Mol Physiol 280: L1189–L1195, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Jardine H, MacNee W, Donaldson K, Rahman I. Molecular mechanism of transforming growth factor (TGF)-beta1-induced glutathione depletion in alveolar epithelial cells. Involvement of AP-1/ARE and Fra-1. J Biol Chem 277: 21158–21166, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappaB-dependent gene expression in interleukin-1beta-stimulated synovial fibroblasts. J Biol Chem 278: 32861–32871, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Knorr-Wittmann C, Hengstermann A, Gebel S, Alam J, Muller T. Characterization of Nrf2 activation and heme oxygenase-1 expression in NIH3T3 cells exposed to aqueous extracts of cigarette smoke. Free Radic Biol Med 39: 1438–1448, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, Rahman I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478–L488, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Kode A, Yang SR, Rahman I. Differential effects of cigarette smoke on oxidative stress and proinflammatory cytokine release in primary human airway epithelial cells and in a variety of transformed alveolar epithelial cells. Respir Res 7: 132, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis 13: 227–232, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Lee DW, Gelein RM, Opanashuk LA. Heme-oxygenase-1 promotes polychlorinated biphenyl mixture aroclor 1254-induced oxidative stress and dopaminergic cell injury. Toxicol Sci 90: 159–167, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res 65: 4789–4798, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Lin CC, Chiang LL, Lin CH, Shih CH, Liao YT, Hsu MJ, Chen BC. Transforming growth factor-beta1 stimulates heme oxygenase-1 expression via the PI3K/Akt and NF-kappaB pathways in human lung epithelial cells. Eur J Pharmacol 560: 101–109, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Maines MD, Trakshel GM. Purification and characterization of human biliverdin reductase. Arch Biochem Biophys 300: 320–326, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 289: L391–L399, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Martey CA, Pollock SJ, Turner CK, O'Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol 287: L981–L991, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Muller T, Gebel S. Heme oxygenase expression in Swiss 3T3 cells following exposure to aqueous cigarette smoke fractions. Carcinogenesis 15: 67–72, 1994. [DOI] [PubMed] [Google Scholar]
- 44.Muller T, Haussmann HJ, Schepers G. Evidence for peroxynitrite as an oxidative stress-inducing compound of aqueous cigarette smoke fractions. Carcinogenesis 18: 295–301, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Nagai T, Kikuchi S, Ohmine K, Miyoshi T, Nakamura M, Kondo T, Furuyama K, Komatsu N, Ozawa K. Hemin reduces cellular sensitivity to imatinib and anthracyclins via Nrf2. J Cell Biochem 104: 680–691, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Nagatomo H, Morimoto Y, Oyabu T, Hirohashi M, Ogami A, Yamato H, Kuroda K, Higashi T, Tanaka I. Expression of heme oxygenase-1 in the lungs of rats exposed to crystalline silica. J Occup Health 48: 124–128, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett 546: 181–184, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Nowell SA, Leakey JE, Warren JF, Lang NP, Frame LT. Identification of enzymes responsible for the metabolism of heme in human platelets. J Biol Chem 273: 33342–33346, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Oguro T, Hayashi M, Nakajo S, Numazawa S, Yoshida T. The expression of heme oxygenase-1 gene responded to oxidative stress produced by phorone, a glutathione depletor, in the rat liver; the relevance to activation of c-jun N-terminal kinase. J Pharmacol Exp Ther 287: 773–778, 1998. [PubMed] [Google Scholar]
- 50.Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol 292: H130–H139, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal 7: 1688–1703, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Rahman I, Li XY, Donaldson K, Harrison DJ, MacNee W. Glutathione homeostasis in alveolar epithelial cells in vitro and lung in vivo under oxidative stress. Am J Physiol Lung Cell Mol Physiol 269: L285–L292, 1995. [DOI] [PubMed] [Google Scholar]
- 54.Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am J Physiol Lung Cell Mol Physiol 277: L1067–L1088, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reddy SP, Mossman BT. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am J Physiol Lung Cell Mol Physiol 283: L1161–L1178, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Reiter TA, Pang B, Dedon P, Demple B. Resistance to nitric oxide-induced necrosis in heme oxygenase-1 overexpressing pulmonary epithelial cells associated with decreased lipid peroxidation. J Biol Chem 281: 36603–36612, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Rizzardini M, Carelli M, Cabello Porras MR, Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glutathione depletion and protection by N-acetylcysteine. Biochem J 304: 477–483, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roux P, Blanchard JM, Fernandez A, Lamb N, Jeanteur P, Piechaczyk M. Nuclear localization of c-Fos, but not v-Fos proteins, is controlled by extracellular signals. Cell 63: 341–351, 1990. [DOI] [PubMed] [Google Scholar]
- 60.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal 7: 80–91, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro SD Smoke gets in your cells. Am J Respir Cell Mol Biol 31: 481–482, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Silvers AL, Bachelor MA, Bowden GT. The role of JNK and p38 MAPK activities in UVA-induced signaling pathways leading to AP-1 activation and c-Fos expression. Neoplasia 5: 319–329, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Slebos DJ, Ryter SW, Choi AM. Heme oxygenase-1 and carbon monoxide in pulmonary medicine. Respir Res 4: 7, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol 36: 409–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J 13: 1800–1809, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Suttner DM, Sridhar K, Lee CS, Tomura T, Hansen TN, Dennery PA. Protective effects of transient HO-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am J Physiol Lung Cell Mol Physiol 276: L443–L451, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med 22: 269–285, 1997. [DOI] [PubMed] [Google Scholar]
- 69.Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969. [PubMed] [Google Scholar]
- 70.Tietze F Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27: 502–522, 1969. [DOI] [PubMed] [Google Scholar]
- 71.Vargas MR, Pehar M, Cassina P, Martinez-Palma L, Thompson JA, Beckman JS, Barbeito L. Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J Biol Chem 280: 25571–25579, 2005. [DOI] [PubMed] [Google Scholar]
- 72.Wang HD, Yamaya M, Okinaga S, Jia YX, Kamanaka M, Takahashi H, Guo LY, Ohrui T, Sasaki H. Bilirubin ameliorates bleomycin-induced pulmonary fibrosis in rats. Am J Respir Crit Care Med 165: 406–411, 2002. [DOI] [PubMed] [Google Scholar]
- 73.Wu CC, Hsieh CW, Lai PH, Lin JB, Liu YC, Wung BS. Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol Appl Pharmacol 214: 244–252, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Yang G, Shegog ML, Dennery PA. Effect of glutathione on lung activator protein-1 activation and heme oxygenase-1 induction in the immature rat. Pediatr Res 52: 34–39, 2002. [DOI] [PubMed] [Google Scholar]