Abstract
Matrix metalloprotease-9 (MMP-9) is increased in lung injury following hyperoxia exposure in neonatal mice, in association with impaired alveolar development. We studied the role of MMP-9 in the mechanism of hyperoxia-induced functional and histological changes in neonatal mouse lung. Reduced alveolarization with remodeling of ECM is a major morbidity component of oxidant injury in developing lung. MMP-9 mediates oxidant injury in developing lung causing altered lung remodeling. Five-day-old neonatal wild-type (WT) and MMP-9 (−/−) mice were exposed to hyperoxia for 8 days. The lungs were inflation fixed, and sections were examined for morphometry. The mean linear intercept and alveolar counts were evaluated. Immunohistochemistry for MMP-9 and elastin was performed. MMP-2, MMP-9, type I collagen, and tropoelastin were measured by Western blot analysis. Lung quasistatic compliance was studied in anaesthetized mice. MMP-2 and MMP-9 were significantly increased in lungs of WT mice exposed to hyperoxia compared with controls. Immunohistochemistry showed an increase in MMP-9 in mesenchyme and alveolar epithelium of hyperoxic lungs. The lungs of hyperoxia-exposed WT mice had less gas exchange surface area and were less compliant compared with room air-exposed WT and hyperoxia-exposed MMP-9 (−/−) mice. Type I collagen and tropoelastin were increased in hyperoxia-exposed WT with aberrant elastin staining. These changes were ameliorated in hyperoxia-exposed MMP-9 (−/−) mice. MMP-9 plays an important role in the structural changes consequent to oxygen-induced lung injury. Blocking MMP-9 activity may lead to novel therapeutic approaches in preventing bronchopulmonary dysplasia.
Keywords: morphometry, elastin, bronchopulmonary dysplasia
the pathophysiological features of the newer forms of bronchopulmonary dysplasia (BPD) are characterized by increased airway responsiveness to direct stimuli, with evidence of minimal alveolarization, variable alveolar wall cellularity and fibrosis, and alveolar-capillary dysplasia (14). Alveolarization begins in the distal saccules of the lung in parallel with development of the alveolar capillary bed in infants born at 24–28 wk of gestation (28). The major manifestation of hyperoxic lung injury in neonates is an interference with normal lung development, particularly alveolar development. In the new BPD, fewer and larger alveoli are present. Hyperoxia-exposed preterm baboons showed a complete arrest of alveolar septation with fewer and larger alveoli. Even though the normal signal for septation of the saccules is unknown, hyperoxia has been shown to delay alveolar development (14).
Interactions between epithelial and mesodermal cells during lung development and repair from injury lead to characteristic morphological and functional changes in the neonatal lung (41). The ECM is an important site of lung injury and repair processes in different developmental stages of the lung. Remodeling of the ECM is a major morbidity component in oxidant injury contributing to the development of BPD (1). Matrix metalloproteases (MMPs) are proteolytic enzymes that degrade ECM components. They have important roles during normal lung development but when produced in excess may lead to altered lung remodeling and change in architecture (10). Both human and animal studies suggest that MMPs are involved in neonatal lung injury. Sweet et al. (37) showed increased MMP-9 levels in the bronchoalveolar lavage fluid of preterm infants who developed BPD. Increased levels of MMP-9 are present in the lungs of premature baboons exposed to hyperoxia in association with changes in lung architecture (38). Although previous studies demonstrated an association between increased levels of MMPs and BPD, there is little information directly addressing the role of MMP-9 in mediating changes in lung function and morphology following hyperoxic injury.
Elastin is an important component of alveolar structures, where it is normally abundant in alveolar septal tips. Disturbed elastin deposition is one of the pathological hallmarks of BPD in premature infants. Alveolar myofibroblasts contain contractile elements and express α-smooth muscle actin (α-SMA). They are believed to be the source of elastin in the form of its soluble precursor tropoelastin (7) and play an important role in alveogenesis. In chronic lung disease in premature infants and after hyperoxia exposure in animal models, there are increased amounts (39) and abnormal distribution of elastin fibers (3) and increased α-SMA expression (13, 43).
The pathogenesis of BPD is multifactorial and not completely understood. We propose that metalloproteases are critical mediators of oxidant injury in the developing lung contributing to remodeling in the subepithelial compartment of peribronchiolar airways and alveolar simplification in response to hyperoxia. We hypothesize that MMP-9 mediates oxidant injury in developing lung causing altered lung remodeling. We specifically studied the effect of loss of MMP-9 expression on hyperoxia-induced changes in neonatal mice.
MATERIALS AND METHODS
Reagents were obtained as follows: α-SMA monoclonal antibody was purchased from R&D Systems (Minneapolis, MN). Rabbit anti-collagen antibody was purchased from Rockland Immunochemical (Gilbertsville, PA). Goat anti-rabbit IgG was purchased from Upstate Biotechnology (Waltham, MA). Penicillin G, acrylamide, DMSO, SDS, pepstatin, and leupeptin were purchased from Sigma Chemical (St. Louis, MO). l-glutamine, HBSS, Dulbecco's modified PBS, DMEM, and FCS were obtained from GIBCO (Grand Island, NY). Antibodies to MMP-2, MMP-9, anti-rabbit IgG, and anti-mouse IgG were obtained from Cell Signaling (Danvers, MA). Mayer's hematoxylin was from Dako (Carpentaria, CA). Tropoelastin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Animal model.
We used neonatal wild-type (WT; CD1) and MMP-9 (−/−) mice of CD1 background for all our studies. WT mice were purchased from Taconic Farm. Our MMP-9 (−/−) mouse colony was a kind gift from Dr. Andrew Camille (Tufts Univ., Boston, MA) and originated from the line developed by Dr. Robert Senior (Univ. of Chicago, Chicago, IL). Animals were housed and cared for by the Division of Laboratory Animal Medicine at New England Medical Center. This facility conforms strictly to the current National Institutes of Health guidelines for animal care. They provide veterinary support and evaluation as needed. The animal use protocol was approved by the Institutional Animal Care and Use Committee.
Hyperoxia exposure of neonatal mice.
Five-day-old neonatal WT and MMP-9 (−/−) mice were exposed to room air or hyperoxia for 8 days as follows. At day 1 age, the pups were assigned to new litters consisting of six pups each. Litters were similar in weight and number of pups assigned to each mother. From days 5–13 of life (corresponding to the major period of murine alveolarization), one litter was exposed to 90% oxygen, and one litter was exposed to room air using Plexiglas chambers as we have previously described (5). The exposure chamber was opened briefly twice a day to replenish food and water, clean the chambers, and rotate the mothers between the hyperoxic and normoxic environment to prevent maternal oxygen toxicity (5). On day 13, pups were used for studies as described below.
Lung fixation and morphometry.
Lungs from room air and hyperoxia-exposed WT and MMP-9 (−/−) mouse pups were used for morphometry studies. Lungs were removed gently and inflation fixed with 4% paraformaldehyde through a tracheal cannula at a pressure of 20 cmH2O. The lung was then placed in 4% paraformaldehyde for 24 h and paraffin embedded, and 5-μm sections were prepared and stained with hematoxylin. Morphometry studies were performed using Scion Image software (National Institutes of Health).
Measurements were taken from the terminal airways and alveoli for mean linear intercept (MLI) and thickness of the airway wall. MLI, a measure of alveolar diameter that is inversely proportional to the alveolar surface area (45), was measured as described (25, 48). MLI was determined in five separate terminal respiratory units in lung tissue. Terminal airways were differentiated from the conducting airways based on the presence of low cuboidal epithelium vs. the columnar epithelium in the conducting airways.
Alveolar counts were done in five random areas in the lungs from WT and MMP-9 (−/−) mice after room air and hyperoxia exposures. Alveolar number across terminal respiratory units was estimated by the radial alveolar count method described (15). A line was drawn from the center of a respiratory bronchiole to the nearest interlobular septum to which an intercept line was drawn perpendicularly. We counted the number of distal air spaces that were transected by the line. We repeated this assessment for five terminal respiratory units in one random tissue section per mouse.
Elastin deposition after hyperoxia exposure.
Deparaffinized lung sections from hyperoxia- and room air-exposed neonatal pups were stained with elastin stain and Van Gieson solutions (Sigma-Aldrich). These sections were then viewed by light microscopy. The distribution, structure, and abundance of elastin were estimated as described (31).
Measurement of lung function.
At the end of the 8 days of hyperoxia exposure (day 13 of life), lung mechanics were measured in control and hyperoxia-exposed animals. Pups were weighed, deeply anesthetized by an intraperitoneal injection of xylazine (8 mg/kg), and the trachea cannulated. The cannula was connected to a computer-controlled small animal ventilator (FlexiVent; SCIREQ, Montreal, PQ, Canada), and regular ventilation was delivered at a frequency of 150 breaths/min and a tidal volume of 5 ml/kg as we and others have described (36, 40). We measured total lung compliance instead of central airway resistance since the major contribution to airway resistance in neonatal oxygen injury is from the distal airways, which are greater in number and lack cartilage.
Western blot analysis.
Western blotting was performed to analyze MMP-2, MMP-9, type I collagen, tropoelastin, and α-SMA in the lung lysates. Tissues were harvested into an ice-cold solution of PBS, pH 7.4, containing protease inhibitors (2 μg leupeptin/ml, 1 μg aprotinin/ml, 1 mM PMSF, and 2 μg antipain/ml). After sonication and centrifugation, aliquots were removed for protein determination, and the samples were stored at −80°C until use. Total protein in the lysates was measured using a BCA protein microassay kit (Pierce, Rockford, IL). A 30-μg protein sample was separated by PAGE on a 10% acrylamide-SDS gel and then transferred to a nitrocellulose membrane, probed using a monoclonal anti-mouse MMP-2 antibody (1:1,000) or anti-mouse MMP-9 antibody (1:1,000 overnight at 4°C). After being washed, the blots were incubated with goat anti-rabbit HRP-conjugated antibody (1:4,000) and developed using chemiluminescence to demonstrate changes in protein levels of MMP-2 and MMP-9 in the lungs after hyperoxia exposure. Anti-collagen type I antibody (1:1,000) and anti-α-SMA antibody (1: 100) followed by anti-rabbit and anti-mouse IgG (1:5,000), respectively, were used to determine levels of type I collagen and α-SMA. Anti-tropoelastin antibody (1: 100) followed by anti-goat IgG (1:4,000) was used to determine the level of tropoelastin. Actin was used as loading control, and all signals were normalized to the corresponding actin signal. Comparisons of relative changes were made by densitometry scanning.
Statistical methods.
Statistical analysis for studies of WT and transgene negative study groups was done by ANOVA with post hoc multiple comparisons using either the Dunnett (20) or Bonferroni procedures as we have done (35, 46) using Graphpad InStat software (San Diego, CA).
RESULTS
Effect of hyperoxia on growth of neonatal mice.
Hyperoxia adversely affected the survival of both WT and MMP-9 (−/−) neonatal mice. However, the mortality was higher in WT (20%) compared with MMP-9 (−/−) (10%) after exposure to 90% oxygen for 8 days (P < 0.05). The majority of the deaths occurred in the first 3 days of oxygen exposure. However, activity levels did not appear different between hyperoxia- and room air-exposed animals. The 8-day exposure to hyperoxia adversely affected growth of WT and MMP-9 (−/−) pups. The average weight of the WT mouse on 13 days of age was 8.5 ± 0.4 after hyperoxia compared with 10 ± 0.1 g after room air (P = 0.03) and 8 ± 0.8 g after hyperoxia compared with 11.6 ± 0.8 g after room air exposure in MMP-9 (−/−) (P = 0.002). However, there was no significant difference in weight between the WT and MMP-9 (−/−) pups groups after hyperoxia exposure. These results are consistent with the studies done in adult mice exposed to hyperoxia that showed that MMP-9 (−/−) mice survived longer compared with WT mice, suggesting overexpression of MMP-9 is partially responsible for pathogenic destruction during hyperoxic condition (30). In the absence of MMP-9, the damaging effect on the alveolar structure was reduced.
Effect of hyperoxia on MMP-9.
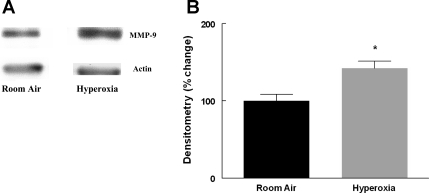
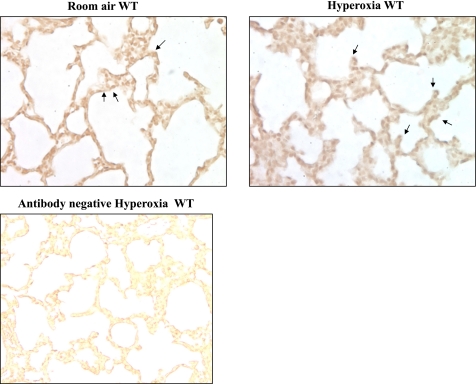
MMP-9 levels were significantly increased in the lungs of WT mice exposed to hyperoxia for 8 days compared with room air (Fig. 1). This is in agreement with the increase in MMP-9 seen in lung homogenates of premature baboons exposed to hyperoxia for 140 days (38). Furthermore, immunostaining for MMP-9 showed increased expression in the epithelium of terminal air spaces in WT mice after hyperoxia exposure (Fig. 2).
Fig. 1.
A: representative Western blot of matrix metalloprotease (MMP)-9 in 13-day-old wild-type (WT) mouse lung following exposure to room air or hyperoxia for 8 days. B: densitometry of Western blots, n = 8 in each condition. Bars are means ± SE. The MMP-9 signal was normalized to actin. *P = 0.01 compared with room air.
Fig. 2.
Photomicrographs of 5-μm lung sections from lungs of room air- or hyperoxia-exposed WT mice stained for MMP-9 (×40 magnification). Arrows point to the positive MMP-9 stain. There is an increase in immunostaining for MMP-9 in hyperoxia-exposed lungs, mainly in the alveolar epithelium.
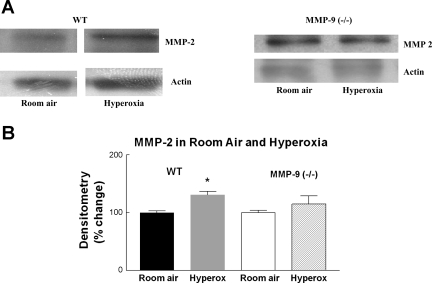
We examined MMP-2 in MMP-9 knockout mice to confirm that the changes we observed in MMP-9 (−/−) mice are not related to changes in MMP-2 expression. We exposed MMP-9 (−/−) mice to hyperoxia and examined for MMP-2 expression by Western blot analysis. We did not see any significant change in MMP-2 in MMP-9 (−/−) mice exposed to hyperoxia compared with room air (Fig. 3), implying that MMP-9 may regulate MMP-2 levels.
Fig. 3.
A: representative Western blot for MMP-2 in 13-day-old mouse lung WT and MMP-9 (−/−) exposed to room air or hyperoxia. B: densitometry of Western blots of lungs from WT and MMP-9 (−/−) mouse pups. N = 8; bars are means ± SE. MMP-2 signal was normalized to actin. *P = 0.02 compared with room air. Oxygen exposure did not significantly change the level of MMP-2 protein in MMP-9 (−/−) mice.
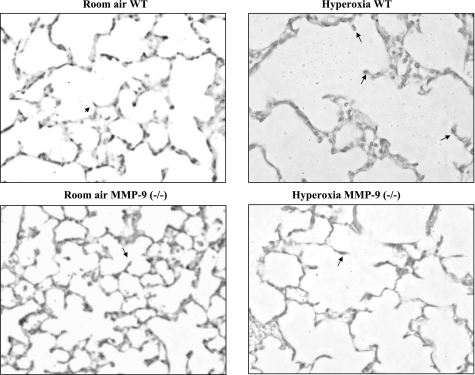
Effect of hyperoxia on lung alveolarization in neonatal mice.
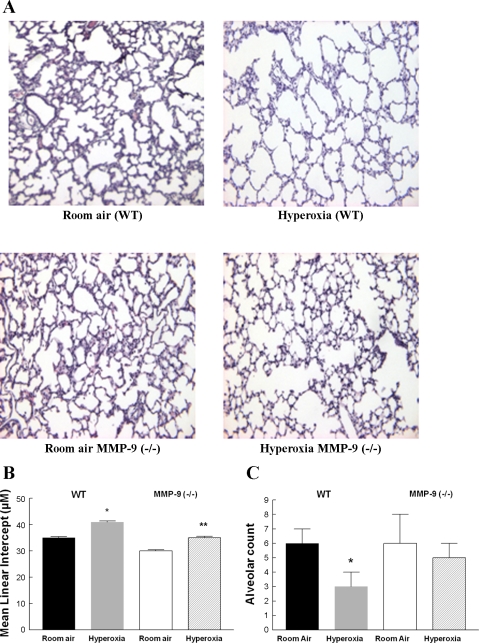
Morphometry studies were done in the lungs of 13-day-old WT and MMP-9 (−/−) neonatal mice exposed to room air or hyperoxia for the previous 8 days. Five-micrometer lung sections were examined for alveolarization. In WT pups, alveolar simplification, characterized by larger and fewer air spaces and decreased septation, was seen in oxygen-exposed compared with room air-exposed lungs. The alveolar simplification seen in WT lungs after hyperoxia was significantly attenuated in MMP-9 (−/−) mouse lungs exposed to hyperoxia (Fig. 4, A and B). The MLI, a measure of alveolar diameter, was increased in hyperoxia-exposed WT mice compared with MMP-9 (−/−) mice indicating that the gas exchange surface area was less affected by hyperoxia exposure in the MMP-9 (−/−) lungs compared with WT lungs. There was no significant difference in the MLI of room air-exposed MMP-9 (−/−) and WT lungs. In addition, alveolar number across terminal respiratory units was estimated by the radial alveolar counts. Figure 4C shows a significant reduction in alveolar counts in hyperoxia-exposed WT mice compared with room air controls. This reduction was not seen in MMP-9 (−/−) mice after hyperoxia compared with room air controls.
Fig. 4.
A: photomicrograph of 5-μm lung sections from paraffin-embedded room air- and hyperoxia-exposed 13-day-old WT and MMP-9 (−/−) neonatal mice. Hematoxylin & eosin stain, ×20 magnification. B: mean linear intercept (MLI) measured in lungs from WT and MMP-9 (−/−) mice after 8 days of exposure to either room air or hyperoxia. WT mice exposed to hyperoxia have less gas exchange surface area (i.e., higher MLI) compared with room air-exposed mice. Lungs from MMP-9 (−/−) mice exposed to hyperoxia have a larger gas exchange surface area (i.e., lower MLI) compared with the lungs from hyperoxia-exposed WT mice. Bars are means ± SE; n = 4 per condition. *Compared with room air P < 0.05; **compared with hyperoxia-exposed WT P < 0.05. C: alveolar number across terminal respiratory units as determined by radial alveolar counts. There is a significant reduction in alveolar counts in hyperoxia-exposed WT mice compared with room air controls; n = 3; bars are means ± SE. *P = 0.02 compared with room air WT.
Effect of hyperoxia on lung function in neonatal mice.
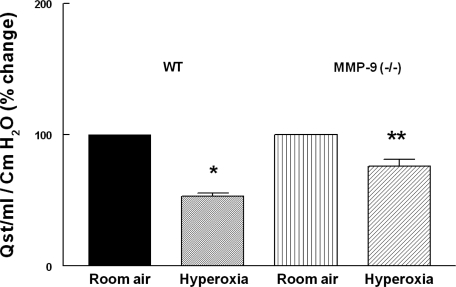
After 8 days of hyperoxia or room air exposure, quasistatic lung compliance (Qst) was measured using the forced oscillation technique in deeply anesthetized, tracheotomized mice. Qst represents the static elastic recoil pressure of the lungs at a given lung volume. We measured the Qst to determine functional correlates to hyperoxia-induced changes in lung morphometry and histology in WT and MMP-9 (−/−) neonatal mice. Figure 5 shows Qst of WT mice and MMP-9 (−/−) neonatal mice after room air or hyperoxia exposure. Lung compliance at 30 cmH2O was significantly decreased after 8 days of hyperoxia exposure. This reduction was significantly less in MMP-9 (−/−) mice after hyperoxia [24% reduction in MMP-9 (−/−) compared with a 47% reduction in WT after hyperoxia]. The decrease in lung compliance in WT mice following hyperoxia is very similar to the decrease seen in infants with BPD (42). These data show that hyperoxic injury alters compliance in a manner consistent with alveolar loss and airway remodeling and that MMP-9 expression is involved in this remodeling process.
Fig. 5.
Quasistatic compliance (Qst) from anesthetized 13-day-old WT and MMP-9 (−/−) pups after 8 days of hyperoxia or room air exposure measured by forced oscillation technique. The lungs of oxygen-exposed mice were less compliant than those of room air-exposed WT and MMP-9 (−/−) neonatal mice. This reduction was significantly less in MMP-9 (−/−) mice after hyperoxia (24% reduction compared with 47% reduction in WT after hyperoxia; n = 4 mouse pups per condition). *P < 0.03 compared with room air-exposed WT; bars are means ± SE. **P = 0.001 compared with room air-exposed MMP-9 (−/−).
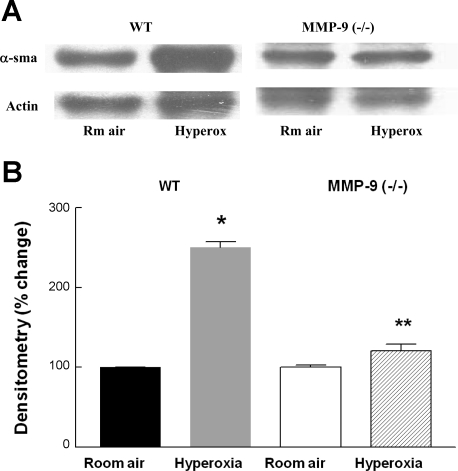
Effect of hyperoxia on α-SMA.
Normal fetal and neonatal lungs have numerous myofibroblasts within the alveolar septa that are immunopositive for α-SMA. After injury in the neonatal lung, α-SMA-positive cells are increased in the center of the septum and invade beyond the basement membrane. These are myofibroblasts as originally described in wound healing (17). We studied the changes in α-SMA expression following hyperoxic injury lung homogenates using Western blot followed by densitometry. The lungs of hyperoxia-exposed WT mice had significantly more α-SMA compared with room air-exposed WT mouse pups (Fig. 6). In contrast, this increase was not seen in MMP-9 (−/−) mice exposed to hyperoxia.
Fig. 6.
A: representative Western blot for α-smooth muscle actin (α-SMA) in 13-day-old WT and MMP-9 (−/−) neonatal mouse lungs following exposure to room air or hyperoxia for 8 days. B: densitometry of Western blots from 4 mouse pups per condition. Bars are means ± SE. α-SMA signal was normalized to actin. *P < 0.05 compared with WT room air. **P < 0.05 compared with hyperoxia-exposed WT.
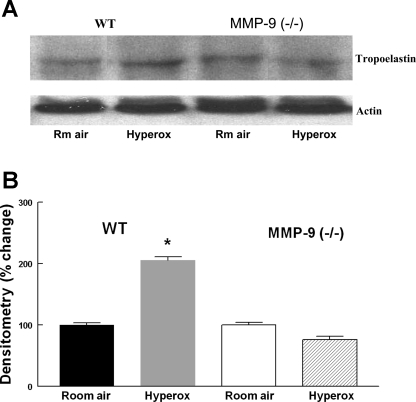
Effect of hyperoxia on lung elastin.
Lungs from WT and MMP-9 (−/−) neonatal mice exposed to 8 days of hyperoxia were sectioned and stained for elastin. Lung tissues from each experimental group were stained simultaneously so that incubation times were equivalent for the treatment groups. The stain revealed a disorganized pattern of elastin following hyperoxia exposure. Thick bands of elastin were seen in distal air spaces in hyperoxia-exposed compared with room air-exposed WT mice. As would be expected, elastin staining was noted at the tips of alveolar crests in room air-exposed WT mice. In hyperoxia-exposed WT mice, the elastin stain was extended into and was more prominent within the body of the alveolar crests compared with the tips (Fig. 7). In addition, we found a significant increase in tropoelastin, a precursor of elastin in the lungs from hyperoxia-exposed compared with room air-exposed WT mice by Western blot analysis. This increase was not seen in hyperoxia-exposed lungs from MMP-9 (−/−) mice compared with room air controls (Fig. 8). These findings are in agreement with the increase in elastin stain described in lungs from premature lambs exposed to hyperoxia and mechanical ventilation (1).
Fig. 7.
Elastin stain. Photomicrographs of 5-μm lung sections from 13-day-old hyperoxia-exposed WT and MMP-9 (−/−) neonatal mice stained with Van Gieson stain for elastin (×40 magnification). Arrows point to the positive stain for elastin in the tip of the crest in room air-exposed lungs and in the body of the alveolar crests in hyperoxia exposed lungs.
Fig. 8.
A: representative Western blot for tropoelastin in 13-day-old WT and MMP-9 (−/−) mouse lungs following exposure to room air or hyperoxia for 8 days. B: densitometry of Western blots. Bars are means ± SE; n = 4. Tropoelastin signal was normalized to actin. *P < 0.05 compared with WT room air.
Effect of hyperoxia on type I collagen.
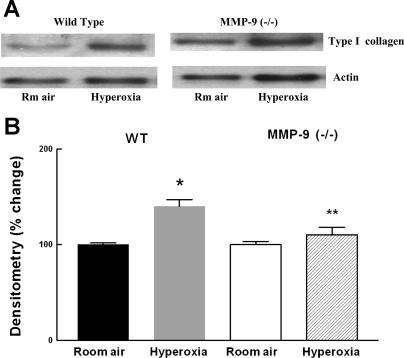
An increase in type I collagen has been reported in the baboon model of BPD with an accumulation of collagen in alveolar ducts (38). We therefore studied collagen expression in WT and MMP-9 (−/−) mice exposed to room air and hyperoxia using an affinity-purified rabbit anti-collagen type I antibody with minimum cross-reactivity to types II to VI collagens. There was a significant increase in type I collagen after hyperoxia exposure compared with room air-exposed WT mice. This increase was not seen in hyperoxia-exposed MMP-9 (−/−) mice (Fig. 9).
Fig. 9.
A: representative Western blot for type I collagen in 13-day-old WT and MMP-9 (−/−) mouse lungs following exposure to room air or hyperoxia for 8 days. B: densitometry of Western blots. Bars are means ± SE; n = 4. Type I collagen signal was normalized to actin. *P < 0.05 compared with room air. **P < 0.05 compared with hyperoxia-exposed WT.
DISCUSSION
Lung development and alveolarization continues after birth in humans and rodents. Clinical interventions, such as oxygen therapy in the first week of life, can adversely impact alveolar formation. Type II alveolar epithelial cells play a pivotal role in alveolar basement membrane remodeling in normal alveolarization and angiogenesis (29) and after hyperoxic injury (10). The ECM is composed of type I collagen, a major substrate for MMP-9. Destruction of the ECM may result in impaired alveolar development and vasculogenesis. Migration of alveolar epithelial cells is necessary for remodeling of the developing lung and effecting repair after lung injury.
During lung development, epithelial cell migration is observed during the glandular stage of organogenesis (26). MMP-2 and MMP-9 protease activities increase during lung development. In the recovery phase of acute lung injury, reepithelialization of the denuded alveolar basement membrane is achieved by migration and proliferation of the alveolar epithelial progenitor cells. Buckley et al. (9) have shown that alveolar epithelial cells derived from adult rats exposed to hyperoxia for 48 h were more migratory and secreted more MMP-9 compared with control rats.
We chose not to expose the mouse pups to hyperoxia soon after birth since hyperoxia-induced morbidity and mortality are much higher in this early neonatal period due to a combination of hyperoxia and poor nutrition (32). Since we wanted to evaluate the lungs at the end of the saccular and at the beginning of the alveolar stage, we exposed the pups to hyperoxia starting on day 5 of life.
Increased metalloprotease activity has been reported after hyperoxic injury. We chose to focus on MMP-9 in this study because of reports showing elevated MMP-9 in bronchoalveolar lavage fluid collected from preterm infants who subsequently developed BPD (19) and in alveolar type II epithelial cells in adult rats after 85% hyperoxia exposure (33) and in the lungs of newborn rat pups after hyperoxic exposure (18, 34).
The increased MMP-9 activity that has been reported in neonatal hyperoxic lungs may be due to several possible modes of activation including uPA activity. MT-I MMP (MMP-14) may also be active since it is expressed in higher levels in lung injuries (10). It is also possible that the reduction in hyperoxia-induced MMP activity during the recovery period may be mediated through increased amounts of tissue inhibitor of metalloprotease (TIMP-1). Increased levels of MMP-8 and -9 were found in the tracheal aspirates of preterm infants with respiratory distress syndrome who subsequently developed BPD (11). MMP-8 was mostly expressed in macrophages.
Of importance to this study, increased MMP-9 was seen in preterm infants who had antenatal lung inflammation associated with chorioamnionitis leading to development of chronic lung disease (16). The MMP-9/TIMP-1 ratio in the bronchoalveolar lavage was found to be higher in babies who developed BPD, implying a proteinase/antiproteinase imbalance in these preterm babies exposed to hyperoxia (37). An imbalance between MMP-9 and TIMP-1 leading to excessive MMP-9 activity has been reported to contribute to lung inflammation and edema in BPD (21, 38).
Studies in adult mice suggest that MMP-9 influx into the lung plays an important role in contributing to alveolar structural damage (30). The MMP-9 (−/−) mice survived longer after 95% oxygen exposure compared with WT mice. Overexpression of MMP-9 was found to be partly responsible for pathogenic destruction during hyperoxic condition. MMP-9 polymorphism has been associated with upper lobe emphysema in COPD patients (27).
There are several reports of increased MMP-9 in the bronchoalveolar lavage fluid and in type II cells in adult rats after 85% hyperoxia exposure (33) and in the lungs of newborn rat pups (18, 34). Our study confirms reports of increased MMP-9 with hyperoxia and further shows that deletion of MMP-9 improves the response to hyperoxia of the developing lung. On the other hand, one study described decreased levels of MMP-9 mRNA and pro-MMP-9 protein and diminished pro-MMP-9 enzyme activity in neonatal rat pups exposed to >95% oxygen (24). The different findings in that study compared with ours could be related to the fact that the authors of that study measured pro-MMP-9 protein and activity, whereas we focused on mature MMP-9. There also could be differences related to the somewhat higher level of oxygen exposure as well as differences in response between neonatal rats and mice.
In this study, we demonstrated that hyperoxia exposure in neonatal mice resulted in decreased alveolarization and disordered elastin accumulation in the lung. These changes were significantly less in MMP-9 (−/−) neonatal mice. We also found a decrease in lung compliance in WT mice exposed to hyperoxia that improved with loss of MMP-9. Metalloproteinase activity regulates the development of organs through ECM remodeling. Our observation that hyperoxia increases MMP-9 in normal lungs and that deletion of MMP-9 protects against biological and functional changes secondary to hyperoxia suggests that inhibition of MMP-9 during hyperoxia could minimize the oxygen-induced damage in neonatal lungs.
In addition to MMP-9, we also found an increase of MMP-2 in lungs of WT mice exposed to hyperoxia. This is in accordance with increased MMP-2 protein expression in the bronchoalveolar lavage fluid of preterm babies and in animal models of hyperoxia (49), as well as in other organs of animals exposed to hyperoxia (12). When we further examined MMP-2 levels in MMP-9 (−/−) mice exposed to hyperoxia, we found no increase, suggesting that inhibition of MMP-9 could also ameliorate the contribution of MMP-2 to altered morphological and functional changes in hyperoxia-induced neonatal mice. It is unclear whether MMP-9 regulates expression of MMP-2 or whether MMP-2 is secondarily induced in response to ongoing damage initiated by MMP-9. Further studies of the interactive regulation of MMPs are needed to resolve this question.
With this in vivo hyperoxia exposure mouse lung model, we have found decreased alveolarization and enlarged alveoli with diminished gas exchange surface area in WT mice. This is consistent with the studies in mice exposed to hyperoxia for 4 wk (47). The changes in lung alveolarization, volume, and compliance that we identified in neonatal mice following hyperoxia are very similar to changes seen in human infants with BPD. An increase in alveolar size with fewer alveoli was found in postmortem specimens of surfactant-treated subjects by Husain et al. (25) in which the MLI was significantly increased in BPD subjects compared with non-BPD. Hislop et al. (23) and others (6, 44) have shown that the lungs of preterm infants who received mechanical ventilation and oxygen had nonuniform inflation, reduced numbers of alveoli, and increased accumulation of elastic fibers in alveolar walls and muscularization of distal airways.
In this study, we found a decrease in total lung compliance with hyperoxia exposure that is very similar to that seen in infants with BPD (42). Low lung compliance was seen on the 7th and 10th days of life in preterm infants. We measured the Qst that reflects the static elastic recoil pressure of the lungs at a given lung volume. We observed that the reduction in compliance seen in hyperoxia-exposed WT mice is ameliorated in MMP-9 (−/−) mice, suggesting a role of MMP-9 in the hyperoxia-induced lung remodeling that later manifests as reactive airway disease.
Distal airways have smooth muscle that is increased in abundance in BPD. Myofibroblasts that are normally present in the terminal airways in the developing lung are increased in number after lung injury, especially around the terminal air spaces as shown both in humans (43) and in animals (1). In agreement with this, we found an increase in α-SMA, a marker for myofibroblasts, in hyperoxia-exposed WT neonatal mouse lung.
MMP-9 has been shown to be an elastolytic protease in emphysema in adult mice. Adult transgenic mice with MMP-9 overexpression in the alveolar macrophages developed enlarged alveoli with elastin breakdown (22). A paucity of elastic fibers in the wall of the alveoli was seen due to destruction of elastic fibers as a result of protease-antiprotease imbalance (6, 8). We found in hyperoxia-exposed WT animals an increase in elastin in the body of the alveolar crests instead at their tips where elastin is normally located during septation. These changes in elastin localization were ameliorated in hyperoxia-exposed MMP-9 (−/−) mice. Alveolar secondary crest formation may be impaired by excessive accumulation of elastic fibers in the stunted alveolar crests and in the alveolar walls as reported by other investigators (4). Decreased alveolar secondary crest volume density, increased amount and abnormal distribution of elastic fibers, and increased smooth muscle around terminal bronchioles have been described in large animals exposed to hyperoxia (1).
To determine whether the increase in elastin in the lungs is associated with changes in ECM elements, we assessed type I collagen protein in the lungs of mice exposed to hyperoxia. Our finding of increased type I collagen in hyperoxia-exposed WT neonatal mice agrees with the increased type I collagen seen in primary cultures of lung fibroblasts exposed to hyperoxia and the increased expression and accumulation in the alveolar ducts described by others in the lungs of neonatal mice exposed to hyperoxia (2, 47) and in premature baboons with BPD (38). Longer durations of hyperoxia exposure lead to changes in terminal air spaces accompanied by increased collagen deposition and increased cellular infiltration with an increase in interstitial thickness.
In conclusion, hyperoxia-exposed WT mice had less gas exchange surface area compared with room air-exposed mice. In addition, hyperoxia-exposed WT mouse lungs had increased MMP-9, increased collagen and α-SMA, and altered elastin deposition. Lungs from MMP-9 (−/−) mice exposed to hyperoxia have a larger gas exchange surface area compared with the lungs from hyperoxia-exposed WT mice. Functional studies revealed a reduction in lung compliance in WT mice after hyperoxia exposure. This reduction was ameliorated in hyperoxia-exposed MMP-9 (−/−) mice. Hyperoxia-induced changes in collagen, α-SMA, and elastin were also improved in MMP-9 (−/−) mice. These findings implicate a mechanistic role of MMP-9 in neonatal hyperoxic lung injury and suggest that this genetically altered hyperoxia mouse model may be of value in future studies to determine the mechanism that creates the BPD phenotype. Blocking MMP-9 activity may lead to novel therapeutic approaches in preventing BPD.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-67089, HL-074859, and HL-37930.
Acknowledgments
We thank Dr. Caroline Owen, Brigham and Women's Hospital, Harvard Medical School, for providing expertise in lung morphometry in relation to oxidant injury.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med 159: 945–958, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med 173: 318–326, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice. Prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol 294: L3–L14, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Bruce MC, Bruce EN, Janiga K, Chetty A. Hyperoxic exposure of developing rat lung decreases tropoelastin mRNA levels that rebound postexposure. Am J Physiol Lung Cell Mol Physiol 265: L293–L300, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bruce MC, Schuyler M, Martin R, Starcher B, Tomashefski JF Jr, Wedig K. Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis 146: 204–212, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Bruce MC Developmental changes in tropoelastin mrna levels in rat lung: evaluation by in situ hybridization. Am J Respir Cell Mol Biol 5: 344–350, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Bruce MC, Lo PY. A morphometric quantitation of developmental changes in elastic fibers in rat lung parenchyma: variability with lung region and postnatal age. J Lab Clin Med 117: 226–233, 1991. [PubMed] [Google Scholar]
- 9.Buckley S, Driscoll B, Shi W, Anderson K, Warburton D. Migration and gelatinases in cultured fetal, adult, and hyperoxic alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L427–L434, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Buckley S, Warburton D. Dynamics of metalloproteinase-2 and -9, TGF-β, and uPA activities during normoxic vs. hyperoxic alveolarization. Am J Physiol Lung Cell Mol Physiol 283: L747–L754, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Cederqvist K, Sorsa T, Tervahartiala T, Maisi P, Reunanen K, Lassus P, Andersson S. Matrix metalloproteinases-2, -8, and -9 and TIMP-2 in tracheal aspirates from preterm infants with respiratory distress. Pediatrics 108: 686–692, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Chen PS, Zhai WR, Zhou XM, Zhang JS, Zhang YE, Ling YQ, Gu YH. Effects of hypoxia, hyperoxia on the regulation of expression and activity of matrix metalloproteinase-2 in hepatic stellate cells. World J Gastroenterol 7: 647–651, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chetty A, Andersson S, Lassus P, Nielsen HC. Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr Pulmonol 37: 128–136, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Coalson JJ Pathology of bronchopulmonary dysplasia. Semin Perinatol 30: 179–184, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37: 572–579, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curley AE, Sweet DG, Thornton CM, O'Hara MD, Chesshyre E, Pizzotti J, Wilbourn MS, Halliday HL, Warner JA. Chorioamnionitis and increased neonatal lung lavage fluid matrix metalloproteinase-9 levels: implications for antenatal origins of chronic lung disease. Am J Obstet Gynecol 188: 871–875, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Darby I, Skalli O, Gabbiani G. α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63: 21–29, 1990. [PubMed] [Google Scholar]
- 18.Devaskar UP, Taylor W, Govindrajan R, Malicdem M, Heyman S, deMello DE. Hyperoxia induces interstitial (type I) and increases type IV collagenase mRNA expression and increases type I and IV collagenolytic activity in newborn rat lung. Biol Neonate 66: 76–85, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Dik WA, van Kaam AH, Dekker T, Naber BA, Janssen DJ, Kroon AA, Zimmermann LJ, Versnel MA, Lutter R. Early increased levels of matrix metalloproteinase-9 in neonates recovering from respiratory distress syndrome. Biol Neonate 89: 6–14, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Dunnett CW A multiple comparison procedure for several treatments with a control. J Am Stat Assoc 50: 1096–1121, 1955. [Google Scholar]
- 21.Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE. Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics 113: 1709–1714, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Foronjy RF, Nkyimbeng T, Wallace AM, Thankachen J, Okada Y, Lemaitre V, D'Armiento JM. The transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol 294: L1149–L1157, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hislop AA, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum Dev 15: 147–164, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Hosford GE, Fang X, Olson DM. Hyperoxia decreases matrix metalloproteinase-9 and increases tissue inhibitor of matrix metalloproteinase-1 protein in the newborn rat lung: association with arrested alveolarization. Pediatr Res 56: 26–34, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Infeld MD Cell-matrix interactions in gland development in the lung. Exp Lung Res 23: 161–169, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Ito I, Nagai S, Handa T, Muro S, Hirai T, Tsukino M, Mishima M. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med 172: 1378–1382, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Jobe AJ The new BPD: an arrest in lung development. Pediatr Res 46: 641–643, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Kraling BM, Wiederschain DG, Boehm T, Rehn M, Mulliken JB, Moses MA. The role of matrix metalloproteinase activity in the maturation of human capillary endothelial cells in vitro. J Cell Sci 112: 1599–1609, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol 174: 7250–7256, 2005. [DOI] [PubMed] [Google Scholar]
- 31.McGowan SE, Holmes AJ, Smith J. Retinoic acid reverses the airway hyperresponsiveness but not the parenchymal defect that is associated with vitamin A deficiency. Am J Physiol Lung Cell Mol Physiol 286: L437–L444, 2004. [DOI] [PubMed] [Google Scholar]
- 32.McGrath-Morrow SA, Stahl J. Apoptosis in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol Biol 25: 150–155, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Pardo A, Barrios R, Maldonado V, Melendez J, Perez J, Ruiz V, Segura-Valdez L, Sznajder JI, Selman M. Gelatinases A and B are up-regulated in rat lungs by subacute hyperoxia: pathogenetic implications. Am J Pathol 153: 833–844, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radomski A, Sawicki G, Olson DM, Radomski MW. The role of nitric oxide and metalloproteinases in the pathogenesis of hyperoxia-induced lung injury in newborn rats. Br J Pharmacol 125: 1455–1462, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramadurai SM, Chen WY, Yerozolimsky GB, Zagami M, Dammann CE, Nielsen HC. Cell-specific and developmental expression of phospholipase C-gamma and diacylglycerol in fetal lung. Am J Physiol Lung Cell Mol Physiol 284: L808–L816, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol 178: 6191–6199, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Sweet DG, Curley AE, Chesshyre E, Pizzotti J, Wilbourn MS, Halliday HL, Warner JA. The role of matrix metalloproteinases -9 and -2 in development of neonatal chronic lung disease. Acta Paediatr 93: 791–796, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD. Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol 39: 5–14, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 93: 263–270, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Torday JS, Rehan VK. Developmental cell/molecular biologic approach to the etiology and treatment of bronchopulmonary dysplasia. Pediatr Res 62: 2–7, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Tortorolo L, Vento G, Matassa PG, Zecca E, Romagnoli C. Early changes of pulmonary mechanics to predict the severity of bronchopulmonary dysplasia in ventilated preterm infants. J Matern Fetal Neonatal Med 12: 332–337, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Toti P, Buonocore G, Tanganelli P, Catella AM, Palmeri ML, Vatti R, Seemayer TA. Bronchopulmonary dysplasia of the premature baby: an immunohistochemical study. Pediatr Pulmonol 24: 22–28, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Van Lierde S, Cornelis A, Devlieger H, Moerman P, Lauweryns J, Egermont E. Different patterns of pulmonary sequelae after hyaline membrane disease: heterogeneity of bronchopulmonary dysplasia? A clinicopathological study. Biol Neonate 60: 152–162, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA. Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia? Am J Respir Cell Mol Biol 31: 650–656, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Villanueva D, McCants D, Nielsen HC. Effects of epidermal growth factor (EGF) on the development of EGF-receptor (EGF-R) binding in fetal rabbit lung organ culture. Pediatr Pulmonol 29: 27–33, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol Lung Cell Mol Physiol 275: L110–L117, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XF, Ding SF, Gao YM, Liang Y, Foda HD. Expression of various matrix metalloproteinases in mice with hyperoxia-induced acute lung injury. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 18: 449–451, 2006. [PubMed] [Google Scholar]