Abstract
This study investigated the effect of age on recovery of skeletal muscle from an ischemia-reperfusion (I/R)-induced injury. Young (6 mo old) and old (24–27 mo old) Sprague-Dawley rats underwent a 2-h bout of hindlimb ischemia induced by a pneumatic tourniquet (TK). The TK was released to allow reperfusion of the affected limb, and animals were divided into 7- and 14-day recovery groups. Maximum plantar flexor force production was assessed in both 7- and 14-day recovery groups of both ages, followed by histological evaluation. Subsequent analysis of IGF-I gene expression and intracellular signaling in 7-day recovery muscles was performed by RT-PCR and Western blotting, respectively. Old rats had significantly greater deficits in force production and exhibited more evidence of histological pathology than young at both 7 and 14 days postinjury. In addition, old rats demonstrated an attenuated upregulation of IGF-I mRNA and induction of proanabolic signaling compared with young in response to injury. We conclude that aged skeletal muscle exhibits more damage and/or defective regeneration following I/R and identify an age-associated decrease in local IGF-I responsiveness as a potential mechanism for this phenomenon.
Keywords: aging, insulin-like growth factor-I, ischemia-reperfusion, muscle regeneration, sarcopenia
over 20,000 operating room tourniquet (TK) applications are performed per day worldwide as a standard, necessary practice to create a bloodless surgery field and prevent excessive blood loss from patients (50). However, this practice itself is potentially damaging to skeletal muscle due to the prolonged ischemia and subsequent reperfusion injury that accompany TK use. During ischemia, tissue is faced with a cascade of ATP depletion, acidosis, and ion imbalance. The reentry of blood flow to the affected area, reperfusion, is much more damaging, as it causes production of reactive oxygen species (ROS) and rapid calcium influx into the cell; both are causes of mitochondrial dysfunction that lead to cellular apoptosis and/or necrosis (32). Since the severity of this ischemia-reperfusion (I/R) injury increases with the length of the ischemic period (57), it is clinically recommended that TK application not exceed 2 h (8).
Skeletal muscle in aged individuals is characterized by a decline in mass and loss of functional capacity in both rodents and humans (see Ref. 1 for review). In addition, aged skeletal muscle demonstrates more susceptibility to eccentric damage, the damage caused by eccentric contraction of the muscle (14, 72), as well as attenuated regenerative capacity compared with adult animals following various modes of injury, including eccentric damage (13), bupivicaine injection (62), and cardiotoxin injection (52). The greater susceptibility to eccentric damage and the impaired rate of functional recovery has been successfully reduced by superoxide dismutase injection (72) and overexpression of heat shock protein 70 (49), suggesting enhanced vulnerability of aged skeletal muscle to oxidative stress.
Carlson and Faulkner demonstrated in a heterochronic muscle transplant model that damaged muscles from an old donor regenerate more efficiently when transplanted into a young host than do young muscles transplanted into an old host (17); thus regeneration depends on the age of the host and not the age of the transplanted tissue. The hypothesis that the age of the systemic environment affects regeneration was further supported by the work of Conboy et al. (20), which achieved a similar result using the parabiosis model of young and old mice sharing a common circulatory system. This evidence suggests the regenerative capacity of muscle is greatly affected by blood-borne factors in the host organism and is not solely intrinsic to the muscle cells.
Evidence of diffusible factors being responsible for the slowed regeneration of aged skeletal muscle suggests a potential role of the circulating endocrine growth factor insulin-like growth factor I (IGF-I). IGF-I is also released in autocrine/paracrine fashion from skeletal muscle in response to injury and mechanical stimuli and is crucial to muscle regeneration and hypertrophy because it stimulates both proliferation and differentiation in satellite cells (see Refs. 1, 2, 26 for review). Binding of the IGF-I peptide to its tyrosine kinase receptor promotes positive net anabolic activity by both promoting protein synthesis through activation of a protein kinase B (Akt)/mammalian target of rapamycin (mTOR)-dependent pathway (10, 59) and inhibiting protein degradation via Akt-dependent inactivation of forkhead transcription factor (FoxO) proteins (42, 64). The local upregulation of IGF-I has been induced in skeletal muscle by a variety of injury models, including myotoxin injection (30, 31, 36), eccentric damage (30, 31, 38, 51), and I/R (25, 37). Although it is not well documented whether muscle-localized expression of IGF-I in response to traumatic injury declines with age, IGF-I overexpression and supplementation models have reversed the age-related loss of muscle mass (7, 18) and promoted normal regeneration of aged muscle (52), suggesting an inverse relationship between age and IGF-I response to injury.
With a disproportionate number of orthopedic surgeries occurring in elderly patients, the postsurgical I/R injury ensuing TK application is a notable concern to the elderly population. With this demographic in mind, the present investigation was conducted to determine the age-related differences in recovery of muscle function from 2-h TK-induced I/R injury in rats. It was found that at both 7 and 14 days of recovery, aged rats demonstrated greater functional deficits than their young counterparts. In an effort to provide a potential mechanism for this finding, we subsequently examined IGF-I gene expression and the abundance of key phosphoproteins in the IGF-I signaling cascade. Our results indicate that an age-related reduction in IGF-I gene expression and proanabolic signaling is a possible explanation for the impaired recovery of muscle function in aged rats following I/R injury.
METHODS AND MATERIALS
Animals.
Male Sprague-Dawley rats from colonies maintained by the University of Texas at Austin Animal Resource Center were used in this study. The rats were 6 mo (young) and 24–27 mo (old) of age and weighed approximately 425 and 500 g, respectively. Rats were housed two per cage, maintained on a 12:12-h light-dark cycle, and allowed ad libitum access to food and water. Rats were assigned to one of four TK recovery groups: young 7-day recovery (n = 4), young 14-day recovery (n = 4), old 7-day recovery (n = 5), and old 14-day recovery (n = 4). All experimental procedures were approved and conducted in accordance with guidelines set by the University of Texas at Austin Institutional Animal Care and Use Committee.
TK application.
Rats were anesthetized with 2% isoflurane gas before and for the duration of TK application. A single, randomly selected hindlimb was elevated, and a pneumatic TK (Hokanson) was wrapped snuggly against the proximal portion of the limb and inflated to 250 mmHg by the Portable Tourniquet System (Delfi Medical Innovations) to ensure complete occlusion of blood flow to the limb for a duration of 2 h (68). Body temperature was maintained with the use of a heat lamp during this procedure. After 2 h, the pneumatic TK was removed, and the rat was returned to its cage for recovery.
In vivo force measurement.
Contractile properties were assessed at either 7 or 14 days following I/R injury. Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (80 mg/kg) before any surgical procedures. A nerve cuff constructed of Coonar wire, Silastic tubing, and Silastic cement was placed around the tibial nerve using a method described by Walters et al. (69). An in vivo model similar to that designed by Ashton-Miller et al. (6) was used to measure the force generated by the triceps surae muscle group (gastrocnemius, soleus, and plantaris). The knee of the anesthetized rat was secured at the tibial condyles by a modified clamp apparatus, while the foot was strapped to a foot pedal connected to a servomotor (Aurora model 310) to allow measurement of the force produced by plantar flexion. The triceps surae group was activated through the nerve cuff implanted about the tibial nerve with a stimulus of ∼20 V at a frequency of 150 Hz from a Grass transducer (S88). The stimulus was enough to invoke a maximal tetanic contraction of the triceps surae group. Force produced was recorded with a computer (Dell 8250), interfaced with a data-acquisition board (National Instruments). The data were stored and analyzed using LabView software programs (National Instruments). Both the TK and contralateral control legs were tested. Measurements were taken in triplicate, one leg at a time, with at least 1 min of rest between each measurement.
Tissue harvesting.
Immediately following completion of the force measurements, the gastrocnemius, plantaris, and soleus muscles were quickly harvested from both the TK and control legs, and muscle wet weights were recorded. Muscle samples were then frozen in liquid nitrogen-cooled isopentane and stored at −80°C until later analysis. Rats were euthanized with an overdose of pentobarbital sodium.
Histology.
Plantaris muscles from control and TK legs of all animals used for this study were fixed in 10% formalin and embedded in paraffin wax. Muscle cross sections were cut 5 μm thick and stained with hematoxylin and eosin (H and E). All slides were evaluated both subjectively and quantitatively by a board-certified veterinary pathologist using an Olympus BX41 microscope at 4, 10, and 40× magnification. Images (see Fig. 2) were captured at 40× magnification using an Olympus BX41 microscope and an Olympus DP71 digital camera.
Fig. 2.
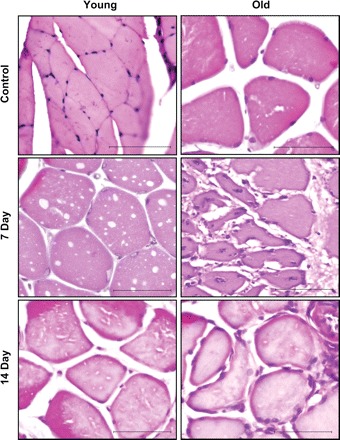
Hematoxylin and eosin (H and E)-stained slides of representative control, 7-day, and 14-day recovery plantaris muscles from young and old rats following I/R-induced damage. Photographs are taken at a magnification of ×40. The inset bar represents 100 μm.
RNA isolation and cDNA synthesis.
RNA was extracted from portions of gastrocnemius muscles using RNA-STAT (Tel-Test, Friendswood, TX). Samples underwent chloroform extraction and centrifugation, followed by precipitation in isopropanol at −20°C. Precipitated RNA was centrifuged, the supernatant was removed, and the pellet was dissolved in nuclease-free water. RNA was quantified on a spectrophotometer at a wavelength of 260 nm. Conversion of total RNA to single-strand cDNA was accomplished using the High-Capacity cDNA Archive Kit (P/N 4322171; Applied Biosystems, Foster City, CA). Briefly, 5–10 μg total RNA were reverse transcribed using random primers for the following incubation times: 25°C for 10 min, then 37°C for 2 h. cDNA samples were stored at −80°C until use.
RT-PCR.
TaqMan-MGB IGF-I and eukaryotic 18S probe and primers were purchased from Applied Biosystems. IGF-I and 18S probes were purchased as “Gene Expression Assays” (ID numbers Mm00439561_m1 and Hs99999901_s1, respectively). The real-time PCR reaction was performed within an ABI 7500 thermal cycler. The fluorescence of 3–15 cycles was set up as background. Data were collected at the annealing step of each cycle, and the threshold cycle (Ct) for each sample calculated by determining the point at which the fluorescence exceeded the threshold limit. The standard curve was calculated automatically via software by plotting the Ct values against each standard of known concentration and calculation of the linear regression line of this curve. Serially diluted amounts of RNA were used to establish standard curves for IGF-I mRNA and 18S rRNA. All samples and standards were run in triplicate or quadruplicate.
Western blotting.
Approximately 500 mg of muscle tissue was removed from frozen gastrocnemius muscles of both control and TK limbs, trimmed of all visible connective tissue, and homogenized in a buffer containing 50 mM HEPES (pH 7.6), 150 mM NaCl, 1% Triton X-100, 20 mM β-glycerol phosphate, 10 mM NaF, 1 mM Na3VO4, 10 ng/ml each of leupeptin and aprotinin, 1 mM PMSF, and 1:100 dilutions of phosphatase inhibitor cocktails 1 and 2 (Sigma-Aldrich). The resulting homogenate was centrifuged at 12,000 g for 30 min, and the supernatant was kept for analysis. Protein concentrations of all samples were determined as described by Bradford (11).
Samples were boiled in 4× Laemmli's sample buffer at a ratio of 3:1, and equal amounts of total protein were loaded into each well of a 5% stacking-15% separating polyacrylamide gel. Following SDS-PAGE, proteins were transferred to a PVDF membrane (Millipore) and blocked with 5% milk in 0.1% Tween-20 in TBS (TBST) for 1 h. Membranes were incubated in 1:1,000 dilutions of either anti-phospho-FoxO3a (Ser 253; Cell Signaling Technology), anti-phospho-mTOR (Ser 2448; Cell Signaling Technology), or anti-phospho-p70 S6 kinase (Thr 389; Santa Cruz) primary antibodies in 5% BSA-TBST overnight at 4°C, then in 1:1,000 dilutions of goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Pierce) in 5% milk-TBST for 2 h. Membranes were then stripped and reprobed with anti-FoxO3a, anti-mTOR, or anti-p70 S6 kinase (Cell Signaling Technology), and then again with anti-GAPDH (Santa Cruz) for a loading control. Blots were imaged with the Chemidoc XRS system (Bio-Rad). Band volumetric analysis was performed using Quantity One software and is expressed as arbitrary units of volume [intensity (INT) × mm2]. Phosphorylation status of each protein species was calculated by dividing the abundance of the phosphorylated form by the total protein abundance. All experiments were repeated in triplicate to verify results.
Statistical analysis.
All values are expressed as means ± SE. Mass, force, mRNA, and protein abundance data were analyzed using two-way ANOVA. Significant differences between means were determined using Student's t-tests. Data analysis was performed using SPSS 16.0 statistical software. Statistical significance was set at P ≤ 0.05.
RESULTS
Recovery of muscle function.
To determine the consequences of the I/R perturbation on muscle contractility, in vivo plantar flexor force measurements were performed on both control and TK legs for 7- and 14-day recovery groups. The results of these experiments are summarized in Fig. 1. There were reductions of 78 and 21% in force production and combined mass of the triceps surae group compared with contralateral control values (P ≤ 0.01 for both measures), respectively, in young rats following 7 days of recovery from the TK-induced injury. After 14 days of recovery, the young rats regained ∼50% of the force deficit seen at day 7 (Fig. 1C), but mean values were still significantly below control values in terms of force (43%; P ≤ 0.001) and muscle mass (17%; P ≤ 0.001). Old rats also demonstrated significant 85% and 13% decreases in force (P ≤ 0.001) and mass (P ≤ 0.05), respectively, from control values following 7 days of recovery from TK-induced I/R. By day 14, old rats showed substantial recovery of force production over 7-day recovery values; however, there were still significant reductions in force (50%; P ≤ 0.001) and muscle mass (15%; P ≤ 0.01) compared with control values. Muscle masses of old control and 14-day recovery rats were significantly lower than those of young rats (P ≤ 0.01 and P ≤ 0.05, respectively), as were the force production values for control, 7-day recovery, and 14-day recovery animals (P ≤ 0.01, P ≤ 0.05, and P ≤ 0.001, respectively). Additionally, old rats demonstrated significantly higher functional deficits at both 7- and 14-day recovery time points (P ≤ 0.05 for both groups) than young.
Fig. 1.
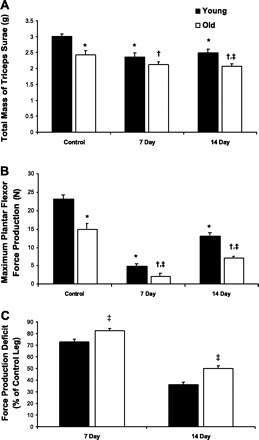
Combined mass of the triceps surea group (gastrocnemius, plantaris, and soleus muscles) (A), maximum plantar flexor force production (B), and force production deficit (C) of young and old Sprague-Dawley rats following 7 and 14 days recovery from 2-h tourniquet (TK)-induced ischemia-reperfusion (I/R). Values are expressed as means ± SE. *P ≤ 0.05 vs. young control; †P ≤ 0.05 vs. old control; ‡P ≤ 0.05 vs. day-matched young.
Histology.
H and E-stained cross sections of plantaris muscles were subjectively and qualitatively evaluated to identify morphological differences between young and old skeletal muscle during recovery from I/R. Representative slides from each group are found in Fig. 2, and mean pathologist ratings are found in Table 1. At 7 days following TK application, there is extensive evidence of tissue injury in both young and old rats. Among the old animals there is moderate to severe degeneration and necrosis of the muscle fibers, mild to severe inflammatory infiltrates, mild to moderate edema, and mild early fibrosis, whereas the young animals have only minimal degeneration of the muscle fibers, minimal inflammatory infiltrates, mild edema, and no fibrosis. At 14 days following TK application, the recovery in the old animals was poor compared with the young, showing moderate degeneration of muscle fibers, mild to moderate inflammatory infiltrates, and mild to moderate fibrosis, whereas young animals have only mild degeneration of the muscle fibers, minimal inflammatory infiltrates, no edema, and no fibrosis.
Table 1.
Pathological evaluation of hematoxylin and eosin-stained plantaris following recovery from I/R
| Control |
7 Day |
14 Day |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Young | Old | Young | Old | Young | Old | ||||
| Muscle pathology | |||||||||
| Degeneration | |||||||||
| Area of slide | 0.0 | 0.0 | 4.0±0.0 | 4.0±0.0 | 3.3±0.3 | 3.3±0.3 | |||
| Severity | Min | Mod-Sev | Mld | Mod | |||||
| Necrosis | 0.0 | 0.0 | 0.5±0.4 | 3.0±0.6 | 0.0 | 0.0 | |||
| Edema | 0.0 | 0.0 | 2.0±0.0 | 2.3±0.3 | 0.0 | 0.0 | |||
| Hemorrhage | 0.0 | 0.0 | 0.0 | 1.7±0.7 | 0.0 | 0.0 | |||
| Fibrosis | 0.0 | 0.0 | 0.0 | 1.7±0.3 | 0.0 | 2.7±0.3 | |||
| Inflammation | |||||||||
| Neutrophils | 0.0 | 0.0 | 0.5±0.4 | 2.7±0.7 | 0.0 | 0.0 | |||
| Macrophages | 0.0 | 0.0 | 0.0 | 2.7±0.7 | 0.0 | 2.3±0.3 | |||
| Lymphocytes/plasma cells | 0.0 | 0.0 | 0.0 | 2.7±0.7 | 1.3±0.3 | 2.3±0.3 | |||
Values are means ± SE. For measures of degeneration and necrosis: 0 = normal muscle, 1 = 0–5%, 2 = 5–20%, 3 = 20–40%, 4 = >40% of slide area; severity of degeneration is denoted as Min (minimal), Mld (mild), Mod (moderate), or Sev (severe). For edema, hemorrhage, fibrosis, neutrophils, macrophages, and lymphocytes/plasma cells: 0 = normal, 1= minimal, 2 = mild, 3 = moderate, and 4 = severe. I/R, ischemia-reperfusion.
IGF-I gene expression.
The upregulation of IGF-I in regenerating skeletal muscle is well documented (25, 30, 31, 36, 37). To determine whether the decline in the functional and morphological qualities of the old TK tissue correlated with reduced IGF-I expression, RT-PCR analysis was performed on portions of gastrocnemius muscles from both age groups following 7 days of recovery from I/R injury (Fig. 3). In this experiment, young TK muscles demonstrated a robust 40-fold increase in IGF-I gene expression compared with young control muscle (P ≤ 0.0001). There was also a significant, fivefold elevation in IGF-I gene expression in old TK muscles compared with old control muscle (P ≤ 0.0001), which was significantly less than that of young TK muscle (P ≤ 0.0001). The expression of 18S rRNA was unchanged in all instances.
Fig. 3.
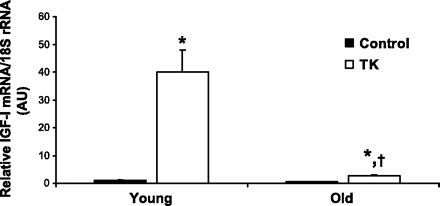
IGF-I mRNA expression relative to 18S rRNA content in 6-mo-old (Young) and 24-mo-old (Old) Sprague-Dawley rats following 7 days recovery from 2-h TK-induced I/R. *P ≤ 0.0001 vs. age-matched control; †P ≤ 0.0001 vs. young TK.
Akt-dependent signaling.
To determine if the difference in IGF-I gene expression relates to functional differences in cellular processes, particularly with regard to the mechanisms of translational control and net protein synthesis, select phosphoproteins in the IGF-I/phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascade were evaluated in 7-day recovery animals of both age groups by Western blotting. A summary of the results is found in Fig. 4.
Fig. 4.
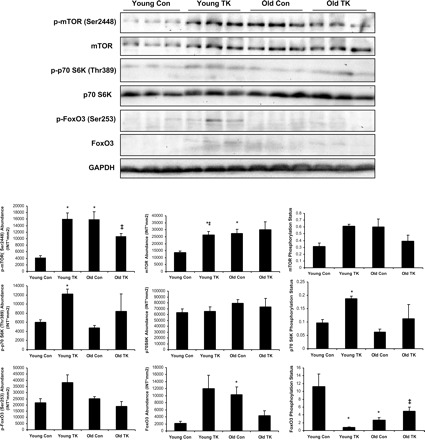
Western blots and corresponding volumetric analyses of phosphorylated (p) protein abundance, total protein abundance, and phosphorylation status of mammalian target of rapamycin (mTOR), p70 S6 kinase (p70 S6K), and forkhead transcription factor (FoxO)-3 in 6-mo-old (Young) and 24-mo-old (Old) Sprague-Dawley rats following 7 days recovery from 2-h TK-induced I/R. Values are expressed as means ± SE. *P ≤ 0.05 vs. Young control (Con); ‡P ≤ 0.05 vs. Young TK.
Phosphorylation of mTOR by Akt stimulates protein synthesis by promoting inhibition of eukaryotic initiation factor 4E binding protein (4E-BP) and activation of p70 S6 kinase (p70S6K; 9, 10, 15, 35, 58, 59); therefore increased quantities of phosphorylated mTOR (p-mTOR) can indicate anabolic activity. In the regenerating muscle from young rats, there were significant 280 and 93% elevations in p-mTOR (P ≤ 0.01) and total mTOR abundance (P ≤ 0.05), respectively, compared with control muscles. TK muscles from old rats demonstrated no significant change in p-mTOR or total mTOR content compared with contralateral control muscles. Interestingly, control muscles from aged rats showed significantly higher abundances of both p-mTOR (280%; P ≤ 0.01) and total mTOR (101%; P ≤ 0.05) than young control tissues; these values were roughly equivalent in abundance to the young TK group. A comparison of p-mTOR in young and old TK muscles was less surprising, as old had a significant 33% decrease in abundance compared with young (P ≤ 0.05). Both age and TK treatments had no significant effect on mTOR phosphorylation status.
One downstream target of mTOR analyzed in this study is p70S6K. When induced into an active state, p70S6K activates ribosomal protein S6, allowing translation initiation to occur (35). Phosphorylation of p70S6K (p-p70S6K) in the young rats increased significantly in abundance by 102% (P ≤ 0.01) in response to TK, total p70S6K did not change, and p70S6K phosphorylation status increased a significant 95% (P ≤ 0.01). The values for p-p70S6K, total p70S6K, and phosphorylation status did not change in old TK tissue compared with contralateral controls. There were no differences with age in control or TK muscles in measures of p-p70S6K, total p70S6K, or phosphorylation status.
Phosphorylation of FoxO3, a member of the forkhead transcription factor family of proteins, was also investigated. When active, FoxO proteins are localized in the nucleus and promote the transcription of atrophy-inducing genes, such as the ubiquitin ligase atrogin-1 (63), as well as prosurvival genes, such as the antioxidant enzymes superoxide dismutase and catalase (40, 48). The actions of FoxOs are negatively regulated by Akt (55, 63, 64), by which they are phosphorylated, excluded from the nucleus, ubiquitinated, and degraded by the proteasome (34, 55). TK-induced I/R had no significant effect on abundance of p-FoxO3 or total FoxO3 in either age group. However, the FoxO3 phosphorylation status, which is inversely indicative of active FoxO3, decreased 93% as a result of the perturbation in the young (P ≤ 0.01). Control muscles from old rats showed no difference in p-FoxO3 abundance, a significant 368% increase in total FoxO3 (P ≤ 0.05), and a significant 76% reduction in phosphorylation status (P ≤ 0.05) compared with young control values. Muscles from the old TK group revealed an 86% increase in FoxO3 phosphorylations status (P ≤ 0.05) compared with young TK muscles.
DISCUSSION
The age-related decline of muscular strength in the elderly population can lead to loss of independence, as the individual loses the ability to perform necessary daily routines. This affliction is a major concern among elderly postoperative individuals, since subsequent TK-induced I/R may exacerbate the loss of skeletal muscle mass and function (21). The results from this study indicate that aged skeletal muscle displays less morphological recovery and larger functional deficits than young at 7 and 14 days of recovery from 2-h TK-induced I/R injury. This suggests that aged muscle exhibits more initial damage and/or a slowed rate of recovery following the induction of injury. In addition, we provide evidence that this phenomenon may be caused by an attenuation of local IGF-I gene expression and its downstream anabolic signaling in regenerating muscle of the aged animals.
The pathology of reperfusion injury involves extensive ROS damage and ensuing inflammatory response of the affected skeletal muscle and supporting microvasculature. This leads to a vicious cycle of cellular malfunctions that ultimately causes apoptosis and/or necrosis of the resident cells (8, 29, 32). The key finding of the present study is that old rats have greater deficits in force production than young rats following I/R injury. This observation may be explained by 1) an age-related deficiency in the regenerative mechanisms of skeletal muscle following I/R injury, and/or 2) greater susceptibility of aged skeletal muscle to I/R injury (i.e. greater initial damage). The work of Brooks and Faulkner (13, 14) using the eccentric damage injury model supports a combination of these two hypotheses. They demonstrate that aged animals require longer regeneration periods following an equal amount of damage (13), as well as exhibit greater damage for a given injury stimulus (14). However, whether this holds true for the I/R injury model warrants further investigation.
Uninjured limbs of old rats from the present study demonstrated 19% less mass and 35% less force production than their young counterparts. This agrees with the established paradigm that aging is characterized by a decline of both skeletal muscle mass and force production (1, 12, 16, 28, 45, 46, 67). However, the extent that the loss of force exceeds loss of mass is surprising and may indicate a decline in specific force with age, a phenomenon reported in the literature (12, 28), but this cannot be verified by the methods used in this study.
IGF-I is a unique growth factor in that its actions on skeletal muscle are mitogenic (5, 22), myogenic (4, 19, 22), hypertrophic (3, 19, 42, 44, 59), and antiapoptotic/prosurvival (39, 56). This makes it a prime molecule of interest with regard to the decline of skeletal muscle with age. Muscle-specific IGF-I overexpression reverses the loss of mass and force (7), as well as the decline in regenerative capacity with age (52). To our knowledge, however, this study is the first to demonstrate that the capability for locally induced IGF-I gene expression declines with age in regenerating skeletal muscle. Previously, an age-related reduction in the mRNA of a single mechanically sensitive splice variant of IGF-I, mechanogrowth factor, was reported following 5 days of compensatory overload in rats (53); however, the authors reported no difference in the predominantly expressed IGF-I Ea splice variant. Conversely, our results are limited by the fact that only a single time point was measured during the recovery process. To confirm that this age-related reduction in IGF-I expression is indeed quantitative and not temporal, it is important that future studies investigate the time course of its expression. Additionally, information pertaining to the gene expression of the individual IGF-I Ea and Eb splice variants could provide potential mechanistic detail to this phenomenon, as evidence suggests that these individual splice variants have differential roles in muscle regeneration (30, 31, 71).
Systemic IGF-I is modulated by growth hormone (GH) (26), and circulating levels of GH and IGF-I decrease with age in humans (41, 60). Therefore, a reduction in the GH/IGF-I axis is one possible explanation for the age-dependent decrease in IGF-I gene expression in regenerating skeletal muscle. However, the skeletal muscle of hypophysectomized animals demonstrates no difference in the induction of IGF-I mRNA compared with control animals in response to I/R injury (25) and compensatory overload (70). This evidence suggests the injury-induced, local upregulation of IGF-I mRNA in skeletal muscle is GH independent, weakening the hypothesis that decreased GH production with age is responsible for our findings.
A large portion of IGF-I activity in skeletal muscle is mediated through the PI3K/Akt signaling pathway by promoting hypertrophy via activation of mTOR and deactivation of FoxOs (10, 42, 59, 61, 64). Thus activity of Akt-dependent signaling (Fig. 5) can indicate if the blunted IGF-I mRNA response in regenerating aged muscle correlates to the functional level. In this study, regenerating tissue of old rats failed to show the significant increases in phosphorylation of mTOR or p70S6K that were seen in young. This indicates deficits in proanabolic signaling in regenerating aged muscle, a possible consequence of less local expression of IGF-I and/or other important growth factors. Another possible explanation for these findings are age-related defects in the signaling mechanisms per se. Similar age-related declines in the responsiveness of the Akt/mTOR/p70S6K pathway in compensatory overload (65), high-frequency electrical stimulation (27), and amino acid supplementation (23) models support the notion of signaling defects with age. Additionally, Li et al. (47) demonstrated intraperitoneally injected des IGF-I phosphorylates Akt-1 equally in nonspecified muscles of young and old mice, while p-p70S6K (Thr421/Ser424) abundance increases significantly in young but not in old. This suggests age-related alterations in signaling may exist downstream of Akt.
Fig. 5.
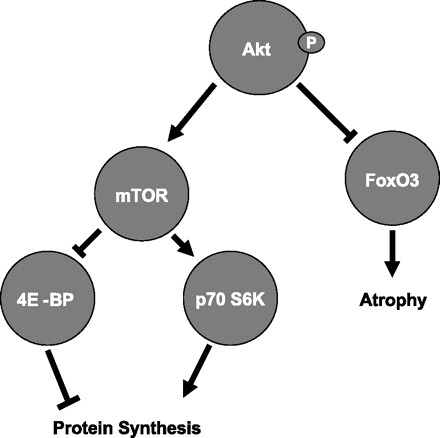
Schematic depicting the model of Akt-dependent initiation of net protein synthesis. This model is the rationale for the choice of phosphoproteins investigated in this study. 4E-BP, eukaryotic initiation factor 4E binding protein.
An unexpected finding was the 2.8-fold higher basal activity of mTOR in the control muscles of aged rats. This agrees with a similar finding of the tibialis anterior muscle from 30-mo-old rats by Parkington et al. (54). Interestingly, constitutively elevated mTOR activation has been shown to sensitize cells to stress-induced apoptosis through a p53-mediated mechanism in vitro (43). This phenomenon may be linked to reports of increased rates of apoptosis in aged skeletal muscle (24), making it an interesting, although speculative, topic for future investigation.
Due to the role of FoxOs in the process of atrophy (61, 63, 64), we hypothesized old skeletal muscle to have a decreased FoxO3 phosphorylation status, as a result of higher abundance of active FoxO3 transcription factor and a lower abundance of the inactive p-FoxO3 (Ser253) in both control and TK muscles. This was the case in control muscles, where aging resulted in significantly less p-FoxO3 relative to total FoxO3 abundance. In TK muscles, however, the significant decrease in FoxO3 phosphorylation status in young, but not old, suggests higher levels of FoxO3 transcription-promoting activity in young regenerating muscles. Recent evidence shows that FoxOs are critical to the oxidative stress response in hematopoietic (66) and erythroid (48) cells, presumably by regulating expression of key antioxidant enzymes (40). If FoxOs play such a role in skeletal muscle, then a differential FoxO3 response in aged skeletal muscle may indicate an alteration in the capacity to respond to the oxidative stress caused by I/R injury.
In the present study, aged rats demonstrated larger deficits in muscle function than young rats following 7 and 14 days of recovery from 2-h TK-induced I/R injury. We provide evidence that this may be due to age-associated decreases in the induction of IGF-I mRNA and subsequent anabolic signaling in response to I/R perturbation. These results indicate that IGF-I supplementation following TK use is a potential intervention against the exacerbated loss of mass and function seen in the old animals. A clinically attractive method of achieving this is via a post-TK intramuscular injection of a biodegradable polymer capable of releasing IGF-I in a time-controlled manner. However, before such an intervention can be tested, it is important to first characterize the time course of IGF-I expression and evaluate the contribution of other important growth factors, such as hepatocyte growth factor and vascular endothelial growth factor, in the regeneration of young and old animals. Alternatively, a regimen of passive stretching of I/R-injured muscles is a possible nonpharmacological intervention to promote protein synthesis, as stretch alone can stimulate protein synthesis by activating mTOR and its downstream targets in skeletal muscle through a phosphatidic acid-dependent mechanism (33). Our present results also do not clarify the role of circulating factors vs. local factors in the decline of recovery from injury that occurs with age. For example, we do not yet know the source of the upregulated IGF-I. If it is derived mainly from circulating monocytes/macrophages, then it is possible that these cells are the circulating factor found in the circulatory system of young animals that promotes recovery of damaged muscle. Future studies remain to be done that focus on determining the source of the IGF-I and that clarify whether the deficits in IGF-I signaling result from decreased ligation of the IGF-I receptor secondary to reduced IGF-I bioavailability or whether there are age-related defects intrinsic to the signaling enzymes themselves.
GRANTS
This work was funded in part by U.S. Army Medical Research and Materiel Command Grant DAMD17-03-1-0735 to R. P. Farrar and National Institute on Aging Grant R01-AG-026012 to M. L. Adamo.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res Rev 5: 310–331, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Adams GR. Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol 93: 1159–1167, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Adi S, Bin-Abbas B, Wu NY, Rosenthal SM. Early stimulation and late inhibition of extracellular signal-regulated kinase 1/2 phosphorylation by IGF-I: a potential mechanism mediating the switch in IGF-I action on skeletal muscle cell differentiation. Endocrinology 143: 511–516, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989 [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Miller JA, He Y, Kadhiresan VA, McCubbrey DA, Faulkner JA. An apparatus to measure in vivo biomechanical behavior of dorsi- and plantarflexors of mouse ankle. J Appl Physiol 72: 1205–1211, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 10: 620–630, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc 38: 1950–1957, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 12.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol 497: 573–580, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc Natl Acad Sci USA 95: 1432–1437, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson BM, Dedkov EI, Borisov AB, Faulkner JA. Skeletal muscle regeneration in very old rats. J Gerontol A Biol Sci Med Sci 56: B224–B233, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol 256: C1262–C1266, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89: 1365–1379, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4: 407–410, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272: 6653–6662, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol 282: R519–R527, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Edwall D, Schalling M, Jennische E, Norstedt G. Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology 124: 820–825, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 290: R1080–R1086, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178: 175–183, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 255: H1269–H1275, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Hill M, Goldspink G. Expression and splicing of the insulin-like growth factor gene in rodent muscle is associated with muscle satellite (stem) cell activation following local tissue damage. J Physiol 549: 409–418, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203: 89–99, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann NY Acad Sci 1047: 248–258, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA 103: 4741–4746, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′-TOP mRNA translation through inhibition of p70s6k. EMBO J 16: 3693–3704, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennische E, Hansson HA. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol Scand 130: 327–332, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Jennische E, Skottner A, Hansson HA. Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand 129: 9–15, 1987 [DOI] [PubMed] [Google Scholar]
- 38.Keller HL, St. Pierre Schneider B, Eppihimer LA, Cannon JG. Association of IGF-I and IGF-II with myofiber regeneration in vivo. Muscle Nerve 22: 347–354, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Kooijman R. Regulation of apoptosis by insulin-like growth factor (IGF)-I. Cytokine Growth Factor Rev 17: 305–323, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419: 316–321, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science 278: 419–424, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem 280: 2737–2744, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, Guan KL. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J 26: 4812–4823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol 96: 1097–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Li M, Li C, Parkhouse WS. Age-related differences in the des IGF-I-mediated activation of Akt-1 and p70 S6K in mouse skeletal muscle. Mech Ageing Dev 124: 771–778, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117: 2133–2144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18: 355–357, 2004 [DOI] [PubMed] [Google Scholar]
- 50.McEwen JA, Inkpen K. Surgical tourniquet technology adapted for military and prehospital use. NATO-RTO-MP-HFM-Proc 109: 1–12, 2004 [Google Scholar]
- 51.McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516: 583–592, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett 505: 259–263, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol 97: 243–248, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem 278: 12361–12366, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, Shenaq SM, Schwartz RJ. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J 17: 53–55, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Racz I, Illyes G, Sarkadi L, Hamar J. The functional and morphological damage of ischemic reperfused skeletal muscle. Eur Surg Res 29: 254–263, 1997 [DOI] [PubMed] [Google Scholar]
- 58.Reynolds THt, Bodine SC, Lawrence JC Jr. Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol 3: 1009–1013, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest 67: 1361–1369, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287: E591–E601, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Sadeh M. Effects of aging on skeletal muscle regeneration. J Neurol Sci 87: 67–74, 1988 [DOI] [PubMed] [Google Scholar]
- 63.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117: 399–412, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14: 395–403, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol 574: 291–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 25: 17–25, 2002 [DOI] [PubMed] [Google Scholar]
- 68.Walters TJ, Kragh JF, Kauvar DS, Baer DG. The combined influence of hemorrhage and tourniquet application on the recovery of muscle function in rats. J Orthop Trauma 22: 47–51, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Walters TJ, Sweeney HL, Farrar RP. Influence of electrical stimulation on a fast-twitch muscle in aging rats. J Appl Physiol 71: 1921–1928, 1991 [DOI] [PubMed] [Google Scholar]
- 70.Yamaguchi A, Fujikawa T, Shimada S, Kanbayashi I, Tateoka M, Soya H, Takeda H, Morita I, Matsubara K, Hirai T. Muscle IGF-I Ea, MGF, and myostatin mRNA expressions after compensatory overload in hypophysectomized rats. Pflügers Arch 453: 203–210, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522: 156–160, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol Cell Physiol 258: C429–C435, 1990 [DOI] [PubMed] [Google Scholar]


