Abstract
We have previously reported that obese db/db mice exhibit innate airway hyperresponsiveness. These mice also have enhanced inflammatory responses to ozone, a common air pollutant that exacerbates asthma. Since db/db mice are diabetic as well as obese, the purpose of the present study was to determine whether metformin, an antihyperglycemic agent, alters the pulmonary phenotype of db/db mice. Lean wild-type (C57BL/6J) and obese db/db mice were treated by gavage with water or metformin (300 μg/g) once a day for 2 wk. Twenty-four hours after the last treatment, in mice of both genotypes, we either measured airway responsiveness to methacholine by forced oscillation, or we exposed the mice to ozone (2 parts per million for 3 h) and examined the ensuing inflammatory response. Compared with water, treatment with metformin caused a significant decrease in fasting blood glucose in obese mice. Airway responsiveness was increased in db/db versus wild-type mice, but metformin did not affect responsiveness in either group. Four hours after exposure to ozone, there was a significant increase in bronchoalveolar lavage fluid neutrophils and chemokines in mice of both genotypes, but the magnitude of these changes was greater in db/db than wild-type mice. Metformin did not affect ozone-induced inflammation in mice of either genotype. The results indicate that hyperglycemia is unlikely to account for the pulmonary phenotype of obese mice.
Keywords: airway responsiveness, chemokine, inflammation, neutrophil, adiponectin
obesity is a risk factor for asthma. Multiple cross-sectional studies in large populations of adults and children of multiple ethnic backgrounds indicate that the prevalence of asthma is higher in obese and overweight individuals. In addition, several large prospective studies show that obesity antedates asthma (see recent reviews in Refs. 16, 61, and 63). Obese asthmatic patients who lose weight experience fewer asthma symptoms, increased airflow rates, reduced peak flow variability, and better asthma control (21, 43, 68). Obesity also appears to impact asthma control and the efficacy of certain asthma medications (13, 36, 51, 55).
Data from animal models also support a relationship between obesity and asthma. Obese mice exhibit airway hyperresponsiveness (AHR), a characteristic feature of asthma, even in the absence of any inciting exposure (31, 32, 34, 41, 54, 66). This innate AHR is observed in ob/ob and db/db mice that are obese because of a genetic deficiency in either the satiety hormone, leptin, or its receptor; in Cpefat mice that are obese because of a genetic deficiency in carboxypeptidase E (Cpe), an enzyme involved in processing neuropeptides involved in eating behaviors; and in mice with obesity induced by a high-fat diet (31, 32, 41, 62, 66). Obese mice have another feature consistent with asthma—exaggerated pulmonary inflammatory responses following exposure to ozone (O3), a common air pollutant (32, 41, 62, 66). Exaggerated responses to O3 are also observed in obese human subjects (1, 3). Increased O3-induced inflammation is observed in ob/ob, db/db, and Cpefat mice and in mice with dietary obesity (31, 32, 41, 66). While these mice are markedly obese, they are also diabetic: fasting hyperglycemia is a feature of each of these models, although the magnitude of hyperglycemia varies with the modality of obesity (8, 37, 48, 59). It is conceivable that this hyperglycemia contributes to the proasthmatic phenotype of the obese mouse. Oxidative stress, which can be a consequence of hyperglycemia, contributes to many aspects of the obese phenotype (26, 58), and oxidative stress has also been linked to asthma (35). In addition, a recent report indicates a higher prevalence of insulin resistance among obese asthmatic patients versus obese nonasthmatic patients, suggesting that insulin resistance may contribute to this phenotype (2).
The biguanide metformin is an oral insulin-sensitizing agent commonly used in the treatment of type 2 diabetes. Treatment with metformin also ameliorates the diabetic phenotype of obese mice, reducing fasting glucose and attenuating the expression of gluconeogenic enzymes in liver (7, 17, 18, 23, 39, 70). In human subjects, metformin also attenuates aspects of the systemic inflammation that is associated with obesity (4, 10–12, 20). The purpose of the present study was to determine whether hyperglycemia contributes to the pulmonary phenotype of obese mice. Accordingly, we treated lean wild-type and obese db/db mice orally with either water or metformin every day for 2 wk. Two cohorts of metformin- and water-treated mice were used. In one cohort, airway responsiveness was assessed 24 h after the last treatment to determine whether metformin could attenuate the innate AHR of db/db mice. In the other cohort, 24 after the last metformin or water treatment, mice were exposed acutely to O3 [2 parts per million (ppm) for 3 h] and the ensuing inflammatory response was examined.
MATERIALS AND METHODS
Animals.
This study was approved by the Harvard Medical Area Standing Committee on Animals. Obese female db/db and ob/ob mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Because these mice were on a C57BL/6J background, wild-type, age-matched, and sex-matched C57BL/6J mice were used as controls. Both types of mice are hyperphagic, hypometabolic, hyperinsulinemic, hyperglycemic, and massively obese (37). To confirm the efficacy of the metformin treatment on serum glucose, we used both ob/ob and db/db mice. For all other experiments, we used db/db mice only.
Protocol.
Obese and lean mice were administered either water or metformin (300 μg/g) by gavage daily for 2 wk. Others have reported reductions in fasting blood glucose in db/db mice with a similar treatment protocol (17, 18). Three cohorts were used. In one cohort, after the last treatment, ob/ob and db/db mice were fasted overnight for the measurement of fasting blood glucose using the Prestige Smart System IQ blood glucose meter (Home Diagnostics, Fort Lauderdale, FL) (4 metformin- and 4 water-treated mice). In the second cohort, 24 h after the last treatment, mice were anesthetized for the measurement of airway responsiveness, followed by collection of serum for assessment of systemic inflammation (n = 6–8 mice per group). Note that we were unable to catheterize the vein for delivery of methacholine in one mouse, but we still collected a blood sample via cardiac puncture in this mouse. In the last cohort, mice were exposed to O3 (2 ppm for 3 h) 24 h after the last gavage treatment. Four hours after exposure, the mice were euthanized, and bronchoalveolar lavage (BAL) was performed (n = 5–6 mice/group).
O3 exposure.
Awake mice were placed unrestrained in individual wire mesh cages inside a stainless steel and plexiglass exposure chamber and exposed to O3 (2 ppm for 3 h) as previously described (29, 66). The O3 exposure protocol was chosen to allow for comparison with data of other investigators studying acute O3-induced inflammation in mice (5, 6, 25, 27, 49, 56, 72), including db/db mice (41). This concentration is higher than typical concentrations used for human exposures. However, the inhaled dose of O3 is the product not only of ozone concentration and exposure time, but also minute ventilation (75). Human exposures are typically carried out with subjects exercising to increase their minute ventilation (3). In contrast, mice undergo a profound decrease in metabolism upon O3 exposure, and at 2 ppm O3, their minute ventilation decreases to values only one third of those measured before exposure (64). Hence, while concentrations used for human and mouse studies may differ substantially, the actual inhaled dose of ozone is likely much more comparable.
Pulmonary mechanics.
Unexposed mice were anesthetized with xylazine (7 mg/kg) and pentobarbital sodium (50 mg/kg). The trachea was cannulated with a tubing adaptor, and the tail vein was cannulated for the delivery of acetyl-β-methylcholine chloride (methacholine; Sigma-Aldrich, St. Louis, MO). The mice were ventilated with a specialized ventilator (flexiVent; SCIREQ, Montreal, QC, Canada). Frequency was set at 150 Hz and 180 Hz in wild-type and db/db mice, respectively. The slightly higher frequency used for the db/db mice was chosen because these mice breathe spontaneously at a higher frequency, but with approximately the same tidal volume as wild-type mice (41). Hence, tidal volume was set at 0.3 ml in both strains. A large window was made on each side of the chest wall by cutting away part of some ribs and the tissue between them. Thus the lungs were mostly exposed to atmospheric pressure, and the measurements made reduced any chest wall contribution to pulmonary mechanics. A positive end-expiratory pressure of 3 cmH2O was applied by placing the expiratory line under water.
Baseline pulmonary mechanics and responses to intravenous methacholine were measured using the forced oscillation technique, as previously described (32, 33, 41, 54, 66). Briefly, the lungs were first inflated to three times tidal volume to establish a standard volume history. One minute later, a bolus of PBS (1 μl/g) was administered via the tail vein over 4–5 s, and pulmonary resistance (RL) was measured with a sinusoidal forcing function at a frequency of 2.5 Hz every 8th breath for the next minute, until RL peaked and began to decline. As soon as RL had peaked, measurements of lung impedance (ZL) were obtained by using forced oscillation with a sinusoidal forcing function containing frequencies ranging from 0.25 to 19.63 Hz. The mouse was then given another inflation to three times tidal volume. The procedure was repeated using doses of methacholine chloride dissolved in PBS increasing in half-log intervals from 0.03 to 1.0 mg/ml at a dose of 1 μl/g. Because both the airways and the lung tissues can contribute to RL, we used measurements of ZL to obtain airway resistance (Raw), and the coefficients of lung tissue damping (G) and lung tissue elastance (H) (32, 54). At very low frequencies, ZL primarily reflects the lung tissue, whereas at high frequencies, the contribution from the tissues becomes negligible and airway resistance dominates (50). A parameter estimation model (22) was used to partition ZL into components representing airway resistance Raw, G, and H as described below:
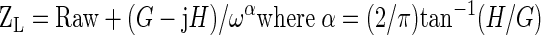 |
We and others have reported that real and imaginary parts of ZL as a function of frequency conform well to this model within this frequency range (24, 53, 54).
Bronchoalveolar lavage.
Four hours after cessation of O3 exposure, mice were euthanized with an overdose of pentobarbital sodium. The trachea was cannulated, and the lungs were lavaged twice with 1 ml PBS/0.6 mM EDTA. The BAL was centrifuged, and total BAL cells and differentials were assessed as previously described (28–30, 32). The BAL supernatant was frozen at −80°C and subsequently analyzed for keratinocyte-derived chemokine (KC), macrophage inflammatory protein 2 (MIP-2), interferon-inducible protein 10 (IP-10), LPS-induced C-X-C chemokine (LIX), and monocyte chemoattractant protein 1 (MCP-1), using DuoSet enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). These neutrophil chemotactic factors were chosen because they are characteristically induced by acute O3 exposure and because they have been reported to contribute to the neutrophil influx that accompanies acute O3 exposure in mice (14, 29, 32, 33, 41, 45, 66, 71). Importantly, in the presence of hyperglycemia, other acute inflammatory stimuli also result in greater induction of many of these chemokines (19).
Markers of systemic inflammation.
Blood samples were obtained by cardiac puncture, serum isolated, and stored at −20°C until assayed by ELISA for MCP-1, leptin, and adiponectin (R&D Systems). Since O3 exposure per se may alter these factors, assays were performed only on serum of mice used in the mechanics studies.
Statistical analysis.
Differences in body weight, RL, Raw, G, H, and BAL parameters and in serum markers of inflammation were assessed by factorial ANOVA using genotype and drug treatment as main effects. In each case, the Fishers least significant difference test was used for post hoc comparisons. Comparisons of serum glucose were made by unpaired Student's t-tests. Statistical analyses were carried out with SAS software (SAS Institute, Cary, NC). All results are presented as means ± SE unless otherwise indicated. P < 0.05 was considered statistically significant.
RESULTS
Body mass and fasting blood glucose.
The db/db mice were extremely obese, with body mass averaging more than twice that of wild-type mice (Table 1). During the 2-wk period during which mice were treated with either water or metformin, factorial ANOVA indicated a significant effect of mouse strain on weight gain: db/db mice gained 1.1 ± 0.3 g, whereas there was no significant increase in body weight in wild-type mice (0.17 ± 0.11 g)(P < 0.05). Compared with water, treatment with metformin had no significant effect on weight gain in either wild-type or db/db mice, consistent with other reports (39, 70).
Table 1.
Baseline measurements of total pulmonary, airway, and parenchymal mechanics in obese db/db and lean wild-type mice treated with water or metformin
| Body Mass, g | RL, cmH2O·ml−1·s | Raw, cmH2O·ml−1·s | G, cmH2O/ml | H, cmH2O/ml | |
|---|---|---|---|---|---|
| WT | |||||
| Water | 26.7±1.7 | 0.50±0.02 | 0.22±0.02 | 2.78±0.14 | 17.3±0.4 |
| Metformin | 26.0±1.4 | 0.51±0.03 | 0.25±0.02 | 2.54±0.17 | 18.7±1.3 |
| db/db | |||||
| Water | 60.8±1.4* | 0.62±0.02* | 0.26±0.02 | 3.52±0.26* | 23.3±1.3* |
| Metformin | 62.0±1.6* | 0.58±0.02* | 0.23±0.02 | 3.69±0.29* | 22.3±1.1* |
Values are means ± SE. Measurements were made 24 h after the last gavage treatment. RL, lung resistance; Raw, airway resistance; G, coefficient of lung tissue damping; H, coefficient of lung tissue elastance.
P < 0.05 compared with wild-type (WT) mice with same exposure.
To confirm the efficacy of the metformin treatment, db/db and ob/ob mice treated with metformin or water were fasted overnight. The obese mice used in the present study exhibited marked fasting hyperglycemia, consistent with results of previous investigators (37, 59). Fasting serum glucose averaged 273 ± 34 mg/dl in mice treated with water and was significantly reduced to 190 ± 23 mg/dl in mice treated with metformin. Using an estimate of 100 mg/dl for normal fasting glucose, this effect of metformin represents an approximate 50% reduction in the hyperglycemia of these mice.
We also measured glucose in the serum of the mice used for the ozone part of the study. These mice were not fasted and had access to food and water from the time they were removed from the ozone chamber until 4 h later when they were studied. In the wild-type mice, serum glucose averaged 185 ± 15 and 155 ± 18 mg/dl in the water- and metformin-treated groups, respectively (not significant). In db/db mice, serum glucose averaged 473 ± 63 and 337 ± 9 mg/dl (P < 0.03) in the water- and metformin-treated mice, respectively (again, an approximate 50% reduction in glucose compared with the values observed in wild-type mice). The data indicate that in db/db mice, metformin was effective in reducing glucose in both the fasting and nonfasting states.
Pulmonary mechanics and responsiveness to methacholine.
Factorial ANOVA indicated a significant effect of genotype (P < 0.01) on RL, G, and H, but not Raw measured before treatment with methacholine. RL, G, and H were each higher in db/db than wild-type mice (Table 1), consistent with the smaller lung size of db/db mice (41). However, there was no effect of metformin treatment and no interaction between genotype and metformin treatment on any of these indices of lung mechanics.
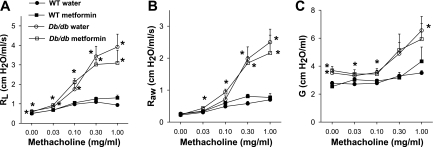
Measurements of airway responsiveness to methacholine were made in mice not exposed to O3 and are shown in Fig. 1. Methacholine caused much more substantial increases in RL in db/db than in wild-type mice (Fig. 1A). The locus of this augmented effect of methacholine in db/db mice is likely to be the airways rather than the lung tissues: there was an approximate 10-fold increase in Raw following administration of the highest concentration of methacholine in db/db mice (Fig. 1B), whereas G increased only minimally (Fig. 1C). The parameter G is related to the resistance of the lung tissues, although inhomogeneous airway constriction can impact the measurement of G without any actual change in the properties of the lung tissue (42) and may account for the small changes in G observed (Fig. 1C). There was no methacholine-induced change in H (data not shown), consistent with previous reports in both wild-type and obese mice (31, 32, 54) and indicating that the elastance of the lung tissue is not affected by methacholine in mice of either genotype. The innate AHR observed in ob/ob mice, Cpefat mice, and mice with diet-induced obesity is also the result of enhanced airway rather than tissue responses (31, 32, 66).
Fig. 1.
Changes in total pulmonary resistance (RL) (A), airway resistance (Raw) (B), and the coefficient of lung tissue damping (G) (C) induced by intravenous methacholine in wild-type (WT) and db/db mice. Mice were treated by gavage with water or metformin once per day for 2 wk. Measurements were made 24 h after the last treatment. Results are means ± SE of data from 6–7 mice in each group. *P < 0.05 vs. WT mice in the same treatment group.
Metformin had no effect on methacholine-induced changes in pulmonary mechanics in either db/db or wild-type mice. Factorial ANOVA indicated significant effects of genotype (P < 0.05) at each dose of methacholine for RL, Raw, and G, but no effect of drug treatment and no interaction between drug treatment and genotype.
Effect of metformin on O3-induced pulmonary inflammation.
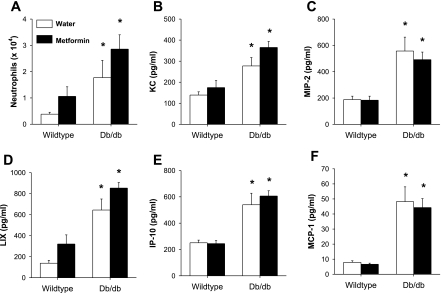
Factorial ANOVA indicated a significant effect of strain (P < 0.005) on BAL neutrophils: regardless of drug treatment, BAL neutrophils were higher in db/db than in wild-type mice exposed to O3 (Fig. 2). (Note that neutrophils were virtually absent from the BAL of wild-type and db/db mice that were used in the mechanics study and not exposed to O3.) There was a trend toward increased BAL neutrophils in lungs of metformin- versus water-treated mice regardless of strain, but this did not reach statistical significance (P = 0.09). There were no significant strain or treatment effects on any other BAL cell type.
Fig. 2.
Bronchoalveolar lavage neutrophils (A), keratinocyte-derived chemokines (KC) (B), macrophage inflammatory protein 2 (MIP-2) (C), LPS-induced C-X-C chemokine (LIX) (D), interferon-inducible protein 10 (IP-10) (E), and monocyte chemoattractant protein 1 (MCP-1) (F) in WT and db/db mice exposed to O3 (2 parts per million for 3 h). Mice were studied 4 h after cessation of O3 exposure and were treated by gavage with either water or metformin every day for 2 wk before exposure. Results are means ± SE of data from 5–6 mice per group. *P < 0.05 vs. WT mice with the same drug treatment.
We also measured BAL concentrations of chemokines that are induced by O3 and have chemotactic activity for neutrophils (Fig. 2). Hyperglycemia has been shown to impact the induction of many of these chemokines that occurs in response to other inflammatory stimuli (19, 40). Factorial ANOVA indicated a significant effect of strain on BAL MIP-2, KC, MCP-1, LIX, and IP-10 (P < 0.05 in each case). In each case, chemokine levels were higher in db/db than in wild-type mice, as previously reported (41). There was no effect of drug treatment on any of these BAL chemokines except for LIX (P < 0.02); there were significantly greater levels of LIX in BAL of metformin- versus water-treated mice regardless of strain. A similar trend toward increased levels of KC in metformin- versus water-treated mice was observed, but this did not reach statistical significance.
Effect of metformin on serum markers of inflammation.
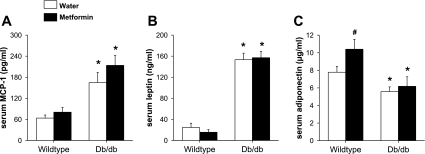
Factorial ANOVA indicated a significant effect of genotype on serum MCP-1, serum leptin, and serum adiponectin (P < 0.01 in each case). Compared with lean wild-type mice, serum MCP-1 and serum leptin were both elevated, whereas serum adiponectin was reduced in db/db mice (Fig. 3). There was no significant effect of drug treatment on either serum MCP-1 or serum leptin (Fig. 3, A and B). In contrast, treatment with metformin caused a significant increase in serum adiponectin in wild-type but not in db/db mice (Fig. 3C).
Fig. 3.
Serum MCP-1 (A), leptin (B), and adiponectin (C) in unexposed WT and db/db mice treated with metformin or water every day for 2 wk. Results are means ± SE of data for 6–8 mice per group. *P < 0.05 vs. WT mice with the same drug treatment; #P < 0.05 vs. mice of the same genotype treated with water.
DISCUSSION
Our data indicate that 2 wk of daily treatment with metformin attenuates hyperglycemia in obese mice without altering either their innate AHR or their increased pulmonary inflammatory responses to O3. The data indicate that the pulmonary phenotype of these obese mice is likely unrelated to their hyperglycemia.
We have previously reported that compared with lean controls, obese mice have increased airway responsiveness, even in the absence of any type of exposure that can cause AHR (31, 32, 34, 41, 54, 66). Although the epithelium has the ability to modify airway responsiveness to inhaled bronchoconstrictors (65), it is unlikely that differences in the epithelium contribute to the innate AHR observed in obese mice, since methacholine was delivered intravenously. Instead, other factors may contribute, as previously described (62). In the present study, we sought to determine whether one of these factors, hyperglycemia, may account for this phenomenon. Innate AHR is a feature of each type of murine obesity we have examined (31, 32, 34, 41, 54, 66), and in each of the models, hyperglycemia is also observed (62). Importantly, hyperglycemia can cause inflammation and oxidative stress, conditions that have been associated with asthma (35). For example, high glucose acts directly on endothelial cells to increase endothelial permeability and increase leukocyte adhesion (57). Acute glucose challenge stimulates monocytes to increase expression of proinflammatory cytokines and chemokines (60) and stimulates release of reactive oxygen species (ROS) from leukocytes (46). Induction of ROS is also enhanced in leukocytes from Akita mice, which are hyperglycemic but are not obese (19). In addition, a recent report indicates a higher prevalence of insulin resistance among obese asthmatic patients than in obese nonasthmatic patients, suggesting that insulin resistance may contribute to this phenotype (2). Interestingly, there is an inverse association between type 1 diabetes and asthma: the prevalence of type 1 diabetes is lower among asthmatic patients than in nonasthmatic patients (44, 73, 74). However, this association is unlikely to be related to issues of blood glucose, since similar inverse associations are observed with asthma and other autoimmune diseases (73). Instead, the inverse association between asthma and type 1 diabetes is consistent with the Th1/Th2 polarization concept wherein the presence of asthma, a Th2 disease, mitigates the likelihood of developing type 1 diabetes, a Th1-mediated disease.
To assess the potential role of hyperglycemia, we treated db/db mice with the antihyperglycemic agent metformin. Metformin caused a marked reduction in fasting blood glucose, consistent with previous studies in obese mice treated in a similar manner (17, 18, 70). Metformin also reduced serum glucose in nonfasting db/db mice, although glucose remained well above wild-type values. The ability of metformin to reduce blood glucose derives from its ability to increase glucose uptake in skeletal muscle (47) and to reduce hepatic gluconeogenesis (69). These actions appear to be mediated via changes in insulin sensitivity (7) induced by activation of AMP-activated protein kinase (AMPK), a cellular sensor of energy status (76).
Consistent with previous reports (41), we observed greater airway responsiveness to intravenous methacholine in obese db/db versus lean wild-type mice (Fig. 1). However, even though metformin substantially reduced fasting hyperglycemia in these mice, it did not alter their innate AHR (Fig. 1). The results suggest that some aspect of the phenotype of these obese mice other than hyperglycemia accounts for their AHR, although it is conceivable that even moderate increases in blood glucose, such as remained in the fasted db/db mice treated with metformin, may contribute to AHR.
We have also reported that obese mice, regardless of the cause of their obesity, have increased pulmonary inflammation following acute exposure to O3. For example, higher levels of cytokines and chemokines that are typically induced by O3 are found in BAL fluid of obese compared with lean mice (31, 32, 41, 66). As described above, these obese mice are also hyperglycemic. Hyperglycemia leads to increased induction of proinflammatory cytokines and chemokines following another type of acute inflammatory stimulus, intraperitoneal injection of zymosan (19). Hyperglycemia induced by glucose infusion also results in oxidative stress in the liver and in increased inflammatory cytokine concentrations in blood of mice challenged with a low dose of endotoxin (40). Hence, to determine whether hyperglycemia could be responsible for the augmented inflammatory response of db/db mice to O3, we treated mice with metformin before O3 exposure to reduce hyperglycemia. Consistent with previous reports, O3-induced inflammation was augmented in db/db mice: the numbers of neutrophils and the concentrations of BAL KC, MIP-2, LIX, MCP-1, and IP-10 were all increased in db/db versus wild-type mice (Fig. 2). However, metformin did not reduce any of these indices of inflammation. Since hyperglycemia was reduced but not abolished in these metformin-treated mice, we cannot rule out the possibility that serum glucose remained above levels that may affect inflammatory responses to O3 or that reductions in glucose of longer duration may have been effective. However, we think this unlikely, since the trend for inflammatory response was toward increased, not decreased, inflammation in the metformin-treated mice (Fig. 2A).
Others have reported effects of metformin on some aspects of the systemic inflammation associated with human obesity. For example, treatment with metformin has been shown to reduce serum migration inhibitor factor, C-reactive protein, soluble E-selectin, and plasminogen activator inhibitor-1 concentrations in obese diabetic subjects (4, 10, 11, 20). Metformin also reduces C-reactive protein and soluble VCAM-1 concentrations in serum of women with polycystic ovary disease (12). Consequently, we examined the effect of metformin on serum MCP-1, a marker of systemic inflammation that is elevated in db/db mice (41). Since our previous data have indicated that the adipose-derived hormones leptin and adiponectin may also play a role in the pulmonary phenotype of obese mice (41, 66, 67), we also examined the effect of metformin treatment on these hormones. Consistent with previous reports (41), serum MCP-1 and leptin were elevated and serum adiponectin was reduced in db/db mice compared with wild-type controls (Fig. 3). Metformin had no effect on any of these outcome indicators in db/db mice, but it caused a significant increase in adiponectin in wild-type mice (Fig. 3). The lack of effect of metformin on serum adiponectin in db/db mice is consistent with previous reports both in these and other types of obese diabetic mice and in obese diabetic patients (9, 15, 17, 52) and suggests that metformin is unlikely to mediate its antihyperglycemic effects by inducing this insulin-sensitizing hormone. However, the metformin-induced increase in serum adiponectin in wild-type mice suggests that metformin can alter adiponectin expression and/or release from adipose tissue under some circumstances. Indeed, another AMPK-activating agent, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR), has been shown to increase adiponectin mRNA expression in human adipose tissue in vitro (38). It may be that in the obese and/or diabetic state, other factors that suppress adiponectin expression dominate any potential adiponectin-inducing effects of metformin.
In summary, our data indicate that 1) innate AHR and 2) increased O3-induced neutrophil influx and chemokine expression are observed in obese mice. These obesity-related differences were not attenuated by metformin treatment, despite reductions in fasting blood glucose, suggesting that hyperglycemia is unlikely to account for the pulmonary phenotype of these obese mice.
GRANTS
This study was supported by National Institute of Environmental Health Sciences Grants ES-013307 and ES-00002, by National Heart, Lung, and Blood Institute Grants HL-084044 and HL-33009, and by a generous gift from Paul and Mary Finnegan.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: effect modified by obesity and airways hyperresponsiveness in the VA Normative Aging Study. Chest 132: 1890–1897, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Al-Shawwa BA, Al-Huniti NH, DeMattia L, Gershan W. Asthma and insulin resistance in morbidly obese children and adolescents. J Asthma 44: 469–473, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal Toxicol 19: 1147–1154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter AM, Bennett CE, Bostock JA, Grant PJ. Metformin reduces C-reactive protein but not complement factor C3 in overweight patients with type 2 diabetes mellitus. Diabet Med 22: 1282–1284, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Gavett SH, Wills-Karp M. CD4+ T lymphocyte modulation of ozone-induced murine pulmonary inflammation. Am J Respir Cell Mol Biol 12: 396–403, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Cho HY, Zhang LY, Kleeberger SR. Ozone-induced lung inflammation and hyperreactivity are mediated via tumor necrosis factor-α receptors. Am J Physiol Lung Cell Mol Physiol 280: L537–L546, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SE, Tseng YH, Michael MD, Kahn CR. Effects of insulin-sensitising agents in mice with hepatic insulin resistance. Diabetologia 47: 407–411, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Coleman DL, Eicher EM. Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered 81: 424–427, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O'Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology 143: 998–1007, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Aljada A, Ghanim H, Mohanty P, Tripathy C, Hofmeyer D, Chaudhuri A. Increased plasma concentration of macrophage migration inhibitory factor (MIF) and MIF mRNA in mononuclear cells in the obese and the suppressive action of metformin. J Clin Endocrinol Metab 89: 5043–5047, 2004. [DOI] [PubMed] [Google Scholar]
- 11.De Jager J, Kooy A, Lehert P, Bets D, Wulffele MG, Teerlink T, Scheffer PG, Schalkwijk CG, Donker AJ, Stehouwer CD. Effects of short-term treatment with metformin on markers of endothelial function and inflammatory activity in type 2 diabetes mellitus: a randomized, placebo-controlled trial. J Intern Med 257: 100–109, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, Katsilambros N, Kreatsas G, Panidis D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod 21: 1426–1431, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, Lima JJ, Allayee H, Irvin CG, Wise RA. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma 43: 553–558, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Dye JA, Madden MC, Richards JH, Lehmann JR, Devlin RB, Costa DL. Ozone effects on airway responsiveness, lung injury, and inflammation. Comparative rat strain and in vivo/in vitro investigations. Inhal Toxicol 11: 1015–1040, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson A, Attvall S, Bonnier M, Eriksson JW, Rosander B, Karlsson FA. Short-term effects of metformin in type 2 diabetes. Diabetes Obes Metab 9: 483–489, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Ford ES The epidemiology of obesity and asthma. J Allergy Clin Immunol 115: 897–909, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Fujita H, Fujishima H, Koshimura J, Hosoba M, Yoshioka N, Shimotomai T, Morii T, Narita T, Kakei M, Ito S. Effects of antidiabetic treatment with metformin and insulin on serum and adipose tissue adiponectin levels db/db mice. Endocr J 52: 427–433, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Fujita H, Fujishima H, Morii T, Koshimura J, Narita T, Kakei M, Ito S. Effect of metformin on adipose tissue resistin expression in db/db mice. Biochem Biophys Res Commun 298: 345–349, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol 177: 7250–7256, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 54: 1566–1572, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 118: 1315–1321, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Heishi M, Ichihara J, Teramoto R, Itakura Y, Hayashi K, Ishikawa H, Gomi H, Sakai J, Kanaoka M, Taiji M, Kimura T. Global gene expression analysis in liver of obese diabetic db/db mice treated with metformin. Diabetologia 49: 1647–1655, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hjoberg J, Shore S, Kobzik L, Okinaga S, Hallock A, Vallone J, Subramaniam V, De Sanctis GT, Elias JA, Drazen JM, Silverman ES. Expression of nitric oxide synthase-2 in the lungs decreases airway resistance and responsiveness. J Appl Physiol 97: 249–259, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hollingsworth JW 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 170: 126–132, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Jang AS, Choi IS, Yang SY, Kim YG, Lee JH, Park SW, Park CS. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration 72: 79–84, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Johnston RA, Mizgerd JP, Flynt L, Quinton LJ, Williams ES, Shore SA. Type I interleukin-1 receptor is required for pulmonary responses to subacute ozone exposure in mice. Am J Respir Cell Mol Biol 37: 477–484, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnston RA, Mizgerd JP, Shore SA. CXCR2 is essential for maximal neutrophil recruitment and methacholine responsiveness after ozone exposure. Am J Physiol Lung Cell Mol Physiol 288: L61–L67, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol 288: L390–L397, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol 104: 1727–1735, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Johnston RA, Theman TA, Shore SA. Augmented responses to ozone in obese carboxypeptidase E-deficient mice. Am J Physiol Regul Integr Comp Physiol 290: R126–R133, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Johnston RA, Theman TA, Terry RD, Williams ES, Shore SA. Pulmonary responses to acute ozone exposure in fasted mice: effect of leptin administration. J Appl Physiol 102: 149–156, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med 176: 650–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther 111: 476–494, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lavoie KL, Bacon SL, Labrecque M, Cartier A, Ditto B. Higher BMI is associated with worse asthma control and quality of life but not asthma severity. Respir Med 100: 648–657, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Leibel RL, Chung WK, Chua SC Jr. The molecular genetics of rodent single gene obesities. J Biol Chem 272: 31937–31940, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Lihn AS, Jessen N, Pedersen SB, Lund S, Richelsen B. AICAR stimulates adiponectin and inhibits cytokines in adipose tissue. Biochem Biophys Res Commun 316: 853–858, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6: 998–1003, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Ling PR, Smith RJ, Bistrian BR. Hyperglycemia enhances the cytokine production and oxidative responses to a low but not high dose of endotoxin in rats. Crit Care Med 33: 1084–1089, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Lu FL, Johnston RA, Flynt L, Theman TA, Terry RD, Schwartzman IN, Lee A, Shore SA. Increased pulmonary responses to acute ozone exposure in obese db/db mice. Am J Physiol Lung Cell Mol Physiol 290: L856–L865, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol 80: 1841–1849, 1996. [DOI] [PubMed] [Google Scholar]
- 43.Maniscalco M, Zedda A, Faraone S, Cerbone MR, Cristiano S, Giardiello C, Sofia M. Weight loss and asthma control in severely obese asthmatic females. Respir Med 102: 102–108, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Meerwaldt R, Odink RJ, Landaeta R, Aarts F, Brunekreef B, Gerritsen J, Van Aalderen WM, Hoekstra MO. A lower prevalence of atopy symptoms in children with type 1 diabetes mellitus. Clin Exp Allergy 32: 254–255, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol 168: 846–852, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Mohanty P, Hamouda W, Garg R, Aljada A, Ghanim H, Dandona P. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab 85: 2970–2973, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51: 2074–2081, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Nishina PM, Lowe S, Wang J, Paigen B. Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43: 549–553, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Park JW, Taube C, Swasey C, Kodama T, Joetham A, Balhorn A, Takeda K, Miyahara N, Allen CB, Dakhama A, Kim SH, Dinarello CA, Gelfand EW. Interleukin-1 receptor antagonist attenuates airway hyperresponsiveness following exposure to ozone. Am J Respir Cell Mol Biol 30: 830–836, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Petak F, Hantos Z, Adamicza A, Daroczy B. Partitioning of pulmonary impedance: modeling vs. alveolar capsule approach. J Appl Physiol 75: 513–521, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J 27: 495–503, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Phillips SA, Ciaraldi TP, Kong AP, Bandukwala R, Aroda V, Carter L, Baxi S, Mudaliar SR, Henry RR. Modulation of circulating and adipose tissue adiponectin levels by antidiabetic therapy. Diabetes 52: 667–674, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Pillow JJ, Korfhagen TR, Ikegami M, Sly PD. Overexpression of TGF-α increases lung tissue hysteresivity in transgenic mice. J Appl Physiol 91: 2730–2734, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Rivera-Sanchez YM, Johnston RA, Schwartzman IN, Valone J, Silverman ES, Fredberg JJ, Shore SA. Differential effects of ozone on airway and tissue mechanics in obese mice. J Appl Physiol 96: 2200–2206, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Saint-Pierre P, Bourdin A, Chanez P, Daures JP, Godard P. Are overweight asthmatics more difficult to control? Allergy 61: 79–84, 2006. [DOI] [PubMed] [Google Scholar]
- 56.Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol 31: 69–77, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of mu-calpain. Diabetes 56: 1842–1849, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Scherer PE Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 55: 1537–1545, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Schreyer SA, Chua SC Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor- deficient mice. J Clin Invest 102: 402–411, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52: 1256–1264, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Shore SA Obesity and asthma: implications for treatment. Curr Opin Pulm Med 13: 56–62, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Shore SA Obesity and asthma: lessons from animal models. J Appl Physiol 102: 516–528, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther 110: 83–102, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Shore SA, Johnston RA, Schwartzman IN, Chism D, Krishna Murthy GG. Ozone-induced airway hyperresponsiveness is reduced in immature mice. J Appl Physiol 92: 1019–1028, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Shore SA, Kariya ST, Anderson K, Skornik W, Feldman HA, Pennington J, Godleski J, Drazen JM. Sulfur-dioxide-induced bronchitis in dogs. Effects on airway responsiveness to inhaled and intravenously administered methacholine. Am Rev Respir Dis 135: 840–847, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol 95: 938–945, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol 118: 389–395, 2006. [DOI] [PubMed] [Google Scholar]
- 68.Stenius-Aarniala B, Poussa T, Kvarnstrom J, Gronlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ 320: 827–832, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med 333: 550–554, 1995. [DOI] [PubMed] [Google Scholar]
- 70.Tang T, Reed MJ. Exercise adds to metformin and acarbose efficacy in db/db mice. Metabolism 50: 1049–1053, 2001. [DOI] [PubMed] [Google Scholar]
- 71.Tankersley CG, Kleeberger SR. Ozone-induced inflammation and altered ventilation in genetically susceptible mice: a comparison of acute and subacute exposures. Toxicol Lett 72: 279–289, 1994. [DOI] [PubMed] [Google Scholar]
- 72.Tankersley CG, Kleeberger SR. Ozone-induced inflammation and altered ventilation in genetically susceptible mice: a comparison of acute and subacute exposures. Toxicol Lett 72: 279–289, 1994. [DOI] [PubMed] [Google Scholar]
- 73.Tirosh A, Mandel D, Mimouni FB, Zimlichman E, Shochat T, Kochba I. Autoimmune diseases in asthma. Ann Intern Med 144: 877–883, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Tzeng ST, Hsu SG, Fu LS, Chi CS. Prevalence of atopy in children with type 1 diabetes mellitus in central Taiwan. J Microbiol Immunol Infect 40: 74–78, 2007. [PubMed] [Google Scholar]
- 75.Weister MJ, Williams TB, King E, Menache MG, Muller FJ. Ozone uptake in awake Sprague-Dawley rats. Toxicol Appl Pharmacol 89: 429–437, 1987. [DOI] [PubMed] [Google Scholar]
- 76.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]